Abstract
Hypoxia at birth represents a very stressful event that can result in severe lifelong consequences in different tissues, including those of the heart. Heat shock and other associated stress proteins are involved in cellular protection, but their roles are not clearly defined at the time of birth. Newborn piglets were subjected to 5% oxygen and 95% nitrogen for either 1 or 4 hours. They were allowed to recover over periods of 1 to 68 hours. The relative levels of αB-crystallin, HspB8, Hsp20, Hsp27, Hsp60, and Hsp70 as well as nitric oxide synthases (NOS) (endothelial NOS, inducible NOS, neuronal NOS) were examined by Western blot analysis. Surprisingly, αB-crystallin expression was drastically increased in animals submitted to hypoxia. The hypoxia-associated factor HIFIα was also strongly and rapidly overexpressed. Heme oxygenase 1 was also increased. To a lesser extent, neuronal NOS was also increased in the left ventricle of animals submitted to hypoxia. This work clearly shows that the Hsp chaperone αB-crystallin is strongly overexpressed in the left ventricle of animals submitted to hypoxia. This observation dissociates the response to low oxygenation of αB-crystallin and other stress-associated proteins including Hsp27, and it indicates that heme oxygenase is not alone among HSPs in its oxygen-related gene expression.
INTRODUCTION
Hypoxia in newborn infants results in severe lifelong consequences. The kidneys, lungs, heart, and brain are particularly sensitive to low oxygenation (Li and Jackson 2002). In the brain, hypoxia at birth can result in severe auditory problems (Jiang 1995) and, as recently shown, can even result in schizophrenia at later stages (Dalman et al 2001). Asphyxia and hypoxia-ischemia can also result in cognitive impairment and developmental delay in infants (Dixon et al 2002). Sudden infant death has also been associated with low oxygenation (Southall and Samuels 1989). As a result of low oxygenation, extensive redistribution of blood takes place in the brain and heart (Kamitomo et al 1993). In particular, left ventricular functions are significantly depressed under such conditions. After hypoxia, compensatory myocytes hypertrophy is observed in the ventricles, and this is associated with angiogenesis (Kamimoto et al 1996; Lewis et al 1999).
At the molecular level, impairment due to hypoxia at birth includes the transient reduction in the number of GABA receptors and the reduced expression of glutamate transporters (Viapiano et al 2001). To counteract such harmful effects on heart functions, cardioprotective agents have been developed both for newborn and adult animals. Because heat shock proteins (Hsps) offer this type of protection, the study of the expression and induction of these proteins is of particular interest, especially after hypoxia at birth. These proteins are induced as a result of a variety of stresses including elevated temperatures (Arrigo and Landry 1994; Kiang and Tsokos 1998) and hypoxia (Hammerer-Larcher et al 2001). HSPs are classified into large families according to their molecular weights (Arrigo and Landry 1994; Kiang and Tsokos 1998) as Hsp110, Hsp90, Hsp70, Hsp60, and small Hsps (sHsps) between 20 and 30 kDa. Very few studies have described their expression in the heart and other tissues at the time of birth (Tanguay et al 1993; David et al 2001b).
This study will focus on Hsp70 and sHsps. Hsp70 (not Hsc70) is strongly expressed in the heart and brain after hypoxia-ischemia (Kawana et al 2000). The main representative of mammalian sHsps is Hsp27. This protein is strongly expressed in the cardiac muscle (David et al 2001a; Tallot et al 2003) where it is involved in several pathologies (Snoeckx et al 2001). Alpha crystallins are chaperoning proteins of the same sHsp family abundantly expressed in the lens (Horwitz 2003). αB-crystallin is present in different tissues including skeletal muscle and the heart (Tallot et al 2003). Related to α-crystallin, a new series of sHSPs has recently been described (Horwitz 2003; Verschuure et al 2003). Two of them (HSPB2 and HSPB20) are expressed in different tissues, particularly in muscle and the heart (Verschuure et al 2003). To our knowledge, the expression of these proteins has never been studied in the heart of animals subjected to conditions of hypoxia. This lack of information strongly justified the study of these small molecular weight Hsps.
Besides Hsps, other stress proteins are of interest in the neonatal infant or in animals after hypoxia. Hypoxia-inducible factor 1α (HIF1α) participates in the immediate early response to oxygen deprivation (Gassmann and Wenger 1997) and triggers the induction of several hypoxia-associated molecules (Agani et al 2002). Among these molecules, it has recently been demonstrated that HIF1α is associated with neuronal nitric oxide synthase (nNOS) and (i)Hsp70 is associated with the inducible form (iNOS) (Bellmann et al 2000). Because of the significance of NO released by NOS for vasorelaxation, the study of the expression of the isoforms of NOS, in association with Hsp in the newborn, is important because of its cardioprotective effect.
Our group has developed a piglet model for the study of early neonatal development. This model presents several important molecular features for the study of stress-associated cerebral outcomes (David et al 2001a, 2001b). It displays several common similarities with the human infant after a hypoxic episode. The aim of this study is to determine the possible effects of birth hypoxia on stress proteins in the heart of piglets. Based on the close relationship among Hsps, HIF1α, NOS, and the lack of information about the effects of hypoxia at birth on αB-crystallin and HSP27, the aim of this study is to determine the effects of hypoxia on the expression of heat shock and stress-associated proteins in the piglet heart at birth.
MATERIALS AND METHODS
All experimental procedures were carried out in accordance with the National Institute of Health Guide and the French Ministry of Agriculture's Guide for the use and care of laboratory animals.
Animal preparation and hypoxia protocol
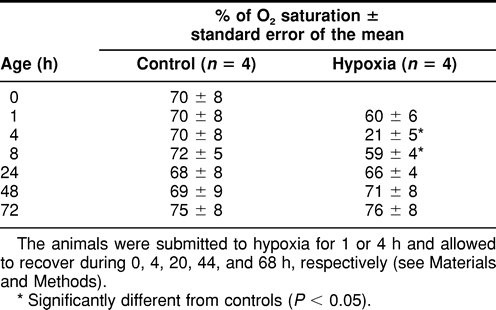
Large white piglets were born at term and immediately placed in the hypoxia chamber. A total number of 54 piglets were used. They were divided into 2 groups: 28 animals were chosen at random and kept as controls, at an ambient temperature of 20°C in normoxic conditions, separated from their mothers. Twenty-six animals were chosen at random and submitted to hypoxia under a continuous flow of 5% O2 and 95% N2. Their body temperature was maintained at 37 ± 1°C during the duration of hypoxia by the use of infrared heating lamps. The resulting oxygen saturations of hemoglobin in venous blood taken at the ear are given in Table 1. They were measured with a hemoxymeter (Type OSM-2, Radiometer Corp, Villeurbanne, France). The volume of the chamber was 214 L. The complete renewal of the chamber atmosphere occurred 5 times per hour. The animals were kept for 4 hours or 1 hour as indicated. They were allowed to recover in the ambient atmosphere and temperature for the indicated times. For experiments longer than 8 hours, the animals were given a maximum of 40 mL of 40% fat milk every 2 hours. Animals were obtained from INRA, St Gilles, France. Two animals (out of 6) died between 48 and 72 hours of age in the second group. There was no mortality in the other group. After 5 minutes inhalation of chloroform, the piglets were killed at indicated times. The left ventricle was dissected, immediately frozen in liquid nitrogen, and kept at −80°C for no longer than 2 weeks.
Table 1.
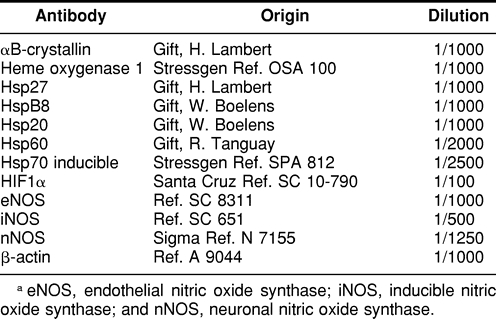
Primary antibodiesa
Western blot analysis
For protein extraction, tissues were minced and placed in extraction buffer (TEX buffer 1×; 60-mM Tris base, pH 6, 8, 10% glycerol, and 3% sodium dodecyl sulfate (SDS)) added with 5% mercaptoethanol and the protease inhibitor Trasyol (Bayer Pharma, Puteaux, France) just before use. Tissues were immediately homogenized in Ultra Turrax at maximum speed for 1 minute in ice and centrifuged at 26 500 × g for 15 minutes. Supernatants were collected in a series of Eppendorf tubes kept at −20°C for no longer than 1 week before use. Protein concentrations were measured as previously described (David et al 2001a, 2001b; Chiral et al 2004) using an ELISA plate reader (Argus 300, Packard, St Cyr, France).
Equal amounts of proteins were loaded on 13% acrylamide gel with a 4% stacking acrylamide gel. Migration was performed in a buffer containing 25-mM Tris, pH 7.6, 0.1% SDS, and 0.2 mglycine. After migration, proteins were transferred on Hybond C membranes (Amersham Bioscience, Little Chalfont, Bucks, UK) for 75 minutes using a buffer containing 25-mM Tris base, pH 7.6, 0, 1% SDS, 0.2 M glycine, and 20% methanol. Blots were washed 4 times in washing buffer (20-mM Tris base, pH 7.6, 12.5 mM NaCl, and 0 5% Tween-20 [TBST buffer]), soaked in rouge Ponceau for 1 minute for marker revelation, and washed again 4 times in TBST buffer for 5 minutes each. Molecular weight markers were purchased from Sigma (L'Isle d'Abeau, France). Membranes were blocked for 1 hour at room temperature in TBST buffer containing 5% milk powder at room temperature and then incubated overnight with the primary antibody. The sources and dilutions of antibodies are indicated in Table 1. Specificity of anti-Hsp27 (David et al 2001a), Hsp70 (David et al 2001b), and αB-crystallin (Tallot et al 2003), as well as anti-HspB8, B20 (Verschuure et al 2003) have been previously established. After overnight incubation at room temperature, the blots were washed 5 × 5 minutes in the TBST buffer and 5% skim milk powder containing the second antibody (1:1000 anti-rabbit immunoglobulin G peroxidase coupled from Sigma). After 5 × 5 minute washes in TBST buffer, bands were revealed using diaminobenzidine (Sigma) in a 30-mL buffer containing 60 mM Tris, pH 6.8, 0.2% hydrogen peroxide, and 200 μL 0.8% Ni CI2. After staining, the membranes were washed in distilled water and dried at 37°C in an oven. The representative Westerns are given in the figures.
Standardization and statistical analysis
To check for equal protein loading of each lane, the same membranes were tested for the expression of β-actin. The secondary antibody was peroxidase coupled (Sigma). Secondary antibodies were used at dilution of 1:1000 and were anti-rabbit with the exception of anti-mouse for β-actin. This experiment was repeated for every membrane. To compare densities, the membranes were scanned using a phosphor imager (Quantum Appligene, Illkirch, France). The density of each band of interest was expressed as a percentage of the density of the β-actin band in the same gel. Statistical analysis was performed as follows: after global analysis of variance, the mean values were compared as indicated in the legends by a nonparametric analysis for variance (Kruskal-Wallis test). When significant, 2 by 2 comparisons were made according to Conover as already described (Conover 1980; Chiral et al 2004) for the highly significant multiple comparison test with P values less than 0.01 indicating significance.
RESULTS
Venous oxygen saturation
As shown in Table 2, oxygen saturation did not vary in neonatal pigs and remained around 70%. Oxygen saturation in the venous blood was unchanged after 1 hour of hypoxia. However, hypoxia for 4 hours reduced oxygen saturation to very low levels. The return of oxygen to the baseline or control value was still incomplete after 4 hours of recovery. Twenty hours after hypoxia, no significant difference in oxygen saturation was detected between control and hypoxic animals.
Table 2.
Venous oxygen saturation of hemoglobin
Relative expression of HIF1α and heme oxygenase 1 in the heart after hypoxia
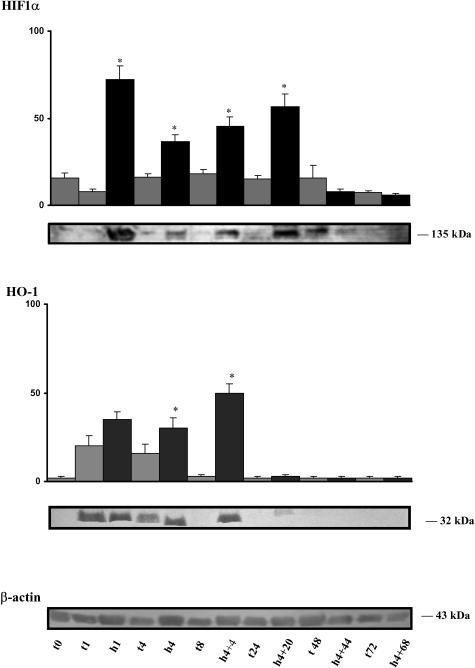
As depicted in Figure 1, detectable HIF1α expression was observed at birth. Eightfold increases were observed after 1 hour of hypoxia. However, the increase in the expression of HIF1α was least (2.5 times) after 4 hours of hypoxia and after 4 hours of hypoxia followed by 4 hours of recovery (3 times). Expression increased again in the hearts of animals submitted to hypoxia for 4 hours and allowed to recover for 20 hours. Starting from nondetectable levels at birth, heme oxygenase 1 (HO-1) expression increased slightly after 1 hour of newborn life and hypoxia. HO-1 was strongly overexpressed after 4 hours of hypoxia and after 4 hours of hypoxia followed by 4 hours of recovery.
Fig 1.
Relative expression of HIF1α and heme oxygenase 1 (HO-1) in the heart after hypoxia of the piglet. Lane t0 is for the newborn control. Lanes t1, h1, t4, h4, t8, h4 + 4, t24, h4 + 20, t48, h4 + 44, t72, h4 + 68 are for control 1 hour, hypoxia 1 hour, control 4 hours, hypoxia 4 hours, control 8 hours, hypoxia 4 hours followed by 4 hours of recovery, control 24 hours, hypoxia 4 hours + 20 hours recovery, control 48 hours, hypoxia 4 hours + 44 hours recovery, control 72 hours, and hypoxia 4 hours + 68 hours recovery, respectively. Light columns are for controls and dark columns are for animals submitted to hypoxia. Hundred micrograms of protein was loaded in each lane. The last representative membrane shows the level of β-actin. Values are the mean ± standard error of mean (SEM) of separate determinations, n = 4. Asterisks indicate significant difference from the corresponding normoxic control (P < 0.001)
Relative expression of αB-crystallin, Hsp27, HspB8, and Hsp20 in the heart after hypoxia
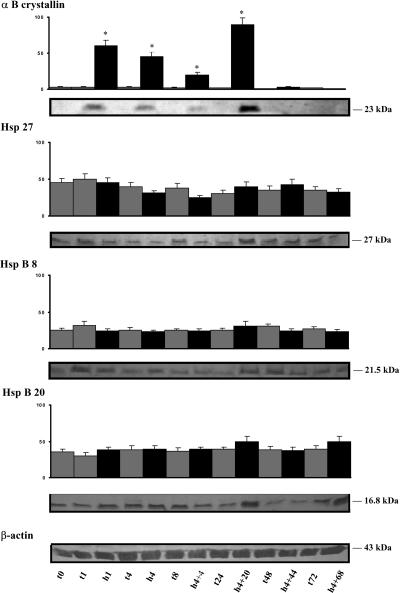
As shown in Figure 2, αB-crystallin levels of expression were drastically increased in animals submitted to low oxygenation. A significant increase in expression was already observed after 1 hour (10 times), and smaller increases were observed after 4 hours of hypoxia (8 times) and after 4 hours of hypoxia followed by 4 hours of recovery (3.5 times). The increase was greatest after 4 hours of hypoxia followed by 20 hours recovery (15 times). No significant change was observed in the expression of Hsp27 either in the control animals or animals submitted to hypoxia during the entire 72-hour experiment. No significant differences were found in the expressions of either HspB8 or Hsp20 in the hearts of animals submitted to hypoxia.
Fig 2.
Relative expression of αB-crystallin, Hsp27, HspB8, and Hsp20 after hypoxia of the piglet. Lanes are as described in the legend of Figure 1. Fifty micrograms of protein was used for each lane. The level of β-actin in the heart is shown in a representative membrane. Values are the mean ± standard error of mean (SEM) of separate determinations, n = 4. Asterisks indicate significant difference from the corresponding normoxic control (P < 0.001)
Relative expression of Hsp60 and inducible Hsp70 in the heart after hypoxia
No significant differences were found between controls and animals submitted to low oxygenation under the same conditions as in Figure 2 for Hsp60 and Hsp70 (data not shown).
Relative expression of endothelial NOS, iNOS, and nNOS in the heart after hypoxia
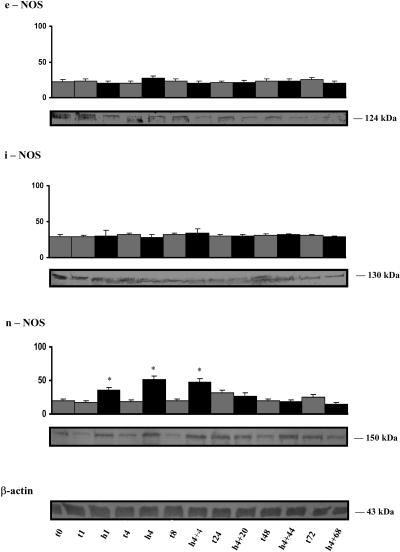
As shown in Figure 3, no significant difference was observed between controls and animals submitted to hypoxia in the expression of endothelial NOS (eNOS) and iNOS. However, for nNOS, expression at birth was higher when compared with the immediate newborn and increased by 2-fold after 1 hour of hypoxia. The increase in expression was greater (2.8-fold) after 4 hours and 2.4-fold after 4 hours of hypoxia followed by 4 hours of recovery. This higher level of expression of nNOS declined in both the controls and in the animals submitted to low oxygenation throughout the 72-hour experiment.
Fig 3.
Relative expression of endothelial nitric oxide synthase (eNOS), inducible NOS (iNOS), and neuronal NOS (nNOS) in the heart of animals submitted to hypoxia. Lanes are as described in the legend of Figure 1. Hundred micrograms was used for each lane. The level of β-actin in the heart is shown in a representative membrane. Values are the mean ± standard error of mean (SEM) of separate determinations, n = 4. Asterisks indicate significant difference from the corresponding control (P < 0.001)
DISCUSSION
The physiological consequences of hypoxia in mammals have been well documented. Basically, after hypoxia, the following 4 different parameters are reduced: (1) body temperature, (2) heart rate, (3) respiratory rate, and (4) blood pH.
Because of their small size and low metabolic rate, neonates are prone to hypothermia resulting in a lowering of the metabolic rate. However, this decrease is associated with a protective effect (Singer 1999).
The reduction in the heart rate, also known as the “diving reflex,” is accompanied by a diversion of blood flow from the peripheral tissues to the central organs. Such changes are also associated with reduced glucose and increased blood lactate levels (Singer 1999).
The reduction in the respiratory rate should be regarded as an immature feature of the respiratory network.
The reduction of blood pH is associated with respiratory acidosis because of an inhibition in the overall enzymatic machinery (Singer 1999).
Hyperthermia through the induction of Hsps has long been described as cardioprotective (Singer 1999). There are many implications of Hsp function in cardiac (Currie et al 1988) and vascular physiology (Snoeckx et al 2001). Although considered as cytoprotective in ischemia, high levels of HSP (particularly Hsp70) (Currie et al 1988) are associated with heart hypertrophy (left ventricle) and observed in spontaneously hypertensive rats (Tanonaka et al 2001). Interestingly, in relation to their strong expression in muscle cells, HSPs (ie, 27, 60, and 70) have been found to be associated with atherosclerosis (Tanonaka et al 2001).
This study is the first to investigate concomitant variations and possible relationships between Hsps and molecules induced by hypoxia in the newborn. Because of the abundant documentation in the literature, cardiac frequency, arterial pressure, blood CO2, glucose, and pH were not determined after hypoxia in this study. The selected criterion for hypoxia was the oxygen saturation of hemoglobin. The hypoxic conditions (4 hours, 5% O2) resulted in a drastic decrease in the hemoglobin saturation of venous blood (Table 2). A strong expression of HIF1α was detected during hypoxia and slowly returned to baseline during the recovery period. Changes in the expression of HIF1α after hypoxia are indicative of alterations of expression of other genes particularly those affecting cellular energy, like the glucose transporter. It should also be noted that, despite the fact that HIF1α is quickly degraded (Gassmann and Wenger 1997), increased expressions can last several hours after the hypoxic insult, thereby indicating a possibly significant requirement for biosynthesis. The increase in HO-1 expression is also indicative of the activation of this second oxygen deficiency marker.
The major finding of this work is the significant increase of αB-crystallin from a low relative value of expression in the left ventricle. This low expression appears to be limited to the unstressed left ventricle. It should be noted that, in the left ventricle, the kinetics of αB-crystallin expression in the animals submitted to hypoxia closely parallels that of the HIF1α expression. We hypothesize that αB-crystallin is closely associated with the oxygen supply, with, possibly, gene activation followed by de novo protein synthesis. Counting HO as the first, this is the second indication of oxygen dependency of HSP. This observation warrants further studies at molecular levels. Two aspects, at least, can be considered: messenger ribonucleic acid (mRNA) expression would be indicative of the activation step for αB-crystallin. The investigation can also be completed by the study of the expression of transcription factors including Hsf1. Surprisingly, Hsp27 does not display any apparent increase in the left ventricle after hypoxia despite its similar chaperoning properties. Hsp27 has been found to be increased in the myocardium during the development of heart failure (Tanonaka et al 2001). However, HSP27 has recently been found to respond differently from αB-crystallin in vascular endothelial cells (Golenhofen et al 2002). These observations warrant investigations at the subcellular level by immunohistochemistry. Possible translocation of HSPs can also be investigated after separation of the nuclei.
HspB8 and Hsp20 are newly described sHSPs (Verschuure et al 2003). HSPB8 displays a low level of expression in the heart during the first few days after birth. Hsp20 was more strongly expressed in the left ventricle than HspB8. In the heart, Hsp20 is biochemically associated with αB-crystallin but localizes in distinct bands as does αB-crystallin and sarcomeric actin. This report appears to dissociate the effects of hypoxia on αB-crystallin and Hsp20 in the heart (Pipkin et al 2003). Hsp60 is a mitochondrial HSP associated with Hsp10. The expression of Hsp60 was not changed after hypoxia under the present conditions. It has been associated with some pathophysiologies of the heart (Singer 1999), and it is well known that Hsp70 is overexpressed in the myocardium after stress (Karmazyn et al 1990). These results are indicative of the absence of the effects of hypoxia on this Hsp in the left ventricle. Although these observations have been made on the left ventricle, it should be noted that such observations may be extended to the right ventricle and atrium. Moreover, they also warrant studies at the immunocytochemical level for cellular localization.
The other proteins studied in this investigation include the isoforms of NOS, namely endothelial, inducible, and neuronal. This study reveals that eNOS and iNOS expressions are not increased in the left ventricle after hypoxia, whereas nNOS is considerably increased after 1 and 4 hours of hypoxia. eNOS is related to vascular hemodynamics and hypertension (Huang et al 1995), whereas iNOS is related to Hsp70 (Bellmann et al 2000). Their expressions are not modified after hypoxia under present conditions, thereby indicating that a low oxygen supply has no effect on these NO-producing enzymes. Because it would also be of interest to study the expression of Hsp90, a protein associated with eNOS, such an investigation is concurrently in progress. nNOS responds rapidly to hypoxia by an increase in the heart rate. This observation appears of important because mice deficient in this isoform show altered cardiac and respiratory responses to hypoxia. Comparing the effect of hypoxia on eNOS and nNOS in relation to Hsp90 could be of particular interest. Determining the expression of the transcription factor (HSF) would also be important. The unexpected overexpression of αB-crystallin emphasizes the need for investigations of the regulatory mechanisms of this activation. Determining mRNA levels would give important insights into the relationship between oxygen and αB-crystallin. These observations also show that an increase in specific HSP (αB-crystallin) expression can still occur several hours after hypoxia. This increase has to be related to delayed protection of tissues observed after several days by manipulating the expression of related enzymes, like NOS (Huang et al 1995).
In conclusion, our study reveals that the newborn piglet model can be used to evaluate the impact of hypoxia at the molecular level. This piglet model can also be used for studying the effect of hypo- or hyperthermia associated with hypoxia as well as early cardiac development. Further characterization at the molecular and cellular levels will determine the extent of the usefulness of our model and its limitations. The use of different animal models and measurements of several hemodynamic parameters in association with HSP expression can result in a better understanding of cardiac damage resulting from hypoxia on myocardial performance. Further studies, such as evaluation of Hsf activation, are required to delineate the precise mechanisms of the specific αB-crystallin activation.
Acknowledgments
The authors are grateful to J. Lareynie for technical assistance and to G. David for preparing the manuscript. The authors also thank Air Liquide for the generous gift of the nitrogen used in the study. R.M.T. was supported by a grant from the Canadian Institutes of Health Research (Grant MOP 43958).
REFERENCES
- Agani FH, Puchowicz M, Chavez JC, Pichiule P, LaManna J. Role of nitric oxide in the regulation of HIF1 Alpha expression during hypoxia. Am J Physiol. 2002;283:C178–C186. doi: 10.1152/ajpcell.00381.2001.0002-9513(2002)283<C178:RONOIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Arrigo AP, Landry J. 1994 Expression and function of the low molecular weight heat shock proteins. In: The Biology of Heat Shock Proteins and Molecular Chaperones, ed Morimoto L, Georgopoulos C. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 335–373. [Google Scholar]
- Bellmann K, Burkart V, Bruckhoff J, Kolb H, Landry J. p38-dependant enhancement of cytokine-induced nitric-oxide synthase gene expression by heat shock protein 70. J Biol Chem. 2000;275:18172–18179. doi: 10.1074/jbc.M000340200.0021-9258(2000)275<18172:PEOCNS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chiral M, Grongnet JF, Plumier JC, David JC. Effect of hypoxia on stress proteins in the brain at birth. Pediatr Res. 2004;56:775–782. doi: 10.1203/01.PDR.0000142732.09325.61.0031-3998(2004)056<0775:EOHOSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Conover WJ. 1980 Practical Nonparametrical Statistics. Wiley, New York. [Google Scholar]
- Currie RW, Kacmazyn M, Kloc M, Mailer K. Heat shock response is associated with enhanced postischemic ventricular recovery. Circ Res. 1988;63:543–549. doi: 10.1161/01.res.63.3.543.0009-7330(1988)063<0543:HSRIAW>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dalman C, Thomas HV, Davis AS, Gentz J, Lewis G, Allebeck P. Signs of asphyxia at birth and risk of schizophrenia. Br J Psychiatry. 2001;179:403–408. doi: 10.1192/bjp.179.5.403.0007-1250(2001)179<0403:SOAABA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- David JC, Grongnet JF. Effect of hypoxia on DNA fragmentation in different brain regions of the newborn piglet. Mol Reprod Dev. 2000;57:153–158. doi: 10.1002/1098-2795(200010)57:2<153::AID-MRD6>3.0.CO;2-7.1040-452X(2000)057<0153:EOHODF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- David JC, Landry J, Grongnet JF. Perinatal expression of HSP27 in brain regions and non-neuronal tissues of the piglet. J Mol Neurosci. 2001a;15:109–120. doi: 10.1385/jmn:15:2:109.0895-8696(2001)015<0109:PEOHIB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- David JC, Tanguay RM, Grongnet JF. Perinatal expression of HSC70 and HSP70 in neural and non neural tissues of the piglet. Dev Brain Res. 2001b;128:91–99. doi: 10.1016/s0165-3806(01)00143-2.0165-3806(2001)128<0091:PEOHAH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Dixon G, Badawi N, Kurinczuk JJ, Keogh JM, Silburn SR, Zubrick SR, Stanley PG. Early developmental outcomes after newborn encephalopathy. Pediatrics. 2002;109:26–33. doi: 10.1542/peds.109.1.26.0031-4005(2002)109<0026:EDOANE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gassmann M, Wenger RH. HIF1, a mediator of the molecular response to hypoxia. News Physiol Sci. 1997;12:214–218.0886-1714(1997)012<0214:HAMOTM>2.0.CO;2 [Google Scholar]
- Golenhofen N, Ness W, Warvrousek EW, Drenckahn D. Expression and induction of the stress protein alpha-B-crystallin in vascular endothelial cells. Histochem Cell Biol. 2002;117:203–209. doi: 10.1007/s00418-001-0378-7.0948-6143(2002)117<0203:EAIOTS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hammerer-Larcher A, Mair J, Bonatti J, Watzka SB, Puschendorf B, Dirnhofer S. Hypoxia induces heat shock protein expression in human coronary artery bypass grafts. Cardiovasc Rev. 2001;50:115–124. doi: 10.1016/s0008-6363(01)00198-5.0197-3118(2001)050<0115:HIHSPE>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6.0014-4835(2003)076<0145:AC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Huang PL, Huang Z, Mashimo H, Bloch KD, Moskowitz MA, Bevan JA. Hypertension in mice lacking the gene for endothelial nitric oxide synthase. Nature. 1995;377:239–242. doi: 10.1038/377239a0.0028-0836(1995)377<0239:HIMLTG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jiang ZD. Long term effect of perinatal and postnatal asphyxia on developing human auditory brainstem responses: peripheral hearing losses. Int J Pediatr Otorhinolaryngol. 1995;33:225–235. doi: 10.1016/0165-5876(95)01213-3.0165-5876(1995)033<0225:LTEOPA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kamimoto M, Longo MD, Gilbert RD. Cardiac functions in fetal sheep during two weeks of hypoxaemia. Am J Physiol. 1996;35:R1778–R1785. doi: 10.1152/ajpregu.1994.266.6.R1778.0002-9513(1996)035<R1778:CFIFSD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kamitomo M, Alonso JG, Okai T, Longo LD, Gilbert RD. Effects of long-term high altitruit hypoxaemia on ovine fetal cardiac output and blood flow distribution. Am J Obstet Gynecol. 1993;169:701–707. doi: 10.1016/0002-9378(93)90646-z.0002-9378(1993)169<0701:EOLHAH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Karmazyn K, Mailer K, Currie RW. Acquisition and decay of heat shock-enhanced post ischemic ventricular recovery. Am J Physiol. 1990;259:H424–H431. doi: 10.1152/ajpheart.1990.259.2.H424.0002-9513(1990)259<H424:AADOHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kawana K, Miyamoto Y, Tanonaka K, Han-No Y, Yoshida H, Takahashi M, Takeo S. Cytoprotective mechanism of HSP70 against hypoxia/reoxygenation injury. J Mol Cell Cardiol. 2000;32:2229–2237. doi: 10.1006/jmcc.2000.1250.0022-2828(2000)032<2229:CMOHAR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kiang JG, Tsokos GC. Heat shock proteins 70 kDa: molecular biology, biochemistry, and physiology. Pharmacol Ther. 1998;80:183–201. doi: 10.1016/s0163-7258(98)00028-x.0163-7258(1998)080<0183:HSPKMB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lewis AM, Mathieu-Costello O, McMilan PJ, Gilbert RD. Effects of long-term high-altitude hypoxia on the capillarity of the ovine fetal heart. Am J Physiol. 1999;277:H756–H762. doi: 10.1152/ajpheart.1999.277.2.H756.0002-9513(1999)277<H756:EOLHHO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Li C, Jackson RM. Reactive species mechanisms of cellular hypoxic-reoxygenation injury. Am J Physiol. 2002;282:C227–C241. doi: 10.1152/ajpcell.00112.2001.0002-9513(2002)282<C227:RSMOCH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Pipkin W, Johnson JA, Creazzo TL, Burch J, Komalavilas P, Brophy C. Localization, macromolecular associations and function of the small heat shock related protein HSP20 in rat heart. Circulation. 2003;107:469–476. doi: 10.1161/01.cir.0000044386.27444.5a.0009-7322(2003)107<0469:LMAAFO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Singer D. Neonatal tolerance to hypoxia: a comparative physiological approach [review] Comp Biochem Physiol A Mol Integr Physiol. 1999;123:221–234. doi: 10.1016/s1095-6433(99)00057-4.1095-6433(1999)123<0221:NTTHAC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Snoeckx LH, Cornelussen RN, Van Nieuwenhoven FA, Reneman RS, Van Der Vusse GJ. Heat shock proteins and cardiovascular physiopathology. Physiol Rev. 2001;81:1461–1497. doi: 10.1152/physrev.2001.81.4.1461.0031-9333(2001)081<1461:HSPACP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Southall DP, Samuels MP. Respiratory infection, hypoxaemic episodes and sudden infant deaths—possible pathways in prevention. Matern Child Health J. 1989;210:17–21. [Google Scholar]
- Tallot P, Grongnet JF, David JC. Dual perinatal and developmental expression of the small heat shock protein αB-crystallin and HSP27 in different tissues of the developing piglet. Biol Neonat. 2003;83:281–288. doi: 10.1159/000069488.0006-3126(2003)083<0281:DPADEO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tanguay RM, Wu B, Khandjian EW. Tissue specific expression of heat shock proteins of the mouse in the absence of stress. Dev Genet. 1993;14:112–118. doi: 10.1002/dvg.1020140205.0192-253X(1993)014<0112:TSEOHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tanonaka K, Yoshida H, Toga W, Furuhama K, Takeo S. Myocardial heat shock proteins during the development of heart failure. Biochem Biophysiol Res Comm. 2001;283:520–525. doi: 10.1006/bbrc.2001.4801. [DOI] [PubMed] [Google Scholar]
- Verschuure P, Tatard C, Boelens WC, Grongnet JF, David JC. Expression of small heat shock protein HSPB2, HSPB8, HSP20 and cvHsp in different tissues of the perinatal developing pig. Eur J Cell Biol. 2003;82:523–530. doi: 10.1078/0171-9335-00337.0171-9335(2003)082<0523:EOSHSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Viapiano MS, Mitridate de Novara AM, Fiszer de Plazas SF, Bozzini CE. Prolonged exposure to hypobaric hypoxia transiently reduces GABAA receptor number in mice cerebral cortex. Brain Res. 2001;894:31–36. doi: 10.1016/s0006-8993(00)03194-2.0006-8993(2001)894<0031:PETHHT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]