Abstract
Objectives
Deprived living environments contribute to greater heart failure (HF) risk among non-Hispanic Black persons, who disproportionately occupy disadvantaged neighborhoods. The mechanisms for these effects are not fully explicated, partially attributable to an insufficient understanding of the individual factors that contribute additional risk or resilience to the impact of neighborhood disadvantage on health. The objective of this study was, therefore, to clarify the complex pathways over which such exposures act to facilitate more targeted, effective interventions. Given the evidence for a mediating role of biological age and a moderating role of individual psychosocial characteristics in the neighborhood disadvantage–HF link, we tested a moderated mediation mechanism.
Methods
Using multilevel causal moderated mediation models, we prospectively examined whether the association of neighborhood disadvantage with incident HF mediated through accelerated biological aging, captured by the GrimAge epigenetic clock, is moderated by hypothesized psychosocial risk (negative affect) and resilience (optimism) factors.
Results
Among a sample of 1,448 Black participants in the shared Jackson Heart Study–Atherosclerosis Risk in Communities cohort (mean age 64.3 years), 334 adjudicated incident hospitalized HF events occurred over a median follow-up of 18 years. In models adjusted for age and sex, the indirect (GrimAge-mediated) effect of neighborhood disadvantage was moderated by psychosocial risk such that for every standard deviation increase in negative affect the hazards of HF was 1.18 (95% confidence interval = 1.05, 1.36). No moderated mediation effect was detected for optimism.
Discussion
Findings support the necessity for multilevel interventions simultaneously addressing neighborhood and individual psychosocial risk in the reduction of HF among Black persons.
Keywords: African Americans, Cardiovascular disease Health disparities, Negative affect, Optimism
Accelerated Aging and Ethnoracial Disparities in Heart Failure
Although life expectancy and overall health have improved in the United States in recent years, these gains are not equally distributed across populations of persons of different racial, ethnic, gender, and socioeconomic backgrounds (Chang et al., 2018; Nayak et al., 2020). The contrast in morbidity and mortality between demographic groups is increasingly attributed to the distinct social conditions in which these groups age (Diez Roux, 2016; Williams et al., 2019). Processes of accelerated physiological aging in comparison with chronologic age have long been described by concepts like weathering (Geronimus et al. 2006) and allostatic load (McEwen, 1998) as driven by cumulative exposure to social stressors. Such cumulative experiences of adversity, which often stem from social inequity, have likewise been shown to have lifelong consequences on health (Geronimus et al. 2006; McEwen, 1998; Williams et al., 2019).
Ethnoracial disparities in chronic disease are particularly evident in heart failure (HF). By some estimates, non-Hispanic (NH) Black persons experience over double the incidence of hospitalized HF events compared with NH Whites; among men, disparities are even more striking (Chang et al., 2018). Challenges in efforts to mitigate this disparity include both the complexity of the HF syndrome, with incompletely characterized physiological mechanisms, and the interdependent social and psychological factors influencing risk within this population (Nayak et al., 2020). Consequently, as continued disparities in age-related disease outcomes remain a central public health concern, there is an increased need for research addressing the multifaceted aging processes that yield unequal health outcomes over the life course.
Neighborhood Disadvantage as a Causal Factor
A large body of evidence has established deprived living environments as a contributor to chronic disease risk. Whether operationalized in objective measures such as the widely used Area Deprivation Index (ADI; Kind & Buckingham, 2018), or subjective measures capturing the perception of disadvantage (Clark et al., 2013), geographically defined social spaces such as neighborhoods have been shown to cluster health and disease through acting as stressors and shaping health behaviors and access to health care (Diez Roux, 2016). The historical and current practices that disproportionately concentrate Black persons in socially and economically disadvantaged neighborhoods are increasingly recognized as an important contributor to disproportionately high burdens of chronic diseases within this population (Williams et al., 2019). Still, effective methods for addressing ethnoracial disparities in HF morbidity and mortality are confounded by a dearth of understanding of HF pathophysiology in diverse populations (Nayak et al., 2020) as well as potential sources of within-group variation in risk and resilience specific to Black persons (Bey et al., 2019).
Mediation Through Accelerated Biological Aging
Despite what is known about the role of neighborhoods in influencing HF risk, the manner in which these social environmental factors drive the physiological changes underpinning the development of HF is not fully clear. With empirical evidence supporting neighborhood disadvantage as a chronic stressor, epigenetic aging processes such as those captured by DNA methylation (DNAm)-based measures show promise as potential mediators of the neighborhood disadvantage–HF pathway (Lu et al., 2019; Roetker et al., 2018). Chronic stress-induced epigenetic alterations, including premature changes in the expression of genes modulating inflammatory and metabolic processes (Fiorito et al., 2017; Gomez-Alonso et al., 2021; Zannas et al., 2019), link DNAm to the primary cardiovascular and cardiometabolic precursors to HF: coronary artery disease, type 2 diabetes, and hypertension. Further, emerging evidence supports a direct role for DNAm in HF (Roetker et al., 2018), and as a mediator of the effect of neighborhood disadvantage on HF (Bey et al., 2022).
One such DNAm-based construct, the GrimAge epigenetic “clock,” is a composite of DNAm-based markers for seven plasma proteins and a DNAm-based estimator of self-reported smoking pack years. Of the validated epigenetic clocks, GrimAge has been demonstrated to capture a broader range of DNAm surrogates for mortality and morbidity biomarkers (Levine, 2020; Lu et al., 2019). There are inherent limitations to any measure designed to assess a single dimension of what a strong theoretical and empirical evidence base is increasingly recognizing as the multidimensional process of aging (Sierra, 2016). Still, there is also compelling evidence of GrimAge’s increased utility as a predictor of both life span and health span (Levine, 2020; Lu et al., 2019), positioning the measure as a viable construct for capturing the biological aging processes hypothesized as one mechanism linking neighborhood stressors to increased HF risk.
Furthermore, the emerging discipline of geroscience challenges the notion of diseases as distinct pathologies and instead reconceptualizes various chronic conditions as pleiotropic manifestations sharing a core set of biological mechanisms which act over the life course (Sierra, 2016). Within this framework, epigenetic measures of biological aging can be conceptualized less as traditional mediators and more as metrics capturing an ongoing process of cumulative biological injury resulting from cumulative exposure to environmental stress, which aligns with other prevailing theories of aging.
Moderation by Individual Psychosocial Characteristics
The literature identifying psychosocial characteristics—psychological phenotypes shaped by social experiences—such as negative affect or optimistic disposition as influential in cardiovascular risk and disease (Dhar & Barton, 2016; Park et al., 2022; Rozanski et al., 2019; Sims et al., 2017, 2019) point to potential sources of variation in the association of neighborhood disadvantage with HF. A recent meta-analysis found dispositional optimism, a generalized tendency to expect good life outcomes, to be associated with reduced risk, and pessimism with increased risk, of cardiovascular events (Rozanski et al., 2019). Building on this work, we recently found that optimism moderates the direct effect of objective (but not subjective) neighborhood disadvantage on incident HF among NH Black persons (Bey et al., 2023), with those endorsing a more optimistic outlook less likely to develop HF when exposed to neighborhood socioeconomic adversities.
This evidence for the role of individual psychosocial characteristics in conferring risk or resilience to the impact of disadvantaged social conditions on HF among Black persons is consistent with multiple empirically supported theoretical frameworks. The Identity Vitality-Pathology (IVP) model (Bey, 2022; Bey et al., 2019), for example, posits a reduced likelihood of perceiving and experiencing stress associated with social inequity among those with a more hopeful outlook, and, alternatively, an increased perception of stress among those endorsing a tendency to experience elevated emotional distress associated with unpleasant feelings (a negative affective disposition). This psychological modulation of stress appraisal and response is hypothesized as influential in several physiological processes associated with HF risk.
It is important to note that while often considered traits characteristics, psychological factors such as negative affect can be modifiable. Although a clear consensus does not exist, several prominent frameworks (e.g., Seligman, 2006) position what are often characterized as affective traits of optimism and pessimism as modifiable characteristics, that, while stable and indicative of one’s disposition, are influenced by environmental exposures shaped by structural inequity. Such exposures include, for example, experiences of abuse, trauma, neglect, deprivation, and race-based discrimination (Carter et al., 2020; Chen et al., 2021) that disproportionately affect ethnoracially minoritized populations over the life course (Mersky et al., 2021).
A Moderated Mediation Approach to Understanding the Relationship of Cumulative Social Stress, Individual Psychological Characteristics, and Accelerated Aging in HF Risk
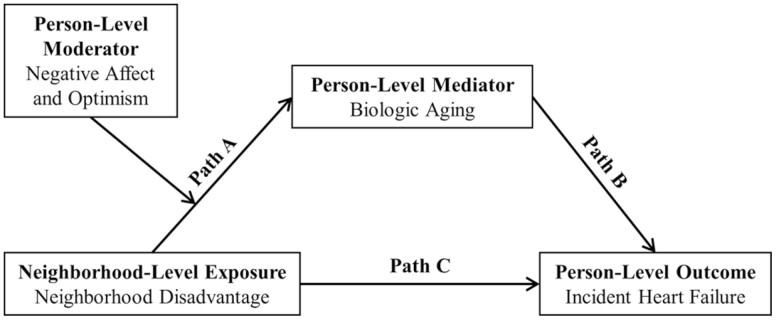
This study aimed to provide insight into the relationship between cumulative experiences of stressful social conditions, individual psychosocial characteristics, and accelerated aging in risk for an age-related disease prevalent among Black persons. Given the evidence for a mediating role of biological age and a moderating role of individual psychosocial characteristics in the relationship of neighborhood disadvantage with HF, a moderated mediation analysis (Figure 1) may capture the complex interplay of neighborhood- and individual-level factors influencing HF risk among Black persons. We, therefore, conducted a moderated mediation analysis among a sample of Black adults to prospectively assess whether hypothesized indicators of psychosocial risk (negative affect) and resilience (optimism) moderated the relationship between neighborhood disadvantage and biological aging (Figure 1, Path A), which, in turn, indirectly alters the risk of incident HF (Figure 1, Path B).
Figure 1.
Hypothesized pathways linking neighborhood disadvantage to incident heart failure .
Two of our previous studies offered evidence that (a) biological age mediates the association of subjective neighborhood disadvantage with incident HF (Figure 1, Paths A and B), and (b) that optimism but not negative affect moderates the effect (Figure 1, Path C) of objective neighborhood disadvantage on incident HF (Bey et al., 2022, 2023). These studies indicate potentially distinct moderating pathways of psychosocial risk and resilience, where optimism may be more likely to shape health behaviors and accessing care, whereas negative affect may be more likely to directly influence one’s physiological stress response to perceived neighborhood disadvantage. We, therefore, hypothesized that the indirect (mediated) effect of subjective neighborhood disadvantage acting through biological age on incident HF would be moderated by negative affect but not optimism (Figure 1, moderation of Path A).
Method
Study Population
Data used for this study come from self-identified NH Black residents of Jackson, MS, enrolled in the Atherosclerosis Risk in Communities (ARIC) study and the Jackson Heart Study (JHS). Our analysis was based on participants dually enrolled in both studies so that the DNAm and outcome data available in ARIC could be examined in the context of the exposure and moderating variables available in JHS.
The ARIC study is a community-based longitudinal investigation of atherosclerosis and its risk factors in four geographic areas: Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD. From 1987 to 1989, 15,792 mostly Black and White persons aged 45–64 years of age were selected using probability sampling and participated in a baseline examination (Wright et al., 2021). Follow-up examinations occurred at discrete intervals and participants are contacted annually by telephone between clinic examinations and semiannually since 2012. Similarly, JHS is a prospective, community-based study of cardiovascular disease among Black persons in Jackson, MS. Four sampling approaches were used to select 5,306 adults aged 21–84 years for participation: a random sampling of adults drawn from a commercially available list of households with adults, volunteers recruited through participant referral or outreach activities, participants from the Jackson field center of the ARIC study, and relatives of JHS participants. The baseline examination occurred between 2000 and 2004 and involved the collection of data on clinical, demographic, social, cultural, and behavioral information through a home interview, an onsite clinical examination, and 42-hr follow-up data. Two follow-up clinical visits took place in 2005–2008 and 2009–2012, and study staff contacted participants each year via telephone for an annual follow-up survey. The JHS design and methods have been previously described in greater detail (Taylor et al., 2005).
Of the 5,306 Black persons enrolled in JHS at baseline in 2000–2004, 1,662 participants were already enrolled in ARIC at the Jackson, MS, site. With exclusions for an HF diagnosis prior to the JHS baseline (n = 139) and lack of linkage to a 1990 census tract (n = 35), our final analytic sample included 1,448 participants (Supplementary Figure 1) dually enrolled in ARIC and JHS residing in 67 neighborhoods as defined by U.S. census tracts. Participants were geocoded to their neighborhood of residence by their home address at the ARIC baseline examination.
Study Variables
Exposure: neighborhood disadvantage
Our exposure to neighborhood disadvantage was operationalized in a measure of objective neighborhood disadvantage, the National ADI, and a measure of subjective neighborhood disadvantage, the perceived neighborhood problems (PNP) scale.
The ADI, used extensively in research on neighborhood health effects, has been previously calculated for every U.S. census block group (Kind & Buckingham, 2018). The measure includes 17 metrics for the theoretical domains of income, education, employment, and housing quality. For additional details on the construction of the ADI, see Kind and Buckingham (2018). Each census block group is assigned a rank ranging from 1 to 100, representing the least and most deprived neighborhoods of the United States, respectively. For this study, we used data from the 1990 census and standardized census tract rankings by subtracting the sample mean and dividing by the standard deviation (SD). We also categorized unstandardized scores from the 67 census tracts spanning the ARIC–JHS catchment area into quartiles, with Quartile 1 including the lowest 25% and Quartile 4 the highest 25% ranked tracts.
The validated PNP scale (Ross & Mirowsky, 1999) is comprised of six survey items assessing perceptions of neighborhood noise, traffic and speeding, access to food, shopping, or parks, and the persistence of trash and litter which were measured in JHS at the baseline examination (2000–2004). The measure has been shown to correlate highly with objective measures of neighborhood quality (Elo et al., 2009) but can be used to contrast the health effects of objective disadvantage with those which may be attributable to subjective experiences of disadvantage. An examination of the distribution of responses in SAS 9.4 (SAS Institute, Cary, NC) revealed sizable skew (>1) and kurtosis (>6). Consequently, each item was treated as a categorical indicator in a multilevel confirmatory factor analysis model that computed census tract-level PNP. To produce valid neighborhood-level estimates, we combined tracts with fewer than 10 participants with neighboring tracts, resulting in 32 analytic census tracts. Adjustments for sex and age were made at the person level to produce neighborhood-level measurements that accounted for demographic imbalances among participants sampled from a specific tract. The computed census tract scores were standardized and discretized into quartiles.
Outcome: incident hospitalized HF
The outcome of interest was adjudicated incident hospitalized HF. We included as incident HF any events occurring between January 1, 2000, and December 31, 2017. HF was ascertained in the ARIC study by reviewing medical records from local hospitals with a discharge ICD code of HF (ICD9: 398.91, 402.01, 402.11, 402.91, 404.01, 404.03, 404.11, 404.13, 404.91, 404.93, 415.0, 416.9, 425.4, 428.x, 518.4, 786.0x; ICD10: J18.9, J96.01, J98.11, J21.9, T17.890A, E11.65, T38.0X5A, I13.0, E11.22, N18.3, I50.9, I48.0, I71.2, I95.1, M19.011, M25.551, I44.0, I44.4, E78.5, R42, Z91.81, E66.9, F32.9, Z68.29, Z79.4, Z86.73). Events occurring at nonlocal hospitals were identified by self-report and from death certificates, and the corresponding medical records were obtained and reviewed.
Mediator: accelerated biological aging
We operationalized biological age using the epigenetic clock, GrimAge (Lu et al., 2019). Methods of determining methylation status and calculation of GrimAge in ARIC have been previously documented (Nguyen et al., 2021). Briefly, methylation status was measured using the Illumina Infinium Human Methylation 450 Bead Chip array in the ARIC participants beginning at Visit 2 (1990–1991). Methylation status was determined for each participant only once. We centered GrimAge at the census tract mean to reflect the difference in years between a specific individual in a census tract and the average across individuals in the same census tract. This analytic approach facilitated the estimation of cross-level moderated mediation.
Moderators: individual psychosocial risk and resilience characteristics
Optimism was assessed using the validated six-item Life Orientation Test—Revised (Scheier et al., 1994). Consistent with JHS methodology, negative affect was assessed as a composite of cynicism, depressive symptoms, and anger expression. Cynicism was measured using Items 1–13 of the Cook–Medley Hostility scale (Barefoot et al., 1989), depressive symptoms using the 20-item Center for Epidemiologic Studies—Depression scale, and anger expression using the 8-item inward and 8-item outward subscales of the 16-item Spielberger State–Trait Anger Expression Inventory (Spielberger et al., 1988). We used a single-level confirmatory factor analysis model to compute a standardized composite score from the mean of each scale and subscale. All measures have been shown as valid and reliable (Sims et al., 2017) and were administered at the JHS baseline visit.
Covariates
We limited covariates in our primary analyses to chronologic age at baseline (continuous years) and sex (binary female, male). This approach was used because we conceptualize other standard confounders as potential mediators of the neighborhood—HF pathway. Models with additional sets of confounders are included in sensitivity analyses (described later) to examine potential residual confounding bias. Baseline age was included in all models to ensure that accelerated biological aging was not confounded with chronological aging.
Statistical Analysis
Trends in the baseline characteristics of the participants across quartiles of ADI and PNP were evaluated in SAS 9.4 utilizing linear regression, Cochran–Armitage trend tests, and Cochran–Mantel–Haenszel trend tests. A causal (VanderWeele, 2011) moderated mediation (Hayes & Rockwood, 2020) structural equation model (Muthén & Asparouhov, 2015) that employed cause-specific, Cox proportional hazards regression was fit in Mplus version 8.8. (Muthen & Muthen, 1998). The analysis examined whether psychosocial risk and resilience moderated the indirect (mediated) effect of neighborhood disadvantage on incident HF. Follow-up time was measured from January 1, 2000, until the first documented instance of hospitalized HF, censoring due to a cause unrelated to HF, or administrative censoring on December 31, 2017. Ties were handled via a Breslow approximation. The assumption of linearity was evaluated by examining Martingale residuals and the proportional hazards assumption was assessed by inspecting Schoenfeld residuals. Neither modeling assumption was violated.
Prior to model fitting, all continuous neighborhood-level measures were grand-mean centered so that the value represented the degree to which a census tract differed from the sample mean. Continuous individual-level measures were centered at the census tract mean to reflect the extent to which an individual differed from the average in a census tract. Centering in this manner improved parameter estimation by removing conflation between level-specific effects. The multilevel nature of the analysis, which integrated a neighborhood-level exposure with an individual-level moderator, mediator, and outcome, was further accounted for by specifying a sandwich estimator that calculated robust standard errors. The resulting parameter estimates were transformed into hazard ratios (HRs) with 95% confidence intervals (95% CIs) of incident HF with the exception of the standardized coefficients (β) from Path A (Figure 1) which document associations with GrimAge estimated from a linear regression.
To account for missing data, full-information maximum likelihood (FIML; Allison, 2003) was employed which prior studies have shown to be comparable to other common missing data techniques such as multiple imputation (Lee & Shi, 2021). The application of FIML permitted the Mplus software to perform a 1,000-iteration bias-corrected bootstrap (Hayes & Rockwood, 2020) when calculating CIs, which was advantageous given that the distribution of the index of moderated mediation (Hayes, 2015) used to evaluate the proposed moderated mediation was not expected to be asymptotically normal (Williams & MacKinnon, 2008). The initial model estimated crude, unadjusted effects. The adjusted model incorporated sex and chronologic age at baseline as time-invariant covariates. The Johnson–Neyman (Johnson & Fay, 1950) technique was applied to visualize the covariate-adjusted moderated mediation effect of neighborhood disadvantage on incident HF.
A sensitivity analysis was conducted to evaluate the robustness of the results. After fitting the model that adjusted for chronologic age and sex, a new model was fit to the data that additionally adjusted for behavioral cardiovascular risk factors including smoking and physical activity at the baseline. The next model accounted for baseline demographics by adjusting for sex, chronologic age, years of formal education ranging from 0 to 19, and whether the participant was living below the federal poverty line. A subsequent model addressed lifestyle factors and comorbidities by adjusting for baseline smoking, physical activity, binge drinking, diabetes, and the use of hypertension medication. The final model incorporated all covariates.
Results
Among the 1,448 participants in the analytic sample, the mean (SD) chronologic age at the baseline visit was 64.3 (5.5) years, 66.3% (960/1,448) were female, 14.3% (184/1,290) were classified as living below the federal poverty line; 27.4% (393/1,434) and 22.0% (316/1,434) resided in the highest and lowest quartiles of area deprivation, respectively; 24.5% (279/1,138) and 22.9% (261/1,138) resided in neighborhoods with the highest and lowest PNP, respectively. A modest percentage of participants, 12.8% (144/1,127), resided in the highest quartile of both area deprivation and PNP illustrating the strong correlation (r = 0.72) and the slight discrepancy between objective and subjective measures. Participants residing in census tracts with higher levels of ADI or PNP were more likely to be older, female, have less years of formal education (Table 1), report higher levels of negative affect, and report lower levels of optimism. The median (interquartile range) follow-up was 18.0 (6.1) years. By the time of administrative censoring at the end of 2017, 334 instances of adjudicated incident HF hospitalizations were recorded.
Table 1.
Characteristics of the Study Population at Baseline by Quartiles: ARIC–JHS Cohort 2000–2017 (n = 1,448)
| ADI | N | All | Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | p-Trend |
|---|---|---|---|---|---|---|---|
| 31–77 | 78–87 | 88–93 | 94–100 | ||||
| Age, mean (SD), years | 1,448 | 64.3 (5.5) | 62.4 (4.9) | 63.7 (5.2) | 65.1 (5.6) | 65.6 (5.6) | <.001 |
| Female sex, no. (%) | 1,448 | 960 (66.3) | 197 (62.3) | 226 (66.1) | 257 (67.1) | 273 (69.5) | .05 |
| Education, mean (SD), years | 1,439 | 13.4 (4.5) | 15.3 (4.2) | 13.2 (4.0) | 13.5 (4.8) | 11.9 (4.4) | <.001 |
| Below federal poverty line, no. (%) | 1,290 | 184 (14.3) | 22 (7.9) | 38 (12.3) | 50 (14.8) | 73 (20.7) | <.001 |
| Smoking history, no. (%) | |||||||
| Current | 1,294 | 197 (15.2) | 27 (9.4) | 60 (20.1) | 46 (13.2) | 62 (17.9) | .008 |
| Former | 466 (36.0) | 111 (38.5) | 108 (36.1) | 122 (35.1) | 119 (34.3) | ||
| Never | 631 (48.8) | 150 (52.1) | 131 (43.8) | 180 (51.7) | 166 (47.8) | ||
| Physical activity, mean (SD), min/week | 1,308 | 110.9 (150.3) | 130.2 (155.2) | 111.2 (149.5) | 109.4 (148.3) | 94.8 (142) | .03 |
| Binge drinking, no. (%) | 951 | 145 (15.2) | 26 (11.8) | 33 (14.0) | 41 (16.9) | 44 (17.9) | .05 |
| Diabetes, no. (%) | 1,277 | 283 (22.2) | 47 (16.6) | 62 (20.9) | 84 (24.6) | 88 (25.6) | .004 |
| Hypertension medication, no. (%) | 1,326 | 728 (54.9) | 139 (47.4) | 180 (58.6) | 201 (56.6) | 203 (56.9) | .04 |
| Negative affect, mean (SD) | 930 | 0.0 (0.8) | -0.2 (0.7) | 0.0 (0.8) | 0.1 (0.9) | 0.1 (0.8) | .003 |
| Optimism, mean (SD) | 1,208 | 19.0 (3.5) | 19.9 (3.2) | 18.9 (3.3) | 18.8 (3.5) | 18.5 (3.6) | <.001 |
| DNA Methylation GrimAge, mean (SD) | 495 | 64.8 (6.5) | 62.5 (6.0) | 65.3 (6.9) | 64.4 (6.0) | 66.4 (6.5) | <.001 |
| Heart failure, no. (%) | 1,448 | 334 (23.1) | 55 (17.4) | 66 (19.3) | 100 (26.1) | 110 (28.0) | <.001 |
| Death, no. (%) | 1,448 | 640 (44.2) | 113 (35.8) | 149 (43.6) | 165 (43.1) | 205 (52.2) | <.001 |
| Perceived neighborhood problems | −2.04 to −0.56 | −0.55 to 0.00 | 0.01–0.55 | 0.56–1.4 | |||
| Age, mean (SD), years | 1,448 | 64.3 (5.5) | 63.3 (5.1) | 64.5 (5.4) | 64.1 (5.3) | 65.3 (5.8) | <.001 |
| Female sex, no. (%) | 1,448 | 960 (66.3) | 164 (62.8) | 217 (71.1) | 207 (70.6) | 212 (76.0) | .002 |
| Education, mean (SD), years | 1,439 | 13.4 (4.5) | 14.7 (4.3) | 14.9 (4.4) | 12.8 (3.9) | 12.1 (4.4) | <.001 |
| Below federal poverty line, no. (%) | 1290 | 184 (14.3) | 22 (9.4) | 20 (7.1) | 45 (17.2) | 43 (17.6) | <.001 |
| Smoking history, no. (%) | |||||||
| Current | 1,294 | 197 (15.2) | 33 (13.9) | 25 (9.0) | 55 (20.6) | 29 (11.5) | <.001 |
| Former | 466 (36.0) | 97 (40.8) | 89 (32.1) | 99 (37.1) | 86 (34.0) | ||
| Never | 631 (48.8) | 108 (45.4) | 163 (58.8) | 113 (42.3) | 138 (54.5) | ||
| Physical activity, mean (SD), min/week | 1,308 | 110.9 (150.3) | 131.1 (162.0) | 124 (163.3) | 96 (140.4) | 93 (142.5) | .006 |
| Binge drinking, no. (%) | 951 | 145 (15.2) | 27 (14.4) | 16 (8.7) | 28 (14.1) | 30 (17.0) | .25 |
| Diabetes, no. (%) | 1,277 | 283 (22.2) | 45 (19.2) | 52 (19.0) | 62 (23.6) | 52 (20.9) | .40 |
| Hypertension medication, no. (%) | 1,326 | 728 (54.9) | 115 (47.7) | 153 (54.1) | 158 (57.9) | 147 (57.0) | .03 |
| Negative affect, mean (SD) | 930 | 0.0 (0.8) | −0.1 (0.8) | −0.1 (0.7) | 0.0 (0.8) | 0.1 (0.9) | .01 |
| Optimism, mean (SD) | 1,208 | 19.0 (3.5) | 19.7 (3.5) | 19.4 (3.2) | 18.7 (3.4) | 18.5 (3.6) | <.001 |
| DNA Methylation GrimAge, mean (SD) | 495 | 64.8 (6.5) | 63.4 (5.5) | 64.3 (6.2) | 64.3 (6.1) | 64.4 (6.8) | .63 |
| Heart failure, no. (%) | 1,448 | 334 (23.1) | 59 (22.6) | 55 (18.0) | 72 (24.6) | 78 (28.0) | .04 |
| Death, no. (%) | 1,448 | 640 (44.2) | 99 (37.9) | 112 (36.7) | 122 (41.6) | 131 (47.0) | .01 |
Notes: ADI = Area Deprivation Index; ARIC = Atherosclerosis Risk in Communities; JHS = Jackson Heart Study; SD = standard deviation. Study baseline defined as January 1, 2000. The Area Deprivation Index ranges from 1 to 100. Quartiles in the analytic sample ranged from 31 to 100. Perceived neighborhood problems were calculated from a standardized multilevel categorical confirmatory factor analysis model. Quartiles in the analytic sample ranged from −2.04 to 1.46. Univariate baseline differences in study variables were assessed using linear regression, Cochran–Armitage trend tests, Cochran–Mantel–Haenszel trend tests as appropriate.
The bolded words indicate the primary exposure of interest.
The unadjusted model fit the data well (McFadden’s R-squared > 0.7). Continuous, standardized measures of ADI and PNP (Tables 2 and 3) exhibited a statistically significant association with incident HF (Path C) but not biological aging (Path A). However, increased biological aging was associated with elevated risk of HF (Path B), and joint significance (Paths A and B) was observed for the indirect effect of ADI and PNP on incident HF in models that included negative affect as a moderator. A 1-SD higher level of negative affect was associated with an increase in the indirect (mediated) effect of continuous, standardized PNP on incident HF in both unadjusted (HR 1.06, 95% CI 1.02–1.11) and adjusted (HR 1.05, 95% CI 1.01–1.09) models. The effect was even more pronounced when comparing the highest quartile of PNP to the lowest. For each 1-SD higher level of negative affect, the unadjusted hazard was 26% higher (HR 1.26, 95% CI 1.14–1.50) and the adjusted hazard was 18% higher (HR 1.18, 95% CI 1.05–1.36). A similar point estimate was observed for ADI but the index of moderated mediation had wider CIs, preventing a definitive conclusion. In contrast, there was no indication that optimism moderated the indirect (mediated) effect of neighborhood disadvantage. Analogous but attenuated estimates were evident in sensitivity analyses that incorporated additional covariates (Supplementary Tables 1–4).
Table 2.
Moderated Mediation Analysis Examining Whether Indirect Effects via Biologic Aging of Area Deprivation Index on Incident Heart Failure Are Moderated by Psychosocial Risk and Resilience: ARIC–JHS Cohort 2000–2017 (N = 1,448)
| Moderator: Negative affect | Moderator: Optimism | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Path A | β (95% CI) | β (95% CI) | ||
| ADI (standardized) | 0.01 (−0.17, 0.30) | 0.41 (−0.04, 0.95) | −0.10 (−0.78, 0.00) | 0.40 (−0.32, 0.67) |
| Moderator (standardized) | 0.55 (−0.76, 1.40) | 0.37 (−0.83, 1.05) | −0.89 (−2.44, −0.01) | −0.20 (−1.43, 0.29) |
| ADI × Moderator | 0.27 (−1.26, 1.64) | 0.64 (−0.45, 1.91) | 0.39 (−0.31, 2.92) | 0.12 (−0.23, 1.81) |
| Path B | HR (95% CI) | HR (95% CI) | ||
| Biological aging (GrimAge) | 1.07 (1.04, 1.10) | 1.06 (1.02, 1.11) | 1.06 (1.03, 1.09) | 1.06 (1.00, 1.10) |
| Path C | HR (95% CI) | HR (95% CI) | ||
| ADI (standardized) | 1.32 (1.15, 1.86) | 1.29 (1.14, 1.79) | 1.33 (1.17, 1.87) | 1.29 (1.14, 1.82) |
| Moderated mediation | HR (95% CI) | HR (95% CI) | ||
| Direct effect | 1.32 (1.15, 1.86) | 1.29 (1.14, 1.79) | 1.33 (1.17, 1.87) | 1.29 (1.14, 1.82) |
| Indirect Effect | 1.00 (0.99, 1.02) | 1.03 (1.00, 1.07) | 0.99 (0.96, 1.00) | 1.02 (0.98, 1.05) |
| Index of moderated mediation | 1.02 (0.93, 1.10) | 1.04 (0.99, 1.15) | 1.02 (0.98, 1.18) | 1.01 (0.98, 1.14) |
| Path A | β (95% CI) | β (95% CI) | ||
| ADI (Q2 vs Q1) | 0.06 (−8.65, 1.95) | 0.87 (−2.30, 2.76) | −0.03 (−1.35, 0.93) | 0.96 (−1.38, 3.48) |
| ADI (Q3 vs Q1) | 0.11 (−0.29, 3.65) | 0.77 (−1.62, 2.92) | −0.04 (−0.79, 0.60) | 0.81 (−1.57, 1.62) |
| ADI (Q4 vs Q1) | 0.08 (−0.19, 3.79) | 1.17 (−1.07, 3.47) | −0.01 (−0.31, 1.44) | 1.20 (−1.03, 2.06) |
| Moderator (Standardized) | −0.47 (−8.62, 0.00) | −1.05 (−8.06, −0.24) | −0.43 (−2.91, 0.83) | 0.08 (−4.65, 0.60) |
| ADI (Q2 vs Q1) × Moderator | 1.35 (−1.37, 7.73) | 1.32 (−2.49, 5.98) | −1.88 (−5.78, 0.51) | −1.07 (−6.87, 3.28) |
| ADI (Q3 vs Q1) × Moderator | 1.59 (−0.24, 8.02) | 2.11 (−0.08, 7.28) | −1.10 (−3.44, 0.86) | −0.50 (−5.87, 3.57) |
| ADI (Q4 vs Q1) × Moderator | 0.78 (−3.46, 6.32) | 1.79 (−0.72, 6.33) | 0.99 (-1.85, 3.01) | 0.20 (−0.94, 4.69) |
| Path B | HR (95% CI) | |||
| Biological aging (GrimAge) | 1.07 (1.03, 1.10) | 1.06 (1.02, 1.11) | 1.07 (1.04, 1.10) | 1.06 (1.02, 1.11) |
| Path C | HR (95% CI) | |||
| ADI (Q2 vs Q1) | 1.02 (0.47, 2.02) | 0.98 (0.45, 1.72) | 1.02 (0.53, 2.00) | 0.98 (0.52, 1.73) |
| ADI (Q3 vs Q1) | 1.36 (0.59, 2.32) | 1.31 (0.58, 2.19) | 1.37 (0.70, 2.37) | 1.31 (0.71, 2.17) |
| ADI (Q4 vs Q1) | 1.83 (0.83, 3.23) | 1.72 (0.77, 2.98) | 1.84 (0.93, 3.24) | 1.72 (0.91, 2.83) |
| Moderated mediation | HR (95% CI) | HR (95% CI) | ||
| Direct effect (Q2 vs Q1) | 1.02 (0.47, 2.02) | 0.98 (0.45, 1.72) | 1.02 (0.53, 2.00) | 0.98 (0.52, 1.73) |
| Direct effect (Q3 vs Q1) | 1.36 (0.59, 2.32) | 1.31 (0.58, 2.19) | 1.37 (0.70, 2.37) | 1.31 (0.71, 2.17) |
| Direct effect (Q4 vs Q1) | 1.83 (0.83, 3.23) | 1.72 (0.77, 2.98) | 1.84 (0.93, 3.24) | 1.72 (0.91, 2.83) |
| Indirect effect (Q2 vs Q1) | 1.00 (0.85, 1.11) | 1.06 (0.90, 1.22) | 1.00 (0.94, 1.06) | 1.06 (0.94, 1.41) |
| Indirect effect (Q3 vs Q1) | 1.01 (0.98, 1.24) | 1.05 (0.89, 1.20) | 1.00 (0.96, 1.04) | 1.05 (0.93, 1.15) |
| Indirect effect (Q4 vs Q1) | 1.01 (0.99, 1.29) | 1.07 (0.94, 1.27) | 1.00 (0.98, 1.09) | 1.08 (0.95, 1.22) |
| Index of moderated mediation (Q2 vs Q1) | 1.09 (0.89, 1.56) | 1.09 (0.87, 1.50) | 0.89 (0.64, 1.02) | 0.94 (0.67, 1.19) |
| Index of moderated mediation (Q3 vs Q1) | 1.11 (0.97, 1.60) | 1.14 (0.94, 1.52) | 0.93 (0.81, 1.04) | 0.97 (0.71, 1.22) |
| Index of moderated mediation (Q4 vs Q1) | 1.05 (0.81, 1.46) | 1.12 (0.98, 1.52) | 1.07 (0.90, 1.21) | 1.01 (0.93, 1.35) |
Notes: β = linear regression coefficient; ADI = Area Deprivation Index; ARIC = Atherosclerosis Risk in Communities; CI = confidence intervals; HR = hazard ratio; JHS = Jackson Heart Study. The exposure Area Deprivation Index is a continuous measure that ranges from 1 to 100. One standard deviation is equal to 14.77 with quartiles (Q) in the analytic sample ranging from 31 to 100 (Q1 = 31–77, Q2 = 78–87, Q3 = 88–93, Q4 = 94–100). The mediator GrimAge was computed from an epigenetic clock composite of DNAm-based markers for seven plasma proteins and self-reported smoking pack years. The moderator negative affect ranged from −1.71 to 4.83, and was calculated from a standardized single-level continuous confirmatory factor analysis model. The moderator optimism ranged from 6 to 24. One standard deviation is equal to 3.3. The outcome of adjudicated incident heart failure was determined from medical records. Parameter estimates generated by fitting a causal moderated mediation structural equation model. Coefficients (β) from Path A represent estimates from linear regression. Hazard ratios from all other paths represent estimates from cause-specific, Cox proportional hazards regression. Full-information maximum likelihood was employed to account for missing values. Adjusted model included sex and chronologic age at baseline.
Table 3.
Moderated Mediation Analysis Examining Whether Indirect Effects via Biologic Aging of Perceived Neighborhood Problems on Incident Heart Failure Are Moderated by Psychosocial Risk and Resilience: ARIC–JHS Cohort 2000–2017 (N = 1,448)
| Moderator: Negative affect | Moderator: Optimism | |||
|---|---|---|---|---|
| Unadjusted | Adjusted | Unadjusted | Adjusted | |
| Path A | β (95% CI) | β (95% CI) | ||
| PNP (standardized) | −0.08 (−0.31, −0.02) | 0.30 (−0.11, 0.61) | −0.02 (−0.17, 0.10) | 0.36 (−0.03, 0.70) |
| Moderator (standardized) | 0.70 (0.01, 1.35) | 0.69 (0.16, 1.16) | −0.72 (−1.42, 0.23) | −0.11 (−0.56, 0.42) |
| PNP × Moderator | 0.85 (0.34, 1.88) | 0.77 (0.37, 1.26) | 0.04 (−0.55, 1.11) | −0.08 (−0.48, 0.59) |
| Path B | HR (95% CI) | HR (95% CI) | ||
| Biological aging (GrimAge) | 1.07 (1.04, 1.10) | 1.06 (1.02, 1.10) | 1.06 (1.03, 1.09) | 1.06 (1.01, 1.10) |
| Path C | HR (95% CI) | HR (95% CI) | ||
| PNP (standardized) | 1.18 (1.01, 1.38) | 1.16 (0.99, 1.34) | 1.18 (1.00, 1.37) | 1.16 (0.99, 1.34) |
| Moderated mediation | HR (95% CI) | HR (95% CI) | ||
| Direct effect | 1.18 (1.01, 1.38) | 1.16 (0.99, 1.34) | 1.18 (1.00, 1.37) | 1.16 (0.99, 1.34) |
| Indirect effect | 1.00 (0.98, 1.00) | 1.02 (1.00, 1.04) | 1.00 (0.99, 1.01) | 1.02 (1.00, 1.05) |
| Index of moderated mediation | 1.06 (1.02, 1.11) | 1.05 (1.01, 1.09) | 1.00 (0.97, 1.07) | 1.00 (0.98, 1.04) |
| Path A | β (95% CI) | β (95% CI) | ||
| PNP (Q2 vs Q1) | 0.02 (−0.53, 0.34) | 0.58 (−0.37, 2.27) | −0.07 (−1.09, 0.20) | 0.59 (−0.44, 2.28) |
| PNP (Q3 vs Q1) | −0.17 (−0.72, 0.04) | 0.77 (−0.06, 1.56) | −0.12 (−0.64, 0.27) | 0.87 (0.00, 1.66) |
| PNP (Q4 vs Q1) | −0.03 (−1.06, 0.38) | 1.00 (−0.18, 2.09) | 0.08 (−0.59, 0.52) | 1.11 (0.10, 2.39) |
| Moderator (standardized) | −0.66 (−2.53, −0.16) | −0.38 (−1.29, 0.10) | −0.91 (−2.33, −0.39) | 0.11 (−1.06, 0.61) |
| PNP (Q2 vs Q1) × Moderator | 1.21 (−0.59, 4.28) | 0.92 (−1.05, 2.09) | −0.47 (−3.69, 1.83) | −0.58 (−1.77, 1.14) |
| PNP (Q3 vs Q1) × Moderator | 0.94 (-0.44, 2.64) | 0.76 (-0.30, 1.98) | 1.44 (-0.50, 3.31) | 0.25 (-0.43, 1.40) |
| PNP (Q4 vs Q1) × Moderator | 3.58 (2.08, 5.90) | 2.71 (1.54, 3.93) | -0.72 (-2.69, 1.29) | -0.73 (-2.23, 0.83) |
| Path B | HR (95% CI) | HR (95% CI) | ||
| Biological aging (GrimAge) | 1.07 (1.04, 1.10) | 1.06 (1.02, 1.10) | 1.06 (1.03, 1.09) | 1.06 (1.01, 1.11) |
| Path C | HR (95% CI) | HR (95% CI) | ||
| PNP (Q2 vs Q1) | 0.91 (0.53, 1.36) | 0.89 (0.52, 1.40) | 0.91 (0.53, 1.37) | 0.90 (0.53, 1.42) |
| PNP (Q3 vs Q1) | 1.12 (0.64, 1.67) | 1.09 (0.62, 1.63) | 1.11 (0.64, 1.64) | 1.08 (0.63, 1.63) |
| PNP (Q4 vs Q1) | 1.40 (0.76, 2.45) | 1.35 (0.76, 2.22) | 1.40 (0.77, 2.50) | 1.34 (0.77, 2.22) |
| Moderated mediation | HR (95% CI) | HR (95% CI) | ||
| Direct effect (Q2 vs Q1) | 0.91 (0.53, 1.36) | 0.89 (0.52, 1.40) | 0.91 (0.53, 1.37) | 0.90 (0.53, 1.42) |
| Direct effect (Q3 vs Q1) | 1.12 (0.64, 1.67) | 1.09 (0.62, 1.63) | 1.11 (0.64, 1.64) | 1.08 (0.63, 1.63) |
| Direct effect (Q4 vs Q1) | 1.40 (0.76, 2.45) | 1.35 (0.76, 2.22) | 1.40 (0.77, 2.50) | 1.34 (0.77, 2.22) |
| Indirect effect (Q2 vs Q1) | 1.00 (0.98, 1.02) | 1.04 (0.99, 1.20) | 1.00 (0.92, 1.01) | 1.04 (0.98, 1.21) |
| Indirect effect (Q3 vs Q1) | 0.99 (0.95, 1.00) | 1.05 (1.00, 1.13) | 0.99 (0.96, 1.02) | 1.05 (1.00, 1.15) |
| Indirect effect (Q4 vs Q1) | 1.00 (0.93, 1.02) | 1.06 (0.99, 1.17) | 1.01 (0.96, 1.03) | 1.07 (1.00, 1.19) |
| Index of moderated mediation (Q2 vs Q1) | 1.08 (0.96, 1.28) | 1.06 (0.94, 1.19) | 0.97 (0.77, 1.10) | 0.97 (0.86, 1.05) |
| Index of moderated mediation (Q3 vs Q1) | 1.06 (0.98, 1.21) | 1.05 (0.99, 1.17) | 1.09 (0.97, 1.26) | 1.01 (0.97, 1.13) |
| Index of moderated mediation (Q4 vs Q1) | 1.26 (1.14, 1.50) | 1.18 (1.05, 1.36) | 0.96 (0.85, 1.10) | 0.96 (0.87, 1.04) |
Notes: β = linear regression coefficient; ARIC = Atherosclerosis Risk in Communities; CI = confidence intervals; HR = hazard ratio; JHS = Jackson Heart Study; PNP = perceived neighborhood problems. The continuous exposure perceived neighborhood problems was calculated from a standardized multilevel categorical confirmatory factor analysis model with quartiles in the analytic sample ranging from −2.04 to 1.46 (Q1 = −2.04 to −0.56, Q2 = −0.55–0.00, Q3 = 0.01–0.55, Q4 = 0.56–1.4). The mediator GrimAge was computed from an epigenetic clock composite of DNAm-based markers for seven plasma proteins and self-reported smoking pack years. The moderator negative affect ranged from −1.71 to 4.83 and was calculated from a standardized single-level continuous confirmatory factor analysis model. The moderator optimism ranged from 6 to 24. One standard deviation is equal to 3.3. The outcome of adjudicated incident heart failure was determined from medical records. Parameter estimates generated by fitting a causal moderated mediation structural equation model. Coefficients (β) from Path A represent estimates from linear regression. Hazard ratios from all other paths represent estimates from cause-specific, Cox proportional hazards regression. Full-information maximum likelihood was employed to account for missing values. Adjusted model included sex and chronologic age at baseline.
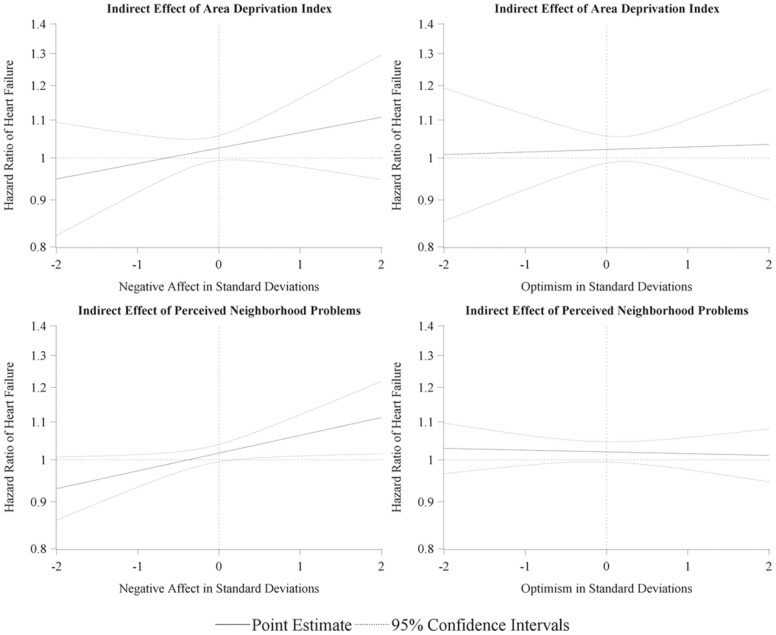
The nature of the hypothesized moderated mediation mechanism is apparent when visualized utilizing the Johnson–Neyman technique (Figure 2). The region of significance in the relationship between the moderator and the indirect (mediated) effect of PNP begins as the negative affect exceeds 0.17 SDs. Within the analytic sample, 38.3% of participants had a negative affect score above this threshold, suggesting that the observed mechanism operated in more than a third of the cohort.
Figure 2.
Visualization of indirect (mediated) effect of neighborhood disadvantage on incident heart failure when moderated by psychosocial negative affect and optimism: ARIC–JHS cohort 2000–2017 (N = 1,448). Johnson–Neyman visualization of conditional indirect (mediated) effect of exposure generated from a covariate-adjusted, cause-specific, Cox proportional hazards, moderated mediation structural equation model. ARIC = Atherosclerosis Risk in Communities; JHS = Jackson Heart Study.
Discussion
In a sample of Black adults in a southern urban city, we found evidence consistent with our hypothesis regarding the relationship of neighborhood disadvantage, biological aging, and psychosocial risk and resilience in HF incidence. Our results indicate that the process by which subjective neighborhood disadvantage, measured as PNP, acts to confer an increased risk of HF through advancing biological age is conditional on a psychosocial risk factor, dispositional negative affect, such that the greater an individual’s negative affect, the greater the indirect (mediated) effect of neighborhood disadvantage on the risk of HF. We did not find support for a moderating role of optimism, a psychosocial resilience factor, in this relationship.
In a previous study, we demonstrated that accelerated biological age mediated a majority of the effect of PNP on HF risk (Bey et al., 2022). We took this finding as evidence that the subjective perception of disadvantage may act to influence cardiovascular health by acting as a chronic stressor. This exposure to chronic stress has been suggested to trigger lasting epigenetic alterations, as those measured by DNAm-based biological clocks such as GrimAge, that can contribute to age-related physiological decline (Palma-Gudiel et al., 2020), aberrant inflammatory signaling, and cardiometabolic worsening (Fiorito et al., 2017; Gomez-Alonso et al., 2021; Stringhini et al., 2015), ultimately manifesting in HF risk. Here, we identified how the effect of perceiving neighborhood problems on age-related physiological decline may be contingent upon an individual’s degree of dispositional negative affect, which aligns with a large body of evidence for a role of psychosocial factors in cardiovascular outcomes (Rozanski et al., 2019; Sims et al., 2019).
Negative affect is defined as feelings of emotional distress stemming from a host of unpleasant emotions including anxiety, sadness, fear, anger, guilt, shame, and irritation. The tendency to experience such a state is characterized as dispositional (or trait) negative affect (Stringer, 2013) and has been shown to contribute to a variety of poor health outcomes (Bleil et al., 2008). The IVP framework (Bey, 2022; Bey et al., 2019) draws from a well-established evidence base, including evidence of underlying inflammation in depressive disorders (Dhar & Barton, 2016) and an association between negative affect and inflammation (Miyamoto et al., 2013; Slavish et al., 2020), in positing that this psychosocial risk factor acts on HF through amplifying the perception, and subsequently, the physiological consequences, of stress associated with potentially stressful experiences. Accordingly, we found evidence that the indirect (mediated) effect of subjective neighborhood disadvantage, hypothesized as a chronic stressor, on HF varies across levels of negative affect.
We did not find evidence that optimism similarly moderates the mediated effect, building on the literature which suggests that psychosocial risk and resilience factors operate along distinct pathways to affect health (Watson et al., 1988). In addition to potentially increasing the likelihood of appraised stress associated with adverse experiences (Watson et al., 1988), negative affect has been shown to promote engagement in maladaptive coping behaviors in the context of stress, including cigarette smoking and excessive alcohol consumption (Ellis et al., 2015; Sims et al., 2017) but has been found to have less association with health-promoting behaviors. When examining differences by race, researchers found that dispositional negative affect predicted lower fruit and vegetable consumption among Whites, but was not associated with any health behaviors among Blacks (Ellis et al., 2015). In contrast, robust evidence suggests that higher levels of optimism yield greater engagement in health-promoting behaviors such as physical activity and fruit and vegetable consumption across ethnoracial groups, including studies also among the JHS cohort (Sims et al., 2019). Further indicating distinct pathways, research has also demonstrated that optimism is not associated with biological aging (Kim et al., 2018). Our current findings lack support for optimism as a moderator of the pathway from subjective neighborhood disadvantage to HF through biological aging and builds on this evidence for distinct mechanistic actions of negative affect and optimism in the development of HF. Thus, these results point to the necessity of methods for addressing the adverse social experiences to which Black persons are disproportionately exposed that increase the risk of developing negative affective dispositions.
This analysis has many strengths. We employed creative use of data from two prospective, community-based cohorts in Jackson, MS. This analysis also used causal, multilevel models to capture the complex processes linking neighborhood disadvantage with the development of HF outlined by a comprehensive theoretical framework. Additionally, we took an innovative approach to investigate within-race variability in HF risk and resilience factors among Black persons. Still, some important limitations should be considered in interpreting the results of this study. Our findings are not based on a nationally representative sample and should only be cautiously generalized beyond Black persons in Jackson, MS. As with most models, there is potential for bias from residual confounding. Consistency in the effect estimates across the various sets of covariate models included as sensitivity analyses, however, should assuage major concerns regarding confounding bias. While the DNAm data used to calculate GrimAge were collected prior to the baseline examination in which the PNP exposure was assessed and prior to when the census data were collected for the ADI measure, we conceptualize the effect of neighborhood disadvantage as a cumulative process that began long before the single instance it was measured in this study. With this conceptualization of the PNP and ADI exposures as indicative of chronic rather than acute stress, for this analysis, we assumed that level of neighborhood stress was consistent over time. Even considering potential changes to the neighborhood environment over time, the low level of residence transition (~14%, Wang et al., 2017) within our study population over the study period as well as studies showing that stressors tend to accumulate over the life course (Sternthal et al., 2011) suggests the validity of this assumption.
In summary, this study provides evidence for an important role of psychosocial risk—rather than resilience—in influencing the extent to which the perception of persistent neighborhood disadvantage will lead to premature aging and subsequent development of HF among Black persons. These results add to a growing body of literature supporting the geroscientific perspective of aging as a key target for mitigating chronic disease risk. The variety of factors, including specific individual psychological predispositions and subjective experiences of neighborhood-level conditions, that influence the pace of aging demonstrate the need for multilevel interventions targeting both individuals and their contexts. Given the modifiable nature of negative affect, a primary contribution of this study is additional insight into actionable influences on the age-related chronic conditions that disproportionately affect ethnoracially minoritized populations.
Supplementary Material
Acknowledgment
The authors would like to extend their deepest gratitude to Gerardo Heiss for his invaluable contribution to this work.
Contributor Information
Ganga S Bey, Department of Epidemiology, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
James R Pike, Bloomberg School of Public Health, Johns Hopkins University, Baltimore, Maryland, USA.
Anthony S Zannas, Department of Psychiatry and Neuroscience, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina, USA.
Qian Xiao, University of Texas Health Sciences Center at Houston, Houston, Texas, USA.
Bing Yu, School of Public Health, University of Texas Health Sciences Center at Houston, Houston, Texas, USA.
Amil M Shah, Department of Medicine, Harvard Medical School, Boston, Massachusetts, USA.
Priya Palta, Department of Neurology, University of North Carolina at Chapel Hill School of Medicine Chapel Hill, North Carolina, USA.
Funding
The Atherosclerosis Risk in Communities (ARIC) study has been funded in whole or in part with Federal funds from the National Heart, Lung, and Blood Institute (NHLBI), National Institutes of Health (NIH), Department of Health and Human Services, under Contract nos. (75N92022D00001, 75N92022D00002, 75N92022D00003, 75N92022D00004, 75N92022D00005). Funding was also supported by 5RC2HL102419 and R01NS087541. The authors thank the staff and participants of the ARIC study for their important contributions.
The Jackson Heart Study (JHS) is supported and conducted in collaboration with Jackson State University (HHSN268201800013I), Tougaloo College (HHSN268201800014I), the Mississippi State Department of Health (HHSN268201800015I) and the University of Mississippi Medical Center (HHSN268201800010I, HHSN268201800011I, and HHSN268201800012I) contracts from the NHLBI and the National Institute on Minority Health and Health Disparities. The authors also wish to thank the staffs and participants of the JHS.
G. Bey was in part supported by NIH/ National Institute on Aging (NIA) grants K99AG075327-01, 5P30AG059303-04, and R01AG066134. P. Palta was in part supported by NIH/NIA grant R01AG066134.
Conflict of Interest
None.
Data Availability
Data used for this analysis may be obtained through requests to the JHS and ARIC coordinating centers. This is not a preregistered study.
Disclaimer
The views expressed in this manuscript are those of the authors and do not necessarily represent the views of the National Heart, Lung, and Blood Institute; the National Institutes of Health; or the U.S. Department of Health and Human Services.
References
- Allison, P. D. (2003). Missing data techniques for structural equation modeling. Journal of Abnormal Psychology, 112(4), 545–557. 10.1037/0021-843X.112.4.545 [DOI] [PubMed] [Google Scholar]
- Barefoot, J. C., Dodge, K. A., Peterson, B. L., Dahlstrom, W. G., & Williams, R. B. (1989). The Cook–Medley hostility scale: Item content and ability to predict survival. Psychosomatic Medicine, 51(1), 46–57. 10.1097/00006842-198901000-00005 [DOI] [PubMed] [Google Scholar]
- Bey, G., Pike, J., & Palta, P. (2023). Distinct moderating pathways for psychosocial risk and resilience in the association of neighborhood disadvantage with incident heart failure among Black persons. Social Science and Medicine—Population Health. 10.1016/j.ssmph.2023.101475 [DOI] [PMC free article] [PubMed]
- Bey, G., Pike, J., Palta, P., Zannas, A., Xiao, Q., Love, S.-A., & Heiss, G. (2022). Biological age mediates the effects of perceived neighborhood problems on heart failure risk among Black persons. Journal of Racial and Ethnic Health Disparities. 10.1007/s40615-022-01476-3 [DOI] [PMC free article] [PubMed]
- Bey, G. S. (2022). The Identity Vitality-Pathology model: A novel theoretical framework proposing “identity state” as a modulator of the pathways from structural to health inequity. Social Science & Medicine, 314, 115495. 10.1016/j.socscimed.2022.115495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey, G. S., Jesdale, B., Forrester, S., Person, S. D., & Kiefe, C. (2019). Intersectional effects of racial and gender discrimination on cardiovascular health vary among black and white women and men in the CARDIA study. SSM—Population Health, 8, 100446. 10.1016/j.ssmph.2019.100446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bey, G. S., Ulbricht, C. M., & Person, S. D. (2019). Theories for race and gender differences in management of social identity-related stressors: A systematic review. Journal of Racial and Ethnic Health Disparities, 6(1), 117–132. 10.1007/s40615-018-0507-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleil, M. E., Gianaros, P. J., Jennings, J. R., Flory, J. D., & Manuck, S. B. (2008). Trait negative affect: Toward an integrated model of understanding psychological risk for impairment in cardiac autonomic function. Psychosomatic Medicine, 70(3), 328–337. 10.1097/PSY.0b013e31816baefa [DOI] [PubMed] [Google Scholar]
- Carter, R. T., Kirkinis, K., & Johnson, V. E. (2020). Relationships between trauma symptoms and race-based traumatic stress. Traumatology, 26(1), 11–18. 10.1037/trm0000217 [DOI] [Google Scholar]
- Chang, P. P., Wruck, L. M., Shahar, E., Rossi, J. S., Loehr, L. R., Russell, S. D., Agarwal, S. K., Konety, S. H., Rodriguez, C. J., & Rosamond, W. D. (2018). Trends in hospitalizations and survival of acute decompensated heart failure in four US communities (2005–2014): The Atherosclerosis Risk in Communities (ARIC) Study Community Surveillance. Circulation, 138(1), 12–24. 10.1161/CIRCULATIONAHA.117.027551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, J., Christ, N. M., Shih, C. H., Xie, H., Grider, S. R., Lewis, C., Elhai, J. D., & Wang, X. (2021). Dispositional optimism mediates relations between childhood maltreatment and PTSD symptom severity among trauma-exposed adults. Child Abuse & Neglect, 115, 105023. doi: 10.1016/j.chiabu.2021.105023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, C. R., Ommerborn, M. J., Hickson, D. A., Grooms, K. N., Sims, M., Taylor, H. A., & Albert, M. A. (2013). Neighborhood disadvantage, neighborhood safety and cardiometabolic risk factors in African Americans: Biosocial Associations in the Jackson Heart Study. PLoS One, 8(5), e63254. 10.1371/journal.pone.0063254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhar, A. K., & Barton, D. A. (2016). Depression and the link with cardiovascular disease. Frontiers in Psychiatry, 7, 33. 10.3389/fpsyt.2016.00033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diez Roux, A. V. (2016). Neighborhoods and health: What do we know? What should we do? American Journal of Public Health, 106(3), 430–431. 10.2105/AJPH.2016.303064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, E. M., Orom, H., Giovino, G. A., & Kiviniemi, M. T. (2015). Relations between negative affect and health behaviors by race/ethnicity: Differential effects for symptoms of depression and anxiety. Health Psychology, 34(9), 966–969. 10.1037/hea0000197 [DOI] [PubMed] [Google Scholar]
- Elo, I. T., Mykyta, L., Margolis, R., & Culhane, J. F. (2009). Perceptions of neighborhood disorder: The role of individual and neighborhood characteristics. Social Science Quarterly, 90(5), 1298–1320. 10.1111/j.1540-6237.2009.00657.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiorito, G., Polidoro, S., Dugué, P. -A., Kivimaki, M., Ponzi, E., Matullo, G., Guarrera, S., Assumma, M. B., Georgiadis, P., Kyrtopoulos, S. A., Krogh, V., Palli, D., Panico, S., Sacerdote, C., Tumino, R., Chadeau-Hyam, M., Stringhini, S., Severi, G., Hodge, A. M., … Vineis, P. (2017). Social adversity and epigenetic aging: A multi-cohort study on socioeconomic differences in peripheral blood DNA methylation. Scientific Reports, 7(1), 16266. 10.1038/s41598-017-16391-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geronimus, A. T., Hicken, M., Keene, D., & Bound, J. (2006). “Weathering” and age patterns of allostatic load scores among blacks and whites in the United States. American Journal of Public Health, 96(5), 826–833. 10.2105/AJPH.2004.060749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Alonso, M. D. C., Kretschmer, A., Wilson, R., Pfeiffer, L., Karhunen, V., Seppälä, I., Zhang, W., Mittelstraß, K., Wahl, S., Matias-Garcia, P. R., Prokisch, H., Horn, S., Meitinger, T., Serrano-Garcia, L. R., Sebert, S., Raitakari, O., Loh, M., Rathmann, W., Müller-Nurasyid, M., … Waldenberger, M. (2021). DNA methylation and lipid metabolism: An EWAS of 226 metabolic measures. Clinical Epigenetics, 13(1), 7. 10.1186/s13148-020-00957-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayes, A. F. (2015). an index and test of linear moderated mediation. Multivariate Behavioral Research, 50(1), 1–22. 10.1080/00273171.2014.962683 [DOI] [PubMed] [Google Scholar]
- Hayes, A. F., & Rockwood, N. J. (2020). Conditional process analysis: Concepts, computation, and advances in the modeling of the contingencies of mechanisms. American Behavioral Scientist, 64(1), 19–54. 10.1177/0002764219859633 [DOI] [Google Scholar]
- Johnson, P. O., & Fay, L. C. (1950). The Johnson–Neyman technique, its theory and application. Psychometrika, 15(4), 349–367. 10.1007/BF02288864 [DOI] [PubMed] [Google Scholar]
- Kim, E. S., DeMeo, D. L., Fong, K. C., Lee, L. O., Grodstein, F., & Kubzansky, L. D. (2018). Optimism and biological aging. Innovation in Aging, 2(Suppl 1), 842–842. 10.1093/geroni/igy023.3138 [DOI] [Google Scholar]
- Kind, A. J. H., & Buckingham, W. R. (2018). Making neighborhood-disadvantage metrics accessible—The neighborhood atlas. The New England Journal of Medicine, 378(26), 2456–2458. 10.1056/NEJMp1802313 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, T., & Shi, D. (2021). A comparison of full information maximum likelihood and multiple imputation in structural equation modeling with missing data. Psychological Methods, 26(4), 466–485. 10.1037/met0000381 [DOI] [PubMed] [Google Scholar]
- Levine, M. E. (2020). Assessment of epigenetic clocks as biomarkers of aging in basic and population research. The Journals of Gerontology, Series A: Biological Sciences and Medical Sciences, 75(3), 463–465. 10.1093/gerona/glaa021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, A. T., Quach, A., Wilson, J. G., Reiner, A. P., Aviv, A., Raj, K., Hou, L., Baccarelli, A. A., Li, Y., Stewart, J. D., Whitsel, E. A., Assimes, T. L., Ferrucci, L., & Horvath, S. (2019). DNA methylation GrimAge strongly predicts lifespan and healthspan. Aging, 11(2), 303–327. 10.18632/aging.101684 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen, B. S. (1998). Stress, adaptation, and disease. Allostasis and allostatic load. Annals of the New York Academy of Sciences, 840, 33–44. 10.1111/j.1749-6632.1998.tb09546.x [DOI] [PubMed] [Google Scholar]
- Mersky, J. P., Choi, C., Lee, C. P., & Janczewski, C. E. (2021). Disparities in adverse childhood experiences by race/ethnicity, gender, and economic status: Intersectional analysis of a nationally representative sample. Child Abuse & Neglect, 117, 105066. doi: 10.1016/j.chiabu.2021.105066. [DOI] [PubMed] [Google Scholar]
- Miyamoto, Y., Boylan, J. M., Coe, C. L., Curhan, K. B., Levine, C. S., Markus, H. R., Park, J., Kitayama, S., Kawakami, N., Karasawa, M., Love, G. D., & Ryff, C. D. (2013). Negative emotions predict elevated interleukin-6 in the United States but not in Japan. Brain, Behavior, and Immunity, 34, 79–85. 10.1016/j.bbi.2013.07.173 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthen & Muthen. (1998). Mplus user’s guide (8th ed.). Muthen & Muthen. [Google Scholar]
- Muthén, B., & Asparouhov, T. (2015). Causal effects in mediation modeling: An introduction with applications to latent variables. Structural Equation Modeling: A Multidisciplinary Journal, 22(1), 12–23. 10.1080/10705511.2014.935843 [DOI] [Google Scholar]
- Nayak, A., Hicks, A. J., & Morris, A. A. (2020). Understanding the complexity of heart failure risk and treatment in black patients. Circulation: Heart Failure, 13(8), e007264. 10.1161/CIRCHEARTFAILURE.120.007264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, S., Northuis, C. A., Guan, W., Bressler, J., Grove, M., Xia, R., Wang, Z., Fernandez-Rhodes, L., Justice, A. E., Li, Y., Whitsel, E., North, K. E., Fornage, M., Boerwinkle, E., Pankow, J. S., & Demerath, E. W. (2021). Abstract 033: Epigenetic clocks and incident heart failure: The Atherosclerosis Risk in Communities (ARIC). Circulation, 143(Suppl_1), A033–A033. 10.1161/circ.143.suppl_1.033 [DOI] [Google Scholar]
- Palma-Gudiel, H., Fañanás, L., Horvath, S., & Zannas, A. S. (2020). Psychosocial stress and epigenetic aging. In Clow A. & Smyth N. (Eds.), International Review of Neurobiology (Vol. 150, pp. 107–128). Elsevier. 10.1016/bs.irn.2019.10.020 [DOI] [PubMed] [Google Scholar]
- Park, J. W., Mealy, R., Saldanha, I. J., Loucks, E. B., Needham, B. L., Sims, M., Fava, J. L., Dulin, A. J., & Howe, C. J. (2022). Multilevel resilience resources and cardiovascular disease in the United States: A systematic review and meta-analysis. Health Psychology, 41(4), 278–290. 10.1037/hea0001069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roetker, N. S., Pankow, J. S., Bressler, J., Morrison, A. C., & Boerwinkle, E. (2018). A prospective study of epigenetic age acceleration and incidence of cardiovascular disease outcomes in the Atherosclerosis Risk in Communities (ARIC) Study. Circulation. Genomic and Precision Medicine, 11(3), e001937. 10.1161/CIRCGEN.117.001937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross, C. E., & Mirowsky, J. (1999). Disorder and decay: The concept and measurement of perceived neighborhood disorder. Urban Affairs Review, 34(3), 412–432. 10.1177/10780879922184004 [DOI] [Google Scholar]
- Rozanski, A., Bavishi, C., Kubzansky, L. D., & Cohen, R. (2019). Association of optimism with cardiovascular events and all-cause mortality: A systematic review and meta-analysis. JAMA Network Open, 2(9), e1912200. 10.1001/jamanetworkopen.2019.12200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheier, M. F., Carver, C. S., & Bridges, M. W. (1994). Distinguishing optimism from neuroticism (and trait anxiety, self-mastery, and self-esteem): A reevaluation of the Life Orientation Test. Journal of Personality and Social Psychology, 67(6), 1063–1078. 10.1037//0022-3514.67.6.1063 [DOI] [PubMed] [Google Scholar]
- Seligman, M. E. P. (2006). Learned optimism: How to change your mind and your life. Knopf Doubleday Publishing Group. [Google Scholar]
- Sierra, F. (2016). The emergence of geroscience as an interdisciplinary approach to the enhancement of health span and life span. Cold Spring Harbor Perspectives in Medicine, 6(4), a025163. 10.1101/cshperspect.a025163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims, M., Glover, L. M., Norwood, A. F., Jordan, C., Min, Y.-I., Brewer, L. C., & Kubzansky, L. D. (2019). Optimism and cardiovascular health among African Americans in the Jackson Heart Study. Preventive Medicine, 129, 105826. 10.1016/j.ypmed.2019.105826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims, M., Lipford, K. J., Patel, N., Ford, C. D., Min, Y. -I., & Wyatt, S. B. (2017). Psychosocial factors and behaviors in African Americans: The Jackson Heart Study. American Journal of Preventive Medicine, 52(1S1), S48–S55. 10.1016/j.amepre.2016.09.020 [DOI] [PubMed] [Google Scholar]
- Slavish, D. C., Jones, D. R., Smyth, J. M., Engeland, C. G., Song, S., McCormick, N. M., & Graham-Engeland, J. E. (2020). Positive and negative affect and salivary markers of inflammation among young adults. International Journal of Behavioral Medicine, 27(3), 282–293. 10.1007/s12529-019-09795-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spielberger, C. D., Krasner, S. S., & Solomon, E. P. (1988). The experience, expression, and control of anger. In Janisse M. P. (Ed.), Individual differences, stress, and health psychology (pp. 89–108). Springer. 10.1007/978-1-4612-3824-9_5 [DOI] [Google Scholar]
- Sternthal, M. J., Slopen, N., & Williams, D. R. (2011). Racial disparities in health: How much does stress really matter? Du Bois Review: Social Science Research on Race, 8(1), 95–113. 10.1017/S1742058X11000087 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stringer, D. M. (2013). Negative affect. In Gellman M. D. & Turner J. R. (Eds.), Encyclopedia of behavioral medicine (pp. 1303–1304). Springer. 10.1007/978-1-4419-1005-9_606 [DOI] [Google Scholar]
- Stringhini, S., Polidoro, S., Sacerdote, C., Kelly, R. S., van Veldhoven, K., Agnoli, C., Grioni, S., Tumino, R., Giurdanella, M. C., Panico, S., Mattiello, A., Palli, D., Masala, G., Gallo, V., Castagné, R., Paccaud, F., Campanella, G., Chadeau-Hyam, M., & Vineis, P. (2015). Life-course socioeconomic status and DNA methylation of genes regulating inflammation. International Journal of Epidemiology, 44(4), 1320–1330. 10.1093/ije/dyv060 [DOI] [PubMed] [Google Scholar]
- Taylor, H. A., Wilson, J. G., Jones, D., Sarpong, D., Srinivasan, A., Garrison, R., Nelson, C., & Wyatt, S. (2005). Toward resolution of cardiovascular health disparities in African Americans: Design and methods of the Jackson Heart Study. Ethnicity & Disease, 15(4 Suppl 6), 4–17. https://pubmed.ncbi.nlm.nih.gov/16320381/ [PubMed] [Google Scholar]
- VanderWeele, T. J. (2011). Causal mediation analysis with survival data. Epidemiology (Cambridge, Mass.), 22(4), 582–585. 10.1097/EDE.0b013e31821db37e [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, X., Auchincloss, A. H., Barber, S., Mayne, S. L., Griswold, M. E., Sims, M., & Diez Roux, A. V. (2017). Neighborhood social environment as risk factors to health behavior among African Americans: The Jackson Heart Study. Health & Place, 45, 199–207. 10.1016/j.healthplace.2017.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson, D., Clark, L. A., & Tellegen, A. (1988). Development and validation of brief measures of positive and negative affect: The PANAS scales. Journal of Personality and Social Psychology, 54(6), 1063–70. 10.1037//0022-3514.54.6.1063 [DOI] [PubMed] [Google Scholar]
- Williams, D. R., Lawrence, J. A., & Davis, B. A. (2019). Racism and health: Evidence and needed research. Annual Review of Public Health, 40(1), 105–125. 10.1146/annurev-publhealth-040218-043750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams, J., & MacKinnon, D. P. (2008). Resampling and distribution of the product methods for testing indirect effects in complex models. Structural Equation Modeling: A Multidisciplinary Journal, 15(1), 23–51. 10.1080/10705510701758166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright, J. D., Folsom, A. R., Coresh, J., Sharrett, A. R., Couper, D., Wagenknecht, L. E., Mosley, T. H., Ballantyne, C. M., Boerwinkle, E. A., Rosamond, W. D., & Heiss, G. (2021). The ARIC (Atherosclerosis Risk In Communities) Study: JACC Focus Seminar 3/8. Journal of the American College of Cardiology, 77(23), 2939–2959. 10.1016/j.jacc.2021.04.035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zannas, A. S., Jia, M., Hafner, K., Baumert, J., Wiechmann, T., Pape, J. C., Arloth, J., Ködel, M., Martinelli, S., Roitman, M., Röh, S., Haehle, A., Emeny, R. T., Iurato, S., Carrillo-Roa, T., Lahti, J., Räikkönen, K., Eriksson, J. G., Drake, A. J., … Binder, E. B. (2019). Epigenetic upregulation of FKBP5 by aging and stress contributes to NF-κB-driven inflammation and cardiovascular risk. Proceedings of the National Academy of Sciences of the United States of America, 116(23), 11370–11379. 10.1073/pnas.1816847116 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data used for this analysis may be obtained through requests to the JHS and ARIC coordinating centers. This is not a preregistered study.