Abstract
Carotenoids are important natural pigments and have medical and health functions for humans. Carotenoid cleavage dioxygenase 4 (CCD4) and ethylene responsive factor (ERF) participate in carotenoid metabolism, but their roles in Lycium have not been discovered. Here, we annotated LbCCDs from the Lycium reference genome and found that LbCCD4.1 expression was significantly correlated with the carotenoid metabolites during Lycium five fruit developmental stages. Over-expression of LbCCD4.1 in NQ’s leaves resulted in a series of significantly lower contents of carotenoid metabolites, including β-carotene and β-cryptoxanthin. Moreover, LbERF5.1, a transcription factor belonging to the ERF family that was located in the nucleus, was isolated. Significant reductions in the carotenoids, especially lutein, violaxanthin and their derivatives, were observed in over-expressing ERF5.1 transgenic NQ’s leaves. Over-expression or virus-induced gene silencing of LbERF5.1 in NQ’s leaves induced a consistent up- or down-expression, respectively, of LbCCD4.1. Furthermore, yeast one-hybrid and dual-luciferase reporter assays showed that ERF5.1 interacted with the promoter of CCD4.1 to increase its expression, and LbERF5.1 could bind to any one of the three predicted binding sites in the promoter of LbCCD4.1. A transcriptome analysis of LbERF5.1 and LbCCD4.1 over-expressed lines showed similar global transcript expression, and geranylgeranyl diphosphate synthase, phytoene synthase, lycopene δ-cyclase cytochrome, cytochrome P450-type monooxygenase 97A, cytochrome P450-type monooxygenase 97C, and zeaxanthin epoxidase in the carotenoid biosynthesis pathway were differentially expressed. In summary, we uncovered a novel molecular mechanism of carotenoid accumulation that involved an interaction between ERF5.1 and CCD4.1, which may be used to enhance carotenoid in Lycium.
Introduction
Wolfberry (Lycium barbarum Linn.) is a traditional Chinese herbal medicine having a bright red color and soft pulp fruit, which contains rich bioactive ingredients, including carotenoids [1]. Specifically, goji (the dried Lycium fruit) contains monohydroxylutein and dihydroxylutein, α-carotene, β-carotene, β-cryptoxanthin, and zeaxanthin; and the carotenoid fatty acid esters are mainly zeaxanthin dipalmitate, zeaxanthin monopalmitate, and β-cryptoxanthin palmitate, as well as other metabolites [2–5]. During the fruit development and ripening of Lycium, carotenoids gradually accumulate, beginning with the discoloration stage, and the content can reach up to 400 μg/g (fresh fruit), indicating that the fruit is a potentially important source of carotenoids [6]. The carotenoid content in fruit is diverse among different accessions [7] and the metabolite diversity can be driven by environmental factors [8]. In addition, the leaf of wolfberry is also an organ for storing carotenoid [9, 10].
Carotenoids are important natural pigments widely distributed in plants, algae, fungi and a few animals. They are the source of gorgeous colors, like yellow, orange and red, in plants, fruits and flowers [11]. To date, over 750 natural carotenoids have been discovered [12]. In the food industry, carotene can be used as an additive for food coloring and nutritional fortification. In the cosmetics industry, carotene is mainly added to lipstick and rouge. In the pharmaceutical industry, carotene is used owing to its physiological functions of stimulating immunity and preventing metastasis and cardiovascular diseases [13–15]. It can also be used to treat diseases caused by vitamin A deficiency. In addition, carotenoids have important functions in plants, like responding to environmental stimuli and enhancing salt and drought-stress tolerance levels by boosting oxidative resistance, as seen in Arabidopsis and tobacco [16, 17].
Over the past decade, significant progress has been made in carotenoid biosynthesis pathway in plants [18, 19], including several enzyme catalysis steps. However, the carotenoid degradation pathway is more complicated than the biosynthesis pathway [20]. In plants, the enzymes involved in carotenoid degradation are termed carotenoid cleavage dioxygenases (CCDs). At present, 13 CCD family members, including six CCD subfamilies (CCD1, −2, −4, −7, −8, and −10) and seven subfamilies of NCED (NCED1–6 and NCED9), have been found in plants [21, 22]. Many CCD enzymes can cleave the conjugated C-C double bonds in carotenoids to produce different apocarotenoids [21]. CCD1 and CCD2 are responsible for carotenoid degradation and the depletion of the carotenoid pools in saffron and spring crocuses [23, 24]. In addition to CCD1 and CCD2, CCD4 is the most reported subfamily involved in carotenoid degradation. In the herb chrysanthemum, CmCCD4a contributes to carotenoid degradation, resulting in a white color [25]. In peach fruit, the sequence and expression associations between PpCCD4 and flesh color, carotenoid metabolites phenotype have been observed, indicating PpCCD4’s function in flesh color formation [26, 27]. The genome-wide identification of CCDs in honeysuckle reveals that LjCCD4 and LjCCD1b are highly expressed in petals. Expressed LjCCD4 and LjCCD1b proteins can convert β-carotene, lutein and 10′-apo-β-carotene into colorless and volatile substances, resulting in color changes [28]. In carrot, DcCCD4 affects the accumulation of carotenoids through the cleavage of α-carotene and β-carotene in carrot taproots [29]. Genome-wide association studies (GWAS) reveal that ZEP and CCD4 are responsible for seed carotenoid degradation, and ZEP is an upstream contributor to carotenoid degradation in Arabidopsis seeds [30, 31]. Using map-based cloning, GmCCD4 was isolated and functionally characterized. It can degrade carotenoid into β-ionone, and it is a negative regulator of carotenoid accumulation [32]. Recently, a study in gardenia reveals that over-expression of GjCCD4a can significantly reduce the content of colored carotene and xanthophylls [33].
The mechanisms by which transcription factors (TFs) regulate the carotenoid biosynthesis structural genes have also been illustrated over the last several years. GLK1 and GLK2, GARP subfamily MYB TFs, can alter the numbers and activity levels of plastids to positively regulate the biosynthesis of various carotenoids, such as octahydro-lycopene, lutein, and lycopene [34]. Conversely, MYB TFs can reduce carotenoid accumulations in papaya pulp [35] and kiwifruit [36–38]. MADS is a common TF that affects the carotenoid synthesis pathway. MADS-RIN [39], SlMBP15 [40], SlCMB1 [41], CsMADS5 [42], CsMADS6 [43], and CsMADS3 [44] are positive regulators, whereas SlMBP8 [45] and SlFYFL [46] are negative regulators. Among NAC TFs, SlNAC1 and SlNAC9 negatively regulate carotenoid synthesis [47, 48], whereas SlNAC4 positively regulates carotenoid synthesis [49]. In papaya, CpNAC1 and CpNAC2 may act as positive regulators of carotenoid biosynthesis, possibly through the transcriptional activation of carotenoid biosynthesis-related genes [50, 51]. In sweet potato, IbNAC29 was very recently found to be a positive regulator of carotenoid accumulation [52]. Another important TF, ERF associated with ethylene, also plays roles in the carotenoid biosynthesis pathway. In tomato, SlAP2a [53] and SlERF6 [54] weaken carotenoid biosynthesis by regulating the ethylene synthesis pathways during fruit ripening. MdAP2–34 promotes carotenoid accumulation in MdAP2–34-OVX transgenic apple calli and fruit by participating in the carotenoid biosynthesis pathway, and it regulates phytoene and β-carotene, but not lutein, accumulations. MdPSY2–1 is a major gene in the carotenoid biosynthesis pathway in apple fruit, and it is directly bound and transcriptionally activated by MdAP2–34 in apple calli, resulting in increased phytoene and total carotenoid contents [55]. Zhu et al. [56] demonstrated that carotenoid accumulation was enhanced by increasing the expression of LCYb2 via ERF TFs, and CsERF061 directly binds to the promoter of LCYb2 and activates its expression in citrus and tomato. Recently, Wang et al. [57] reported that CitERF23 showed significant positively correlation with CCD4, indicating that ERF family played a role in regulating carotenoid metabolism in pummelo. MaERF124 acts as a transcriptional repressor and negatively modulates carotenoid accumulation during banana’s fruit ripening [58].
Ethylene has been reported to be involved in the regulation of carotenoid accumulation [56, 58, 59], and the corresponding usually activated ERF TF or TF complex regulates carotenoid production in plants. We have reported that CCD4.1 might have a potential relationship with ERF5 [60], but whether ERFs and CCD4.1 are involved in regulating carotenoid accumulation remains unclear in wolfberry. In the present study, we isolated LbCCD4.1 through CCD family expression during fruit development and correlations with carotenoids, followed by function characterization using a transient over-expression assay. This paper verified that LbERF5.1 can bound to the promoter of LbCCD4.1, enhancing its expression, which might accelerate carotenoid degradation. These results provided new insights into the regulation of carotenoid accumulation in wolfberry’s fruit.
Results
LbCCD family identification and expression profile characterization
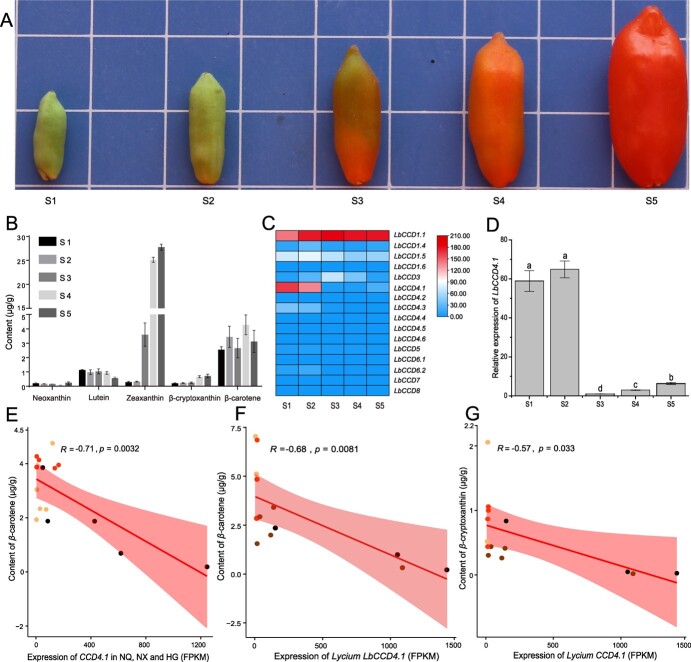
The fruit color of ‘Ningqi No.1’ (NQ) gradually changes from green to yellow-green and then to red during the ripening process (Fig. 1A). Fruit samples at five key developmental stages (from S1 to S5) were used for the quantitative analysis of five carotene metabolites. The zeaxanthin kept a relatively low content during stage S1 to S2 but was the highest at stage S5, whereas the neoxanthin remained at a low content during the whole developmental period. β-cryptoxanthin gradually accumulated to a stabilized level from the S1 to S5; and β-carotene gradually accumulated through the S4 stage and then decreased during the S5 stage, and lutein kept a relatively stable content from S1 to S4 but a significant decrease at S5, indicating that β-carotene and lutein might be degraded to a certain extent during late development (Fig. 1B; Table S1, see online supplementary material). On the basis of the Lycium genome sequence, 18 L. barbarum carotenoid cleavage dioxygenase (LbCCD) genes were identified. Among the LbCCD family genes, LbCCD4.6 was the longest at 31358 bp and LbCCD4.4 was the shortest sequence at 818 bp. LbCCD family protein sequences ranged from 171 to 644 aa, and the molecular weights were between 18742.55 and 72607.61 Da (Table S2, see online supplementary material). A phylogenetic analysis (Fig. S1, see online supplementary material) showed that these genes could be divided into three groups: Class I included LbCCD7 and LbCCD8, which were mainly involved in regulating the growth and development of lateral branches; Class II included LbCCD1 and LbCCD4, which were mainly involved in the formation of flavor and volatiles; and Class III included LbCCD3, LbCCD5, and LbCCD6, which were mainly involved in the biosynthesis of abscisic acid (ABA) [61, 62]. In addition, the LbCCD family genes were highly similar to those in Arabidopsis, tobacco, and tomato, indicating that LbCCDs were conserved during species differentiation (Fig. S1, see online supplementary material). Using transcriptome data from the fruits of NQ at stage S1, S2, S3, S4, and S5, only two of the 18 genes were not detected (Fig. 1C). The expression of LbCCD4.1 was higher in the S1 and S2 stages, decreased rapidly after the S3 stage, and then increased slightly in the S5 stage (Fig. 1D). A further correlation analysis using the expression of CCD4.1 and the β-carotene content in the fruits of NQ, NX, and HG from stage S1 to S5 revealed that CCD4.1 expression was extremely negatively correlated with the β-carotene level (R = −0.71, P = 0.0032) (Fig. 1E). Finally, we used a total of 14 Lycium accessions (fruits at stage S5) to explore the correlation between CCD4.1 and carotenoid content. Similarly, extremely negative correlations were observed between Lycium CCD4.1 expression and both β-carotene and β-cryptoxanthin levels (Fig. 1F and G). These results indicated that LbCCD4.1 might be an important gene involved in carotenoid metabolism.
Figure 1.
LbCCD4.1 was associated with the carotenoid content. A Appearance of Lycium barbarum’s fruit at five key developmental stages (S1–S5). B Dynamics of carotenoid metabolites in NQ fruit during five key developmental stages, and the histograms of every metabolite from left to right indicated S1, S2, S3, S4, and S5, respectively. C Heatmap of quantitative expression dynamics of LbCCD family genes in NQ fruit at the five key developmental stages based on transcriptome sequencing. D Relative expression of LbCCD4.1 in NQ fruit during the five key developmental stages. E Correlations between the β-carotene content and CCD4.1 expression among NQ (red dot), NX (yellow dot), and HG (black dot) fruits during stage S1 to S5. F and G Correlations between the β-cryptoxanthin content, β-carotene content, and CCD4.1 expression among 14 Lycium accessions. The 14 Lycium included four NQ (red dot), two NX (yellow dot), three HG (black dot), and five other types (brown dot). The expression and metabolite content measurements included three biological replicates, and values are presented as averages ± standard deviations. Multiple comparisons were performed using one-way ANOVA and Tukey’s multiple range test (P < 0.05).
Functional characterization of LbCCD4.1
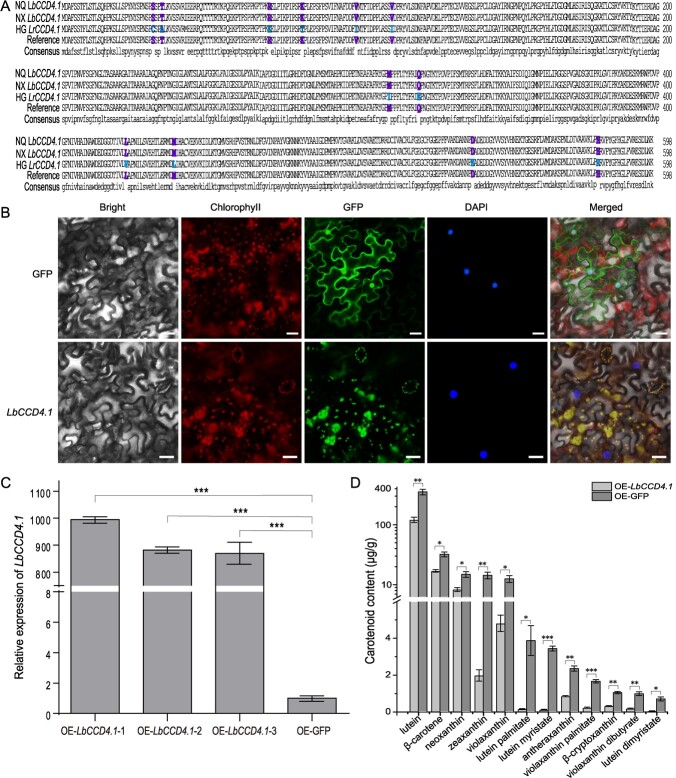
We cloned the coding sequences (CDSs) of CCD4.1 in NQ, NX, and HG, of which the colors of full ripe fruit were red, orange-yellow, and black, respectively (Fig. 1A; Fig. S2, see online supplementary material). The ORF of the CCD4.1 gene was 1800 bp, encoding 599 aa, in each of the three accessions. A multiple sequence alignment showed that the CDSs of LbCCD4.1 in NQ and NX were completely consistent, but that of the LrCCD4.1 in HG had 31 SNP variants, resulting in sequence differences of 12 aa (Fig. 2A; Fig. S3, see online supplementary material), which suggested that CCD4.1 was conserved in the same species, but had differentiated among species. We constructed a GFP fusion marker expression vector containing LbCCD4.1 (Fig. S4, see online supplementary material), and a transient expression analysis was performed to detect the subcellular localization of LbCCD4.1 in tobacco leaves. LbCCD4.1 was localized on the chloroplast (Fig. 2B). Furthermore, an LbCCD4.1 transient over-expression (OE) vector (Fig. S5, see online supplementary material) was constructed and transformed into NQ’s leaves. The expression of OE-LbCCD4.1 plants was significantly increased (Fig. 2C). Carotenes and xanthophylls, such as β-carotene and β-cryptoxanthin, respectively, in the OE-LbCCD4.1 plants were significant or extremely significant down-regulated (Fig. 2D). Thus, LbCCD4.1 negatively mediated the accumulation of carotenoid metabolites.
Figure 2.
Sequence analysis and functional verification of LbCCD4.1. A Multiple sequence alignment of the CCD4.1 genes in NQ, NX and HG. B Subcellular localization of LbCCD4.1 in tobacco leaves. Scale bars = 50 μm. C The qRT-PCR detection of LbCCD4.1 expression in the leaves of NQ OE-LbCCD4.1 plants using GFP as the control. D Detection of carotenoid metabolites in the leaves of NQ LbCCD4.1 over-expression plants. Three biological replicates were used, and values are presented as averages ± standard deviations. Student’s t-test was used to determine significance: *P < 0.05; **P < 0.01; ***P < 0.001.
Identification and genetic variations of LbERF5.1
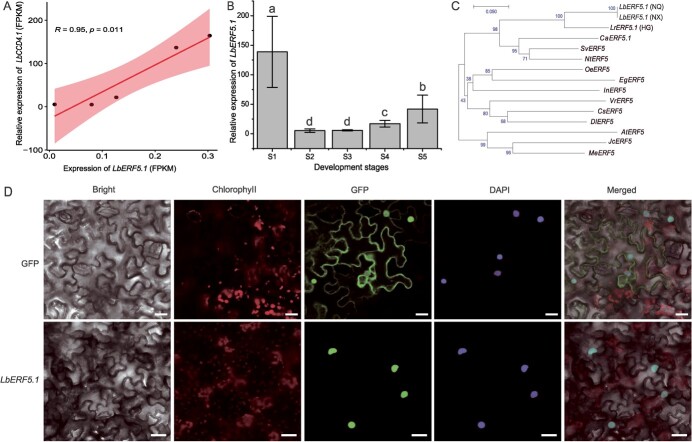
The promoter of LbCCD4.1 contains three predicted binding sites (Fig. S6, see online supplementary material), which may be the TF recognition sites of ethylene responsive factor (ERF). We identified the ERF TF expressed in the fruit of NQ at S5 stage, LbERF5.1, and a screen determined that its expression was significantly positively correlated with LbCCD4.1 expression (R = 0.95, P = 0.011) (Fig. 3A). The LbERF5.1 expression was higher at early stages of fruit development, then decreased rapidly, and finally increased slowly at later stages. The expression pattern was consistent with that of LbCCD4.1 (Fig. 3B). The ORF of LbERF5.1 in NQ was 735 bp, encoding 244 aa, including 35 basic aa (Asp+Glu) and 36 acidic aa (Arg + Lys). In addition, we cloned the LrERF5.1 in HG, and the length was 873 bp, encoding 290 aa, including 41 basic aa (Asp+Glu) and 39 acidic aa (Arg + Lys). An evolutionary tree analysis (Fig. 3C) of 14 diverse species revealed that the ERF5s of Solanaceae species clustered into a single group. Three Lycium ERF5.1 grouped into one branch, with CaERF5 being the closest to LbERF5.1. Furthermore, we constructed a GFP fusion vector (Fig. S7, see online supplementary material) and transiently over-expressed it in tobacco leaves. The GFP and DAPI nuclear staining signals were coincident, indicating that LbERF5.1 was localized in the nucleus (Fig. 3D).
Figure 3.
Identification of LbERF5.1. A Correlation between LbCCD4.1 and LbERF5.1 expression levels in NQ fruit at five key developmental stages. B Expression profile of LbERF5.1 in NQ fruit at five key developmental stages. Multiple comparative analyses were performed using one-way ANOVA and Tukey’s multiple range test (P < 0.05). C Evolutionary tree analysis of ERF5.1 in 14 diverse species. The evolutionary tree was constructed using the Neighbor Joining method of MEGA7 with bootstrap = 1000. D Subcellular localization of LbERF5.1 in tobacco leaves. Scale bars = 50 μm.
Interaction between ERF5.1 and CCD4.1
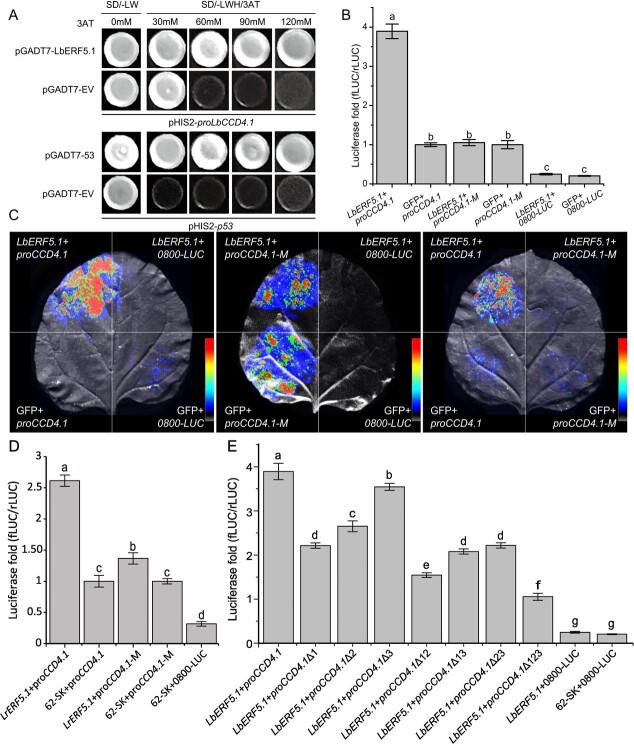
The yeast one-hybrid (Y1H) experiment showed that all Y187 strains co-transfected with pGADT7 and pHIS2 plasmids produced clones on the double amino acid-deficient medium. Yeast co-transformed with plasmids containing LbERF5.1 and LbCCD4.1 promoters grew normally in SD/-His/−Leu/−Trp plates with a high concentration of 3-amino-1,2,4-triazole, whereas no clones grew from the negative control group, which indicated that LbERF5.1 bound to the promoter sequence of LbCCD4.1 in vivo (Fig. 4A). To further explore the specific transcriptional regulation of LbERF5.1 on LbCCD4.1, we recombined LbERF5.1 into the pGreenII-62-SK vector and the LbCCD4.1 promoter into the dual-luciferase vector pGreenII0800-LUC. Three binding site-deleted mutations in LbCCD4.1’s promoter formed proCCD4.1-M, which served as a control (Fig. S8, see online supplementary material). When the system did not contain LbCCD4.1’s promoter, there was almost no expression of the Luc reporter gene; however, when the system contained LbCCD4.1’s normal promoter, the Luc reporter gene was expressed (Fig. 4B and C), indicating that the LbCCD4.1 promoter sequence had activity. The promoter activities of mutant proCCD4.1-M and wild-type proLbCCD4.1 were not obviously different, indicating that mutating the promoter did not change its activity. In addition, when LbERF5.1 was over-expressed in the system, LbERF5.1 recognized the promoter and the downstream reporter gene Luc was expressed. However, when proLbCCD4.1-M was in the system, LbERF5.1 did not promote the expression of the downstream reporter gene Luc, indicating that the deletion of proLbCCD4.1 promoter site resulted in LbERF5.1’s inability to bind, which led to a lack of downstream reporter gene Luc expression, indicating that LbERF5.1 could bind to the promoter of LbCCD4.1 and activate its expression (Fig. 4B and C). To test the bind activity of LrCCD4.1 and LrERF5.1 (Lycium ruthenicum), we conducted the dual-luciferase assay using the same system in HG. Similarly, LrERF5.1 could bind to the promoter of LrCCD4.1 (Fig. 4D). To further investigate whether the transcription factor LbERF5.1 could specifically bind to the three binding sites in the LbCCD4.1’s promoter region, we constructed single, double, and triple mutation vectors for dual-luciferase experiments (Fig. S9, see online supplementary material). The results showed that the luciferase fold (fLUC/rLUC) of wild-type had the highest detection value, while single mutations △1, △2, and △3 performed significantly reduced activity compared with the wild-type but were still significantly higher than that in the control, indicating that all the three sites had binding activity with LbERF5.1 but with different promoter activity abilities (△1 > △2 > △3). This was consistent with the results of double mutations, where △12 had significantly lower promoter activity compared with △23 and △13. The binding activity of the triple mutation △123 was significantly lower than that in the wild-type and either single or double mutations (Fig. 4E).
Figure 4.
Physical interaction between CCD4.1 and ERF5.1. A A yeast one-hybrid experiment showed that LbERF5.1 bound to the promoter region of LbCCD4.1. The pGADT7-p53 and pHIS2-p53 were used as positive quality controls, and pGADT7 and pHIS2-p53 were used as negative quality controls. The pGADT7 and pHIS2-proLbCCD4.1 served as negative controls. B and C A dual-luciferase assay showed that LbERF5.1 enhanced the promoter activity of LbCCD4.1. D A dual-luciferase assay showed that LrERF5.1 enhanced the promoter activity of LrCCD4.1. E A dual-luciferase assay showed LbERF5.1’s binding activity with the three predicted binding sites of LbCCD4.1’s promoter. Luciferase fold-changes in tobacco under different effector and reporter gene combinations were calculated using the ratio of firefly luciferase to renal luciferase (fLUC/rLUC). All the data were calculated as the mean values of three replicates. Error bars represent standard deviations. The data were analysed using one-way ANOVA and Tukey’s multiple range test (P < 0.01).
Functional verification of ERF5.1 in carotenoid accumulation
To verify the function of LbERF5.1 in the regulation of carotenoid biosynthesis, we firstly tested the expression patterns in the fruits of NQ, NX, and HG at S5 stage. The results showed that the ERF5.1 expression in NQ was the lowest, followed by in NX, and the highest was in HG with extremely significant differences (Fig. S10, see online supplementary material), the trend of which was opposite to the carotenoids content of a report [63], indicating its negative regulation role. We further constructed OE (Fig. S11, see online supplementary material) and virus-induced gene silencing (VIGS) vectors, respectively, (Fig. S12, see online supplementary material) and carried out the transient transformation of NQ’s leaves. The expression of LbERF5.1 in VIGS plants decreased significantly (Fig. 5A), as did the expression of LbCCD4.1 (Fig. 5B). In OE plants, the expression of LbERF5.1 was significantly higher than that of GFP (P < 0.001) (Fig. 5C). In addition, the expression of LbCCD4.1 was also significantly increased (Fig. 5D). The carotenoid contents, especially lutein, violaxanthin and their derivatives, in OE plants were significantly or extremely significantly down-regulated (Fig. 5E). Moreover, the β-carotene content decreased significantly in OE plants. Thus, LbERF5.1 appeared to positively regulate LbCCD4.1 expression and then negatively regulate carotenoid metabolite accumulation. As the carotenoid content was low and the expression of ERF5.1 was high in HG, we decided to transfer LrERF5.1 to NQ’s leaves because of the small leaves hampered transient transformation assay in HG (Fig. 5F). Analogously, some carotenoid metabolites (violaxanthin, zeaxanthin, α-cryptoxanthin, and antheraxanthin) were down-regulated in the OE-LrERF5.1 plants (Fig. 5G).
Figure 5.
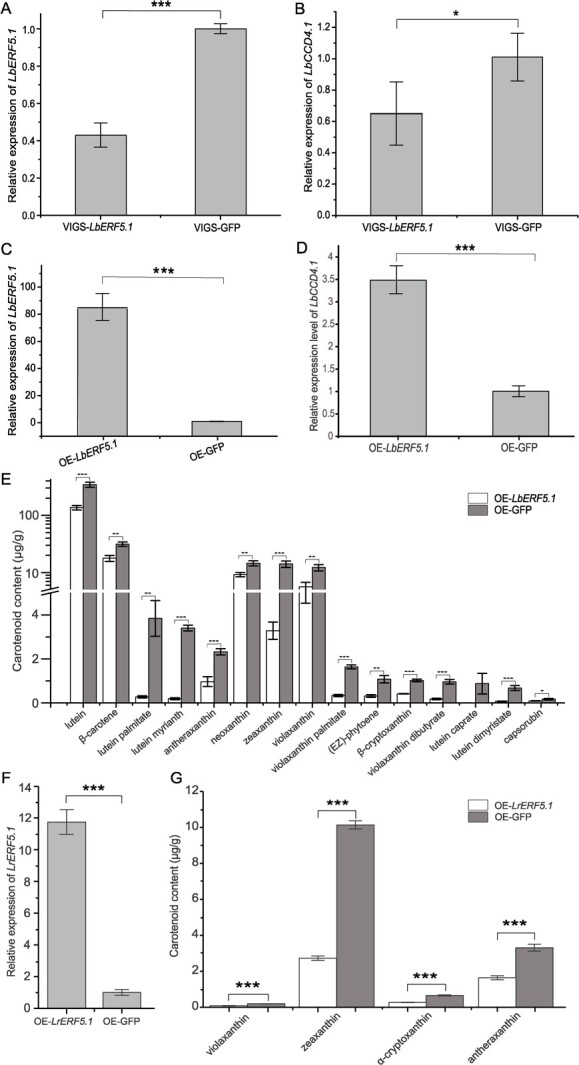
ERF5.1 promoted the expression of CCD5.1 and participated in the regulation of carotenoid accumulation. A Expression of LbERF5.1 and GFP under VIGS of LbERF5.1 conditions as assessed by qRT-PCR. B Expression of LbCCD4.1 and GFP under VIGS of LbERF5.1 conditions as assessed by qRT-PCR. C The levels of LbERF5.1 in LbERF5.1 over-expression plants as assessed by qRT-PCR. D The levels of LbCCD4.1 in LbERF5.1 over-expression plants as assessed by qRT-PCR. E Detection of carotenoid metabolites in the leaves of NQ LbERF5.1 over-expression plants. F The expression levels of LrERF5.1 in LrERF5.1 over-expression plants (NQ’s leaves) as assessed by qRT-PCR. G Detection of carotenoid metabolites in the leaves of OE-LrERF5.1 over-expression plants (NQ’s leaves). All assays contained three biological replicates. Student’s t-test was used for significance comparisons: *P < 0.05; **P < 0.01; ***P < 0.001.
Over-expressing LbERF5.1 and LbCCD4.1 affects the global transcriptome and contributes to the carotenoid biosynthetic pathway in transgenic Lycium.
We performed an RNA-seq analysis of over-expressing LbCCD4.1 and LbERF5.1 leaves in NQ. Compared with the control, there were 9751 differentially expressed genes (DEGs) in OE-LbCCD4.1 plants, of which 5273 were up-regulated and 4478 were down-regulated. In OE-LbERF5.1 plants, there were 7651 DEGs, of which 4417 were up-regulated and 3234 were down-regulated. A total of 5967 DEGs were shared between LbCCD4.1 and LbERF5.1 transformed plants (Tables S3and S4; Fig. S13, see online supplementary material). Among the DEGs in LbCCD4.1 and LbERF5.1, 511 and 409 annotated genes were TFs, respectively. The most differently expressed regulatory genes were in the AP2/ERF, WRKY, MYB, NAC, C2H2 and bHLH families (Tables S3and S4). A GO analysis indicated that the DEGs in the comparisons of LbCCD4.1 vs. control and LbERF5.1 vs. control were similarly enriched in the top three terms, photosynthesis, calmodulin binding, and apoplast (Fig. S14, see online supplementary material). A KEGG analysis indicated that the DEGs in the comparisons LbCCD4.1 vs. control and LbERF5.1 vs. control were all principally associated with the metabolic pathway and biosynthensis of secondary metabolites (Fig. S14, see online supplementary material).
In OE-LbCCD4.1 and OE-LbERF5.1 plants, the upstream genes of the carotene biosynthesis process were differentially expressed to different degrees. Among the GGPS genes, evm.TU.chr02.3627 and evm.TU.chr11.272 were down-regulated by approximately 5.0 times in OE-LbCCD4.1 plants, and by approximately 2.5 times in OE-LbERF5.1 plants. Notably, the expression of evm.TU.chr10.291 increased sharply by 42 times in OE-LbCCD4.1 plants and 72.9 times in OE-LbERF5.1 plants. PSY genes (evm.TU.chr01.2052, evm.TU.chr11.3124, and evm.TU.chr12.1296) were significantly down-regulated in OE-LbCCD4.1 plants, whereas one PSY gene, evm.TU.chr11.3124, was up-regulated in OE-LbERF5.1, which indicated that the over-expression of LbCCD4.1 and LbERF5.1 could change the expression of rate-limiting enzymes, thereby affecting the accumulation of upstream substances in carotenoid synthesis. In the downstream pathway of carotenoid biosynthesis, LCYE (evm.TU.chr04.3738), CYP97A (evm.TU.chr10.1526), CYP97C (evm.TU.chr08.2566), and ZEP (evm.TU.chr03.2986) were significantly down-regulated (2–3 times) in OE-LbCCD4.1 and OE-LbERF5.1 plants, whereas ZEP (evm.TU.chr12.3134) was only down-regulated 2.5 times in OE-LbERF5.1 plants. The expression of ZEP (evm.TU.chr07.2471) in OE-LbCCD4.1 and OE-LbERF5.1 plants was significantly enhanced relative to the wild type, by up to 44.1 times and 29.4 times, respectively. However, the expression of PDS, ZISO, ZDS, CriISO, LCYB, and BCH did not change significantly. Thus, LbCCD4.1 and LbERF5.1 appeared to have similar effects on the overall expression level and on the carotene gene-specific expression in transgenic Lycium leaves.
Discussion
Carotenoids are a series of important secondary metabolites that function mainly in the growth and development fruit of plants. CCDs are structural genes required for carotenoid degradation [23, 24] that may be involved in regulating carotenoid accumulation in Lycium. In the present study, we identified the LbCCD4.1 gene in L. barbarum, and we also identified LbERF5.1, an ERF type TF that interacts with the promoter region of LbCCD4.1. We functionally characterized LbCCD4.1 and LbERF5.1 as being involved in significantly regulating the accumulation of different carotenoid metabolites in Lycium.
LbCCD4.1 decreased carotenoid metabolite accumulation in Lycium.
An analysis of CCD4.1 sequences from HG (Lycium ruthenicum Murr.), NQ (L. barbarum Linn.), and NX (L. barbarum Linn.) wolfberry showed that the sequences of the latter two were the same, whereas that of the former was slightly different. The gene expression patterns of CCD4.1 in red fruit and black fruit wolfberry differed, with the expression in the latter being higher than in the former [6], and the latter also had a low carotenoid content [5], suggesting that CCD4.1 was conserved in the same species of Lycium but had undergone potential functional differentiation among species. Furthermore, we identified 11 SNPs in the promoter region (~2 kb upstream) of CCD4.1 in NQ, NX, and HG (Fig. S15, see online supplementary material). However, no SNPs were located in the three predicted binding sites of ERF5.1, indicating that these SNP may not affect the binding ability. In Lycium chinense, the amino acid sequence of LcCCD4 shares homology with that of CCD4 proteins from other Solanaceae plants [64], further confirming its conservation. The LbCCD4.1 expression was associated with wolfberry fruit ripening and was significantly negatively correlated with the carotenoid metabolite accumulations in fruit (Fig. 1). What should be noted was that although the expression level of LbCCD4.1 was the highest in the stages of S1 and S2 (Fig. 1D) and the carotenoid content was low in these two stages, a lower content of carotenoids might be caused by lower biosynthesis but not by higher degradation by higher expression of CCD4.1 during the early development stages of goji fruit. Furthermore, over-expressing LbCCD4.1 in NQ leaves resulted in a reduction in carotenoid profiles (Fig. 2) and the corresponding expression of geranylgeranyl diphosphate synthase, PSY, LCYE, CYP97A, and CYP97C (Fig. 6), indicating that LbCCD4.1 affected carotenoid accumulation through changing global carotenoid biosynthesis. DcCCD4 affected the accumulation of carotenoids through clearance of α-carotene and β-carotene in carrot taproot [29]. Here, the over-expressing LbCCD4.1 did not demonstrate a change in the α-carotene content, whereas the β-carotene content decreased, suggesting that LbCCD4.1 only acted on the latter. Nevertheless, further direct evidence is needed to determine which LbCCD4.1 enzyme can cleave β-carotene.
Figure 6.
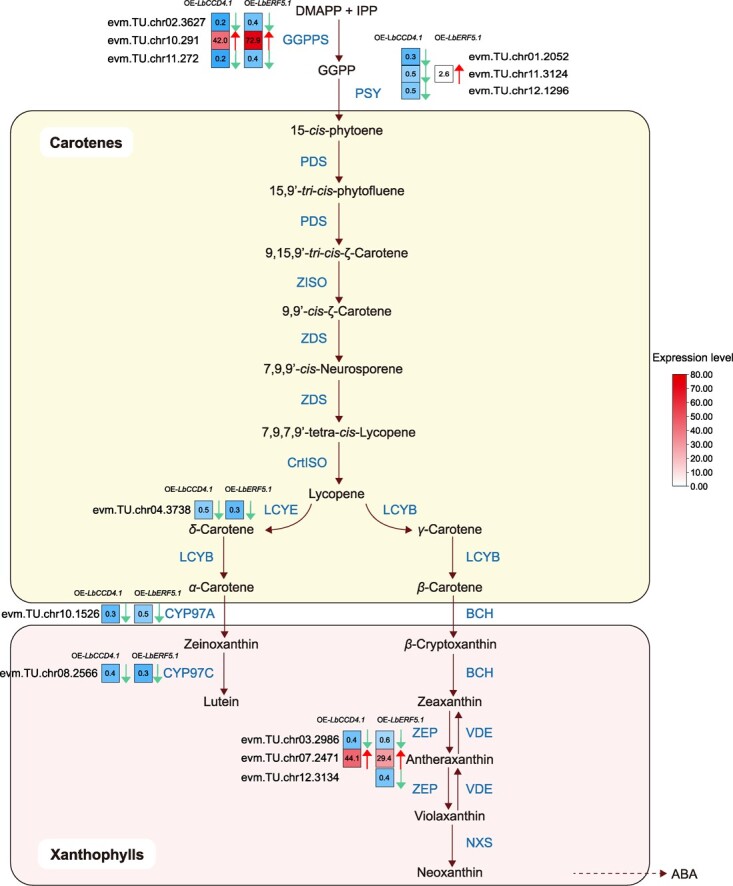
Differential expression of carotenoid pathway-involved genes induced by over-expressing LbCCD5.1 and LbERF5.1. Solid and dashed arrows indicate direct and indirect reaction flows in the pathway, respectively. The enzymes encoded by the related DEGs in the carotenoid pathway are located next to the arrows. The left and right adjacent square heat maps represent the corresponding DEGs in OE-LbCCD4.1 and OE-LbERF5.1 plants, respectively, with fold-change (OE-plants/CK) values. The green-down and red-up arrows indicate significant decreases and increases, respectively. ABA, abscisic acid.
LbERF5.1 is a positive regulator of LbCCD4.1 involved in carotenoid metabolism.
We determined that LbERF5.1 binds to the promoter of LbCCD4.1 (Fig. 4) using Y1H and dual-luciferase assays, and a typical motif [65, 66] promoted the expression of LbCCD4.1 in OE-LbERF5.1 transgenic lines (Fig. 5D). In the OE-LbERF5.1 lines, LbCCD4.1 was up-regulated, in accordance with its negative role in carotenoid, especially lutein and violaxanthin accumulation (Fig. 5E). LbCCD4.1 expression was expectantly inhibited in the LbERF5.1-silenced lines, which was in line with the expression pattern of an AP2/ERF TF, MdAP2–34, in relation to MdCCD4 in the fruit skin of apples [55]. However, MdAP2–34 promotes phytoene and β-carotene, but not lutein, accumulations [55], suggesting that LbERF5.1 and MdAP2–34 have different roles in the carotenoid biosynthesis pathway. Moreover, the binding activity between CCD4.1 and ERF5.1 in HG could also be detected (Fig. 4D), indicating that similar regulatory mechanism in carotenoid accumulation existed between L. barbarum and L. ruthenicum. In addition, the reduced expression of ERFs can regulate carotenoid biosynthesis by enhancing both carotenoid and ethylene levels during fruit ripening [54, 67] and by directly binding to the structural genes required for carotenoid biosynthesis, such as PSY [55] and LCYb2 [56] to regulate carotenoid accumulation. Therefore, to further understand the mechanisms of carotenogenesis in wolfberry, we will identify the direct targets of LbERF5.1 using other assays, such as chromatin immunoprecipitation sequencing analysis in the future.
LbCCD4.1 and LbERF5.1 can affect multiple carotenoid synthesis pathway genes.
Silencing carotenoid biosynthesis structural genes, like PDS, ZDS, βOH, ZEP, and PSY, causes a decrease in the total carotenoid contents [68–70]. In the OE-LbCCD4.1 and OE-LbERF5.1 plants, we also detected that some carotenoid biosynthesis structural genes were down-regulated (Fig. 6), which may partially explain the decline in carotenoid accumulation. However, the expression levels of two genes in OE lines, evm.TU.chr10.291 and evm.TU.chr07.2471, encoding geranylgeranyl diphosphate synthase and ZEP, respectively, were dramatically up-regulated by LbCCD4.1 and LbERF5.1. Thus, a complex inducement mechanism of carotenoid biosynthesis genes may affect carotenoid accumulation, which was similar to a previous report in sweet orange [43]. Notably, the RNA-seq analysis results suggested the involvement of the AP2/ERF, WRKY, MYB, NAC, C2H2, and bHLH families in the regulation of Lycium carotenoid compound accumulation (Tables S3 andS4, see online supplementary material), indicating possible critical links among different TFs in the regulation of carotenoid biosynthesis, and even fruit ripening, in Lycium, as reported by a previous report on the light-specific regulatory mechanism of carotenoid biosynthesis in rice leaves [71].
Materials and methods
Plant materials and fruit sampling
The experimental accessions were from the National Wolfberry Engineering Research Center of China (38°380′N, 106°9′E; altitude 1100 m). The fruits of a wide genetic range of 14 eight-year old Lycium accessions involving over five species (Table S5, see online supplementary material), including NQ (L. barbarum L.), NX (L. barbarum L.), and HG (L. ruthenicum Murr.) at five key developmental stages, 9–12 (S1), 14–19 (S2), 20–26 (S3), 30–37 (S4), and 38–45 (S5) days post-flowering, were sampled with 20 biological repetitions and quickly frozen in liquid nitrogen. They were then stored at −80°C for transcriptome, metabolome, and quantitative real-time PCR (qRT-PCR) experiments. Hydroponic seedlings of NQ were used for transient over-expression (OE) and VIGS experiments.
Carotenoid contents determination
The carotenoids extractions and content determinations were performed as described previously [72].
CCD family identification and sequence analysis
First, CCD family genes were selected as queries from the Arabidopsis genome sequences in line with the report of Tan et al. [62]. Second, BLASTP was used to identify the best match in the genome of Lycium (NQ) with threshold value E < 1e−10. Thereafter, hmmer [73] was used to search for the RPE65 domain of CCD genes among the hit genes using E < 1e−5 as threshold value. Finally, the above hit genes were mapped in SMART [74], CDD [75], Gene3D [76], and PRINTS [77] databases using InterProScan [78] with default parameters. The candidates from all the four databases were used to generate CCD family gene set in wolfberry. ExPASy [79] and WoLF PSORT (https://wolfpsort.hgc.jp/) were used to predict the physicochemical properties and subcellular localizations of the CCD genes. MEGA7 [80] was used for Neighbor Joining phylogenetic tree construction (bootstrap = 1000).
Identification of carotenoid pathway genes in Lycium
To identify homologous genes of the carotenoid pathway in Lycium, we downloaded the protein sequences of reported carotenoid pathway structural genes and TFs from the NCBI and TAIR [81] databases. These protein sequences were used as query to search against the protein annotations of Lycium, and the putative proteins were obtained by BLASTP searching (E < 1e−10). In addition, the putative proteins were submitted to the Pfam database to identify conserved domains having E = 1.0, and proteins without corresponding conserved domains were excluded from further analysis.
Gene and promoter cloning in Lycium
Using Lycium fruit as test material, total RNA was isolated using a RNA extraction kit from Takara (Takara, Dalian, China). Single-strand cDNA of CCD4.1 and ERF5.1 were prepared using a Reverse Aid First-strand cDNA Synthesis Kit (Thermo Fisher Scientific, Waltham, MA, USA). Using the Lycium genome and transcriptome data, primers (Table S6, see online supplementary material) were designed [82] to amplify the CDSs of CCD4.1 and ERF5.1 with the following system component: 25 μL 2× PCR Buffer, 10 μL dNTP (2 mM), 2 μL upstream primer (10 μM), 2 μL downstream primer (10 μM), 5 μL single-stranded cDNA, 1 μL KOD FX Neo (Toyobo Life Science, Osaka, Japan) and 10 μL Millipore H2O. The PCR reaction procedure was set as: pre-denaturation at 98°C for 3 min, and followed by 30 cycles of denaturation for 10 s, 58°C annealing for 30 s, 68°C extension for 2 min; and final extension at 68°C for 5 min. The amplified products were purified, and independently cloned onto the pMD18-T vector (TaKaRa, Tokyo, Japan) for Sanger sequencing. The 2000-bp upstream sequence of the CCD4.1 was treated as a possible promoter sequence. A cis-element was predicted using PlantCARE [83]. DNA from NQ, NX and HG’s fruits were extracted using the hexadecyl trimethyl ammonium bromide method. The primers (Table S6, see online supplementary material) were designed to amplify the promoter sequence of the CCD4.1 with the same primer3 procedure. The PCR cloning reaction system was basically the same as CCD4.1 and ERF5.1 cloning.
Subcellular localization assay
CCD4.1 and ERF5.1 were cloned into the tobacco OE vector pCambia1300–35 s-GFP independently, and transferred into Agrobacterium GV3101. The infective bacterial solution was obtained using infection buffer (5 g/L D-glucose, 50 mM MES, 2 mM Na3PO4·12 H2O and 0.1 mM acetobutanone) at an OD600 = 1.0 at 20–25°C for 1–2 h. At 72 h after injection into the tobacco leaves with the bacterial solution, fluorescence signals were visualized using laser copolymerization fluorescence microscopy. GFP and DAPI excitation were determined at 488 nm and 405 nm, respectively.
Over-expression and VIGS transformation
To obtain OE lines of Lycium, the full-length cDNAs of LbCCD4.1, LbERF5.1, and LrERF5.1 were amplified. Then, the cDNAs were independently cloned into the pCambia 1300–35 s vector to obtain the p35s: LbCCD4.1, p35s: LbERF5.1 vectors and p35s: LrERF5.1 vectors, respectively. The OE vector was transformed into Agrobacterium GV3101 in infection buffer solution (5 g/L D-glucose, 50 mM MES, 2 mM Na3PO4·12 H2O and 0.1 mM acetobutanone) at an adjusted OD600 = 1.0 at 20–25°C for 1–2 h. The infective bacterial solution was injected into NQ leaves to obtain OE-LbCCD5.1, OE-LbERF5.1, and OE-LrERF5.1 plants. The pCambia1300–35 s-GFP was used as the negative control. Samples were taken at 72 h after injection for qRT-PCR, metabolome, and RNA-seq analyses, with each sample having three biological repetitions.
For VIGS assay, 366 bp of the LbERF5.1 (1–366) region was amplified and cloned into the pTRV2 vector. The primers are shown in Table S6 (see online supplementary material). The vector pTRV2-LbERF5.1 was transformed into Agrobacterium GV3101 as described above. The NQ leaves were transfected by Agrobacterium infiltration. The bacterial solution containing the pTRV2 empty carrier was used as a negative control, and pTRV2-GFP was used as a positive control. Fresh leaves of three lines were collected for qRT-PCR testing after transformation 7–10 days.
Yeast one-hybrid (Y1H) assay
The LbCCD4.1 promoter, containing the GCC-box, was cloned and fused to the HIS3 mini-promoter in the pHIS2 vector to obtain the reporter construct. The full-length LbERF5.1 was fused to the GAL4 activation domain in pGADT7, which was co-transformed with the reporter construct into the yeast strain (Y187) in 600 μL of PEG/LiAc solution. The pGADT7–53 and pHIS2-P53 were co-transformed as positive controls, whereas pGADT7 + pHIS2-P53 and pGADT7 + pHIS2-proLbCCD4.1 were used as negative controls. The yeast cells were transferred into SD/−Leu/−Trp medium for positive clone selection. Then, the DNA-protein interaction was surveyed by the appearance of yeast’s growing status on SD/−Trp/−Leu/-His medium supplemented with 30 mM, 60 mM, 90 mM, and 120 mM concentrations of 3-amino-1,2,4-triazole independently.
Dual-luciferase assay
The possible recognition motifs in the LbCCD4.1 promoter (proCCD4.1) and LbERF5.1 TF were predicted using the Jaspar database (https://jaspar.genereg.net/). A total of three possible binding sites, located at −44, −116, and – 277 bp upstream of the TSS (Fig. S6, see online supplementary material), were identified. A total of three combinations, LbERF5.1 & LbCCD4.1’s promoter, LrERF5.1 & LrCCD4.1’s promoter, LbERF5.1 & three binding site of LbCCD4.1’s promoter, were designed to illustrate their binding activities. For combination LbERF5.1 & LbCCD4.1’s promoter, a mutant promoter of proCCD4.1 (proCCD4.1-M) and LbCCD4.1 were cloned into the dual luciferase vector pGreenII 0800-LUC using homologous recombination methods. The mutant promoter proCCD4.1-M was used as a negative control, and LbERF5.1 was homologously recombined onto the pGreenII-62-SK vector. The similar experimental operation was conducted to combination LrERF5.1 & LrCCD4.1’s promoter. For combination LbERF5.1 & three binding site of LbCCD4.1’s promoter, we constructed a total of eight vectors to verify the binding specificity between LbERF5.1 and the three binding sites of LbCCD4.1’s promoter (Fig. S9, see online supplementary material). All the plasmids were transformed into Pichia Pastoris GS115 and then the infective bacterial solution was injected into tobacco leaves. After cultivating 2 days, the injection site of the leaf tissue was used for protein extraction. The contents of firefly luciferase and renilla luciferase were determined using the Pierce™ Renilla-Firefly Dual Luciferase Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA) and compared with the empty vector pGreenII 0800-LUC. The ratio fLUC/rLUC (firefly luciferase/renal luciferase) was used to measure the relative luciferase activity. The fluorescein was injected into tobacco leaves to determine the intensity of luciferase using a 4800 automatic chemiluminescence image analysis system (Tianneng, Shanghai, China).
RNA-seq and qRT-PCR
The fruits of 14 Lycium accessions and the leaves of OE-LbCCD4.1 and OE-LbERF5.1 were sampled (three replicates) for total RNA isolation using RNAprep Pure Plant Kit (Tiangen Biotech, China). RNA-seq libraries were prepared and 150 bp paired-end sequences were performed in MetWare Co., Ltd (Wuhan, China). The clean data was obtained through the removal of reads that did not meet quality standards [84]. STAR package was used to map the clean reads to the reference genome of Lycium under default settings [85], followed by StringTie’s transcripts assembly and expression quantification using fragments per kilobase of transcript per million mapped reads (FPKM) method [86]. Differentially expressed genes (DEGs) were yielded by the following two criteria: (i): P value <0.01; and (ii): fold change ≥1.5 by applying DESeq [87]. The TFs of all the DEGs were annotated using PlantTFDB 5.0 (http://planttfdb.gao-lab.org/index.php). Finally, GO and KEGG enrichment analyses were performed independently based on DEGs from different group comparisons [88, 89].
The qRT-PCR primers of this study were designed using primer3 [82] (Table S6, see online supplementary material). Using BIO-RAD CFX Connect™ Amplification with LbEf1a as internal reference, the qRT-PCR was performed according to our previous report [90], which used the 2−ΔΔCT method to convert the gene expression level [91].
Acknowledgements
This work was sponsored by the National Natural Science Foundation of China (No. 32060359), the Key Research & Development Program of Ningxia Hui Autonomous Region (No. 2021BEF02002,2022BBF01001), the Innovative Research Group Project of Ningxia Hui Autonomous Region (No. 2021AAC01001) and the Innovation Team for Genetic Improvement of Economic Forests (No. 2022QCXTD04).
Author contributions
J.Z. and W.A. conceived and designed the research. H.L., Y.Y., X. Zhang and X. Zhu. prepared the population material. H.L., L.D., X.L., T.H., and B.Z. performed sampling, sequencing, and transcriptome analyses. J.Z., Z.S., X.Q., and Y.C. contributed to the project discussion. J.Z., Y.X., and H.L. wrote the manuscript. J.Z., Y.X., and Y.C. revised the manuscript. All the authors read and approved the final manuscript.
Data availability statement
Data supporting the findings of this work are available within the paper and the Supplementary Tables and Figures. The transcriptome clean sequencing data have been deposited into the National Center for Biotechnology Information Sequence Read Archive database (PRJNA936937).
Conflict of interests
All the authors declare that there is no conflict of interest.
Supplementary data
Supplementary data is available at Horticulture Research online.
Supplementary Material
Contributor Information
Jianhua Zhao, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Yuhui Xu, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Haoxia Li, Institute of Forestry and Grassland Ecology, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Xinlei Zhu, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Yue Yin, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Xiyan Zhang, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Jun Zhou, College of Biological Science & Engineering, North Minzu University, Yinchuan 750021, China.
Linyuan Duan, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Xiaojie Liang, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Ting Huang, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Bo Zhang, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Ru Wan, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Zhigang Shi, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Youlong Cao, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
Wei An, National Wolfberry Engineering Research Center/Wolfberry Science Research Institute, Ningxia Academy of Agriculture and Forestry Sciences, Yinchuan, 750002, China.
References
- 1. Wang CC, Chang SC, Inbaraj BS. et al. Isolation of carotenoids, flavonoids and polysaccharides from Lycium barbarum L. and evaluation of antioxidant activity. Food Chem. 2010;120:184–92 [Google Scholar]
- 2. Chung IM, Ali M, Kim EH. et al. New tetraterpene glycosides from the fruits of Lycium chinense. J Asian Nat Prod Res. 2013;15:136–44 [DOI] [PubMed] [Google Scholar]
- 3. Chung IM, Ali M, Nagella P. et al. Evaluation of antioxidant activity of new constituents from the fruits of Lycium chinense. Med Chem Res. 2014;23:3852–60 [Google Scholar]
- 4. Inbaraj BS, Lu H, Hung CF. et al. Determination of carotenoids and their esters in fruits of Lycium barbarum Linnaeus by HPLC-DAD-APCI-MS. J Pharm Biomed Anal. 2008;47:812–8 [DOI] [PubMed] [Google Scholar]
- 5. Islam T, Yu X, Badwal TS. et al. Comparative studies on phenolic profiles, antioxidant capacities and carotenoid contents of red goji berry (Lycium barbarum) and black goji berry (Lycium ruthenicum). Chem Cent J. 2017;11:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Liu Y, Zeng S, Sun W. et al. Comparative analysis of carotenoid accumulation in two goji (Lycium barbarum L. and L. ruthenicum Murr.) fruits. BMC Plant Biol. 2014;14:269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Patsilinakos A, Ragno R, Carradori S. et al. Carotenoid content of goji berries: CIELAB, HPLC-DAD analyses and quantitative correlation. Food Chem. 2018;268:49–56 [DOI] [PubMed] [Google Scholar]
- 8. Chen M, Huang W, Yin Z. et al. Environmentally-driven metabolite and lipid variations correspond to altered bioactivities of black wolfberry fruit. Food Chem. 2022;372:131342 [DOI] [PubMed] [Google Scholar]
- 9. Lee HW, Bi X, Henry CJ. Carotenoids, tocopherols and phylloquinone content of 26 green leafy vegetables commonly consumed in Southeast Asia. Food Chem. 2022;385:132729 [DOI] [PubMed] [Google Scholar]
- 10. Rosca I, Glijin A, Chiorchina N. et al. Chlorophyll and carotenoid content in wolfberry (Lycium barbarum L.) leaves. Agricultura. 2022;122:1–13 [Google Scholar]
- 11. Alcaíno J, Baeza M, Cifuentes V. Carotenoid distribution in nature. Carotenoids Nat. 2016;79:3–33 [DOI] [PubMed] [Google Scholar]
- 12. Zhong L, Gustavsson KE, Oredsson S. et al. Determination of free and esterified carotenoid composition in rose hip fruit by HPLC-DAD-APCI+-MS. Food Chem. 2016;210:541–50 [DOI] [PubMed] [Google Scholar]
- 13. Desmarchelier C, Borel P. Overview of carotenoid bioavailability determinants: from dietary factors to host genetic variations. Trends Food Sci Tech. 2017;69:270–80 [Google Scholar]
- 14. Eggersdorfer M, Wyss A. Carotenoids in human nutrition and health. Arch Biochem Biophys. 2018;652:18–26 [DOI] [PubMed] [Google Scholar]
- 15. Young AJ, Lowe GL. Carotenoids-antioxidant properties. Antioxidants. 2018;7:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Fanciullino AL, Bidel LPR, Urban L. Carotenoid responses to environmental stimuli: integrating redox and carbon controls into a fruit model. Plant Cell Environ. 2014;37:273–89 [DOI] [PubMed] [Google Scholar]
- 17. Li T, Liu JX, Deng YJ. et al. Over-expression of a carrot BCH gene, DcBCH1, improves tolerance to drought in Arabidopsis thaliana. BMC Plant Biol. 2021;21:475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Hermanns AS, Zhou X, Xu Q. et al. Carotenoid pigment accumulation in horticultural plants. Hortic Plant J. 2020;6:343–60 [Google Scholar]
- 19. Wang XJ, Luo Q, Li T. et al. Origin, evolution, breeding, and omics of Apiaceae: a family of vegetables and medicinal plants. Hortic Res. 2022;9:uhac076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Nisar N, Li L, Lu S. et al. Carotenoid metabolism in plants. Mol Plant. 2015;8:68–82 [DOI] [PubMed] [Google Scholar]
- 21. Ahrazem O, Rubio-Moraga A, Berman J. et al. The carotenoid cleavage dioxygenase CCD2 catalysing the synthesis of crocetin in spring crocuses and saffron is a plastidial enzyme. New Phytol. 2016;209:650–63 [DOI] [PubMed] [Google Scholar]
- 22. Zhong Y, Pan X, Wang R. et al. ZmCCD10a encodes a distinct type of carotenoid cleavage dioxygenase and enhances plant tolerance to low phosphate. Plant Physiol. 2020;184:374–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ahrazem O, Gómez-Gómez L, Rodrigo MJ. et al. Carotenoid cleavage oxygenases from microbes and photosynthetic organisms: features and functions. Int J Mol Sci. 2016;17:1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Frusciante S, Diretto G, Bruno M. et al. Novel carotenoid cleavage dioxygenase catalyzes the first dedicated step in saffron crocin biosynthesis. Proc Natl Acad Sci U S A. 2014;111:12246–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Ohmiya A, Kishimoto S, Aida R. et al. Carotenoid cleavage dioxygenase (CmCCD4a) contributes to white color formation in chrysanthemum petals. Plant Physiol. 2006;142:1193–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Falchi R, Vendramin E, Zanon L. et al. Three distinct mutational mechanisms acting on a single gene underpin the origin of yellow flesh in peach. Plant J. 2013;76:175–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Wen L, Wang Y, Deng Q. et al. Identifying a carotenoid cleavage dioxygenase (CCD4) gene controlling yellow/white fruit flesh color of ‘Piqiutao’ (white fruit flesh) and its mutant (yellow fruit flesh). Plant Mol Biol Rep. 2020;38:513–20 [Google Scholar]
- 28. Pu X, Li Z, Tian Y. et al. The honeysuckle genome provides insight into the molecular mechanism of carotenoid metabolism underlying dynamic flower coloration. New Phytol. 2020;227:930–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Li T, Deng YJ, Liu JX. et al. DcCCD4 catalyzes the degradation of α-carotene and β-carotene to affect carotenoid accumulation and taproot color in carrot. Plant J. 2021;108:1116–30 [DOI] [PubMed] [Google Scholar]
- 30. Gonzalez-Jorge S, Ha SH, Magallanes-Lundback M. et al. Carotenoid cleavage dioxygenase4 is a negative regulator of beta-carotene content in Arabidopsis seeds. Plant Cell. 2013;25:4812–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Gonzalez-Jorge S, Mehrshahi P, Magallanes-Lundback M. et al. Zeaxanthin epoxidase activity potentiates carotenoid degradation in maturing Arabidopsis seed. Plant Physiol. 2016;171:1837–51 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Gao J, Yang S, Tang K. et al. GmCCD4 controls carotenoid content in soybeans. Plant Biotechnol J. 2021;19:801–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Zheng X, Mi J, Balakrishna A. et al. Gardenia carotenoid cleavage dioxygenase 4a is an efficient tool for biotechnological production of crocins in green and non-green plant tissues. Plant Biotechnol J. 2022;20:2202–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Powell AL, Nguyen CV, Hill T. et al. Uniform ripening encodes a Golden 2-like transcription factor regulating tomato fruit chloroplast development. Science. 2012;336:1711–5 [DOI] [PubMed] [Google Scholar]
- 35. Fu C, Chen H, Gao H. et al. Two papaya MYB proteins function in fruit ripening by regulating some genes involved in cell-wall degradation and carotenoid biosynthesis. J Sci Food Agr. 2020;100:4442–8 [DOI] [PubMed] [Google Scholar]
- 36. Ampomah-Dwamena C, Thrimawithana AH, Dejnoprat S. et al. A kiwifruit (Actinidia deliciosa) R2R3-MYB transcription factor modulates chlorophyll and carotenoid accumulation. New Phytol. 2019;221:309–25 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Sagawa JM, Stanley LE, LaFountain AM. et al. An R2R3-MYB transcription factor regulates carotenoid pigmentation in Mimulus lewisii flowers. New Phytol. 2016;209:1049–57 [DOI] [PubMed] [Google Scholar]
- 38. Wu M, Xu X, Hu X. et al. SlMYB72 regulates the metabolism of chlorophylls, carotenoids, and flavonoids in tomato fruit. Plant Physiol. 2020;183:854–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Li S, Chen K, Grierson D. A critical evaluation of the role of ethylene and MADS transcription factors in the network controlling fleshy fruit ripening. New Phytol. 2019;221:1724–41 [DOI] [PubMed] [Google Scholar]
- 40. Yin W, Yu X, Chen G. et al. Suppression of SlMBP15 inhibits plant vegetative growth and delays fruit ripening in tomato. Front Plant Sci. 2018;9:938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zhang J, Hu Z, Yao Q. et al. A tomato MADS-box protein, SlCMB1, regulates ethylene biosynthesis and carotenoid accumulation during fruit ripening. Sci Rep. 2018;8:3413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lu S, Ye J, Zhu K. et al. A fruit ripening-associated transcription factor CsMADS5 positively regulates carotenoid biosynthesis in citrus. J Exp Bot. 2021;72:3028–43 [DOI] [PubMed] [Google Scholar]
- 43. Lu S, Zhang Y, Zhu K. et al. The citrus transcription factor CsMADS6 modulates carotenoid metabolism by directly regulating carotenogenic genes. Plant Physiol. 2018;176:2657–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Zhu K, Chen H, Mei X. et al. Transcription factor CsMADS3 coordinately regulates chlorophyll and carotenoid pools in Citrus hesperidium. Plant Physiol. 2023;193:519–36 [DOI] [PubMed] [Google Scholar]
- 45. Yin W, Hu Z, Cui B. et al. Suppression of the MADS-box gene SlMBP8 accelerates fruit ripening of tomato (Solanum lycopersicum). Plant Physiol Bioch. 2017;118:235–44 [DOI] [PubMed] [Google Scholar]
- 46. Xie Q, Hu Z, Zhu Z. et al. Over-expression of a novel MADS-box gene SlFYFL delays senescence, fruit ripening and abscission in tomato. Sci Rep. 2014;4:4367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Kou X, Zhao Y, Wu C. et al. SNAC4 and SNAC9 transcription factors show contrasting effects on tomato carotenoids biosynthesis and softening. Postharvest Biol Tec. 2018;144:9–19 [Google Scholar]
- 48. Meng C, Yang D, Ma X. et al. Suppression of tomato SlNAC1 transcription factor delays fruit ripening. J Plant Physiol. 2016;193:88–96 [DOI] [PubMed] [Google Scholar]
- 49. Zhu M, Chen G, Zhou S. et al. A new tomato NAC (NAM/ATAF1/2/CUC2) transcription factor, SlNAC4, functions as a positive regulator of fruit ripening and carotenoid accumulation. Plant Cell Physiol. 2014;55:119–35 [DOI] [PubMed] [Google Scholar]
- 50. Fu C, Han Y, Fan Z. et al. The papaya transcription factor CpNAC1 modulates carotenoid biosynthesis through activating phytoene desaturase genes CpPDS2/4 during fruit ripening. J Agr Food Chem. 2016;64:5454–63 [DOI] [PubMed] [Google Scholar]
- 51. Fu C, Han Y, Kuang J. et al. Papaya CpEIN3a and CpNAC2 co-operatively regulate carotenoid biosynthesis-related genes CpPDS2/4, CpLCY-e and CpCHY-b during fruit ripening. Plant Cell Physiol. 2017;58:2155–65 [DOI] [PubMed] [Google Scholar]
- 52. Xing S, Li R, Zhao H. et al. The transcription factor IbNAC29 positively regulates the carotenoid accumulation in sweet potato. Hortic. Res. 2023;10:uhad010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Chung MY, Vrebalov J, Alba R. et al. A tomato (Solanum lycopersicum) APETALA2/ERF gene, SlAP2a, is a negative regulator of fruit ripening. Plant J. 2010;64:936–47 [DOI] [PubMed] [Google Scholar]
- 54. Lee JM, Joung JG, McQuinn R. et al. Combined transcriptome, genetic diversity and metabolite profiling in tomato fruit reveals that the ethylene response factor SlERF6 plays an important role in ripening and carotenoid accumulation. Plant J. 2012;70:191–204 [DOI] [PubMed] [Google Scholar]
- 55. Dang Q, Sha H, Nie J. et al. An apple (Malus domestica) AP2/ERF transcription factor modulates carotenoid accumulation. Hortic Res. 2021;8:223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Zhu K, Sun Q, Chen H. et al. Ethylene activation of carotenoid biosynthesis by a novel transcription factor CsERF061. J Exp Bot. 2021;72:3137–54 [DOI] [PubMed] [Google Scholar]
- 57. Wang N, Sun Y, Lian R. et al. Genome-wide screening of AP2/ERF transcription factors involved in Citrus maxima "Sanhongmiyou" exocarp coloring. Sci Hortic. 2023;318:112041 [Google Scholar]
- 58. Cai D, Xu H, Liu Z. et al. Banana MaERF124 negatively modulates carotenoid accumulation during fruit ripening through repression of carotenogenesis genes. Postharvest Biol Tec. 2023;195:112151 [Google Scholar]
- 59. Xiao X, Shi L, Dong W. et al. Ethylene promotes carotenoid accumulation in peach pulp after harvest. Sci Hortic. 2022;304:111347 [Google Scholar]
- 60. Zhao J, Li H, Yin Y. et al. Fruit ripening in Lycium barbarum and Lycium ruthenicum is associated with distinct gene expression patterns. FEBS Open Bio. 2020;10:1550–67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Auldridge ME, Block A, Vogel JT. et al. Characterization of three members of the Arabidopsis carotenoid cleavage dioxygenase family demonstrates the divergent roles of this multifunctional enzyme family. Plant J. 2006;45:982–93 [DOI] [PubMed] [Google Scholar]
- 62. Tan BC, Joseph LM, Deng WT. et al. Molecular characterization of the Arabidopsis 9-cis epoxycarotenoid dioxygenase gene family. Plant J. 2003;35:44–56 [DOI] [PubMed] [Google Scholar]
- 63. Li HX, Yin Y, An W. et al. Dynamics of carotenoids during the development of Goji berry fruit. J Northwest Forestry College. 2015;30:139–42(In Chinese) [Google Scholar]
- 64. Tian X, Ji J, Wang G. et al. Cloning and functional characterisation of carotenoid cleavage dioxygenase 4 from wolfberry. Trans Tianjin Univ. 2017;23:62–9 [Google Scholar]
- 65. Lestari R, Rio M, Martin F. et al. Over-expression of Hevea brasiliensis ethylene response factor HbERF-IXc5 enhances growth and tolerance to abiotic stress and affects laticifer differentiation. Plant Biotechnol J. 2018;16:322–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Shoji T, Yuan L. ERF gene clusters: working together to regulate metabolism. Trends Plant Sci. 2021;26:23–32 [DOI] [PubMed] [Google Scholar]
- 67. Feng K, Hou XL, Xing GM. et al. Advances in AP2/ERF super-family transcription factors in plant. Crit Rev Biotechnol. 2020;40:750–76 [DOI] [PubMed] [Google Scholar]
- 68. Liu JX, Chiou CY, Shen CH. et al. RNA interference-based gene silencing of phytoene synthase impairs growth, carotenoids, and plastid phenotype in Oncidium hybrid orchid. Springerplus. 2014;3:478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Wang M, Wang G, Ji J. et al. The effect of pds gene silencing on chloroplast pigment composition, thylakoid membrane structure and photosynthesis efficiency in tobacco plants. Plant Sci. 2009;177:222–6 [Google Scholar]
- 70. Zhou J, Hunter DA, Lewis DH. et al. Insights into carotenoid accumulation using VIGS to block different steps of carotenoid biosynthesis in petals of California poppy. Plant Cell Rep. 2018;37:1311–23 [DOI] [PubMed] [Google Scholar]
- 71. Mohanty B, Lakshmanan M, Lim SH. et al. Light-specific transcriptional regulation of the accumulation of carotenoids and phenolic compounds in rice leaves. Plant Signal Behav. 2016;11:3002–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Yin Y, Shi H, Mi J. et al. Genome-wide identification and analysis of the BBX gene family and its role in carotenoid biosynthesis in wolfberry (Lycium barbarum L.). Int J Mol Sci. 2022;23:8440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Wheeler TJ, Eddy SR. Nhmmer: DNA homology search with profile HMMs. Bioinformatics. 2013;29:2487–9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Schultz J, Copley RR, Doerks T. et al. SMART: a web-based tool for the study of genetically mobile domains. Nucleic Acids Res. 2000;28:231–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Marchler-Bauer A, Bo Y, Han L. et al. CDD/SPARCLE: functional classification of proteins via subfamily domain architectures. Nucleic Acids Res. 2017;45:D200–3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Lees J, Yeats C, Perkins J. et al. Gene3D: a domain-based resource for comparative genomics, functional annotation and protein network analysis. Nucleic Acids Res. 2012;40:D465–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Attwood TK, Croning MDR, Flower DR. et al. PRINTS-S: the database formerly known as PRINTS. Nucleic Acids Res. 2000;28:225–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Jones P, Binns D, Chang HY. et al. InterProScan 5: genome-scale protein function classification. Bioinformatics. 2014;30:1236–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Artimo P, Jonnalagedda M, Arnold K. et al. ExPASy: SIB bioinformatics resource portal. Nucleic Acids Res. 2012;40:W597–603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Kumar S, Stecher G, Tamura K. MEGA7: Molecular Evolutionary Genetics Analysis version 7.0 for bigger datasets. Mol Biol Evol. 2016;33:1870–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Swarbreck D, Wilks C, Lamesch P. et al. The Arabidopsis Information Resource (TAIR): gene structure and function annotation. Nucleic Acids Res. 2007;36:D1009–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Untergasser A, Cutcutache I, Koressaar T. et al. Primer3-new capabilities and interfaces. Nucleic Acids Res. 2012;40:e115–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lescot M, Déhais P, Thijs G. et al. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002;30:325–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Zhao J, Li H, Xu Y. et al. A consensus and saturated genetic map provides insight into genome anchoring, synteny of Solanaceae and leaf-and fruit-related QTLs in wolfberry (Lycium Linn.). BMC Plant Biol. 2021;21:350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Dobin A, Davis CA, Schlesinger F. et al. STAR: ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Pertea M, Pertea GM, Antonescu CM. et al. StringTie enables improved reconstruction of a transcriptome from RNA-seq reads. Nat Biotechnol. 2015;33:290–5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Anders S, Huber W. Differential expression analysis for sequence count data. Nat Preceedings. 2010;11:R106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Zheng Q, Wang XJ. GOEAST: a web-based software toolkit for gene ontology enrichment analysis. Nucleic Acids Res. 2008;36:W358–63 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Kanehisa M, Goto S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 2000;28:27–30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Zhao J, Sun C, Shi F. et al. Comparative transcriptome analysis reveals sesquiterpenoid biosynthesis among 1-, 2-and 3-year old Atractylodes chinensis. BMC Plant Biol. 2021;21:354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–8 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data supporting the findings of this work are available within the paper and the Supplementary Tables and Figures. The transcriptome clean sequencing data have been deposited into the National Center for Biotechnology Information Sequence Read Archive database (PRJNA936937).