Abstract
The mechanism of farnesol (FOH)-induced growth inhibition of Saccharomyces cerevisiae was studied in terms of its promotive effect on generation of reactive oxygen species (ROS). The level of ROS generation in FOH-treated cells increased five- to eightfold upon the initial 30-min incubation, while cells treated with other isoprenoid compounds, like geraniol, geranylgeraniol, and squalene, showed no ROS-generating response. The dependence of FOH-induced growth inhibition on such an oxidative stress was confirmed by the protection against such growth inhibition in the presence of an antioxidant such as α-tocopherol, probucol, or N-acetylcysteine. FOH could accelerate ROS generation only in cells of the wild-type grande strain, not in those of the respiration-deficient petite mutant ([rho0]), which illustrates the role of the mitochondrial electron transport chain as its origin. Among the respiratory chain inhibitors, ROS generation could be effectively eliminated with myxothiazol, which inhibits oxidation of ubiquinol to the ubisemiquinone radical by the Rieske iron-sulfur center of complex III, but not with antimycin A, an inhibitor of electron transport that is functional in further oxidation of the ubisemiquinone radical to ubiquinone in the Q cycle of complex III. Cellular oxygen consumption was inhibited immediately upon extracellular addition of FOH, whereas FOH and its possible metabolites failed to directly inhibit any oxidase activities detected with the isolated mitochondrial preparation. A protein kinase C (PKC)-dependent mechanism was suggested to exist in the inhibition of mitochondrial electron transport since FOH-induced ROS generation could be effectively eliminated with a membrane-permeable diacylglycerol analog which can activate PKC. The present study supports the idea that FOH inhibits the ability of the electron transport chain to accelerate ROS production via interference with a phosphatidylinositol type of signal.
Farnesol (FOH) is an isoprenoid alcohol that may be endogenously generated within the cells by enzymatic dephosphorylation of farnesyl pyrophosphate (FPP), an intermediate of the metabolic pathway yielding sterols and other isoprenoid compounds from mevalonate (4). In addition to geranylgeranyl pyrophosphate, FPP also plays an important role as a precursor of protein prenylation such as in the posttranslational modification of oncogenic RAS proteins and other GTP-binding proteins (11). When exogenously added to the medium, FOH is subjected to either phosphorylation, yielding FPP, or oxidation, to give farnesal, farnesoic acid, and prenyldicarboxylic acid in mammalian cells (4). Recently, FOH has attracted much attention since it causes apoptotic cell death of human acute leukemia CEM-C1 cells (15, 20) and HL-60 cells (23). Interference with a phosphatidylinositol type of signaling has been proposed to be a cause of apoptosis in FOH-treated mammalian cells. In our previous study, FOH was found to exhibit a static growth inhibitory effect on the yeast Saccharomyces cerevisiae in which the intracellular diacylglycerol (DAG) level was significantly decreased and accompanied by downregulation of cell cycle gene expression (16). Mammalian and yeast cells may have a common mechanism in their responses to FOH regardless of whether these organisms are subjected to fatal or static damage from it.
Reactive oxygen species (ROS), including hydrogen peroxide, superoxide anion, and hydroxyl radical, are highly toxic oxidants which are inevitably produced to a certain extent under aerobic conditions. Among them, superoxide anions are generated in a wide variety of enzymatic reactions catalyzed by mitochondrial respiratory chain enzymes, cytochrome P-450 systems, nitric oxide synthase, NADPH oxidase, and lipoxygenase (9, 29, 31, 32). ROS generation is remarkably enhanced in K-ras-transformed murine cells by an inhibitor of protein farnesylation such as (α-hydroxyfarnesyl)phosphonic acid and by lovastatin, which should also affect this reaction by inhibiting 3-hydroxy-3-methylglutaryl coenzyme A reductase (27). However, no evidence has been obtained to elucidate the mechanism of ROS generation under the conditions in which protein farnesylation or the corresponding mevalonate biosynthetic reaction is inhibited. It is highly probable that FOH-induced events depend on oxidative stress in both yeast and mammalian cells since FOH can ultimately participate in the reaction of protein farnesylation. In this case, the mitochondrial respiratory chain seems to play an important role since it has been evaluated as the major source of superoxide anion in yeast cells (13).
In this study, the mechanism of FOH-induced growth inhibition of S. cerevisiae was studied in terms of its promotive effect on ROS generation. The mitochondrial electron transport chain was considered a target for inhibition of ROS generation by FOH. We hereby consider the possibility that FOH can indirectly inhibit the mitochondrial function via interference with a phosphatidylinositol type of signal.
MATERIALS AND METHODS
Yeast strains and media.
The S. cerevisiae strains used in this study were X2180-1A (MATa) (grande) and its isogenic [rho0] petite mutants, which had been generated by ethidium bromide treatment (7). The loss of mitochondrial respiratory chain activity in the mutant cells was confirmed by the absence of molecular oxygen consumption, which was measured as described below. As is often the case with [rho0] mutants lacking only mitochondrial DNA (5), the cells of our mutants could grow in minimal medium where mitochondrial proteins encoded by nuclear DNA have to be functional. Unless stated otherwise, the growth properties of these yeast cells were examined in YPD medium, which contained 10 g of yeast extract, 20 g of polypeptone, and 20 g of glucose per liter at 30°C. For the preparation of mitochondria as well as the assay of cellular respiratory activity, yeast cells were grown in semisynthetic lactate medium (SSM), which contained 3 g of yeast extract, 0.5 g of glucose, 0.5 g of CaCl2 · 2H2O, 0.5 g of NaCl, 0.6 g of MgCl2 · 2H2O, 1.0 g of KH2PO4, 1.0 g of NH4Cl, 22 ml of 90% dl-lactate, and 8.0 g of NaOH pellets per liter, where the pH was appropriately adjusted to 5.5 with 6 N NaOH (10).
Measurement of yeast cell growth.
The yeast cells were grown overnight in YPD medium with vigorous shaking and were inoculated into freshly prepared medium to give an initial cell density of approximately 107 cells/ml. Cells were then grown with or without each effector or inhibitor with vigorous shaking, and portions were withdrawn at various time intervals to measure the cell density at A610. The cell suspension (107 cells/ml) gave an A610 value of approximately 1.0.
Measurement of ROS production.
Cellular ROS production was examined by a method dependent on intracellular deacylation and oxidation of 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) to the fluorescent compound 2′,7′-dichlorofluorescein (DCF). This probe was highly reactive with hydrogen peroxide and has been used in evaluating ROS generation in mammalian (14, 25) and yeast (3, 34) cells. After preincubation of the yeast cells (107 cells/ml) in YPD medium with 40 μM DCFH-DA at 30°C for 60 min, the cell suspensions (1.0 ml) were withdrawn and further treated with each chemical for the indicated time and then washed and resuspended in 100 μl of phosphate-buffered saline. Fluorescence intensity of the cell suspension (100 μl) containing 107 cells was read with a Cytoflow 2300 fluorescence spectrophotometer (Millipore Co.) with excitation at 480 nm and emission at 530 nm. The arbitrary units were based directly on fluorescence intensity.
Preparation of mitochondria and the cytosol fraction.
Cells of strain X2180-1A were aerobically grown in 10 liters of SSM at 30°C for 15 h. Mitochondria were isolated from the cell lysate, which had been prepared by enzymatic digestion with Zymolyase 20 T (Seikagaku Kogyo Co.) according to the method of Glick and Pon (10). Cells from an overnight culture were further incubated in 100 ml of YPD medium (107 cells/ml) containing 200 μM FOH for 15 min, collected, suspended in 1.0 ml of cold lysis buffer (0.3 M d-sorbitol, 0.1 M NaCl, 5 mM MgCl2, 10 mM Tris-HCl, 1 mM phenylmethylsulfonyl fluoride [PMSF; pH 7.4]) at the cell density of 109/ml, and disrupted by repeated vortexing with glass beads (6). The supernatant obtained after removing the beads and cell debris by centrifugation at 1,500 × g for 15 min was used as the cytosol fraction. One milliliter of the fraction was thus expected to contain FOH and its metabolites, if any, from approximately 109 cells. Protein was measured by the method of Bradford (2) by using bovine serum albumin as a standard.
Assay of cellular and mitochondrial respiratory chain activity.
Cells of strain X2180-1A were grown in SSM with vigorous shaking at 30°C for 15 h, collected, and suspended in HEPES buffer (pH 7.4) containing 50 mM glucose at a cell density of 107 cells/ml. After preincubating the cell suspension with shaking at 30°C for 10 min, the respiratory activity of yeast cells was measured polarographically with an oxygen electrode (model 100; Rank Brothers, Ltd., Cambridge, United Kingdom). We also measured cellular oxygen consumption under the condition in which the mitochondrial respiration was fully stimulated with 100 μM 2,4-dinitrophenol (DNP) prior to the addition of FOH.
The rate of oxygen consumption by isolated mitochondria was also measured polargraphically in 2 mM HEPES buffer (pH 7.4) containing 0.6 M mannitol, 1 mM KCl, 2 mM MgCl2, 1 mM EDTA, and each respiratory substrate. The final mitochondrial protein concentration was adjusted to 250 μg/ml, and DNP was added at 40 μM whenever needed. NADH oxidase (complex I), succinate oxidase (complex II), and cytochrome c oxidase (complex IV) activities were defined as the amount of molecular oxygen (in nanomoles) consumed by yeast mitochondria (1 mg) for 1 min with 2 mM β-hydroxybutyrate (β-HB), 2 mM succinate, and a mixture of 2 mM ascorbate and 1 mM N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD), respectively, as the substrate.
Chemicals.
The following chemicals were purchased from Sigma: geraniol (GOH), FOH, farnesylacetate, FPP, geranyl geraniol (GGOH), antimycin A, myxothiazol, thenoyltrifluoroacetone (TTFA), rotenone, α-tocopherolacetate, probucol, N-acetylcysteine (NAC), TMPD, and 1-oleoyl,2-acetyl-sn-glycerol (OAG) as a membrane-permeable DAG analog. DCFH-DA was a product of Molecular Probe. A stock solution of each chemical was routinely prepared in either phosphate-buffered saline, ethanol, acetone, or dimethyl formamide due to its solubility. Farnesoic acid and farnesal were kindly provided by G. Asanuma (Kurare, Co., Osaka, Japan). The other chemicals were of analytical reagent grade.
RESULTS AND DISCUSSION
Correlation between FOH-induced growth inhibition and ROS generation.
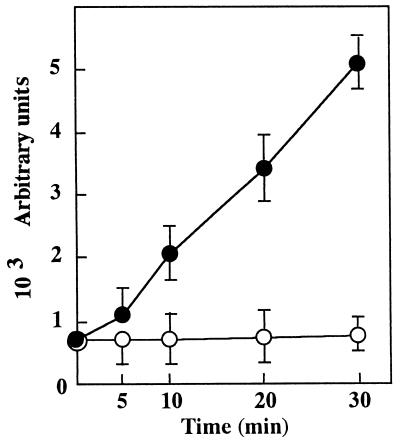
We first examined whether the yeast cells were subjected to oxidative stress due to ROS generation as reflected by DCFH-DA oxidation when the cells were grown or incubated with FOH. DCFH-DA was not directly oxidized even with 200 μM FOH in the absence of the yeast cells (data not shown), indicating that DCFH-DA oxidation depended only on the cellular response to FOH treatment. As summarized in Table 1, FOH significantly promoted cellular ROS generation in a dose-dependent manner, demonstrating its clear relation to the growth inhibitory effect. ROS generation was kept almost at the control level in medium containing other isoprenoid compounds, with growth inhibitory effect demonstrated even at a quite high concentration (200 μM). Surprisingly, GGOH was as effective as FOH in inducing apoptosis (23), suggesting that these isoprenoid alcohols are commonly involved in the mechanism of causing oxidative stress in mammalian cells. The susceptibility of mammalian cells to the cytotoxic effect of farnesylacetate (19) may depend on the ability to hydrolyze it to FOH as an active form. The level of ROS generation was linearly increased up to fivefold of the control level during the initial 30 min of incubation in the medium with 25 μM FOH, as shown in Fig. 1. Such a time-dependent increase in the fluorescence intensity indicated that ROS production occurred at a constant rate in FOH-treated cells.
TABLE 1.
Dose-dependent growth inhibition and induction of ROS production by FOH and related isoprenoid compounds in S. cerevisiae
| Addition | Concn (μM) | Relativea cell growth (%) | ROS productionb (arbitrary units) |
|---|---|---|---|
| None | 100 | 824 ± 122 | |
| GOH | 200 | 91 | 667 ± 84 |
| FOH | 6.25 | 104 | 860 ± 132 |
| 12.5 | 31 | 1,146 ± 156 | |
| 25 | 5 | 5,280 ± 345 | |
| 50 | 0 | 5,703 ± 192 | |
| 100 | 0 | 7,440 ± 310 | |
| GGOH | 200 | 98 | 626 ± 98 |
| Farnesylacetate | 200 | 96 | 421 ± 47 |
| Squalene | 200 | 101 | 633 ± 63 |
Yeast cells were grown with or without each isoprenoid compound at 30°C for 4 h in YPD medium. The initial cell density was adjusted to 107 cells/ml to give an A610 value of around 1.0.
After treatment with 40 μM DCFH-DA, yeast cells were incubated with or without each isoprenoid compound for 30 min and DCF fluorescence was measured as described in Materials and Methods. Values are means ± standard deviations (n = 4).
FIG. 1.
Time course of FOH-induced ROS generation. ROS generation was assayed by using at the indicated times 100 μl of yeast cell suspension (equivalent to 107 cells) which had been incubated in YPD medium with 0 (○) and 25 (•) μM FOH after pretreatment with DCFH-DA for 60 min. Values are means ± standard deviations (n = 4).
We next examined the protective effects of various types of antioxidants against FOH-induced growth inhibition as well as ROS generation and found a clear correlation between protection against these events. These antioxidants did not show any growth-promoting effects by themselves in medium without FOH. As summarized in Table 2, the FOH-induced events could be mostly eliminated by 25 μM α-tocopherol (α-TOH), a naturally occurring lipophilic antioxidant which can easily penetrate the plasma membrane and protect free and membranous lipids against oxidative damage (1). α-TOH is also known to protect against free radical-mediated liver injury (17) and apoptosis of MOLT-4 cells due to membrane-associated oxidation triggered by radiation (18). Of the synthetic antioxidants, probucol was as effective as α-TOH in protecting against FOH-induced growth inhibition, as is the case with protection against generation of hydrogen peroxide in macrophages (8). NAC was only partly effective, even at a concentration of 10 mM. Such a difference should depend mainly on the hydrophilic property of NAC since it is known to be a potent scavenger of hydroxyl free radical (3). l-Ascorbate is an extremely hydrophilic antioxidant that can act at the interface of the plasma membrane, showing alternative biological effects in a dose-dependent fashion (1, 26). In yeast cells, l-ascorbate at concentrations of 0.2 and 10 mM showed neither a protective effect against FOH-induced growth inhibition nor a growth inhibitory effect when added alone. This could be attributed to its poor permeation, especially through the yeast plasma membrane, because increased cellular ROS production was still observed even in the presence of 10 mM l-ascorbate. These findings revealed oxidative stress due to ROS generation inside the cytoplasmic membrane as a primary cause of FOH-induced growth inhibition in the yeast cells.
TABLE 2.
Protective effects of various antioxidants against FOH-induced growth inhibition and ROS production in S. cerevisiae
| Addition | Antioxidant concn (μM) | Relative cella growth (%) | ROS productionb (arbitrary units) |
|---|---|---|---|
| None | 100 | 860 ± 124 | |
| FOH | 1 | 5,686 ± 355 | |
| FOH + α-TOH | 6.25 | 10 | 2,663 ± 133 |
| 12.5 | 37 | 1,367 ± 122 | |
| 25 | 85 | 769 ± 89 | |
| α-TOH | 25 | 102 | 540 ± 133 |
| FOH + probucol | 6.25 | 6 | 2,556 ± 244 |
| 12.5 | 25 | 1,567 ± 136 | |
| 25 | 90 | 669 ± 78 | |
| Probucol | 25 | 97 | 530 ± 121 |
| FOH + NAC | 5 × 103 | 3 | 3,512 ± 233 |
| 1 × 104 | 41 | 1,194 ± 144 | |
| NAC | 1 × 104 | 87 | 660 ± 122 |
| FOH + l-ascorbate | 2 × 102 | 1 | 5,488 ± 346 |
| 1 × 104 | 3 | 3,090 ± 322 | |
| l-ascorbate | 1 × 104 | 92 | 810 ± 124 |
Yeast cells (107 cells/ml) were grown in YPD medium as indicated in Materials and Methods at 30°C for 4 h. The initial cell density was around 1.0 at A610. The results are means ± standard deviations (n = 4).
Yeast cells were preincubated in YPD medium with DCFH-DA (40 μM) for 60 min. The cells were then grown in YPD medium as indicated in Materials and Methods for 30 min, and levels of DCF fluorescence were measured. Results are means ± standard deviations (n = 4).
Resistance of respiration-deficient petite mutant against FOH-induced events.
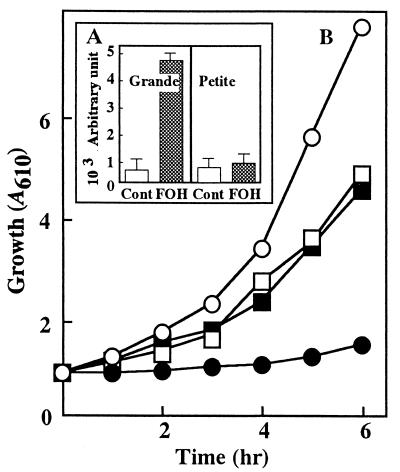
To assess the role of mitochondrial function in FOH-induced ROS generation, the FOH sensitivity of a [rho0] petite mutant was compared with that of the wild-type grande strain. As shown in Fig. 2B, the mutant cells could grow in YPD medium depending on fermentation equally well with or without 200 μM FOH. In accordance with this fact, cellular ROS generation stayed at the control level even after 30 min of incubation of the mutant cells with FOH (Fig. 2A). The same results were obtained with another four petite mutants which had been independently isolated by ethidium bromide treatment of the parent strain. These mutant strains were all characterized by the rate of cellular uptake of FOH in comparison to that of the parent strain (data not shown), which strongly supported the dependence of FOH-induced ROS generation on mitochondrial function alone. The electron transport chain could be a target of FOH since the [rho0] petite mutant generally lacks cytochrome b of complex III and various subunits of cytochrome c oxidase in addition to F1F0-ATPase (5).
FIG. 2.
Effects of respiration competence on ROS production (A) and FOH-induced growth inhibition (B). (A) ROS generation was assayed as described in the legend to Fig. 1 except that FOH was added at 25 μM for cells of the wild-type strain and at 200 μM for cells of the mutant strain. Values are means ± standard deviations (n = 4). (B) Cells of parental grande strain (○ and •) and [rho0] petite mutant (□ and ■) were grown in YPD medium with (• and ■) or without (○ and □) FOH at 30°C for 6 h.
Effect of blocking electron flow in complexes I, II, III, and IV of the respiratory chain on FOH-induced ROS generation.
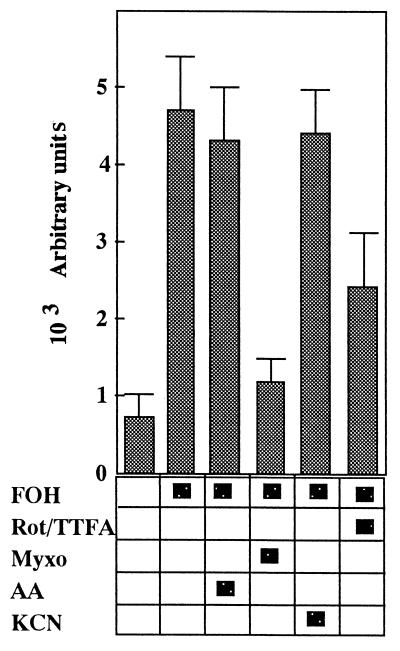
Wild-type cells were pretreated with a respiratory chain inhibitor(s) for 10 min to identify the critical site of ROS generation during the subsequent incubation with 25 μM FOH. Each respiratory chain inhibitor was used at the concentration that entirely inhibited cellular oxygen consumption but that did not influence cell growth upon fermentation in YPD medium. As shown in Fig. 3, ROS generation was reduced by almost 50% when yeast cells were pretreated with rotenone, which specifically inhibits complex I, and TTFA, a specific inhibitor of complex II (24). Protection against ROS generation and growth inhibition was not provided by treatment with either antimycin A, which inhibits cytochrome reductase of complex III, or KCN, a typical inhibitor of complex IV (28). Of the various respiratory chain inhibitors tested, myxothiazol, which inhibits the oxidation of ubiquinol to the ubisemiquinone radical via the Rieske iron-sulfur center of complex III (29), eliminated cellular ROS generation most effectively. With myxothiazol pretreatment, the yeast cells treated with FOH (25 μM) could grow normally upon fermentation in YPD medium, in which the relative cell growth (A610 = 3.9) was around 50% of the control level at 6 h. This strongly supported that the protection against ROS generation provided by mxyothiazol depended solely on its role as a respiratory chain inhibitor.
FIG. 3.
Protective effects provided by blocking the electron transport chain in FOH-induced ROS generation. ROS generation was assayed by using the cell suspension with or without the following treatment, as described in the legend to Fig. 1. Prior to the addition of 25 μM FOH, cells were pretreated with a mixture of 50 μM rotenone (Rot) and 1 mM TTFA, 20 μM antimycin A (AA), 30 μM myxothiazol (Myxo), or 2.5 mM KCN for 10 min. Bars are means ± standard deviations (n = 4).
The overall electron transfer from complex I or II to cytochrome c of complex III is coupled with proton translocation across the mitochondrial inner membrane in which ubiquinone functions as a carrier of both protons and electrons by forming ubiquinol (hydroquinone) (21). The ubisemiquinone radical inevitably appears as a result of the transfer of one electron from ubiquinol to the cytochrome bc1 complex catalyzed by the Rieske iron-sulfur center. Under conditions with no respiratory chain inhibition, however, the radical is further oxidized to ubiquinone by means of sequential reactions with cytochrome b-566 and b-562 in the Q cycle of complex III, thereby protecting its accumulation to a significant extent. In other words, the radical can accumulate only when the electron transport is inhibited in the sequential reactions described above and the extent of radical formation thus should depend on the level of the ubiquinol pool. Inhibitors of cytochrome b reoxidation that bind to the heme of cytochrome b-562 such as antimycin A can directly potentiate the accumulation of the unstable ubisemiquinone radical as the electron donor to molecular oxygen (30). Accumulation of the unstable ubisemiquinone radical in the Q cycle of complex III could also be a cause of FOH-induced ROS generation. This is strongly supported by the fact that only myxothiazol, which can inhibit the formation of the ubisemiquinone radical itself as already mentioned, can effectively protect against FOH-induced ROS generation. In this case, the radical formation cannot be protected with a respiratory chain inhibitor of complex IV but seems to be partly protected by inhibition of electron transport upstream of complex III since such inhibition reduces the level of the ubiquinol pool. It remains to be demonstrated whether FOH affects cytochrome b reoxidation in the Q cycle with the accompanying accumulation of the ubisemiquinone radical.
Effects of FOH on cellular oxygen consumption and mitochondrial oxidase activities.
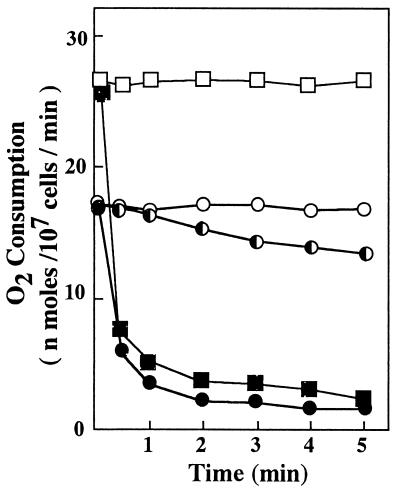
As deduced from the dependence of FOH-induced ROS generation on mitochondrial function, FOH restricted cellular oxygen consumption immediately upon its addition to the cell suspension (Fig. 4). Similar results were obtained under conditions in which mitochondrial respiration was fully stimulated with DNP. This means that FOH inhibited mitochondrial electron transport that was normally coupled with oxygen consumption at complex IV. FOH-induced ROS generation (Fig. 1) should proceed mainly by utilizing the mitochondrial electron flow from the ubiquinol pool which was still remaining since the overall mitochondrial electron transport had been mostly shut off within 5 min of incubation. In fact, FOH could accelerate ROS generation even under conditions in which mitochondrial electron transport was fully repressed with antimycin A (Fig. 3).
FIG. 4.
Effects of FOH on the respiratory activity of a whole-cell suspension. Cells were suspended in HEPES buffer (pH 7.4) containing 50 mM glucose at a density of 107 cells/ml and incubated with shaking at 30°C. The rate of oxygen consumption was measured with 0 (○, □), 12.5 (◐), and 25 (• and ■) μM FOH. Square symbols indicate the cellular oxygen consumption when it was fully stimulated with 100 μM DNP.
Antimycin A induces apoptosis of mammalian cells by accelerating ROS generation with accompanying inhibition of mitochondrial electron transport (14). In our experiment, only a very low level of ROS generation was detected, with fewer than 1,000 arbitrary units in the yeast cells after 30 min of incubation with 20 μM antimycin A. This potent cytotoxic agent exhibited respiration-inhibitory activity only on S. cerevisiae cells, still allowing anaerobic growth on glucose in YPD medium. The mechanism of mitochondrial ROS generation is not fully understood, as reflected by a difference in sensitivity to antimycin A between mammalian and yeast cells. The yeast cells may possess a more advanced ability to eliminate the oxidative stress than that of mammalian cells.
The inhibitory effects of FOH on mitochondrial oxidase activities were examined by measuring the rates of oxygen consumption by isolated mitochondrial preparations (Table 3). Rotenone, antimycin A, and KCN inhibited the corresponding oxidase activities as expected. It was surprising that no significant inhibition of the oxygen-consuming reactions catalyzed by NADH oxidase, succinate oxidase, and cytochrome c oxidase was observed even with 200 μM FOH. These oxidase activities were not inhibited with any of the most probable metabolites of FOH, such as FPP, farnesal, and farnesoic acid. We therefore examined whether the cytosol fraction contained what was necessary to directly inhibit mitochondrial electron transport. This component might be either a metabolite of FOH other than those described above or another molecule which newly appeared within the cells under conditions with FOH. As deduced from the rate of oxygen consumption (25 nmol of O2/min) by using both β-hydroxybutyrate and succinate, the assay mixture was expected to contain the mitochondrial preparation from approximately 107 cells. However, the oxidase activities were not inhibited even if the cytosol fraction from 2 × 107 cells was mixed with the mitochondrial preparation described above in the assay mixtures. These results revealed that FOH-induced ROS generation or inhibition of oxygen consumption was not due to direct inhibition of the mitochondrial electron transport by FOH or its metabolites.
TABLE 3.
Effects of respiratory chain inhibitors, FOH, and related compounds on mitochondrial oxidase activities
| Additiona | Activity (nmol of O2/mg of protein/min)
|
||
|---|---|---|---|
| NADH oxidase | Succinate oxidase | Cytochrome c oxidase | |
| None | 31.8 ± 3.6 | 64.6 ± 9.6 | 216.1 ± 7.9 |
| Rotenone | 4.1 ± 1.1 | 63.3 ± 8.6 | 210.3 ± 6.5 |
| Antimycin A | 1.8 ± 0.7 | 2.2 ± 0.5 | 205.2 ± 7.2 |
| KCN | 1.4 ± 0.3 | 2.1 ± 0.4 | 5.5 ± 0.9 |
| FOH | 29.9 ± 3.9 | 59.6 ± 4.7 | 229.7 ± 8.7 |
| Farnesal | 29.1 ± 1.9 | 62.6 ± 8.6 | 224.1 ± 4.0 |
| Farnesoic acid | 34.2 ± 2.9 | 68.3 ± 8.8 | 240.0 ± 9.7 |
| FPP | 33.9 ± 4.7 | 62.0 ± 6.3 | 235.2 ± 9.6 |
| Cytosol | 31.8 ± 3.7 | 62.3 ± 6.0 | 251.8 ± 9.9 |
The assay mixture contained either 50 μM rotenone, 20 μM antimycin A, 2.5 mM KCN, or 200 μM each FOH and other isoprenoid compounds. The cytosol fraction from 2 × 107 cells was mixed with the mitochondrial preparation from 107 cells in the assay mixtures. Values are means ± standard deviations (n = 4).
Involvement of a phosphatidylinositol type of signal in FOH-induced ROS generation.
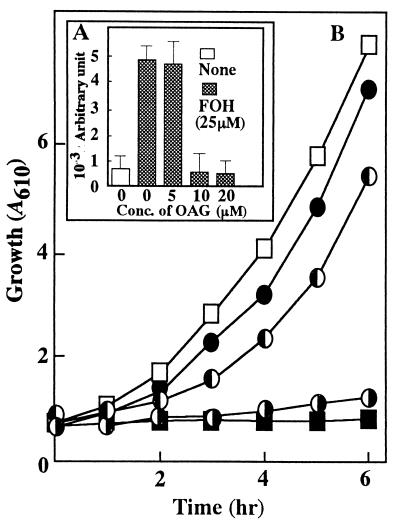
In the experiment with a human acute leukemia cell line, the inhibitory effect of FOH on cell proliferation could be restored with the extracellular addition of OAG, a membrane-permeable analog of DAG which can activate PKC from mammalian (15, 20) and yeast (22) cells. As shown in Fig. 5, FOH-induced ROS generation and growth inhibition were apparently diminished to the control level in the presence of 10 μM OAG. This agreed with our previous finding that the PKC activator restored FOH-induced growth inhibition of S. cerevisiae cells in which the endogenous DAG level had been drastically decreased immediately upon incubation with FOH (16). These findings support the idea that FOH inhibits mitochondrial electron transport via interference with a phosphatidylinositol type of signal without having a direct inhibitory effect on the mitochondrion itself. It seems possible that a PKC-dependent mechanism normally functions in yeast cells to regulate mitochondrial electron transport, especially at the Q cycle of complex III, not to promote ROS generation.
FIG. 5.
Protective effects of OAG against ROS production (A) and FOH-induced growth inhibition (B). (A) ROS generation with or without various concentrations of OAG was assayed as described in the legend to Fig. 1. A control assay was run with YPD medium without any other ingredient. Values are means ± standard deviations (n = 4). (B) Cells (107 cells/ml) were grown in YPD medium with (■) or without (□) 25 μM FOH. FOH was further added at 25 μM to YPD medium containing OAG at either 5 (◑), 10 (◐), or 20 (•) μM.
FOH-induced growth inhibition of S. cerevisiae cells has been characterized as consisting of cell cycle arrest with accompanying downregulation of the corresponding cell cycle gene expression (16). Interference with cellular DAG metabolism was thought to directly influence gene expression. In the present study, we have considered another possibility, that ROS generation can be a signal of cell cycle arrest in yeast cells. This possibility is currently under investigation.
REFERENCES
- 1.Barroso M P, Gomez-Diaz C, Lopez-Lluch G, Malagon M M, Crane F L, Navas P. Ascorbate and α-tocopherol prevent apoptosis induced by serum removal independent of Bcl-2. Arch Biochem Biophys. 1997;343:243–248. doi: 10.1006/abbi.1997.0170. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Brennan R J, Schiestl R H. Aniline and its metabolites generate free radicals in yeast. Mutagenesis. 1997;12:215–220. doi: 10.1093/mutage/12.4.215. [DOI] [PubMed] [Google Scholar]
- 4.Crick D C, Andres D A, Waechter C J. Novel salvage pathway utilizing farnesol and geranylgeraniol for protein isoprenylation. Biochem Biophys Res Commun. 1997;237:483–487. doi: 10.1006/bbrc.1997.7145. [DOI] [PubMed] [Google Scholar]
- 5.Evans I H. Molecular genetic aspects of yeast mitochondria. In: Spencer J F T, Spencer D M, Smith A R W, editors. Yeast genetics. Fundamental and applied aspects. New York, N.Y: Springer-Verlag; 1983. pp. 269–370. [Google Scholar]
- 6.Fishbein J D, Dobrowsky R T, Bielawska A, Garrett S, Hannun Y A. Ceramide-mediated growth inhibition and CAPP are conserved in Saccharomyces cerevisiae. J Biol Chem. 1993;268:9255–9261. [PubMed] [Google Scholar]
- 7.Fox T D, Folley L S, Mulero J J, McMullin T W, Thorsness P E, Hedin L O, Costanzo M C. Analysis and manipulation of yeast mitochondrial genes. Methods Enzymol. 1991;194:149–165. doi: 10.1016/0076-6879(91)94013-3. [DOI] [PubMed] [Google Scholar]
- 8.Fukuda M, Ikegami H, Kawaguchi Y, Sano T, Ogihara T. Antioxidant, probucol, can inhibit the generation of hydrogen peroxide in islet cells induced by macrophages and prevent islet cell destruction in NOD mice. Biochem Biophys Res Commun. 1995;209:953–958. doi: 10.1006/bbrc.1995.1590. [DOI] [PubMed] [Google Scholar]
- 9.Garssen G J, Vliegenthart J F G, Boldingh J. An anaerobic reaction between lipoxygenase, linoleic acid and its hydroperoxide. Biochem J. 1971;122:327–332. doi: 10.1042/bj1220327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glick B S, Pon L A. Isolation of highly purified mitochondria from Saccharomyces cerevisiae. Methods Enzymol. 1995;260:213–217. doi: 10.1016/0076-6879(95)60139-2. [DOI] [PubMed] [Google Scholar]
- 11.Glomset J A, Gelb M H, Farnsworth C C. Prenyl proteins in eukaryotic cells: a new type of membrane anchor. Trends Biochem Sci. 1990;15:139–142. doi: 10.1016/0968-0004(90)90213-u. [DOI] [PubMed] [Google Scholar]
- 12.Goldstein J L, Brown M S. Regulation of the mevalonate pathway. Nature. 1990;343:425–430. doi: 10.1038/343425a0. [DOI] [PubMed] [Google Scholar]
- 13.Grant C M, MacIver F H, Dawes I W. Mitochondrial function is required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. FEBS Lett. 1997;410:219–222. doi: 10.1016/s0014-5793(97)00592-9. [DOI] [PubMed] [Google Scholar]
- 14.Hagar H, Ueda N, Shah S V. Role of reactive oxygen metabolites in DNA damage and cell death in chemical hypoxic injury to LLC-PK1 cells. Am J Physiol. 1996;271:F209–F215. doi: 10.1152/ajprenal.1996.271.1.F209. [DOI] [PubMed] [Google Scholar]
- 15.Hang J S, Goldner C M, Yazolvitskaya E M, Voziyan P A, Melnykovych G. Directed cell killing (apoptosis) in human lymphoblastoid cells incubated in the presence of farnesol: effect of phosphatidylcholine. Biochim Biophys Acta. 1994;1223:133–140. doi: 10.1016/0167-4889(94)90082-5. [DOI] [PubMed] [Google Scholar]
- 16.Machida, K., T. Tanaka, Y. Yano, S. Otani, and M. Taniguchi. Submitted for publication.
- 17.Matsura T, Yamada K, Kawasaki T. Antioxidant role of cellular reduced coenzyme Q homologs and α-tocopherol in free radical-induced injury of hepatocytes isolated from rats fed diets with different vitamin E contents. Biochim Biophys Acta. 1992;1127:277–283. doi: 10.1016/0005-2760(92)90232-k. [DOI] [PubMed] [Google Scholar]
- 18.McClain D E, Kalinich J F, Ramakrishnan N. Trolox inhibits apoptosis in irradiated MOLT-4 lymphocytes. FASEB J. 1995;9:1345–1354. doi: 10.1096/fasebj.9.13.7557025. [DOI] [PubMed] [Google Scholar]
- 19.Meigs T E, Sherwood S W, Simoni R D. Farnesyl acetate, a derivative of an isoprenoid of the mevalonate pathway, inhibits DNA replication in hamster and human cells. Exp Cell Res. 1995;219:461–470. doi: 10.1006/excr.1995.1253. [DOI] [PubMed] [Google Scholar]
- 20.Melnykovych G, Haug J S, Goldner C M. Growth inhibition of leukemia cell line CEM-C1 by farnesol: effects of phosphatidylcholine and diacylglycerol. Biochem Biophys Res Commun. 1992;186:543–548. doi: 10.1016/s0006-291x(05)80842-3. [DOI] [PubMed] [Google Scholar]
- 21.Mitchell P. Protonmotive redox mechanism of the cytochrome b-c1 complex in the respiratory chain: protonmotive ubiquinone cycle. FEBS Lett. 1975;56:1–6. doi: 10.1016/0014-5793(75)80098-6. [DOI] [PubMed] [Google Scholar]
- 22.Ogita K, Miyamoto S, Koide H, Iwai T, Oka M, Ando K, Kishimoto A, Ikeda K, Fukami Y, Nishizuka Y. Protein kinase C in Saccharomyces cerevisiae: comparison with the mammalian enzyme. Proc Natl Acad Sci USA. 1990;87:5011–5015. doi: 10.1073/pnas.87.13.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohizumi H, Masuda Y, Nakajo S, Sakai I, Ohsawa S, Nakaya K. Geranylgeraniol is a potent inducer of apoptosis in tumor cells. J Biochem. 1995;117:11–13. doi: 10.1093/oxfordjournals.jbchem.a124695. [DOI] [PubMed] [Google Scholar]
- 24.Ramsay R R, Ackrell B A, Coles C J, Singer T P, White G A, Thorn G D. Reaction site of carboxanilides and thenoyltrifluoroacetone in complex II. Proc Natl Acad Sci USA. 1981;78:825–828. doi: 10.1073/pnas.78.2.825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rosenkranz A R, Schmaldienst S, Stuhlmeier K M, Chen W, Knapp W, Zlabinger G J. A microplate assay for the detection of oxidative products using 2′,7′-dichlorofluorescin-diacetate. J Immunol Methods. 1992;156:39–45. doi: 10.1016/0022-1759(92)90008-h. [DOI] [PubMed] [Google Scholar]
- 26.Sakagami H, Satoh K, Ohata H, Takahashi H, Yoshida H, Iida M, Kuribayashi N, Sakagami T, Momose K, Takeda M. Relationship between ascorbyl radical intensity and apoptosis-inducing activity. Anticancer Res. 1996;16:2635–2644. [PubMed] [Google Scholar]
- 27.Santillo M, Mondola P, Gioielli A, Seru R, Iossa S, Annella T, Vitale M, Bifulco M. Inhibitors of Ras farnesylation revert the increased resistance to oxidative stress in K-ras transformed NIH 3T3 cells. Biochem Biophys Res Commun. 1996;229:739–745. doi: 10.1006/bbrc.1996.1874. [DOI] [PubMed] [Google Scholar]
- 28.Slater E C. Application of inhibitors and uncouplers for a study of oxidative phosphorylation. Methods Enzymol. 1967;10:48–57. [Google Scholar]
- 29.Thompson J A, Schullek K M, Turnipseed S B, Ross D. Role of cytochrome p450 in the metabolism and toxicity of hydroperoxide in isolated rat hepatocytes. Arch Biochem Biophys. 1995;323:463–470. doi: 10.1006/abbi.1995.0068. [DOI] [PubMed] [Google Scholar]
- 30.Turrens J F, Alexandre A, Lehninger A L. Ubisemiquinone is the electron donor for superoxide formation by complex III of heart mitochondria. Arch Biochem Biophys. 1985;237:408–414. doi: 10.1016/0003-9861(85)90293-0. [DOI] [PubMed] [Google Scholar]
- 31.Umei T, Takeshige K, Minakami S. NADPH-binding component of the superoxide-generating oxidase in unstimulated neutrophils and the neutrophils from the patients with chronic granulomatous disease. Biochem J. 1987;243:467–472. doi: 10.1042/bj2430467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vaziri N D, Ding Y, Ni Z, Gonick H C. Altered nitric oxide metabolism and increased oxygen free radical activity in lead-induced hypertension: effect of lazaroid therapy. Kidney Int. 1997;52:1042–1046. doi: 10.1038/ki.1997.426. [DOI] [PubMed] [Google Scholar]
- 33.Voziyan P A, Haug J S, Melnykovych G. Mechanism of farnesol cytotoxicity: further evidence for the role of PKC-dependent signal transduction in farnesol-induced apoptotic cell death. Biochem Biophys Res Commun. 1995;212:479–486. doi: 10.1006/bbrc.1995.1995. [DOI] [PubMed] [Google Scholar]
- 34.Yurkow E J, Mckenzie M A. Characterization of hypoxia-dependent peroxidase production in cultures of Saccharomyces cerevisiae using flow cytometry: a model for ischemic tissue destruction. Cytometry. 1993;14:287–293. doi: 10.1002/cyto.990140309. [DOI] [PubMed] [Google Scholar]