Abstract
Endoplasmic reticulum (ER) stress plays a pivotal role in adipogenesis, which encompasses the differentiation of adipocytes and lipid accumulation. Sustained ER stress has the potential to disrupt the signaling of the unfolded protein response (UPR), thereby influencing adipogenesis. This comprehensive review illuminates the molecular mechanisms that underpin the interplay between ER stress and adipogenesis. We delve into the dysregulation of UPR pathways, namely, IRE1-XBP1, PERK and ATF6 in relation to adipocyte differentiation, lipid metabolism, and tissue inflammation. Moreover, we scrutinize how ER stress impacts key adipogenic transcription factors such as proliferator-activated receptor γ (PPARγ) and CCAAT-enhancer-binding proteins (C/EBPs) along with their interaction with other signaling pathways. The cellular ramifications include alterations in lipid metabolism, dysregulation of adipokines, and aged adipose tissue inflammation. We also discuss the potential roles the molecular chaperones cyclophilin A and cyclophilin B play in adipogenesis. By shedding light on the intricate relationship between ER stress and adipogenesis, this review paves the way for devising innovative therapeutic interventions.
Keywords: endoplasmic reticulum stress, adipogenesis, unfolded protein response, obesity, metabolic disorders
1. Introduction
The prevalence of obesity and associated metabolic disorders is on the rise as a result of sedentary lifestyles and high caloric intake [1]. Obesity results from genetic, environmental, and lifestyle factors, and excessive endoplasmic reticulum (ER) stress is believed to play a role [2,3,4]. The ER is involved in folding and assembling proteins while facilitating the movement of newly synthesized proteins to their required destinations, lipid synthesis, and regulation of calcium levels [5]. ER stress occurs when there is an influx of proteins into the ER beyond its processing capacity, reaching a limit in protein folding, or depletion of calcium within the ER [6]. Prolonged ER stress leads to cellular apoptosis. To prevent this, the ER regulates homeostasis through the unfolded protein response (UPR) [7].
The UPR responds to ER stress by reducing protein translation [8,9], upregulating chaperones, promoting folding [9], and degrading misfolded proteins [10,11,12]. The inositol-requiring enzyme1—X-box binding protein 1 (IRE1-XBP1), protein kinase RNA-like ER kinase (PERK) and activating transcription factor 6 (ATF6) pathways impact both ER stress and adipogenesis, influencing adipogenic transcription factors such as peroxisome proliferator-activated receptor γ (PPARγ) and CCAAT-enhancer-binding proteins (C/EBPs) [13].
The escalation of ER stress can perpetuate obesity in a vicious cycle [4]. Elevated ER stress has the potential to stimulate inflammatory responses associated with adipocytes and insulin resistance [14]. This stimulation may lead to increased energy storage and exacerbation of obesity, consequently further amplifying ER stress [3,15,16]. These research findings underscore the bidirectional relationship between ER stress and obesity, highlighting that their interaction is not merely a unidirectional cause-and-effect scenario but rather manifests as a mutually reinforcing connection. Understanding ER stress-adipogenesis interplay is crucial for combating obesity, since investigating the relationship between ER stress and adipogenesis provides valuable insights into the origins of obesity and potential treatments. Therefore, understanding ER stress-induced adipogenesis could lead to innovative strategies for combating obesity and its associated complications. Also, targeting ER stress could alleviate adipose tissue dysfunction and metabolic issues.
This review explores the dysregulation of UPR pathway, ER stress’s impact on adipogenesis, and therapeutic interventions for obesity-related metabolic disorders.
2. The Process of Adipogenesis
Adipogenesis is the formation process of adipocytes which entails cell conversion into adipose tissue followed by accumulating lipids within the cells and differentiation into adipocytes. In human physiology and health, adipogenesis holds profound significance since it involves the differentiation of precursor cells into mature adipocytes. These specialized fat cells serve as important energy storage reservoirs, releasing triglycerides during periods of heightened energy demand [17,18,19]. They also act as an insulating layer, regulating body temperature [20,21], and provide crucial protection to internal organs [22,23]. Additionally, fat cells act as endocrine cells, secreting hormones and adipokines such as leptin, adiponectin, and resistin, which play vital roles in regulating metabolism, appetite, and inflammatory processes [24,25,26,27,28]. Dysregulation of adipogenesis contributes to obesity, a significant risk factor for metabolic disorders such as type 2 diabetes, cardiovascular diseases, and fatty liver disease [29,30,31,32]. Therefore, comprehending this complex biological process is imperative for advancing human health.
Adipogenesis involves the formation and differentiation of fat cells or adipocytes, progressing through several critical stages. In theory, the process starts with multipotent mesenchymal stem cells (MSCs), capable of differentiating into various cell types, including adipocytes [33]. These cells commit to becoming adipocytes along one of the numerous differentiation pathways available [34].
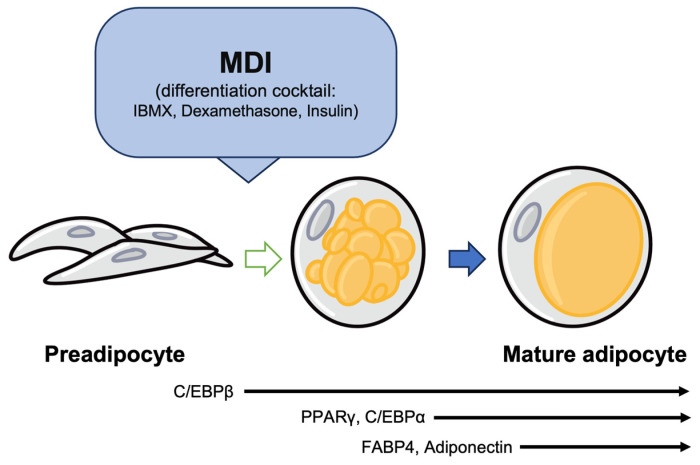
As differentiation begins, crucial genes such as C/EBPs and PPARγ are activated [35,36]. These proteins have a vital role in the initial stages of adipocyte differentiation. As shown in Figure 1, the expression of C/EBPs and PPARγ starts the conversion of MSCs into adipocytes, allowing these cells to obtain the ability to form fat and transform into adipose tissue [37,38].
Figure 1.
Adipogenesis factors involved in the stages of adipogenesis. C/EBPβ plays an important role in activating the expression of PPARγ and C/EBPα during the early stages of differentiation. PPARγ induces the expression of FABP4. Additionally, C/EBPα promotes the expression of adiponectin.
Differentiated adipocytes initiate the synthesis of triacylglycerol (TAG), a major neutral fat stored within fat cells [39,40]. Consequently, the fat content inside the cell increases, culminating in the formation of fully mature adipocytes [41]. These adipocytes typically exhibit a larger, round or spherical shape and contain substantial lipid droplets, serving as critical components of adipose tissue [42,43].
Adipogenesis plays a critical role in energy storage and metabolic regulation, rendering it a prominent research area, notably concerning obesity and metabolic disorders. This process leads to the formation of adipose tissue, which contributes to energy balance and metabolic regulation.
3. UPR Signaling Pathways (IRE1-XBP1, PERK, ATF6) in Obesity
The UPR is conducted by three primary transmembrane proteins present on the ER membrane: IRE1, PERK, and ATF6 [44]. These sensors can detect ER stress and activate adaptive signaling pathways aimed at restoring ER homeostasis [45].
The IRE1 pathway involves the non-traditional splicing of XBP1 mRNA, which produces a spliced form called XBP1s [46]. This transcription factor encourages the expression of chaperones, foldases, and ER-associated degradation (ERAD) pathway constituents [47,48,49]. This leads to improved folding capacity of the ER [50]. The PERK pathway leads to the phosphorylation of eukaryotic translation initiation factor 2 alpha (eIF2α), which reduces overall protein translation [51]. Despite this decrease, it eases the ER protein load and triggers the translation of ATF4 [52]. This promotes the expression of genes related to antioxidant responses and amino acid metabolism [53]. In contrast, the ATF6 pathway involves ATF6 translocating to the Golgi apparatus under ER stress [47]. The cleavage of ATF6 results in a transcriptionally active fragment that enters the nucleus, improving the ER’s ability to manage protein folding demands by enhancing the expression of chaperones and ERAD-related factors [54].
The UPR acts as a balance between adaptive mechanisms that mend ER homeostasis and a terminal response that triggers apoptosis in highly stressed cells [55,56,57]. ER homeostasis is maintained by ER chaperone proteins such as glucose-regulated protein 78 (GRP78), GRP94, calreticulin (CRT), and protein disulfide isomerase (PDI) [58,59,60,61,62]. Particularly, GRP78 (also known as immunoglobulin heavy chain-binding protein or BiP) is a well-characterized member of the heat shock protein 70 kDa (HSP70) family, encoded by the HSPA5 gene, which is essential for proper protein folding, regulation of the UPR signaling, maintaining chaperone balance, and preventing apoptosis [63,64,65,66]. Among its crucial roles, GRP78 facilitates proper protein folding within the ER, maintains proteins in their folded state, prevents aggregation of protein folding intermediates, and directs misfolded proteins to the ERAD pathway [67,68,69].
Additionally, GRP78 plays a significant role in maintaining intracellular calcium (Ca2+) homeostasis within the ER [70]. It regulates intracellular Ca2+ levels and contributes to various cellular processes involving Ca2+ signaling [71]. Furthermore, under specific cellular stress conditions, GRP78 can form complexes with pro-caspases such as caspase-7 and caspase-12 on the ER membrane, providing protective functions for cell survival [72,73].
Through these diverse functions, GRP78 plays a pivotal role in balancing cell survival and apoptosis in cells experiencing ER stress [66,73]. It is also necessary during early embryonic development and exhibits reduced expression during aging [65,74].
Obesity, which is defined as the excessive accumulation of adipose tissue, is linked to a chronic condition of low-grade inflammation and metabolic disorders [4,75,76]. When there is an abundance of nutrients, adipocytes face a challenge to produce and secrete significant amounts of adipokines and cytokines, increasing the risk of ER stress [2,77,78]. To cope with the heightened demand for protein folding, the UPR is activated to restore ER function [79]. This impacts adipocyte function significantly. The activation of the UPR in adipocytes has a dual influence on obesity. Firstly, it strives to restore ER homeostasis by boosting the expression of chaperone proteins and increasing the ER folding capacity [80]. Secondly, persistent ER stress can disrupt UPR signaling, resulting in cellular dysfunction and insulin resistance [30].
3.1. The IRE1-XBP1 Pathway and Lipid Metabolism
The IRE1-XBP1 pathway, a pivotal component of the UPR, is connected with lipid metabolism and adipogenesis [81,82]. Via IRE1, the splicing of XBP1 has a direct impact on the expression of lipogenic genes and lipid droplet dynamics [5]. In such scenarios, IRE1 becomes activated and splices XBP1 mRNA [83]. XBP1 mRNA splicing converts the inactive XBP1 (XBP1u) into its active form, XBP1s, through IRE1’s ribonuclease activity [84]. XBP1s, once activated, functions as a transcription factor that regulates the expression of different genes. Specifically, XBP1s promotes the expression of ERAD genes which facilitate the elimination of unnecessary misfolded proteins [85]. When specifically deleting the XBP1 gene in the adult mouse liver to investigate its function, we observed a significant reduction of approximately 85–90% in hepatic fatty acid and cholesterol synthesis. This led to lowered concentrations of plasma cholesterol and triglycerides [86]. Furthermore, hepatic overexpression of XBP1 directly upregulates the promoters of lipid synthesis genes, including acetyl-CoA carboxylase 2 (ACC2) and sterol regulatory element-binding Protein 1 (SREBP1), thereby promoting lipid synthesis [87]. In mouse hepatic cells, XBP1 exacerbates lipid synthesis and suppresses lipid breakdown, thereby worsening lipid accumulation. However, XBP1 knockout in mice reduces hepatic steatosis, increases lipid breakdown, and decreases lipid accumulation. Consequently, pharmacologically inhibiting XBP1 presents a new potential for treating non-alcoholic fatty liver disease (NAFLD) [88].
XBP1s induces the expression of various genes, such as FAS, SREBP1c, ACC, DGAT, ChREBP, PLIN, CIDE, ATGL, HSL, and others, as shown in Table 1 [47,86,89,90,91]. This regulation allows XBP1s to exert a significant impact on physiological processes related to lipid metabolism and obesity.
Table 1.
XBP1s regulate various genes involved in lipid synthesis and lipid storage by promoting the expression of lipogenic genes. The table displays some of the key lipogenic genes promoted by XBP1s.
| Modulators | Full Name | Roles in Lipogenesis |
|---|---|---|
| FAS | Fatty acid synthase | XBP1s promotes the expression of the FAS gene, contributing to fatty acid synthesis. FAS is an enzyme responsible for generating fatty acids and plays a crucial role in lipid metabolism. |
| SREBP1c | Sterol regulatory element-binding protein 1c | XBP1s regulates the expression of the SREBP1c gene, facilitating lipid synthesis. SREBP1c also activates other important genes related to lipid metabolism. |
| ACC | Acetyl-CoA carboxylase | XBP1s enhances the expression of the ACC gene, increasing the conversion of acetyl-CoA into fatty acids. This process is essential in fatty acid synthesis and is one of the key steps. |
| DGAT | Diacylglycerol O-Acyltransferase | XBP1s regulates the expression of the DGAT gene, promoting processes related to lipid droplets. This is associated with lipid storage |
| ChREBP | Carbohydrate-responsive element-binding protein | XBP1s controls the expression of the ChREBP gene, regulating the interaction between carbohydrate metabolism and fatty acid synthesis. |
| PLIN | Perilipin | XBP1s controls the expression of the PLIN gene, facilitating the perilipin protein found on the surface of lipid droplets. Perilipin stabilizes lipid droplets and regulates lipid storage and movement processes. |
| CIDE | Cell death-inducing DFFA-like effector | XBP1s contributes to the dynamics of lipid droplets by regulating the expression of certain genes within the CIDE gene family. These genes play a role in modulating the structure and function of lipid droplets. |
| ATGL | Adipose triglyceride lipase | XBP1s regulates the expression of the ATGL gene, controlling the breakdown of triglycerides in neutral fat. This process is associated with the movement of lipids within lipid droplets. |
| HSL | Hormone-sensitive lipase | XBP1s further regulates the breakdown of triglycerides in neutral fat by controlling the expression of the HSL gene. This process is related to energy metabolism. |
Collectively, the IRE1-XBP1 pathway detects ER stress and responds by regulating lipid metabolism. XBP1s functions as a transcription factor, overseeing the expression of diverse genes, thereby aiding in lipid generation, storage, and regulation. Disrupting this pathway due to lengthy ER stress can lead to abnormal lipid accumulation and malfunctioning adipocytes, thus promoting obesity-related ailments.
3.2. The PERK Pathway and Insulin Sensitivity
PERK regulates protein synthesis to oversee the correct folding of proteins within the ER and the accumulation of defective proteins [92]. To overcome imbalances caused by ER stress, PERK promotes the phosphorylation of eIF2α, leading to the temporary inhibition of protein synthesis [93]. Consequently, cells can withstand stress, ensuring survival through appropriately regulated protein synthesis [94].
PERK exists in a homomeric form under stable conditions but transitions into a tetrameric structure under stress conditions, leading to trans-autophosphorylation of the PERK domain at the C-terminus [95,96].
Pancreatic islet β cells are specialized secretory cells responsible for insulin storage, and they produce more insulin in insulin-resistant states [97,98]. In this context, processes such as proinsulin folding, ERAD, and mediation of quality/quantity control, as well as trafficking, are regulated to manage metabolic states and insulin demand [99,100,101,102].
Furthermore, the PERK-ATF4 pathway plays a crucial role in β cell biology and diabetes research [103,104]. PERK deficiency induces ER stress and high blood glucose levels, and PERK-mediated phosphorylation of eIFα is associated with glucose intolerance [105,106,107]. However, reduced PERK activity promotes glucose-stimulated insulin secretion (GSIS), and deletion of downstream signaling factors in the PERK-ATF4 pathway helps alleviate ER stress and prevent β cell loss [108,109,110].
Moreover, in the absence of PERK, the activity of enzymes involved in lipid production such as SREBP-1c, FAS, and SCD1 is hindered, and PERK accumulates in lipid droplets [111]. Additionally, during the differentiation process of fat cells, PERK has demonstrated its utilization of diacylglycerol to activate lipid kinases [112]. Research confirms that the downregulation of PERK reduces adipogenesis by decreasing ATF4 [13]. ATF4 has demonstrated active regulation of adipocyte differentiation across various evidence. Overexpression of ATF4 in 3T3-L1 cells enhances adipogenesis, while ATF4 siRNA inhibits pre-adipocyte differentiation into mature adipocytes. Depletion of ATF4 reduces adipocyte differentiation in human mesenchymal stem cells [113]. Recent studies observed elevated phosphorylation of PERK, an ER stress marker, in obese mice on a high-fat diet (HFD). This heightened phosphorylation, compared with normal diet-fed mice, correlates with abnormal protein degradation and increased lipid accumulation [114]. PERK utilizes its intrinsic lipid kinase activity to generate phosphatides, mediating Akt activation, thereby promoting adipocyte differentiation [112,115]. Consequently, PERK can stimulate adipocyte differentiation through Akt activation [116]. Additionally, ATF6α pathway activation also contributes to adipogenesis [117].
Thus, pathways associated with PERK significantly influence insulin sensitivity and β cell function, playing a crucial role in diabetes research and obesity.
3.3. The ATF6 Pathway and Inflammation
ATF6 is a transmembrane transcription factor with an N-terminal domain in the cytoplasm and a C-terminal domain in the ER lumen [118]. ATF6 contributes towards ERAD for resolving incorrect protein folding [54]. In mammalian cells, ATF4 and ATF6 are reported to interact with the 26S proteasome, inducing the ER membrane protein HERP/Mif1 and facilitating efficient ERAD [119]. This arrangement positions the proteasome closer to the ER, enabling smoother protein degradation [44]. The UPR is suggested to function in two stages. In the first stage, it allows time for protein folding, and in the second stage, it targets unfolded proteins for degradation [120]. ATF6’s rapid activation is compared with IRE1, which is believed to occur due to ATF6’s swift activation compared with IRE1, responsible for inducing XBP1 splicing and translation. During this period, ATF6-induced ER chaperones can facilitate protein folding before inducing ERAD genes that promote the degradation of unfolded XBP1 [120,121].
ATF6, initially located on the ER membrane, moves to the Golgi apparatus under ER stress [47], where it is cleaved by site 1 protease (S1P) and site 2 protease (S2P) to form the N-terminal fragment [122,123]. The N-fragment translocates to the nucleus and serves as a transcription factor [124]. One study confirmed that protein kinase B (AKT) phosphorylation mediated by ATF6 contributes to downstream nuclear factor-kappa B (NF-κB) activation [125,126,127]. This interaction assists NF-κB, which regulates inflammation and immune responses, by inducing the expression of inflammatory genes. The association of ATF6 with NF-κB upregulates the expression of inflammation-associated genes, including the cytokines interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α) [128]. Furthermore, ATF6 can induce cell death during prolonged ER stress by activating downstream effectors, including CHOP, c-Jun N-terminal kinase (JNK), and proapoptotic Bcl-2 family proteins [129]. Dysfunctional signaling of ATF6 may contribute to the accumulation of proteins that are misfolded and exacerbate inflammation, which is a characteristic of obesity [2].
Inhibition of ATF6 in mesenchymal stromal C3H10T1/2 cells impedes lipid accumulation, downregulating crucial genes for adipogenesis: PPARγ, SREBP-1c, GLUT4, and aP2 [117]. PPARγ reduction intensifies during adipogenesis in ATF6-deficient cells versus controls [5]. Diminished ATF6 correlates with restrained C/EBPβ, an early adipogenic factor. Although direct regulation is not confirmed, ATF6 overexpression increases acetyl-CoA carboxylase beta (Acacb) and Fasn expression in mouse embryonic fibroblasts (MEF) and enhances FAS in Chinese hamster ovary (CHO) cells [130]. The ER stress pathways (IRE1, PERK, ATF6) collectively drive both lipogenesis and adipogenesis [5,131]. Inhibiting all of them is pivotal to curb lipogenesis and delay the onset of obesity.
4. ER Stress and Adipogenesis
4.1. Effects of ER Stress on Transcription Factor Involed in Adipogenesis (PPARγ, C/EBPs)
The impact of ER stress on transcription factors that control fat, specifically peroxisome PPARγ and C/EBPs, has recently gained significance in research. PPARγ and C/EBPs are crucial in regulating adipocyte differentiation and lipid metabolism [132]. Under normal physiological conditions, these transcription factors coordinate the expression of genes involved in adipogenesis, adipocyte maturation, and lipid storage [36]. However, during ER stress, the phosphorylation of eIF2α in the PERK-eIF2α pathway also increases the translation of C/EBP in an in vitro model [133]. Similarly, reduced phosphorylation of eIF2α achieved by overexpression of GADD34 in the liver decreases the expression of C/EBPα, C/EBPβ, and PPARγ [134].
The typical adipogenesis progression in 3T3-L1 preadipocytes involves three stages: first, they undergo contact inhibition, then mitotic clonal expansion (McE), followed by the final stage of adipogenic differentiation [135]. Initially, preadipocytes express adipogenic transcription factors such as CCAAT-enhancer-binding proteinβ/δ (C/EBPβ/δ) and exhibit low levels of PPARγ [136]. Interestingly, early induced C/EBPβ is inactive in preadipocytes, while PPARγ serves as a crucial master regulator in the transcriptional program of adipocytes [137]. C/EBPβ and C/EBPδ are early inducers of adipocyte differentiation and promote the expression of CCAAT-enhancer-binding Protein α (C/EBPα) and PPARγ, key regulators of mature adipocyte function [36].
C/EBPα is required for the accumulation of lipids and insulin sensitivity in differentiated adipocytes [138]. The transition from preadipocytes to mature adipocytes is initiated by pro-adipogenic signals including insulin, dexamethasone, 3-isobutyl-1-methylxanthine (IBMX), or bone morphogenetic proteins (BMPs). This process entails increasing the expression of adipogenic transcription factors, such as PPARγ and C/EBPα, resulting in morphological alterations from fibroblast-like cells to spherical ones with a solitary prominent lipid droplet [36,139]. Over time, mature adipocytes demonstrate metabolic and endocrine characteristics, supported by genes such as fatty acid-binding protein 4 (FABP4), glucose transporter type 4 (GLUT4), leptin, and adiponectin [140,141].
PPARγ plays a key role in adipocyte differentiation, with two isoforms referred to as PPARγ1 and PPARγ2 [142,143,144]. Both isoforms promote adipocyte differentiation, although PPARγ2 demonstrates more efficiency at lower ligand concentrations [144,145]. Additionally, C/EBPs, a group of transcription factors, are crucial for adipogenesis [132]. They stimulate the production of C/EBPα, which is vital for insulin sensitivity in differentiated adipocytes [146]. The complex molecules involved in adipogenesis propose that mitotic clonal expansion could produce internal ligands for PPARγ [147,148]. Additional study is necessary to completely clarify the complicated connection between peroxisome proliferator-activated receptors (PPARs), C/EBPs, and ER stress in the context of adipogenesis.
Understanding the complex relationship between ER stress and fat transcription factors, namely, PPARγ and C/EBPs, is essential to uncovering the molecular mechanisms that drive adipogenesis and metabolic dysregulation. By elucidating the effects of ER stress on these transcription factors, new therapeutic approaches targeting ER stress reduction and proper function restoration may be developed, providing potential interventions for obesity and related metabolic disorders.
4.2. Relationship between CHOP and a Transcription Factor Involved in Adipogenesis
ER stress induces the expression of interleukin-8 (IL-8), an inflammatory cytokine, and the nuclear translocation of CHOP [149]. This upregulation of IL-8 due to ER stress subsequently leads to an increase NF-κB expression [150,151]. NF-κB is a factor that is negatively regulated by the adipogenic differentiation factor PPARγ [149,152]. The activity of PPARγ serves as a crucial regulator in maintaining balance by inhibiting NF-κB and decreasing inflammatory responses [153,154].
ER stress-induced CHOP expression is induced through the UPR, typically through the PERK pathway [151]. CHOP is a transcriptional regulator within the nucleus and regulates numerous genes involved in cellular processes such as inflammation, differentiation, autism, and apoptosis [155,156,157,158]. CHOP is a stress response element that responds to cellular insults such as ER stress and nutrient deprivation and is dependent on eIF2α phosphorylation [159]. It also plays a role in various inflammatory responses [160]. Moreover, endotoxemia enhances CHOP activity, which leads to caspase processing of interleukin-1β (IL-1β) [161].
CHOP impedes the differentiation of the mesenchymal lineage [162]. It is a crucial regulator of adipogenesis, and this function is supported by various experiments. CHOP is recognized to be a principal inhibitory factor for the adipogenic differentiation factor C/EBPβ, and it can hinder the downstream targets of C/EBPβ, such as PPARγ [154,162]. CHOP has a negative impact on the initial stages of adipogenic differentiation by inhibiting the activation of C/EBPβ, which then affects the activation of C/EBPα and PPARγ [163]. The inhibition of CHOP also fortifies the binding of C/EBPα to PPARγ and increases PPARγ promoter activity in response to intracellular ER stress [149]. For effective fat storage, the final differentiation of adipocytes is necessary [164]. CHOP was first discovered to inhibit the differentiation of adipocytes in response to metabolic stress, hypoxia, and phosphorylation induced by p38 MAPK [159,165]. Subsequent activation of PERK-eIF2α during ER stress results in suppressed biphasic differentiation through CHOP expression [53]. Under conditions of polyamine depletion, CHOP interacts with C/EBPβ to inhibit the execution of the tin dioxide clonal expansion process and transcriptional activation of adipogenesis. This results in an inhibitory effect [163]. Overexpression of CHOP leads to poorly differentiated adipocytes and an increase in undifferentiated adipose tissue in a mouse model [166]. Inhibition of CHOP mRNA is required for full adipocyte differentiation of MEFs [159].
4.3. Crosstalk between ER Stress and Other Signaling Pathways in Adipogenesis
Adipogenesis involves adipocyte differentiation and maturation regulated by a network of signaling pathways [167]. Recent evidence indicates that ER stress, defined as the buildup of misfolded proteins in the ER, collaborates with other signaling pathways to regulate adipogenesis [168,169,170,171]. This section seeks to explore the complex molecular interactions and crosstalk between ER stress and other signaling pathways implicated in adipogenesis.
4.3.1. ER Stress and UPR in Adipogenesis
In an obese environment, fat accumulation within cells can lead to protein folding issues during processes such as fatty acid synthesis in adipocytes [43]. These problems result in a larger number of incomplete protein folds in the ER and the activation of the UPR [4]. ER stress, a component of the UPR, can restrict protein synthesis through signaling pathways, which inhibits adipocyte differentiation and consequently limits adipocyte formation [111]. During ER stress, cells use eIF2α as a protein guide [166]. eIF2α plays a crucial part in the initial stages of protein synthesis and undergoes regulation in a unique way under ER stress conditions [172]. Phosphorylation of eIF2α occurs via guidepost proteins due to ER stress [173]. This blocks protein synthesis by hindering the communication with eIF2B, which facilitates the transfer of methionine from nucleic acid tRNA to the ribosome during the initial phases of protein synthesis [174].
When these mechanisms operate in unison to trigger ER stress, this represses protein synthesis, ultimately hindering the differentiation of adipocytes [175,176,177].
4.3.2. ER Stress and Wingless/Integrated (Wnt) Signaling in Adipogenesis
Wnt signaling is a crucial pathway in adipogenesis, governing the determination of preadipocyte fate and adipocyte maturation [178,179]. Recent research highlights the possibility of crosstalk between ER stress and Wnt signaling, implying the ability of ER stress to regulate the key Wnt signaling components [180,181]. Wnt signaling occurs when Wnt proteins bind to frizzled receptors [182,183,184]. This activates signaling pathways that are both dependent and independent of β-catenin [185]. Importantly, Wnt signaling represses adipocyte differentiation through suppression of adipogenic transcription factors, such as PPARγ and C/EBPα [181]. Wnt10b exhibits constitutive expression, mainly in preadipocytes and stromal vascular cells—not adipocytes—and significantly impedes adipogenesis [179,181,186]. In vivo, transgenic expression of Wnt10b in adipocytes leads to a 50% decrease in total body fat and lack of brown adipose tissue formation, emphasizing the intricate nature of Wnt signaling in adipogenesis [186]. The interplay between Wnt signaling and adipogenesis implies that preadipocytes integrate signals from numerous Wnt pathways, which ultimately influences the expression of vital adipogenic regulators such as PPARγ and C/EBPα, affecting adipocyte differentiation and development.
4.3.3. ER Stress and mTOR Signaling in Adipogenesis
Mammalian target of rapamycin (mTOR) is a significant protein that regulates both cell growth and metabolism, playing a crucial role in adipocyte differentiation and lipid metabolism [187,188]. Recent research has indicated that ER stress modulates mTOR signaling. Moreover, the activation of ER stress can hinder the activation of mTOR complex 1 (mTORC1) [189,190].
The mechanism involved in the interaction between ER stress and mTORC1 is intriguing. Notably, ER stress is associated with AMP-activated protein kinase (AMPK) [191,192]. ER stress triggers a pathway that reduces cellular ATP levels and increases AMP levels, resulting in the activation of AMPK [193]. AMPK senses and regulates cellular energy status [194]. Its activation leads to the inhibition of mTORC1 by activating tuberous sclerosis complex1-tuberous sclerosis complex 2 (TSC1-TSC2), which inhibits Rheb protein required for mTORC1 activation [188,190].
Furthermore, AMPK phosphorylates raptor, one of mTORC1’s subunits, leading to the inhibition of raptor’s activity and control over mTORC1 activation [195,196]. The AKT-mTORC1 pathway regulates lipid synthesis via the sterol regulatory element-binding protein (SREBP) transcription factor [187,197]. When ER stress is triggered, AKT’s activation is impeded [198].
ER stress triggers ATF4 translation, which fosters cellular apoptosis by means of inhibiting AKT through stress-related proteins, such as TRB3 and others [199]. Moreover, ER stress obstructs mTORC2 and AKT via the GSK-3β pathway, leading to the activation of the IRE1-JNK pathway and ultimately inducing cell apoptosis [131,200,201].
In summary, AKT and AMPK function as significant signaling nodes pertaining to the activation and inhibition of mTORC1 [190,200]. Additionally, ER stress plays a regulatory role in these interactions [191].
4.3.4. ER Stress and Insulin Signaling in Adipogenesis
Insulin signaling pathways are closely connected to adipogenesis and metabolic homeostasis [187]. Experimental models [202] confirm ER stress’s role in obesity-related insulin resistance. Increased ER stress has been linked to impaired insulin action in obese mice [203], and chemical or genetic modification of this stress has been shown to improve insulin sensitivity and glucose homeostasis [202]. In cases where tissues, such as liver, skeletal muscle, and fat, become less responsive to insulin, signal transmission is reduced for insulin receptor substrate (IRS) [204], AKT, and glycogen synthase kinase-3β (GSK3β) [205,206]. Previous studies suggest that increased levels of interleukin-6 (IL-6) and TNF-α may be linked to obesity and insulin resistance [207], signifying their involvement in ER stress and reduced insulin sensitivity [208,209]. These results indicate a considerable role for cytokines [210]. Research findings show that ER stress can interfere with insulin signaling pathways by activating serine kinases, including JNK and inhibitor of nuclear factor kappa-B kinase (IKK) [211,212,213]. Impairment of insulin signaling by ER stress can hinder adipocyte differentiation, ultimately contributing to insulin resistance [214].
IRE1 signaling pathway-induced activation of JNK and subsequent activation of inflammatory signaling pathways are pivotal factors in the development of insulin resistance and type 2 diabetes (T2DM) associated with obesity [211,215,216]. A number of studies have emphasized this process as a crucial component of the pathophysiology of insulin resistance related to obesity [217].
ER stress caused by obesity stimulates JNK activation, which acts as a core mediator resulting in modifications in insulin signaling [218,219]. The activation of JNK is primarily responsible for the changes in insulin signaling pathways that contribute to insulin resistance [211].
ER stress and inflammation in obesity result in the elevation of pro-inflammatory cytokines, including IL-6 and TNF-α [4,220]. These raised cytokine levels impair insulin action and promote insulin resistance [220,221].
When cells encounter pro-inflammatory cytokines or high levels of free fatty acids (FFA), they hinder insulin signaling by phosphorylating serine residues on the insulin receptor substrate-1 (IRS-1) [222]. This phosphorylation disrupts insulin signaling downstream and impairs insulin function [220].
Moreover, JNK is activated and phosphorylates IRS-1 when cells encounter stimuli such as ER stress, elevated cytokine levels, or high levels of fatty acids [131,219]. IRS-1 and insulin receptor substrate-2 (IRS-2) play a vital role as substrates for the insulin receptor tyrosine kinase in the insulin signaling pathway [223,224]. This action, in turn, reduces the receptor’s sensitivity to insulin [223]. Consequently, overexpression of inflammatory molecules that result in the removal of IRS-1/2 receptors impede the insulin signaling pathway and lead to insulin resistance [219].
In summary, the activation of JNK by IRE1 disrupts the signaling of the insulin receptor, leading to insulin resistance [225]. This process is facilitated by ER stress and inflammatory cytokines, both playing crucial roles [221].
4.3.5. ER Stress and Nuclear Receptors in Adipogenesis
The nuclear receptors, specifically the PPARs and the liver X receptors (LXRs), assume critical roles in adipogenesis and lipid metabolism [226]. These molecular entities are responsible for regulating lipid metabolism and the development of adipocytes [227]. Notably, the LXRs belong to the class of nuclear receptors that exert a significant impact on both cholesterol metabolism and fat metabolism [228]. When ER stress inhibits LXRs, it may affect cholesterol and fat metabolism processes, resulting in increased cholesterol levels and abnormal fat metabolism [229].
ER stress-induced LXR inhibition negatively impacts cholesterol and lipid metabolic processes [230]. Activated LXRs trigger cholesterol metabolism genes, producing high-density lipoprotein (HDL) particles to contain cholesterol [231]. Furthermore, LXRs play a role in fat metabolism, regulating both oxidation and storage in adipose tissue [232]. Hence, impeding LXR regulation during ER stress can potentially cause abnormalities in fat metabolism, leading to abnormal fat accumulation [233]. Some significant genes involved in cholesterol metabolism are the ATP-binding cassette A1 (ABCA1) gene, which increases when LXRs are activated, and the ATP-binding cassette G1 (ABCG1) gene, which is also regulated by LXRs [234,235]. The ABCA1 gene is responsible for shuttling cholesterol and phospholipids and facilitates HDL particle production; its activation helps move cholesterol from cells to HDL [236]. Additionally, ABCG1 contributes to cholesterol transport. It aids in the transportation of fat phosphate, which is a constituent of HDL particles [237]. Additionally, LXRs regulate the gene expression of cholesteryl ester transfer protein (CETP), which is accountable for the transfer of cholesterol from HDL to other lipid particles [238].
Furthermore, the activation of LXR leads to an increase in lysophosphatidylcholine acyltransferase 3 (Lpcat3) expression [239]. This indicates that LXR identifies polyunsaturated fatty acids that encourage their absorption into phospholipids (PLs), thereby enhancing ER stability [239,240]. The link between Lpcat3 and LXR indicates that LXR activation escalates Lpcat3 expression, promoting the release of polyunsaturated PLs, subsequently contributing to higher ER membrane stability and minimizing ER stress [240]. This process of membrane remodeling decreases the stress on the endothelial membrane caused by saturated fatty acids [241]. Additionally, the LXR–Lpcat3 pathway mitigates hepatitis by regulating the activation of c-Src kinase and controlling the availability of lipid inflammatory mediators [242]. These findings underscore the significance of Lpcat3 regulation for regulating lipid balance in physiology and disease through LXR signaling [243,244].
This interaction is part of the intricate networks involved in adipocyte development [244]. The interaction between ER stress and nuclear receptors is crucial in comprehending and treating metabolic diseases including obesity, diabetes, and non-alcoholic fatty liver disease (NAFLD) [245].
Understanding the interplay between ER stress and other signaling pathways during adipogenesis yields key insights into the molecular mechanisms governing adipocyte development and function [246]. Disrupting these interactions may contribute to metabolic disorders and dysfunction of adipose tissue [247]. Further research is required to elucidate the precise molecular mechanisms relating to the interplay between ER stress and signaling pathways, as well as their potential implications for adipogenesis and metabolic health [248].
5. Cyclophilin Family in Adipogenesis
Cyclophilin A (CypA) and cyclophilin B (CypB) are both members of the cyclophilin protein family. They are peptidyl-prolyl cis-trans isomerases (PPIases) that catalyze peptidyl-prolyl bond isomerization in proteins. Despite their similar functions, they have distinct roles and cellular localizations [249].
CypA is a highly abundant, ubiquitously cytosolic protein present in various cell types and tissues [212]. It is primarily recognized for its function in mediating the immunosuppressive effects of the immunosuppressive medication cyclosporine A (CsA) [250]. CypA plays a pivotal role in the immune response by binding with the protein calcineurin and inhibiting its phosphatase activity, ultimately blocking T-cell activation and the production of pro-inflammatory cytokines [251,252,253].
CypB is predominantly located within the lumen of the ER [254]. Its main roles involve protein folding and ERAD [255,256]. It functions as a molecular chaperone by aiding in the correct folding of newly produced proteins in the ER and supporting their transportation to their intended destinations [257]. Additionally, CypB is involved in numerous cellular processes such as collagen biosynthesis and virus replication [258,259].
Although both CypA and CypB are peptidyl-prolyl isomerases and share some functional similarities, they have distinct roles in different cellular compartments [249]. CypA is primarily involved in immune regulation, while CypB functions in protein folding and quality control within the ER [251].
5.1. CypA
Recent research has confirmed that CypA is a critical regulator of fat production [260,261]. According to the study’s findings, CypA has emerged as a key factor in fat metabolism and its association with obesity. Experimental results have shown that CypA promotes fat production in test tubes and plays a role in contributing to obesity induced by a high-fat diet (HFD) in mice. CypA was also found to be associated with offspring obesity induced by maternal gestational diabetes in mice [260]. The 3T3L1 cells used in the study are progenitor cells that differentiate into adipocytes upon insulin stimulation [262]. An increase in CypA expression was observed on day 6 of the 8-day process of these cells differentiating into adipocytes. It has been reported that insulin affects adipocyte differentiation by regulating the expression of key transcription factors involved in adipogenesis, including CypA and PPARγ, C/EBPα and C/EBPβ. Specifically, silencing or knocking out CypA significantly reduced the expression of C/EBPβ in the early stages of adipocyte differentiation and reduced the expression of PPARγ, C/EBPα and C/EBPβ in the late stages of differentiation.
However, other studies suggest that CypA-CD147 interaction mediates obesity-induced macrophage–adipocyte crosstalk and, thus, may represent a novel target for the treatment of insulin resistance and type 2 diabetes [263]. CypA activates the surface receptor CD147, thereby activating NF-κB signaling, which increases the expression of pro-inflammatory cytokines, inducing adipocyte inflammation. Simultaneously, it hinders adipocyte differentiation by suppressing the expression of PPARγ and C/EBPβ through leucine zipper tumor suppressor 2 (LZTS2) mediated downregulation of β-catenin [263,264]. These findings suggest that CypA may attenuate adipose tissue function and improve insulin sensitivity. However, this study still leaves some unknowns that require further investigation.
In conclusion, these studies shed light on the effects of CypA on fat production and metabolism, suggesting its potential importance in obesity-related diseases. However, further research is needed to clarify the exact roles of these proteins and the underlying mechanisms, particularly the direct interaction mechanism between CypA and ER stress-related proteins, which remains to be elucidated.
5.2. CypB
CypB functions as a molecular chaperone predominantly located in the lumen of the ER, facilitating protein folding through its PPIase activity [256,265]. CypB is known to be related to cellular collagen formation and the growth of various cancer cells [266,267]. However CypB’s influence extends significantly into the intricate realm of adipogenesis, where it interacts with regulatory factors [268]. Recent evidence highlights the transcriptional upregulation of CypB as a response to ER stress. Interestingly, increased CypB expression triggers an enhanced interaction between CHOP and p300, an ER-resident proteasome [269]. This interaction, in turn, initiates the ubiquitination-driven degradation of CHOP, culminating in the attenuation of apoptotic effects during ER stress [269]. Furthermore, CypB reveals an intriguing facet of its functionality in the inflammatory setting, as it intricately modulates ER calcium levels and counteracts the accumulation of ROS, thereby contributing to the amelioration of cellular inflammation [254].
Expanding its functional spectrum, CypB emerges as an important regulator of adipogenesis [268,270]. Existing research has established a compelling link between ER stress and the dampening of adipogenic factors, ultimately leading to reduced fat accumulation [2]. Accumulating evidence in the literature has shown that ER stress contributes to the development and progression of obesity through multiple mechanisms [169]. This phenomenon has been primarily attributed to the inhibitory effects of CHOP on C/EBPβ, a critical regulator in the early stages of preadipocyte development [163]. However, recent investigations have revealed a paradigm shift, as CypB is now recognized for its role in downregulating CHOP expression [271]. Interestingly, the absence of CypB is associated with reduced lipid droplet formation in knockout cells, underscoring an enhanced adipogenic process under conditions of ER stress [268]. Most intriguingly, the alleviation of C/EBPβ repression acts as a catalyst, promoting the activation of C/EBPα and PPARγ, key transcription factors that exert maximal influence during the intermediate to late stages of cellular differentiation [36].
In summary, this comprehensive review holistically synthesizes the evolving understanding of the multifaceted roles of CypB in the intricate landscape of obesity-induced ER stress [268]. From its active participation in ER stress responses to its pivotal role in the regulation of adipogenesis, CypB’s significance reverberates across multiple physiological contexts. This nuanced exploration illuminates the intricate interplay between CypB and ER stress, extends its influence to mitigate inflammation and modulate adipogenesis, and provides a comprehensive view of its multifunctional capabilities.
6. The Cellular Consequences of Excessive ER Stress in the Adipose Tissue
6.1. Altered Lipid Metabolism and Dynamics of the Lipid Droplets
The ER plays a crucial role in lipid metabolism and homeostasis [272]. ER stress, caused by the accumulation of misfolded or unfolded proteins in the ER lumen, is a significant factor that affects lipid metabolism and droplet dynamics [273]. The disruption of lipid metabolism due to ER stress can have significant consequences for cellular lipid homeostasis and may contribute to the development of metabolic disorders [169,273,274,275].
ER stress can have an impact on lipid synthesis, including fatty acids and triglycerides. ER stress can upset the expression and activity of important enzymes, such as FAS and ACC, which are involved in lipogenesis [247]. This imbalance can lead to alterations in lipid synthesis and result in imbalances in the composition and species of lipid [5].
Furthermore, ER stress affects the dynamics and functioning of lipid droplets, which are intracellular organelles involved in lipid storage and metabolism [273]. Changes in lipid droplet-associated proteins, such as perilipins, adipose differentiation-related protein (ADRP), and seipin, induced by ER stress, can potentially impact lipid droplet formation, growth, and turnover, which, in turn, can alter cellular lipid storage capacity, lipolysis, and lipid utilization [276,277,278].
The interplay between ER stress and altered lipid metabolism contributes to lipotoxicity, a condition characterized by the accumulation of toxic lipid species, mitochondrial dysfunction, and cellular damage [274]. ER stress-induced lipotoxicity has been implicated in the pathogenesis of various metabolic disorders, including obesity, insulin resistance, NAFLD, and cardiovascular disease [131].
Understanding the molecular mechanisms underlying the crosstalk between ER stress and altered lipid metabolism is crucial for elucidating the pathogenesis of metabolic disorders and identifying potential therapeutic targets [169]. Modulating ER stress and restoring lipid homeostasis have emerged as potential strategies for mitigating the detrimental effects of altered lipid metabolism associated with ER stress.
In summary, ER stress disrupts lipid metabolism and alters lipid droplet dynamics, leading to imbalances in lipid synthesis, storage, and utilization [273]. These alterations contribute to lipotoxicity and the development of metabolic disorders [275]. Further research is needed to unravel the specific mechanisms underlying these changes and to explore therapeutic interventions that can restore lipid homeostasis and mitigate the adverse effects of ER stress on lipid metabolism [273].
6.2. Adipokine Dysregulation and Metabolic Inflammation
Adipocytes produce their own cytokines, also known as adipokines, causing chronic inflammation in the adipose tissue (AT) [279,280]. AT macrophages (ATM) intensify this metabolic dysfunction of adipocytes, increasing inflammation within the cells [281]. Leptin and adiponectin are major adipokines regulating lipid metabolism and glucose levels within the AT, and dysregulation of adipokines is associated with obesity [27]. A study also reported that the number of macrophages present in the AT is related to the actual adipocyte size [282,283].
The PERK pathway during excessive ER stress also activates cytokines such as TNF-a, IL-6, and IL-1β, a major contributor to the inflammation that induces obesity [4,284,285]. Increases in TNF-a and IL-6 cytokines show morphological changes in adipocytes, forming crown-like structures [79]. However, these cytokines are reported to reduce adipogenesis by inhibiting PPARγ and C/EBPα expression [147]. IL-6 production causes AT dysfunction that impairs differentiation of preadipocytes, and TNF-a alone is sufficient to inhibit the induction of PPARγ and C/EBPα [286].
In addition, activation of M1 macrophages causes proinflammatory effects in AT through secretion of IL-1B and TNF-a cytokines [287]. A study reported that adipocyte apoptosis accumulates macrophages and other immune cells around the dead cell, forming the crown-like shape [79,288]. The increase in crown-like structures in all fat depots has a positive relationship with obesity, and the change in shape causes the overall size of the adipocyte to increase, causing hypertrophic results [289,290]. M1 macrophages also induce insulin resistance, dysregulate AT homeostasis, and further exacerbate obese characteristics [291].
6.3. Correlation between Aged Adipose Tissue and ER Stress
Aging is associated with redistribution of adipose tissue in visceral organs [292]. It is recognized as a major source of chronic systemic inflammatory cytokines during aging (inflammaging) due to abundant inflammatory mediators and M1 pro-inflammatory macrophages [293]. Comprising various cell populations, adipose tissue includes not only adipocytes contributing to fat storage, but also non-adipocytes known as stromal vascular fraction (SVF), derived post-collagenase digestion, forming the extracellular matrix (ECM) and vasculature [294,295]. The majority of cells in the SVF are composed of white blood cells and adipose tissue stromal cells (ATSCs) [296]. Adipose tissue macrophages (ATM) are the major leukocyte population found in adipose tissue, presumed to promote inflammation in obesity and metabolic disorders [297].
Studies strongly suggest that ATSC, a constituent of internal visceral adipose tissue (including pre-adipocytes), is a primary cause of age-related adipose tissue inflammation [296]. Notably, elevated levels of TNFα in aged adipose tissue interfere with fat generation and correlate with increased expression of CHOP, a downstream target of the ER stress response pathway [298,299].
Furthermore, investigation of aged adipose tissue cells in mice revealed decreased autophagy, increased endoplasmic reticulum stress, and heightened inflammation [296]. The expression of the autophagy-related genes, autophagy related 7 (Atg7) and microtubule-associated protein 1A/1B-light chain 3-II (LC3-II) proteins decreases as levels of p62 and polyubiquitin accumulate, which coincides with decreased autophagy in aged rat kidneys [300,301]. Insufficient specific autophagy-related genes compromise cellular maintenance, notably impacting the lifespan of model organisms such as C. elegans and Drosophila, particularly under nutritional and oxidative stress [302]. Key lifespan-regulating pathways (Foxo3, SIRT1, mTOR, NF-κB, P53) modulate autophagy [303,304]. Activating autophagy through rapamycin highlights its potential in extending lifespan, emphasizing insights into aging mechanisms [305]. Notably, fibroblasts from long-lived mutant mice exhibit enhanced autophagy under stress conditions [306]. Autophagy-related genes significantly declined in aged adipose cells and worsened after stress induction [307]. New data now link heightened ER stress response and impaired autophagy alongside the accumulation of senescent cell progenitors to molecular events upstream of age-related adipose tissue inflammation [308,309].
Excessive weight in old age impacts physical function decline, loss of independence, and the development of frailty [308]. Delaying the aging process of adipose tissue is believed to prevent age-related diseases.
7. Conclusions
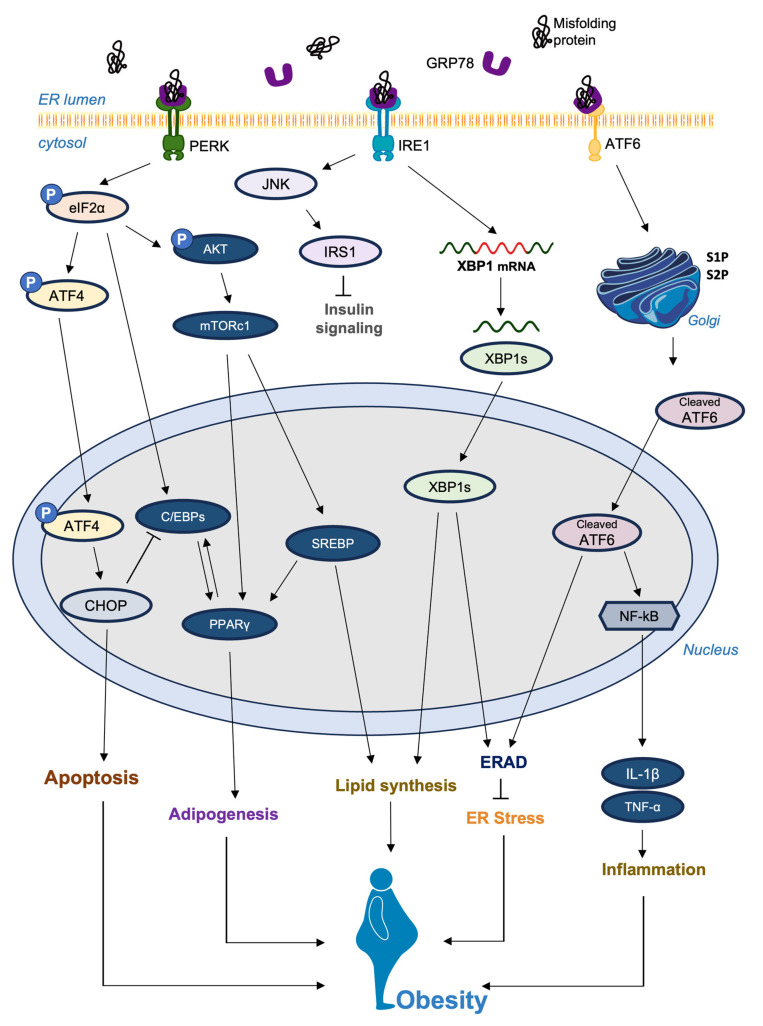
This review highlights the intricate relationship between ER stress and adipogenesis. The published findings in this review suggest that there is scientific evidence supporting the crosstalk between ER stress and adipogenesis. Their potential interplay is illustrated in Figure 2. Targeting ER stress pathways shows promise in treating adipogenesis-related disorders. Dysregulated adipogenesis contributes to obesity and metabolic disorders. Lifestyle changes such as exercise and diet affect ER stress and adipogenesis. Pharmacological options include agents that alleviate ER stress and modulate UPR pathways such as ATF6, IRE1 and PERK. Chemical chaperones and small molecule inhibitors have potential. Lifestyle interventions coupled with pathway modulation offer avenues for progress. However, research gaps remain in understanding mechanisms and clinical feasibility. Safety, efficacy and personalized approaches need to be explored. The link between ER stress and adipogenesis-related disorders provides opportunities for innovative interventions to improve metabolic health and patient outcomes.
Figure 2.
The potential association between the ER stress signaling pathway and obesity. ER stress induced by a variety of factors activates the unfolded protein response (UPR). PERK, IRE1α, and ATF6α, located in the ER membrane, act as UPR messengers and maintain ER stability by coordinating protein production and gene expression. PERK reduces protein synthesis by modifying eIF2α and induces CHOP through ATF4 mRNA translation. IRE1α splices XBP1 mRNA to generate XBP1 and induces genes linked to ER function. XBP1 enhances ER membrane formation, protein folding, transport, and ERAD. ATF6α is processed in the Golgi by S1P and S2P to release p50ATF6α (cleaved ATF6), which is pivotal for genes involved in ER protein folding and processing. All of these processes are multifactorial and highly interlinked.
Abbreviations
| IRE1 | inositol-requiring enzyme1 |
| XBP1 | X-box binding protein 1 |
| PERK | protein kinase RNA-like ER kinase |
| PPARγ | proliferator-activated receptor γ |
| C/EBPα | CCAAT-enhancer-binding protein α |
| C/EBPβ | CCAAT-enhancer-binding protein β |
| TAG | triacylglycerol |
| CHOP | C/EBP homologous protein |
| NF-κB | nuclear factor-kappa B |
| AKT | protein kinase B |
| TNF-α | tumor necrosis factor-alpha |
| JNK | c-Jun N-terminal kinase |
| IL-6 | interleukin-6 |
| IL-8 | interleukin-8 |
| FABP4 | fatty acid-binding protein 4 |
| GLUT4 | glucose transporter type 4 |
| IL-1β | interleukin-1β |
| mTOR | mammalian target of rapamycin |
| mTORC1 | mTOR Complex 1 |
| AMPK | AMP-activated protein kinase |
| TSC1 | tuberous sclerosis complex 1 |
| TSC2 | tuberous sclerosis complex 2 |
| SREBP | sterol regulatory element-binding protein |
| IKK | inhibitor of nuclear factor kappa-B kinase |
| FFA | free fatty acids |
| IRS-1 | insulin receptor substrate-1 |
| IRS-2 | insulin receptor substrate-2 |
| LXRs | liver X receptors |
| HDL | high-density lipoprotein |
| ABCA1 | ATP-binding cassette A1 |
| ABCG1 | ATP-binding cassette G1 |
| CETP | cholesteryl ester transfer protein |
| Lpcat3 | lysophosphatidylcholine acyltransferase 3 |
| PLs | phospholipids |
| CypA | cyclophilin A |
| CypB | cyclophilin B |
| CsA | cyclosporine A |
Author Contributions
Conceptualization, W.C. and G.K.; investigation, writing—original draft preparation, G.K., J.L. and W.C.; writing—review and editing, G.K., J.L., J.H., I.K. and W.C.; supervision, W.C.; project administration, W.C.; funding acquisition, W.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by National Research Foundation of Korea (NRF) grants funded by Korean government (MEST) (NRF-2018R1A6A1A030525124).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Park J.H., Moon J.H., Kim H.J., Kong M.H., Oh Y.H. Sedentary Lifestyle: Overview of Updated Evidence of Potential Health Risks. Korean J. Fam. Med. 2020;41:365–373. doi: 10.4082/kjfm.20.0165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ajoolabady A., Liu S., Klionsky D.J., Lip G.Y.H., Tuomilehto J., Kavalakatt S., Pereira D.M., Samali A., Ren J. ER stress in obesity pathogenesis and management. Trends Pharmacol. Sci. 2022;43:97–109. doi: 10.1016/j.tips.2021.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yilmaz E. Endoplasmic Reticulum Stress and Obesity. Adv. Exp. Med. Biol. 2017;960:261–276. doi: 10.1007/978-3-319-48382-5_11. [DOI] [PubMed] [Google Scholar]
- 4.Kawasaki N., Asada R., Saito A., Kanemoto S., Imaizumi K. Obesity-induced endoplasmic reticulum stress causes chronic inflammation in adipose tissue. Sci. Rep. 2012;2:799. doi: 10.1038/srep00799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moncan M., Mnich K., Blomme A., Almanza A., Samali A., Gorman A.M. Regulation of lipid metabolism by the unfolded protein response. J. Cell Mol. Med. 2021;25:1359–1370. doi: 10.1111/jcmm.16255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kumar V., Maity S. ER Stress-Sensor Proteins and ER-Mitochondrial Crosstalk-Signaling Beyond (ER) Stress Response. Biomolecules. 2021;11:173. doi: 10.3390/biom11020173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Liang W.L., Cai M.R., Zhang M.Q., Cui S., Zhang T.R., Cheng W.H., Wu Y.H., Ou W.J., Jia Z.H., Zhang S.F. Chinese Herbal Medicine Alleviates Myocardial Ischemia/Reperfusion Injury by Regulating Endoplasmic Reticulum Stress. Evid. Based Complement. Altern. Med. 2021;2021:4963346. doi: 10.1155/2021/4963346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yanagitani K., Kimata Y., Kadokura H., Kohno K. Translational pausing ensures membrane targeting and cytoplasmic splicing of XBP1u mRNA. Science. 2011;331:586–589. doi: 10.1126/science.1197142. [DOI] [PubMed] [Google Scholar]
- 9.Gardner B.M., Walter P. Unfolded proteins are Ire1-activating ligands that directly induce the unfolded protein response. Science. 2011;333:1891–1894. doi: 10.1126/science.1209126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Casagrande R., Stern P., Diehn M., Shamu C., Osario M., Zuniga M., Brown P.O., Ploegh H. Degradation of proteins from the ER of S. cerevisiae requires an intact unfolded protein response pathway. Mol. Cell. 2000;5:729–735. doi: 10.1016/S1097-2765(00)80251-8. [DOI] [PubMed] [Google Scholar]
- 11.Friedlander R., Jarosch E., Urban J., Volkwein C., Sommer T. A regulatory link between ER-associated protein degradation and the unfolded-protein response. Nat. Cell Biol. 2000;2:379–384. doi: 10.1038/35017001. [DOI] [PubMed] [Google Scholar]
- 12.Travers K.J., Patil C.K., Wodicka L., Lockhart D.J., Weissman J.S., Walter P. Functional and genomic analyses reveal an essential coordination between the unfolded protein response and ER-associated degradation. Cell. 2000;101:249–258. doi: 10.1016/S0092-8674(00)80835-1. [DOI] [PubMed] [Google Scholar]
- 13.Ko J., Kim J.Y., Chae M.K., Lee E.J., Yoon J.S. PERK mediates oxidative stress and adipogenesis in Graves’ orbitopathy pathogenesis. J. Mol. Endocrinol. 2021;66:313–323. doi: 10.1530/JME-21-0057. [DOI] [PubMed] [Google Scholar]
- 14.Jiao P., Ma J., Feng B., Zhang H., Diehl J.A., Chin Y.E., Yan W., Xu H. FFA-induced adipocyte inflammation and insulin resistance: Involvement of ER stress and IKKbeta pathways. Obesity. 2011;19:483–491. doi: 10.1038/oby.2010.200. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes-da-Silva A., Miranda C.S., Santana-Oliveira D.A., Oliveira-Cordeiro B., Rangel-Azevedo C., Silva-Veiga F.M., Martins F.F., Souza-Mello V. Endoplasmic reticulum stress as the basis of obesity and metabolic diseases: Focus on adipose tissue, liver, and pancreas. Eur. J. Nutr. 2021;60:2949–2960. doi: 10.1007/s00394-021-02542-y. [DOI] [PubMed] [Google Scholar]
- 16.Zatterale F., Longo M., Naderi J., Raciti G.A., Desiderio A., Miele C., Beguinot F. Chronic Adipose Tissue Inflammation Linking Obesity to Insulin Resistance and Type 2 Diabetes. Front. Physiol. 2019;10:1607. doi: 10.3389/fphys.2019.01607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moreno M.J., Martinez J.A. Adipose tissue: A storage and secretory organ. An. Del Sist. Sanit. De Navar. 2002;25((Suppl. S1)):29–39. doi: 10.23938/ASSN.0812. [DOI] [PubMed] [Google Scholar]
- 18.Arrese E.L., Soulages J.L. Insect fat body: Energy, metabolism, and regulation. Annu. Rev. Entomol. 2010;55:207–225. doi: 10.1146/annurev-ento-112408-085356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fain J.N. Release of interleukins and other inflammatory cytokines by human adipose tissue is enhanced in obesity and primarily due to the nonfat cells. Vitam. Horm. 2006;74:443–477. doi: 10.1016/S0083-6729(06)74018-3. [DOI] [PubMed] [Google Scholar]
- 20.Han J.S., Jeon Y.G., Oh M., Lee G., Nahmgoong H., Han S.M., Choi J., Kim Y.Y., Shin K.C., Kim J., et al. Adipocyte HIF2α functions as a thermostat via PKA Cα regulation in beige adipocytes. Nat. Commun. 2022;13:3268. doi: 10.1038/s41467-022-30925-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park A., Kim K.-e., Park I., Lee S.H., Park K.-Y., Jung M., Li X., Sleiman M.B., Lee S.J., Kim D.-S., et al. Mitochondrial matrix protein LETMD1 maintains thermogenic capacity of brown adipose tissue in male mice. Nat. Commun. 2023;14:3746. doi: 10.1038/s41467-023-39106-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rosen E.D., Spiegelman B.M. What we talk about when we talk about fat. Cell. 2014;156:20–44. doi: 10.1016/j.cell.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shuster A., Patlas M., Pinthus J.H., Mourtzakis M. The clinical importance of visceral adiposity: A critical review of methods for visceral adipose tissue analysis. Br. J. Radiol. 2012;85:1–10. doi: 10.1259/bjr/38447238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Coelho M., Oliveira T., Fernandes R. Biochemistry of adipose tissue: An endocrine organ. Arch. Med. Sci. 2013;9:191–200. doi: 10.5114/aoms.2013.33181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Deng Y., Scherer P.E. Adipokines as novel biomarkers and regulators of the metabolic syndrome. Ann. N. Y. Acad. Sci. 2010;1212:E1–E19. doi: 10.1111/j.1749-6632.2010.05875.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Carvalho A.F., Rocha D.Q., McIntyre R.S., Mesquita L.M., Kohler C.A., Hyphantis T.N., Sales P.M., Machado-Vieira R., Berk M. Adipokines as emerging depression biomarkers: A systematic review and meta-analysis. J. Psychiatr. Res. 2014;59:28–37. doi: 10.1016/j.jpsychires.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 27.Landecho M.F., Tuero C., Valenti V., Bilbao I., de la Higuera M., Fruhbeck G. Relevance of Leptin and Other Adipokines in Obesity-Associated Cardiovascular Risk. Nutrients. 2019;11:2664. doi: 10.3390/nu11112664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greenberg A.S., Obin M.S. Obesity and the role of adipose tissue in inflammation and metabolism. Am. J. Clin. Nutr. 2006;83:461S–465S. doi: 10.1093/ajcn/83.2.461S. [DOI] [PubMed] [Google Scholar]
- 29.Bluher M. Adipose tissue dysfunction contributes to obesity related metabolic diseases. Best Pract. Res. Clin. Endocrinol. Metab. 2013;27:163–177. doi: 10.1016/j.beem.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 30.Jung U.J., Choi M.S. Obesity and its metabolic complications: The role of adipokines and the relationship between obesity, inflammation, insulin resistance, dyslipidemia and nonalcoholic fatty liver disease. Int. J. Mol. Sci. 2014;15:6184–6223. doi: 10.3390/ijms15046184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mitrovic B., Gluvic Z.M., Obradovic M., Radunovic M., Rizzo M., Banach M., Isenovic E.R. Non-alcoholic fatty liver disease, metabolic syndrome, and type 2 diabetes mellitus: Where do we stand today? Arch. Med. Sci. 2023;19:884–894. doi: 10.5114/aoms/150639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou X.-D., Cai J., Targher G., Byrne C.D., Shapiro M.D., Sung K.-C., Somers V.K., Chahal C.A.A., George J., Chen L.-L., et al. Metabolic dysfunction-associated fatty liver disease and implications for cardiovascular risk and disease prevention. Cardiovasc. Diabetol. 2022;21:270. doi: 10.1186/s12933-022-01697-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Marquez M.P., Alencastro F., Madrigal A., Jimenez J.L., Blanco G., Gureghian A., Keagy L., Lee C., Liu R., Tan L., et al. The Role of Cellular Proliferation in Adipogenic Differentiation of Human Adipose Tissue-Derived Mesenchymal Stem Cells. Stem. Cells Dev. 2017;26:1578–1595. doi: 10.1089/scd.2017.0071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lefterova M.I., Lazar M.A. New developments in adipogenesis. Trends Endocrinol. Metab. 2009;20:107–114. doi: 10.1016/j.tem.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 35.Chawla A., Schwarz E.J., Dimaculangan D.D., Lazar M.A. Peroxisome proliferator-activated receptor (PPAR) gamma: Adipose-predominant expression and induction early in adipocyte differentiation. Endocrinology. 1994;135:798–800. doi: 10.1210/endo.135.2.8033830. [DOI] [PubMed] [Google Scholar]
- 36.Farmer S.R. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McKnight S.L., Lane M.D., Gluecksohn-Waelsch S. Is CCAAT/enhancer-binding protein a central regulator of energy metabolism? Genes Dev. 1989;3:2021–2024. doi: 10.1101/gad.3.12b.2021. [DOI] [PubMed] [Google Scholar]
- 38.Wu Z., Bucher N.L., Farmer S.R. Induction of peroxisome proliferator-activated receptor gamma during the conversion of 3T3 fibroblasts into adipocytes is mediated by C/EBPbeta, C/EBPdelta, and glucocorticoids. Mol. Cell Biol. 1996;16:4128–4136. doi: 10.1128/MCB.16.8.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Robert A.W., Marcon B.H., Dallagiovanna B., Shigunov P. Adipogenesis, Osteogenesis, and Chondrogenesis of Human Mesenchymal Stem/Stromal Cells: A Comparative Transcriptome Approach. Front. Cell Dev. Biol. 2020;8:561. doi: 10.3389/fcell.2020.00561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmadian M., Duncan R.E., Jaworski K., Sarkadi-Nagy E., Sul H.S. Triacylglycerol metabolism in adipose tissue. Future Lipidol. 2007;2:229–237. doi: 10.2217/17460875.2.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakamura T., Shiojima S., Hirai Y., Iwama T., Tsuruzoe N., Hirasawa A., Katsuma S., Tsujimoto G. Temporal gene expression changes during adipogenesis in human mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2003;303:306–312. doi: 10.1016/S0006-291X(03)00325-5. [DOI] [PubMed] [Google Scholar]
- 42.Pittenger M.F., Mackay A.M., Beck S.C., Jaiswal R.K., Douglas R., Mosca J.D., Moorman M.A., Simonetti D.W., Craig S., Marshak D.R. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 43.Wan Q., Calhoun C., Zahr T., Qiang L. Uncoupling Lipid Synthesis from Adipocyte Development. Biomedicines. 2023;11:1132. doi: 10.3390/biomedicines11041132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Read A., Schroder M. The Unfolded Protein Response: An Overview. Biology. 2021;10:384. doi: 10.3390/biology10050384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sicari D., Delaunay-Moisan A., Combettes L., Chevet E., Igbaria A. A guide to assessing endoplasmic reticulum homeostasis and stress in mammalian systems. FEBS J. 2020;287:27–42. doi: 10.1111/febs.15107. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida H., Matsui T., Yamamoto A., Okada T., Mori K. XBP1 mRNA is induced by ATF6 and spliced by IRE1 in response to ER stress to produce a highly active transcription factor. Cell. 2001;107:881–891. doi: 10.1016/S0092-8674(01)00611-0. [DOI] [PubMed] [Google Scholar]
- 47.Park S.M., Kang T.I., So J.S. Roles of XBP1s in Transcriptional Regulation of Target Genes. Biomedicines. 2021;9:791. doi: 10.3390/biomedicines9070791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gariballa N., Ali B.R. Endoplasmic Reticulum Associated Protein Degradation (ERAD) in the Pathology of Diseases Related to TGFbeta Signaling Pathway: Future Therapeutic Perspectives. Front. Mol. Biosci. 2020;7:575608. doi: 10.3389/fmolb.2020.575608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bhattacharya A., Qi L. ER-associated degradation in health and disease—From substrate to organism. J. Cell Sci. 2019;132:jcs232850. doi: 10.1242/jcs.232850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hwang J., Qi L. Quality Control in the Endoplasmic Reticulum: Crosstalk between ERAD and UPR pathways. Trends Biochem. Sci. 2018;43:593–605. doi: 10.1016/j.tibs.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.DuRose J.B., Scheuner D., Kaufman R.J., Rothblum L.I., Niwa M. Phosphorylation of eukaryotic translation initiation factor 2alpha coordinates rRNA transcription and translation inhibition during endoplasmic reticulum stress. Mol. Cell Biol. 2009;29:4295–4307. doi: 10.1128/MCB.00260-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rozpedek W., Pytel D., Mucha B., Leszczynska H., Diehl J.A., Majsterek I. The Role of the PERK/eIF2alpha/ATF4/CHOP Signaling Pathway in Tumor Progression During Endoplasmic Reticulum Stress. Curr. Mol. Med. 2016;16:533–544. doi: 10.2174/1566524016666160523143937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oyadomari S., Mori M. Roles of CHOP/GADD153 in endoplasmic reticulum stress. Cell Death Differ. 2004;11:381–389. doi: 10.1038/sj.cdd.4401373. [DOI] [PubMed] [Google Scholar]
- 54.Kroeger H., Grimsey N., Paxman R., Chiang W.C., Plate L., Jones Y., Shaw P.X., Trejo J., Tsang S.H., Powers E., et al. The unfolded protein response regulator ATF6 promotes mesodermal differentiation. Sci. Signal. 2018;11:eaan5785. doi: 10.1126/scisignal.aan5785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hetz C., Chevet E., Oakes S.A. Proteostasis control by the unfolded protein response. Nat. Cell Biol. 2015;17:829–838. doi: 10.1038/ncb3184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Szegezdi E., Logue S.E., Gorman A.M., Samali A. Mediators of endoplasmic reticulum stress-induced apoptosis. EMBO Rep. 2006;7:880–885. doi: 10.1038/sj.embor.7400779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Srivastava S.P., Kumar K.U., Kaufman R.J. Phosphorylation of eukaryotic translation initiation factor 2 mediates apoptosis in response to activation of the double-stranded RNA-dependent protein kinase. J. Biol. Chem. 1998;273:2416–2423. doi: 10.1074/jbc.273.4.2416. [DOI] [PubMed] [Google Scholar]
- 58.Ibrahim I.M., Abdelmalek D.H., Elfiky A.A. GRP78: A cell’s response to stress. Life Sci. 2019;226:156–163. doi: 10.1016/j.lfs.2019.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Marzec M., Eletto D., Argon Y. GRP94: An HSP90-like protein specialized for protein folding and quality control in the endoplasmic reticulum. Biochim. Biophys. Acta. 2012;1823:774–787. doi: 10.1016/j.bbamcr.2011.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Grek C., Townsend D.M. Protein Disulfide Isomerase Superfamily in Disease and the Regulation of Apoptosis. Cell Pathol. 2014;1:4–17. doi: 10.2478/ersc-2013-0001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luo B., Lee A.S. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene. 2013;32:805–818. doi: 10.1038/onc.2012.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hecht J.T., Hayes E., Snuggs M., Decker G., Montufar-Solis D., Doege K., Mwalle F., Poole R., Stevens J., Duke P.J. Calreticulin, PDI, Grp94 and BiP chaperone proteins are associated with retained COMP in pseudoachondroplasia chondrocytes. Matrix Biol. 2001;20:251–262. doi: 10.1016/S0945-053X(01)00136-6. [DOI] [PubMed] [Google Scholar]
- 63.Luan W., Li F., Zhang J., Wang B., Xiang J. Cloning and expression of glucose regulated protein 78 (GRP78) in Fenneropenaeus chinensis. Mol. Biol. Rep. 2009;36:289–298. doi: 10.1007/s11033-007-9178-z. [DOI] [PubMed] [Google Scholar]
- 64.Roufayel R., Kadry S. Molecular Chaperone HSP70 and Key Regulators of Apoptosis—A Review. Curr. Mol. Med. 2019;19:315–325. doi: 10.2174/1566524019666190326114720. [DOI] [PubMed] [Google Scholar]
- 65.Wang M., Wey S., Zhang Y., Ye R., Lee A.S. Role of the unfolded protein response regulator GRP78/BiP in development, cancer, and neurological disorders. Antioxid. Redox Signal. 2009;11:2307–2316. doi: 10.1089/ars.2009.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lee A.S. The ER chaperone and signaling regulator GRP78/BiP as a monitor of endoplasmic reticulum stress. Methods. 2005;35:373–381. doi: 10.1016/j.ymeth.2004.10.010. [DOI] [PubMed] [Google Scholar]
- 67.Christianson J.C., Shaler T.A., Tyler R.E., Kopito R.R. OS-9 and GRP94 deliver mutant alpha1-antitrypsin to the Hrd1-SEL1L ubiquitin ligase complex for ERAD. Nat. Cell Biol. 2008;10:272–282. doi: 10.1038/ncb1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lippincott-Schwartz J., Bonifacino J.S., Yuan L.C., Klausner R.D. Degradation from the endoplasmic reticulum: Disposing of newly synthesized proteins. Cell. 1988;54:209–220. doi: 10.1016/0092-8674(88)90553-3. [DOI] [PubMed] [Google Scholar]
- 69.Ushioda R., Hoseki J., Araki K., Jansen G., Thomas D.Y., Nagata K. ERdj5 is required as a disulfide reductase for degradation of misfolded proteins in the ER. Science. 2008;321:569–572. doi: 10.1126/science.1159293. [DOI] [PubMed] [Google Scholar]
- 70.Zhang I.X., Raghavan M., Satin L.S. The Endoplasmic Reticulum and Calcium Homeostasis in Pancreatic Beta Cells. Endocrinology. 2020;161:bqz028. doi: 10.1210/endocr/bqz028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gorkhali R., Tian L., Dong B., Bagchi P., Deng X., Pawar S., Duong D., Fang N., Seyfried N., Yang J. Extracellular calcium alters calcium-sensing receptor network integrating intracellular calcium-signaling and related key pathway. Sci. Rep. 2021;11:20576. doi: 10.1038/s41598-021-00067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Reddy R.K., Mao C., Baumeister P., Austin R.C., Kaufman R.J., Lee A.S. Endoplasmic reticulum chaperone protein GRP78 protects cells from apoptosis induced by topoisomerase inhibitors: Role of ATP binding site in suppression of caspase-7 activation. J. Biol. Chem. 2003;278:20915–20924. doi: 10.1074/jbc.M212328200. [DOI] [PubMed] [Google Scholar]
- 73.Rao R.V., Peel A., Logvinova A., del Rio G., Hermel E., Yokota T., Goldsmith P.C., Ellerby L.M., Ellerby H.M., Bredesen D.E. Coupling endoplasmic reticulum stress to the cell death program: Role of the ER chaperone GRP78. FEBS Lett. 2002;514:122–128. doi: 10.1016/S0014-5793(02)02289-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Salganik M., Sergeyev V.G., Shinde V., Meyers C.A., Gorbatyuk M.S., Lin J.H., Zolotukhin S., Gorbatyuk O.S. The loss of glucose-regulated protein 78 (GRP78) during normal aging or from siRNA knockdown augments human alpha-synuclein (alpha-syn) toxicity to rat nigral neurons. Neurobiol. Aging. 2015;36:2213–2223. doi: 10.1016/j.neurobiolaging.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Khanna D., Khanna S., Khanna P., Kahar P., Patel B.M. Obesity: A Chronic Low-Grade Inflammation and Its Markers. Cureus. 2022;14:e22711. doi: 10.7759/cureus.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Kawai T., Autieri M.V., Scalia R. Adipose tissue inflammation and metabolic dysfunction in obesity. Am. J. Physiol. Cell Physiol. 2021;320:C375–C391. doi: 10.1152/ajpcell.00379.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Alwarawrah Y., Kiernan K., MacIver N.J. Changes in Nutritional Status Impact Immune Cell Metabolism and Function. Front. Immunol. 2018;9:1055. doi: 10.3389/fimmu.2018.01055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Li X. Endoplasmic reticulum stress regulates inflammation in adipocyte of obese rats via toll-like receptors 4 signaling. Iran J. Basic Med. Sci. 2018;21:502–507. doi: 10.22038/IJBMS.2018.27346.6674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tripathi Y.B., Pandey V. Obesity and endoplasmic reticulum (ER) stresses. Front. Immunol. 2012;3:240. doi: 10.3389/fimmu.2012.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Adams C.J., Kopp M.C., Larburu N., Nowak P.R., Ali M.M.U. Structure and Molecular Mechanism of ER Stress Signaling by the Unfolded Protein Response Signal Activator IRE1. Front. Mol. Biosci. 2019;6:11. doi: 10.3389/fmolb.2019.00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhao P., Huang P., Xu T., Xiang X., Sun Y., Liu J., Yan C., Wang L., Gao J., Cui S., et al. Fat body Ire1 regulates lipid homeostasis through the Xbp1s-FoxO axis in Drosophila. iScience. 2021;24:102819. doi: 10.1016/j.isci.2021.102819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sha H., He Y., Yang L., Qi L. Stressed out about obesity: IRE1alpha-XBP1 in metabolic disorders. Trends Endocrinol. Metab. 2011;22:374–381. doi: 10.1016/j.tem.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Madhavan A., Kok B.P., Rius B., Grandjean J.M.D., Alabi A., Albert V., Sukiasyan A., Powers E.T., Galmozzi A., Saez E., et al. Pharmacologic IRE1/XBP1s activation promotes systemic adaptive remodeling in obesity. Nat. Commun. 2022;13:608. doi: 10.1038/s41467-022-28271-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Chalmers F., van Lith M., Sweeney B., Cain K., Bulleid N.J. Inhibition of IRE1alpha-mediated XBP1 mRNA cleavage by XBP1 reveals a novel regulatory process during the unfolded protein response. Wellcome Open Res. 2017;2:36. doi: 10.12688/wellcomeopenres.11764.2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Liu S., Ding H., Li Y., Zhang X. Molecular Mechanism Underlying Role of the XBP1s in Cardiovascular Diseases. J. Cardiovasc. Dev. Dis. 2022;9:459. doi: 10.3390/jcdd9120459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee A.H., Scapa E.F., Cohen D.E., Glimcher L.H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320:1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Peng J., Qin C., Ramatchandirin B., Pearah A., Guo S., Hussain M., Yu L., Wondisford F.E., He L. Activation of the canonical ER stress IRE1-XBP1 pathway by insulin regulates glucose and lipid metabolism. J. Biol. Chem. 2022;298:102283. doi: 10.1016/j.jbc.2022.102283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wang Q., Zhou H., Bu Q., Wei S., Li L., Zhou J., Zhou S., Su W., Liu M., Liu Z., et al. Role of XBP1 in regulating the progression of non-alcoholic steatohepatitis. J. Hepatol. 2022;77:312–325. doi: 10.1016/j.jhep.2022.02.031. [DOI] [PubMed] [Google Scholar]
- 89.Markussen L.K., Rondini E.A., Johansen O.S., Madsen J.G.S., Sustarsic E.G., Marcher A.B., Hansen J.B., Gerhart-Hines Z., Granneman J.G., Mandrup S. Lipolysis regulates major transcriptional programs in brown adipocytes. Nat. Commun. 2022;13:3956. doi: 10.1038/s41467-022-31525-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Wang S., Chen Z., Lam V., Han J., Hassler J., Finck B.N., Davidson N.O., Kaufman R.J. IRE1alpha-XBP1s induces PDI expression to increase MTP activity for hepatic VLDL assembly and lipid homeostasis. Cell Metab. 2012;16:473–486. doi: 10.1016/j.cmet.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Morishita Y., Kellogg A.P., Larkin D., Chen W., Vadrevu S., Satin L., Liu M., Arvan P. Cell death-associated lipid droplet protein CIDE-A is a noncanonical marker of endoplasmic reticulum stress. JCI Insight. 2021;6:e143980. doi: 10.1172/jci.insight.143980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Smedley G.D., Walker K.E., Yuan S.H. The Role of PERK in Understanding Development of Neurodegenerative Diseases. Int. J. Mol. Sci. 2021;22:8146. doi: 10.3390/ijms22158146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Oakes S.A. Endoplasmic Reticulum Stress Signaling in Cancer Cells. Am. J. Pathol. 2020;190:934–946. doi: 10.1016/j.ajpath.2020.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Hetz C., Zhang K., Kaufman R.J. Mechanisms, regulation and functions of the unfolded protein response. Nat. Rev. Mol. Cell Biol. 2020;21:421–438. doi: 10.1038/s41580-020-0250-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Cui W., Li J., Ron D., Sha B. The structure of the PERK kinase domain suggests the mechanism for its activation. Pt 5Acta Crystallogr. D Biol. Crystallogr. 2011;67:423–428. doi: 10.1107/S0907444911006445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Pitale P.M., Gorbatyuk O., Gorbatyuk M. Neurodegeneration: Keeping ATF4 on a Tight Leash. Front. Cell Neurosci. 2017;11:410. doi: 10.3389/fncel.2017.00410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Prentki M., Nolan C.J. Islet beta cell failure in type 2 diabetes. J. Clin. Invest. 2006;116:1802–1812. doi: 10.1172/JCI29103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Boland B.B., Rhodes C.J., Grimsby J.S. The dynamic plasticity of insulin production in beta-cells. Mol. Metab. 2017;6:958–973. doi: 10.1016/j.molmet.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Gupta S., McGrath B., Cavener D.R. PERK (EIF2AK3) regulates proinsulin trafficking and quality control in the secretory pathway. Diabetes. 2010;59:1937–1947. doi: 10.2337/db09-1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Scheuner D., Kaufman R.J. The unfolded protein response: A pathway that links insulin demand with beta-cell failure and diabetes. Endocr. Rev. 2008;29:317–333. doi: 10.1210/er.2007-0039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Liu M., Huang Y., Xu X., Li X., Alam M., Arunagiri A., Haataja L., Ding L., Wang S., Itkin-Ansari P., et al. Normal and defective pathways in biogenesis and maintenance of the insulin storage pool. J. Clin. Invest. 2021;131:e142240. doi: 10.1172/JCI142240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Rohli K.E., Boyer C.K., Blom S.E., Stephens S.B. Nutrient Regulation of Pancreatic Islet beta-Cell Secretory Capacity and Insulin Production. Biomolecules. 2022;12:335. doi: 10.3390/biom12020335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rabhi N., Salas E., Froguel P., Annicotte J.S. Role of the unfolded protein response in beta cell compensation and failure during diabetes. J. Diabetes Res. 2014;2014:795171. doi: 10.1155/2014/795171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kitakaze K., Oyadomari M., Zhang J., Hamada Y., Takenouchi Y., Tsuboi K., Inagaki M., Tachikawa M., Fujitani Y., Okamoto Y., et al. ATF4-mediated transcriptional regulation protects against beta-cell loss during endoplasmic reticulum stress in a mouse model. Mol. Metab. 2021;54:101338. doi: 10.1016/j.molmet.2021.101338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Berry C., Lal M., Binukumar B.K. Crosstalk Between the Unfolded Protein Response, MicroRNAs, and Insulin Signaling Pathways: In Search of Biomarkers for the Diagnosis and Treatment of Type 2 Diabetes. Front. Endocrinol. 2018;9:210. doi: 10.3389/fendo.2018.00210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.de la Cadena S.G., Hernandez-Fonseca K., Camacho-Arroyo I., Massieu L. Glucose deprivation induces reticulum stress by the PERK pathway and caspase-7- and calpain-mediated caspase-12 activation. Apoptosis. 2014;19:414–427. doi: 10.1007/s10495-013-0930-7. [DOI] [PubMed] [Google Scholar]
- 107.Lee J.H., Lee J. Endoplasmic Reticulum (ER) Stress and Its Role in Pancreatic beta-Cell Dysfunction and Senescence in Type 2 Diabetes. Int. J. Mol. Sci. 2022;23:4843. doi: 10.3390/ijms23094843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sharma R.B., Landa-Galvan H.V., Alonso L.C. Living Dangerously: Protective and Harmful ER Stress Responses in Pancreatic beta-Cells. Diabetes. 2021;70:2431–2443. doi: 10.2337/dbi20-0033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kong F.J., Wu J.H., Sun S.Y., Zhou J.Q. The endoplasmic reticulum stress/autophagy pathway is involved in cholesterol-induced pancreatic beta-cell injury. Sci. Rep. 2017;7:44746. doi: 10.1038/srep44746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Tran K., Li Y., Duan H., Arora D., Lim H.Y., Wang W. Identification of small molecules that protect pancreatic beta cells against endoplasmic reticulum stress-induced cell death. ACS Chem. Biol. 2014;9:2796–2806. doi: 10.1021/cb500740d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Turishcheva E., Vildanova M., Onishchenko G., Smirnova E. The Role of Endoplasmic Reticulum Stress in Differentiation of Cells of Mesenchymal Origin. Biochemistry. 2022;87:916–931. doi: 10.1134/S000629792209005X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Bobrovnikova-Marjon E., Pytel D., Riese M.J., Vaites L.P., Singh N., Koretzky G.A., Witze E.S., Diehl J.A. PERK utilizes intrinsic lipid kinase activity to generate phosphatidic acid, mediate Akt activation, and promote adipocyte differentiation. Mol. Cell Biol. 2012;32:2268–2278. doi: 10.1128/MCB.00063-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Cohen D.M., Won K.J., Nguyen N., Lazar M.A., Chen C.S., Steger D.J. ATF4 licenses C/EBPbeta activity in human mesenchymal stem cells primed for adipogenesis. Elife. 2015;4:e06821. doi: 10.7554/eLife.06821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Yuzefovych L.V., Musiyenko S.I., Wilson G.L., Rachek L.I. Mitochondrial DNA damage and dysfunction, and oxidative stress are associated with endoplasmic reticulum stress, protein degradation and apoptosis in high fat diet-induced insulin resistance mice. PLoS ONE. 2013;8:e54059. doi: 10.1371/journal.pone.0054059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Kapetanaki S., Kumawat A.K., Persson K., Demirel I. The Fibrotic Effects of TMAO on Human Renal Fibroblasts Is Mediated by NLRP3, Caspase-1 and the PERK/Akt/mTOR Pathway. Int. J. Mol. Sci. 2021;22:11864. doi: 10.3390/ijms222111864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Wang X., Hai C. Redox modulation of adipocyte differentiation: Hypothesis of “Redox Chain” and novel insights into intervention of adipogenesis and obesity. Free Radic. Biol. Med. 2015;89:99–125. doi: 10.1016/j.freeradbiomed.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 117.Lowe C.E., Dennis R.J., Obi U., O’Rahilly S., Rochford J.J. Investigating the involvement of the ATF6alpha pathway of the unfolded protein response in adipogenesis. Int. J. Obes. 2012;36:1248–1251. doi: 10.1038/ijo.2011.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhang K., Kaufman R.J. Signaling the unfolded protein response from the endoplasmic reticulum. J. Biol. Chem. 2004;279:25935–25938. doi: 10.1074/jbc.R400008200. [DOI] [PubMed] [Google Scholar]
- 119.van Laar T., van der Eb A.J., Terleth C. Mif1: A missing link between the unfolded protein response pathway and ER-associated protein degradation? Curr. Protein Pept. Sci. 2001;2:169–190. doi: 10.2174/1389203013381189. [DOI] [PubMed] [Google Scholar]
- 120.Yoshida H., Matsui T., Hosokawa N., Kaufman R.J., Nagata K., Mori K. A time-dependent phase shift in the mammalian unfolded protein response. Dev. Cell. 2003;4:265–271. doi: 10.1016/S1534-5807(03)00022-4. [DOI] [PubMed] [Google Scholar]
- 121.Qu J., Zou T., Lin Z. The Roles of the Ubiquitin-Proteasome System in the Endoplasmic Reticulum Stress Pathway. Int. J. Mol. Sci. 2021;22:1526. doi: 10.3390/ijms22041526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Chen X., Shen J., Prywes R. The luminal domain of ATF6 senses endoplasmic reticulum (ER) stress and causes translocation of ATF6 from the ER to the Golgi. J. Biol. Chem. 2002;277:13045–13052. doi: 10.1074/jbc.M110636200. [DOI] [PubMed] [Google Scholar]
- 123.Shen J., Prywes R. Dependence of site-2 protease cleavage of ATF6 on prior site-1 protease digestion is determined by the size of the luminal domain of ATF6. J. Biol. Chem. 2004;279:43046–43051. doi: 10.1074/jbc.M408466200. [DOI] [PubMed] [Google Scholar]
- 124.Thuerauf D.J., Morrison L.E., Hoover H., Glembotski C.C. Coordination of ATF6-mediated transcription and ATF6 degradation by a domain that is shared with the viral transcription factor, VP16. J. Biol. Chem. 2002;277:20734–20739. doi: 10.1074/jbc.M201749200. [DOI] [PubMed] [Google Scholar]
- 125.Stengel S.T., Fazio A., Lipinski S., Jahn M.T., Aden K., Ito G., Wottawa F., Kuiper J.W.P., Coleman O.I., Tran F., et al. Activating Transcription Factor 6 Mediates Inflammatory Signals in Intestinal Epithelial Cells Upon Endoplasmic Reticulum Stress. Gastroenterology. 2020;159:1357–1374.e10. doi: 10.1053/j.gastro.2020.06.088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen Z., Liu Y., Yang L., Liu P., Zhang Y., Wang X. MiR-149 attenuates endoplasmic reticulum stress-induced inflammation and apoptosis in nonalcoholic fatty liver disease by negatively targeting ATF6 pathway. Immunol. Lett. 2020;222:40–48. doi: 10.1016/j.imlet.2020.03.003. [DOI] [PubMed] [Google Scholar]
- 127.Nakajima S., Hiramatsu N., Hayakawa K., Saito Y., Kato H., Huang T., Yao J., Paton A.W., Paton J.C., Kitamura M. Selective abrogation of BiP/GRP78 blunts activation of NF-kappaB through the ATF6 branch of the UPR: Involvement of C/EBPbeta and mTOR-dependent dephosphorylation of Akt. Mol. Cell Biol. 2011;31:1710–1718. doi: 10.1128/MCB.00939-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Rao J., Yue S., Fu Y., Zhu J., Wang X., Busuttil R.W., Kupiec-Weglinski J.W., Lu L., Zhai Y. ATF6 mediates a pro-inflammatory synergy between ER stress and TLR activation in the pathogenesis of liver ischemia-reperfusion injury. Am. J. Transplant. 2014;14:1552–1561. doi: 10.1111/ajt.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Senkal C.E., Ponnusamy S., Bielawski J., Hannun Y.A., Ogretmen B. Antiapoptotic roles of ceramide-synthase-6-generated C16-ceramide via selective regulation of the ATF6/CHOP arm of ER-stress-response pathways. FASEB J. 2010;24:296–308. doi: 10.1096/fj.09-135087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Bommiasamy H., Back S.H., Fagone P., Lee K., Meshinchi S., Vink E., Sriburi R., Frank M., Jackowski S., Kaufman R.J., et al. ATF6alpha induces XBP1-independent expansion of the endoplasmic reticulum. Pt 10J. Cell Sci. 2009;122:1626–1636. doi: 10.1242/jcs.045625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Ghemrawi R., Battaglia-Hsu S.F., Arnold C. Endoplasmic Reticulum Stress in Metabolic Disorders. Cells. 2018;7:63. doi: 10.3390/cells7060063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.White U.A., Stephens J.M. Transcriptional factors that promote formation of white adipose tissue. Mol. Cell Endocrinol. 2010;318:10–14. doi: 10.1016/j.mce.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Oyadomari S., Harding H.P., Zhang Y., Oyadomari M., Ron D. Dephosphorylation of translation initiation factor 2alpha enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metab. 2008;7:520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Park S.W., Ozcan U. Potential for therapeutic manipulation of the UPR in disease. Semin. Immunopathol. 2013;35:351–373. doi: 10.1007/s00281-013-0370-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Tang Q.Q., Lane M.D. Adipogenesis: From Stem Cell to Adipocyte. Annu. Rev. Biochem. 2012;81:715–736. doi: 10.1146/annurev-biochem-052110-115718. [DOI] [PubMed] [Google Scholar]
- 136.Tang Q.Q., Otto T.C., Lane M.D. CCAAT/enhancer-binding protein beta is required for mitotic clonal expansion during adipogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:850–855. doi: 10.1073/pnas.0337434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Rosen E.D., MacDougald O.A. Adipocyte differentiation from the inside out. Nat. Rev. Mol. Cell Biol. 2006;7:885–896. doi: 10.1038/nrm2066. [DOI] [PubMed] [Google Scholar]
- 138.El-Jack A.K., Hamm J.K., Pilch P.F., Farmer S.R. Reconstitution of insulin-sensitive glucose transport in fibroblasts requires expression of both PPARgamma and C/EBPalpha. J. Biol. Chem. 1999;274:7946–7951. doi: 10.1074/jbc.274.12.7946. [DOI] [PubMed] [Google Scholar]
- 139.de Winter T.J.J., Nusse R. Running Against the Wnt: How Wnt/beta-Catenin Suppresses Adipogenesis. Front. Cell Dev. Biol. 2021;9:627429. doi: 10.3389/fcell.2021.627429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Funcke J.B., Scherer P.E. Beyond adiponectin and leptin: Adipose tissue-derived mediators of inter-organ communication. J. Lipid Res. 2019;60:1648–1684. doi: 10.1194/jlr.R094060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Straub L.G., Scherer P.E. Metabolic Messengers: Adiponectin. Nat. Metab. 2019;1:334–339. doi: 10.1038/s42255-019-0041-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Wang Q.A., Zhang F., Jiang L., Ye R., An Y., Shao M., Tao C., Gupta R.K., Scherer P.E. Peroxisome Proliferator-Activated Receptor gamma and Its Role in Adipocyte Homeostasis and Thiazolidinedione-Mediated Insulin Sensitization. Mol. Cell Biol. 2018;38:e00677-17. doi: 10.1128/MCB.00677-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Li D., Zhang F., Zhang X., Xue C., Namwanje M., Fan L., Reilly M.P., Hu F., Qiang L. Distinct functions of PPARgamma isoforms in regulating adipocyte plasticity. Biochem. Biophys. Res. Commun. 2016;481:132–138. doi: 10.1016/j.bbrc.2016.10.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Mu F., Jing Y., Ning B., Huang J., Cui T., Guo Y., You X., Yan X., Li H., Wang N. Peroxisome proliferator-activated receptor gamma isoforms differentially regulate preadipocyte proliferation, apoptosis, and differentiation in chickens. Poult. Sci. 2020;99:6410–6421. doi: 10.1016/j.psj.2020.09.086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Wan R., Ding Z., Xia S., Zheng L., Lu J. Effects Of PPARgamma2 Pro12Ala Variant On Adipocyte Phenotype Dependent Of DHA. Diabetes Metab. Syndr. Obes. 2019;12:2273–2279. doi: 10.2147/DMSO.S214526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Wu Z., Rosen E.D., Brun R., Hauser S., Adelmant G., Troy A.E., McKeon C., Darlington G.J., Spiegelman B.M. Cross-regulation of C/EBP alpha and PPAR gamma controls the transcriptional pathway of adipogenesis and insulin sensitivity. Mol. Cell. 1999;3:151–158. doi: 10.1016/S1097-2765(00)80306-8. [DOI] [PubMed] [Google Scholar]
- 147.Jakab J., Miskic B., Miksic S., Juranic B., Cosic V., Schwarz D., Vcev A. Adipogenesis as a Potential Anti-Obesity Target: A Review of Pharmacological Treatment and Natural Products. Diabetes Metab. Syndr. Obes. 2021;14:67–83. doi: 10.2147/DMSO.S281186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Hallenborg P., Petersen R.K., Feddersen S., Sundekilde U., Hansen J.B., Blagoev B., Madsen L., Kristiansen K. PPARgamma ligand production is tightly linked to clonal expansion during initiation of adipocyte differentiation. J. Lipid Res. 2014;55:2491–2500. doi: 10.1194/jlr.M050658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Park S.H., Choi H.J., Yang H., Do K.H., Kim J., Lee D.W., Moon Y. Endoplasmic reticulum stress-activated C/EBP homologous protein enhances nuclear factor-kappaB signals via repression of peroxisome proliferator-activated receptor gamma. J. Biol. Chem. 2010;285:35330–35339. doi: 10.1074/jbc.M110.136259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cucinotta M., Visalli M., Aguennouz M., Valenti A., Loddo S., Altucci L., Teti D. Regulation of interleukin-8 gene at a distinct site of its promoter by CCAAT enhancer-binding protein homologous protein in prostaglandin E2-treated human T cells. J. Biol. Chem. 2008;283:29760–29769. doi: 10.1074/jbc.M803145200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Hayakawa K., Nakajima S., Hiramatsu N., Okamura M., Huang T., Saito Y., Tagawa Y., Tamai M., Takahashi S., Yao J., et al. ER stress depresses NF-kappaB activation in mesangial cells through preferential induction of C/EBP beta. J. Am. Soc. Nephrol. 2010;21:73–81. doi: 10.1681/ASN.2009040432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Weidemann A., Lovas A., Rauch A., Andreas N., von Maltzahn J., Riemann M., Weih F. Classical and alternative NF-kappaB signaling cooperate in regulating adipocyte differentiation and function. Int. J. Obes. 2016;40:452–459. doi: 10.1038/ijo.2015.198. [DOI] [PubMed] [Google Scholar]
- 153.Griffin M.J. On the Immunometabolic Role of NF-kappaB in Adipocytes. Immunometabolism. 2022;4:e220003. doi: 10.20900/immunometab20220003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Siersbaek R., Nielsen R., Mandrup S. PPARgamma in adipocyte differentiation and metabolism—novel insights from genome-wide studies. FEBS Lett. 2010;584:3242–3249. doi: 10.1016/j.febslet.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 155.Accattatis F.M., Caruso A., Carleo A., Del Console P., Gelsomino L., Bonofiglio D., Giordano C., Barone I., Ando S., Bianchi L., et al. CEBP-beta and PLK1 as Potential Mediators of the Breast Cancer/Obesity Crosstalk: In Vitro and In Silico Analyses. Nutrients. 2023;15:2839. doi: 10.3390/nu15132839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.DeZwaan-McCabe D., Riordan J.D., Arensdorf A.M., Icardi M.S., Dupuy A.J., Rutkowski D.T. The stress-regulated transcription factor CHOP promotes hepatic inflammatory gene expression, fibrosis, and oncogenesis. PLoS Genet. 2013;9:e1003937. doi: 10.1371/journal.pgen.1003937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Fujita E., Dai H., Tanabe Y., Zhiling Y., Yamagata T., Miyakawa T., Tanokura M., Momoi M.Y., Momoi T. Autism spectrum disorder is related to endoplasmic reticulum stress induced by mutations in the synaptic cell adhesion molecule, CADM1. Cell Death Dis. 2010;1:e47. doi: 10.1038/cddis.2010.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Li Y., Jiang W., Niu Q., Sun Y., Meng C., Tan L., Song C., Qiu X., Liao Y., Ding C. eIF2alpha-CHOP-BCl-2/JNK and IRE1alpha-XBP1/JNK signaling promote apoptosis and inflammation and support the proliferation of Newcastle disease virus. Cell Death Dis. 2019;10:891. doi: 10.1038/s41419-019-2128-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Yang Y., Liu L., Naik I., Braunstein Z., Zhong J., Ren B. Transcription Factor C/EBP Homologous Protein in Health and Diseases. Front. Immunol. 2017;8:1612. doi: 10.3389/fimmu.2017.01612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Allagnat F., Fukaya M., Nogueira T.C., Delaroche D., Welsh N., Marselli L., Marchetti P., Haefliger J.A., Eizirik D.L., Cardozo A.K. C/EBP homologous protein contributes to cytokine-induced pro-inflammatory responses and apoptosis in beta-cells. Cell Death Differ. 2012;19:1836–1846. doi: 10.1038/cdd.2012.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang P., Qian H., Xiao M., Lv J. Role of signal transduction pathways in IL-1beta-induced apoptosis: Pathological and therapeutic aspects. Immun. Inflamm. Dis. 2023;11:e762. doi: 10.1002/iid3.762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Frith J., Genever P. Transcriptional control of mesenchymal stem cell differentiation. Transfus. Med. Hemotherapy. 2008;35:216–227. doi: 10.1159/000127448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brenner S., Bercovich Z., Feiler Y., Keshet R., Kahana C. Dual Regulatory Role of Polyamines in Adipogenesis. J. Biol. Chem. 2015;290:27384–27392. doi: 10.1074/jbc.M115.686980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Ma X., Lee P., Chisholm D.J., James D.E. Control of adipocyte differentiation in different fat depots; implications for pathophysiology or therapy. Front. Endocrinol. 2015;6:1. doi: 10.3389/fendo.2015.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Hosogai N., Fukuhara A., Oshima K., Miyata Y., Tanaka S., Segawa K., Furukawa S., Tochino Y., Komuro R., Matsuda M., et al. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- 166.Han J., Murthy R., Wood B., Song B., Wang S., Sun B., Malhi H., Kaufman R.J. ER stress signalling through eIF2alpha and CHOP, but not IRE1alpha, attenuates adipogenesis in mice. Diabetologia. 2013;56:911–924. doi: 10.1007/s00125-012-2809-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Cawthorn W.P., Scheller E.L., MacDougald O.A. Adipose tissue stem cells meet preadipocyte commitment: Going back to the future. J. Lipid Res. 2012;53:227–246. doi: 10.1194/jlr.R021089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Zhu W., Niu X., Wang M., Li Z., Jiang H.K., Li C., Caton S.J., Bai Y. Endoplasmic reticulum stress may be involved in insulin resistance and lipid metabolism disorders of the white adipose tissues induced by high-fat diet containing industrial trans-fatty acids. Diabetes Metab. Syndr. Obes. 2019;12:1625–1638. doi: 10.2147/DMSO.S218336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Lemmer I.L., Willemsen N., Hilal N., Bartelt A. A guide to understanding endoplasmic reticulum stress in metabolic disorders. Mol. Metab. 2021;47:101169. doi: 10.1016/j.molmet.2021.101169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Almanza A., Carlesso A., Chintha C., Creedican S., Doultsinos D., Leuzzi B., Luis A., McCarthy N., Montibeller L., More S., et al. Endoplasmic reticulum stress signalling—From basic mechanisms to clinical applications. FEBS J. 2019;286:241–278. doi: 10.1111/febs.14608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Mansour S.Z., Moustafa E.M., Moawed F.S.M. Modulation of endoplasmic reticulum stress via sulforaphane-mediated AMPK upregulation against nonalcoholic fatty liver disease in rats. Cell Stress Chaperones. 2022;27:499–511. doi: 10.1007/s12192-022-01286-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Guan B.J., Krokowski D., Majumder M., Schmotzer C.L., Kimball S.R., Merrick W.C., Koromilas A.E., Hatzoglou M. Translational control during endoplasmic reticulum stress beyond phosphorylation of the translation initiation factor eIF2alpha. J. Biol. Chem. 2014;289:12593–12611. doi: 10.1074/jbc.M113.543215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cnop M., Toivonen S., Igoillo-Esteve M., Salpea P. Endoplasmic reticulum stress and eIF2alpha phosphorylation: The Achilles heel of pancreatic beta cells. Mol. Metab. 2017;6:1024–1039. doi: 10.1016/j.molmet.2017.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Leitman J., Barak B., Benyair R., Shenkman M., Ashery U., Hartl F.U., Lederkremer G.Z. ER stress-induced eIF2-alpha phosphorylation underlies sensitivity of striatal neurons to pathogenic huntingtin. PLoS ONE. 2014;9:e90803. doi: 10.1371/journal.pone.0090803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Park S., Lim Y., Lee D., Elvira R., Lee J.M., Lee M.R., Han J. Modulation of Protein Synthesis by eIF2alpha Phosphorylation Protects Cell from Heat Stress-Mediated Apoptosis. Cells. 2018;7:254. doi: 10.3390/cells7120254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Kasinath B.S., Mariappan M.M., Sataranatarajan K., Lee M.J., Ghosh Choudhury G., Feliers D. Novel mechanisms of protein synthesis in diabetic nephropathy—role of mRNA translation. Rev. Endocr. Metab. Disord. 2008;9:255–266. doi: 10.1007/s11154-008-9091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lang C.H., Frost R.A., Nairn A.C., MacLean D.A., Vary T.C. TNF-alpha impairs heart and skeletal muscle protein synthesis by altering translation initiation. Am. J. Physiol. Endocrinol. Metab. 2002;282:E336–E347. doi: 10.1152/ajpendo.00366.2001. [DOI] [PubMed] [Google Scholar]
- 178.Bagchi D.P., Nishii A., Li Z., DelProposto J.B., Corsa C.A., Mori H., Hardij J., Learman B.S., Lumeng C.N., MacDougald O.A. Wnt/beta-catenin signaling regulates adipose tissue lipogenesis and adipocyte-specific loss is rigorously defended by neighboring stromal-vascular cells. Mol. Metab. 2020;42:101078. doi: 10.1016/j.molmet.2020.101078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Prestwich T.C., Macdougald O.A. Wnt/beta-catenin signaling in adipogenesis and metabolism. Curr. Opin. Cell Biol. 2007;19:612–617. doi: 10.1016/j.ceb.2007.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Palsgaard J., Emanuelli B., Winnay J.N., Sumara G., Karsenty G., Kahn C.R. Cross-talk between insulin and Wnt signaling in preadipocytes: Role of Wnt co-receptor low density lipoprotein receptor-related protein-5 (LRP5) J. Biol. Chem. 2012;287:12016–12026. doi: 10.1074/jbc.M111.337048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Bennett C.N., Ross S.E., Longo K.A., Bajnok L., Hemati N., Johnson K.W., Harrison S.D., MacDougald O.A. Regulation of Wnt signaling during adipogenesis. J. Biol. Chem. 2002;277:30998–31004. doi: 10.1074/jbc.M204527200. [DOI] [PubMed] [Google Scholar]
- 182.Corda G., Sala A. Non-canonical WNT/PCP signalling in cancer: Fzd6 takes centre stage. Oncogenesis. 2017;6:e364. doi: 10.1038/oncsis.2017.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Li F., Chong Z.Z., Maiese K. Vital elements of the Wnt-Frizzled signaling pathway in the nervous system. Curr. Neurovasc. Res. 2005;2:331–340. doi: 10.2174/156720205774322557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.MacDonald B.T., Tamai K., He X. Wnt/beta-catenin signaling: Components, mechanisms, and diseases. Dev. Cell. 2009;17:9–26. doi: 10.1016/j.devcel.2009.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Tran B.M., Flanagan D.J., Phesse T.J., Vincan E. Frizzled(7) Activates beta-Catenin-Dependent and beta-Catenin-Independent Wnt Signalling Pathways During Developmental Morphogenesis: Implications for Therapeutic Targeting in Colorectal Cancer. Handb. Exp. Pharmacol. 2021;269:251–277. doi: 10.1007/164_2021_524. [DOI] [PubMed] [Google Scholar]
- 186.Longo K.A., Wright W.S., Kang S., Gerin I., Chiang S.H., Lucas P.C., Opp M.R., MacDougald O.A. Wnt10b inhibits development of white and brown adipose tissues. J. Biol. Chem. 2004;279:35503–35509. doi: 10.1074/jbc.M402937200. [DOI] [PubMed] [Google Scholar]
- 187.Calejman C.M., Doxsey W.G., Fazakerley D.J., Guertin D.A. Integrating adipocyte insulin signaling and metabolism in the multi-omics era. Trends Biochem. Sci. 2022;47:531–546. doi: 10.1016/j.tibs.2022.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Caron A., Richard D., Laplante M. The Roles of mTOR Complexes in Lipid Metabolism. Annu. Rev. Nutr. 2015;35:321–348. doi: 10.1146/annurev-nutr-071714-034355. [DOI] [PubMed] [Google Scholar]
- 189.Kato H., Nakajima S., Saito Y., Takahashi S., Katoh R., Kitamura M. mTORC1 serves ER stress-triggered apoptosis via selective activation of the IRE1-JNK pathway. Cell Death Differ. 2012;19:310–320. doi: 10.1038/cdd.2011.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Heberle A.M., Prentzell M.T., van Eunen K., Bakker B.M., Grellscheid S.N., Thedieck K. Molecular mechanisms of mTOR regulation by stress. Mol. Cell Oncol. 2015;2:e970489. doi: 10.4161/23723548.2014.970489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Boss M., Newbatt Y., Gupta S., Collins I., Brune B., Namgaladze D. AMPK-independent inhibition of human macrophage ER stress response by AICAR. Sci. Rep. 2016;6:32111. doi: 10.1038/srep32111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Kim H., Moon S.Y., Kim J.S., Baek C.H., Kim M., Min J.Y., Lee S.K. Activation of AMP-activated protein kinase inhibits ER stress and renal fibrosis. Am. J. Physiol. Renal. Physiol. 2015;308:F226–F236. doi: 10.1152/ajprenal.00495.2014. [DOI] [PubMed] [Google Scholar]
- 193.Li H., Min Q., Ouyang C., Lee J., He C., Zou M.H., Xie Z. AMPK activation prevents excess nutrient-induced hepatic lipid accumulation by inhibiting mTORC1 signaling and endoplasmic reticulum stress response. Biochim. Biophys. Acta. 2014;1842:1844–1854. doi: 10.1016/j.bbadis.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Steinberg G.R., Hardie D.G. New insights into activation and function of the AMPK. Nat. Rev. Mol. Cell Biol. 2023;24:255–272. doi: 10.1038/s41580-022-00547-x. [DOI] [PubMed] [Google Scholar]
- 195.Gwinn D.M., Shackelford D.B., Egan D.F., Mihaylova M.M., Mery A., Vasquez D.S., Turk B.E., Shaw R.J. AMPK phosphorylation of raptor mediates a metabolic checkpoint. Mol. Cell. 2008;30:214–226. doi: 10.1016/j.molcel.2008.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Hindupur S.K., Gonzalez A., Hall M.N. The opposing actions of target of rapamycin and AMP-activated protein kinase in cell growth control. Cold Spring Harb. Perspect. Biol. 2015;7:a019141. doi: 10.1101/cshperspect.a019141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Peterson T.R., Sengupta S.S., Harris T.E., Carmack A.E., Kang S.A., Balderas E., Guertin D.A., Madden K.L., Carpenter A.E., Finck B.N., et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell. 2011;146:408–420. doi: 10.1016/j.cell.2011.06.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Qin L., Wang Z., Tao L., Wang Y. ER stress negatively regulates AKT/TSC/mTOR pathway to enhance autophagy. Autophagy. 2010;6:239–247. doi: 10.4161/auto.6.2.11062. [DOI] [PubMed] [Google Scholar]
- 199.Ohoka N., Yoshii S., Hattori T., Onozaki K., Hayashi H. TRB3, a novel ER stress-inducible gene, is induced via ATF4-CHOP pathway and is involved in cell death. EMBO J. 2005;24:1243–1255. doi: 10.1038/sj.emboj.7600596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Lee P.L., Jung S.M., Guertin D.A. The Complex Roles of Mechanistic Target of Rapamycin in Adipocytes and Beyond. Trends Endocrinol. Metab. 2017;28:319–339. doi: 10.1016/j.tem.2017.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Chen C.H., Shaikenov T., Peterson T.R., Aimbetov R., Bissenbaev A.K., Lee S.W., Wu J., Lin H.K., Sarbassov D.D. ER stress inhibits mTORC2 and Akt signaling through GSK-3beta-mediated phosphorylation of rictor. Sci. Signal. 2011;4:ra10. doi: 10.1126/scisignal.2001731. [DOI] [PubMed] [Google Scholar]
- 202.Ozcan U., Yilmaz E., Ozcan L., Furuhashi M., Vaillancourt E., Smith R.O., Gorgun C.Z., Hotamisligil G.S. Chemical chaperones reduce ER stress and restore glucose homeostasis in a mouse model of type 2 diabetes. Science. 2006;313:1137–1140. doi: 10.1126/science.1128294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Ozcan U., Cao Q., Yilmaz E., Lee A.H., Iwakoshi N.N., Ozdelen E., Tuncman G., Gorgun C., Glimcher L.H., Hotamisligil G.S. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306:457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 204.Goldstein B.J. Insulin resistance as the core defect in type 2 diabetes mellitus. Am. J. Cardiol. 2002;90:3G–10G. doi: 10.1016/S0002-9149(02)02553-5. [DOI] [PubMed] [Google Scholar]
- 205.Korbecki J., Bajdak-Rusinek K. The effect of palmitic acid on inflammatory response in macrophages: An overview of molecular mechanisms. Inflamm. Res. 2019;68:915–932. doi: 10.1007/s00011-019-01273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Shi F., Collins S. Regulation of mTOR Signaling: Emerging Role of Cyclic Nucleotide-Dependent Protein Kinases and Implications for Cardiometabolic Disease. Int. J. Mol. Sci. 2023;24:11497. doi: 10.3390/ijms241411497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Jayaraman S., Devarajan N., Rajagopal P., Babu S., Ganesan S.K., Veeraraghavan V.P., Palanisamy C.P., Cui B., Periyasamy V., Chandrasekar K. β-Sitosterol Circumvents Obesity Induced Inflammation and Insulin Resistance by down-Regulating IKKbeta/NF-kappaB and JNK Signaling Pathway in Adipocytes of Type 2 Diabetic Rats. Molecules. 2021;26:2101. doi: 10.3390/molecules26072101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shi C., Zhu L., Chen X., Gu N., Chen L., Zhu L., Yang L., Pang L., Guo X., Ji C., et al. IL-6 and TNF-alpha induced obesity-related inflammatory response through transcriptional regulation of miR-146b. J. Interferon Cytokine Res. 2014;34:342–348. doi: 10.1089/jir.2013.0078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Roy P.K., Islam J., Lalhlenmawia H. Prospects of potential adipokines as therapeutic agents in obesity-linked atherogenic dyslipidemia and insulin resistance. Egypt Heart J. 2023;75:24. doi: 10.1186/s43044-023-00352-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Jayawardena T.U., Kim S.Y., Jeon Y.J. Sarcopenia; functional concerns, molecular mechanisms involved, and seafood as a nutritional intervention—Review article. Crit. Rev. Food Sci. Nutr. 2023;63:1983–2003. doi: 10.1080/10408398.2021.1969889. [DOI] [PubMed] [Google Scholar]
- 211.Yung J.H.M., Giacca A. Role of c-Jun N-terminal Kinase (JNK) in Obesity and Type 2 Diabetes. Cells. 2020;9:706. doi: 10.3390/cells9030706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Handschumacher R.E., Harding M.W., Rice J., Drugge R.J., Speicher D.W. Cyclophilin: A specific cytosolic binding protein for cyclosporin A. Science. 1984;226:544–547. doi: 10.1126/science.6238408. [DOI] [PubMed] [Google Scholar]
- 213.Lee J.M. Nuclear Receptors Resolve Endoplasmic Reticulum Stress to Improve Hepatic Insulin Resistance. Diabetes Metab. J. 2017;41:10–19. doi: 10.4093/dmj.2017.41.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Djedaini M., Peraldi P., Drici M.D., Darini C., Saint-Marc P., Dani C., Ladoux A. Lopinavir co-induces insulin resistance and ER stress in human adipocytes. Biochem. Biophys. Res. Commun. 2009;386:96–100. doi: 10.1016/j.bbrc.2009.05.148. [DOI] [PubMed] [Google Scholar]
- 215.Brown M., Dainty S., Strudwick N., Mihai A.D., Watson J.N., Dendooven R., Paton A.W., Paton J.C., Schroder M. Endoplasmic reticulum stress causes insulin resistance by inhibiting delivery of newly synthesized insulin receptors to the cell surface. Mol. Biol. Cell. 2020;31:2597–2629. doi: 10.1091/mbc.E18-01-0013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Eizirik D.L., Cardozo A.K., Cnop M. The role for endoplasmic reticulum stress in diabetes mellitus. Endocr. Rev. 2008;29:42–61. doi: 10.1210/er.2007-0015. [DOI] [PubMed] [Google Scholar]
- 217.Tong Y., Xu S., Huang L., Chen C. Obesity and insulin resistance: Pathophysiology and treatment. Drug Discov. Today. 2022;27:822–830. doi: 10.1016/j.drudis.2021.11.001. [DOI] [PubMed] [Google Scholar]
- 218.Liang L., Chen J., Zhan L., Lu X., Sun X., Sui H., Zheng L., Xiang H., Zhang F. Endoplasmic reticulum stress impairs insulin receptor signaling in the brains of obese rats. PLoS ONE. 2015;10:e0126384. doi: 10.1371/journal.pone.0126384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Solinas G., Becattini B. JNK at the crossroad of obesity, insulin resistance, and cell stress response. Mol. Metab. 2017;6:174–184. doi: 10.1016/j.molmet.2016.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Chen Y., Wu Z., Zhao S., Xiang R. Chemical chaperones reduce ER stress and adipose tissue inflammation in high fat diet-induced mouse model of obesity. Sci. Rep. 2016;6:27486. doi: 10.1038/srep27486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Wu J., Wu D., Zhang L., Lin C., Liao J., Xie R., Li Z., Wu S., Liu A., Hu W., et al. NK cells induce hepatic ER stress to promote insulin resistance in obesity through osteopontin production. J. Leukoc. Biol. 2020;107:589–596. doi: 10.1002/JLB.3MA1119-173R. [DOI] [PubMed] [Google Scholar]
- 222.Khan S., Wang C.H. ER stress in adipocytes and insulin resistance: Mechanisms and significance (Review) Mol. Med. Rep. 2014;10:2234–2240. doi: 10.3892/mmr.2014.2532. [DOI] [PubMed] [Google Scholar]
- 223.Copps K.D., White M.F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55:2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Mathew D., Barillas-Cerritos J., Nedeljkovic-Kurepa A., Abraham M., Taylor M.D., Deutschman C.S. Phosphorylation of insulin receptor substrates (IRS-1 and IRS-2) is attenuated following cecal ligation and puncture in mice. Mol. Med. 2023;29:106. doi: 10.1186/s10020-023-00703-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Sadeghi A., Niknam M., Momeni-Moghaddam M.A., Shabani M., Aria H., Bastin A., Teimouri M., Meshkani R., Akbari H. Crosstalk between autophagy and insulin resistance: Evidence from different tissues. Eur. J. Med. Res. 2023;28:456. doi: 10.1186/s40001-023-01424-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Dixon E.D., Nardo A.D., Claudel T., Trauner M. The Role of Lipid Sensing Nuclear Receptors (PPARs and LXR) and Metabolic Lipases in Obesity, Diabetes and NAFLD. Genes. 2021;12:645. doi: 10.3390/genes12050645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Zhao Y., Ma D.X., Wang H.G., Li M.Z., Talukder M., Wang H.R., Li J.L. Lycopene Prevents DEHP-Induced Liver Lipid Metabolism Disorder by Inhibiting the HIF-1alpha-Induced PPARalpha/PPARgamma/FXR/LXR System. J. Agric. Food Chem. 2020;68:11468–11479. doi: 10.1021/acs.jafc.0c05077. [DOI] [PubMed] [Google Scholar]
- 228.Lee S.D., Tontonoz P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis. 2015;242:29–36. doi: 10.1016/j.atherosclerosis.2015.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Wang T., Zhao Y., You Z., Li X., Xiong M., Li H., Yan N. Endoplasmic Reticulum Stress Affects Cholesterol Homeostasis by Inhibiting LXRalpha Expression in Hepatocytes and Macrophages. Nutrients. 2020;12:3088. doi: 10.3390/nu12103088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Bilotta M.T., Petillo S., Santoni A., Cippitelli M. Liver X Receptors: Regulators of Cholesterol Metabolism, Inflammation, Autoimmunity, and Cancer. Front. Immunol. 2020;11:584303. doi: 10.3389/fimmu.2020.584303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Zhu R., Ou Z., Ruan X., Gong J. Role of liver X receptors in cholesterol efflux and inflammatory signaling (review) Mol. Med. Rep. 2012;5:895–900. doi: 10.3892/mmr.2012.758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Kalaany N.Y., Gauthier K.C., Zavacki A.M., Mammen P.P., Kitazume T., Peterson J.A., Horton J.D., Garry D.J., Bianco A.C., Mangelsdorf D.J. LXRs regulate the balance between fat storage and oxidation. Cell Metab. 2005;1:231–244. doi: 10.1016/j.cmet.2005.03.001. [DOI] [PubMed] [Google Scholar]
- 233.Alnaaim S.A., Al-Kuraishy H.M., Alexiou A., Papadakis M., Saad H.M., Batiha G.E. Role of Brain Liver X Receptor in Parkinson’s Disease: Hidden Treasure and Emerging Opportunities. Mol. Neurobiol. 2023;60:1–17. doi: 10.1007/s12035-023-03561-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Tavoosi Z., Moradi-Sardareh H., Saidijam M., Yadegarazari R., Borzuei S., Soltanian A., Goodarzi M.T. Cholesterol Transporters ABCA1 and ABCG1 Gene Expression in Peripheral Blood Mononuclear Cells in Patients with Metabolic Syndrome. Cholesterol. 2015;2015:682904. doi: 10.1155/2015/682904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.He P., Gelissen I.C., Ammit A.J. Regulation of ATP binding cassette transporter A1 (ABCA1) expression: Cholesterol-dependent and—Independent signaling pathways with relevance to inflammatory lung disease. Respir. Res. 2020;21:250. doi: 10.1186/s12931-020-01515-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Liu Y., Tang C. Regulation of ABCA1 functions by signaling pathways. Biochim. Biophys. Acta. 2012;1821:522–529. doi: 10.1016/j.bbalip.2011.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Rezaei F., Farhat D., Gursu G., Samnani S., Lee J.Y. Snapshots of ABCG1 and ABCG5/G8: A Sterol’s Journey to Cross the Cellular Membranes. Int. J. Mol. Sci. 2022;24:484. doi: 10.3390/ijms24010484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Liu M., Chen Y., Zhang L., Wang Q., Ma X., Li X., Xiang R., Zhu Y., Qin S., Yu Y., et al. Regulation of Hepatic Cholesteryl Ester Transfer Protein Expression and Reverse Cholesterol Transport by Inhibition of DNA Topoisomerase II. J. Biol. Chem. 2015;290:14418–14429. doi: 10.1074/jbc.M115.643015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Jalil A., Bourgeois T., Menegaut L., Lagrost L., Thomas C., Masson D. Revisiting the Role of LXRs in PUFA Metabolism and Phospholipid Homeostasis. Int. J. Mol. Sci. 2019;20:3787. doi: 10.3390/ijms20153787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Rong X., Albert C.J., Hong C., Duerr M.A., Chamberlain B.T., Tarling E.J., Ito A., Gao J., Wang B., Edwards P.A., et al. LXRs regulate ER stress and inflammation through dynamic modulation of membrane phospholipid composition. Cell Metab. 2013;18:685–697. doi: 10.1016/j.cmet.2013.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Wang B., Tontonoz P. Phospholipid Remodeling in Physiology and Disease. Annu. Rev. Physiol. 2019;81:165–188. doi: 10.1146/annurev-physiol-020518-114444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Shao G., Qian Y., Lu L., Liu Y., Wu T., Ji G., Xu H. Research progress in the role and mechanism of LPCAT3 in metabolic related diseases and cancer. J. Cancer. 2022;13:2430–2439. doi: 10.7150/jca.71619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Sun M.M., Beier F. Liver X Receptor activation regulates genes involved in lipid homeostasis in developing chondrocytes. Osteoarthr. Cartil. Open. 2020;2:100030. doi: 10.1016/j.ocarto.2020.100030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Feng C., Lou B., Dong J., Li Z., Chen Y., Li Y., Zhang X., Jiang X.C., Ding T. Lysophosphatidylcholine acyltransferase 3 deficiency impairs 3T3L1 cell adipogenesis through activating Wnt/beta-catenin pathway. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2018;1863:834–843. doi: 10.1016/j.bbalip.2018.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zhang X.Q., Xu C.F., Yu C.H., Chen W.X., Li Y.M. Role of endoplasmic reticulum stress in the pathogenesis of nonalcoholic fatty liver disease. World J. Gastroenterol. 2014;20:1768–1776. doi: 10.3748/wjg.v20.i7.1768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.De Luca M., Mandala M., Rose G. Towards an understanding of the mechanoreciprocity process in adipocytes and its perturbation with aging. Mech. Ageing Dev. 2021;197:111522. doi: 10.1016/j.mad.2021.111522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Zha B.S., Zhou H. ER Stress and Lipid Metabolism in Adipocytes. Biochem. Res. Int. 2012;2012:312943. doi: 10.1155/2012/312943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Amen O.M., Sarker S.D., Ghildyal R., Arya A. Endoplasmic Reticulum Stress Activates Unfolded Protein Response Signaling and Mediates Inflammation, Obesity, and Cardiac Dysfunction: Therapeutic and Molecular Approach. Front. Pharmacol. 2019;10:977. doi: 10.3389/fphar.2019.00977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Wang P., Heitman J. The cyclophilins. Genome Biol. 2005;6:226. doi: 10.1186/gb-2005-6-7-226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Takahashi N., Hayano T., Suzuki M. Peptidyl-prolyl cis-trans isomerase is the cyclosporin A-binding protein cyclophilin. Nature. 1989;337:473–475. doi: 10.1038/337473a0. [DOI] [PubMed] [Google Scholar]
- 251.Gegunde S., Alfonso A., Alvarino R., Alonso E., Botana L.M. Cyclophilins A, B, and C Role in Human T Lymphocytes Upon Inflammatory Conditions. Front. Immunol. 2021;12:609196. doi: 10.3389/fimmu.2021.609196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Jin Z.G., Lungu A.O., Xie L., Wang M., Wong C., Berk B.C. Cyclophilin A is a proinflammatory cytokine that activates endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2004;24:1186–1191. doi: 10.1161/01.ATV.0000130664.51010.28. [DOI] [PubMed] [Google Scholar]
- 253.Dawar F.U., Xiong Y., Khattak M.N.K., Li J., Lin L., Mei J. Potential role of cyclophilin A in regulating cytokine secretion. J. Leukoc. Biol. 2017;102:989–992. doi: 10.1189/jlb.3RU0317-090RR. [DOI] [PubMed] [Google Scholar]
- 254.Kim J., Choi T.G., Ding Y., Kim Y., Ha K.S., Lee K.H., Kang I., Ha J., Kaufman R.J., Lee J., et al. Overexpressed cyclophilin B suppresses apoptosis associated with ROS and Ca2+ homeostasis after ER stress. Pt 21J Cell Sci. 2008;121:3636–3648. doi: 10.1242/jcs.028654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Bernasconi R., Solda T., Galli C., Pertel T., Luban J., Molinari M. Cyclosporine A-sensitive, cyclophilin B-dependent endoplasmic reticulum-associated degradation. PLoS ONE. 2010;5:e13008. doi: 10.1371/journal.pone.0013008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Stocki P., Chapman D.C., Beach L.A., Williams D.B. Depletion of cyclophilins B and C leads to dysregulation of endoplasmic reticulum redox homeostasis. J. Biol. Chem. 2014;289:23086–23096. doi: 10.1074/jbc.M114.570911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Bagola K., Mehnert M., Jarosch E., Sommer T. Protein dislocation from the ER. Biochim. Biophys. Acta. 2011;1808:925–936. doi: 10.1016/j.bbamem.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 258.DeBoer J., Madson C.J., Belshan M. Cyclophilin B enhances HIV-1 infection. Virology. 2016;489:282–291. doi: 10.1016/j.virol.2015.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Khong M.L., Li L., Solesio M.E., Pavlov E.V., Tanner J.A. Inorganic polyphosphate controls cyclophilin B-mediated collagen folding in osteoblast-like cells. FEBS J. 2020;287:4500–4524. doi: 10.1111/febs.15249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Zhang L., Li Z., Zhang B., He H., Bai Y. PPIA is a novel adipogenic factor implicated in obesity. Obesity. 2015;23:2093–2100. doi: 10.1002/oby.21208. [DOI] [PubMed] [Google Scholar]
- 261.Qu Y., Lin Q., Yuan Y., Sun Z., Li P., Wang F., Jiang H., Chen T. Cyclosporin A inhibits adipogenic differentiation and regulates immunomodulatory functions of murine mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2018;498:516–522. doi: 10.1016/j.bbrc.2018.03.012. [DOI] [PubMed] [Google Scholar]
- 262.Rosen E.D. The transcriptional basis of adipocyte development. Prostaglandins Leukot. Essent. Fat. Acids. 2005;73:31–34. doi: 10.1016/j.plefa.2005.04.004. [DOI] [PubMed] [Google Scholar]
- 263.Banerjee D., Patra D., Sinha A., Roy S., Pant R., Sarmah R., Dutta R., Kanta Bhagabati S., Tikoo K., Pal D., et al. Lipid-induced monokine cyclophilin-A promotes adipose tissue dysfunction implementing insulin resistance and type 2 diabetes in zebrafish and mice models of obesity. Cell Mol. Life Sci. 2022;79:282. doi: 10.1007/s00018-022-04306-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Zhang T., Li H., Wang K., Xu B., Chen Z.N., Bian H. Deficiency of CD147 Attenuated Non-alcoholic Steatohepatitis Progression in an NLRP3-Dependent Manner. Front. Cell Dev. Biol. 2020;8:784. doi: 10.3389/fcell.2020.00784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Price E.R., Zydowsky L.D., Jin M.J., Baker C.H., McKeon F.D., Walsh C.T. Human cyclophilin B: A second cyclophilin gene encodes a peptidyl-prolyl isomerase with a signal sequence. Proc. Natl. Acad. Sci. USA. 1991;88:1903–1907. doi: 10.1073/pnas.88.5.1903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Fang F., Zheng J., Galbaugh T.L., Fiorillo A.A., Hjort E.E., Zeng X., Clevenger C.V. Cyclophilin B as a co-regulator of prolactin-induced gene expression and function in breast cancer cells. J. Mol. Endocrinol. 2010;44:319–329. doi: 10.1677/JME-09-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Choi J.W., Sutor S.L., Lindquist L., Evans G.L., Madden B.J., Bergen H.R., 3rd, Hefferan T.E., Yaszemski M.J., Bram R.J. Severe osteogenesis imperfecta in cyclophilin B-deficient mice. PLoS Genet. 2009;5:e1000750. doi: 10.1371/journal.pgen.1000750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Yoon J.S., Kim S.S., Ha J., Kang I., Choe W. Cyclophilin B, a molecule chaperone, promotes adipogenesis in 3T3-L1 preadipocytes via AKT/mTOR pathway. Int. J. Mol. Med. 2023;51:6. doi: 10.3892/ijmm.2022.5209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Jeong K., Kim H., Kim K., Kim S.J., Hahn B.S., Jahng G.H., Yoon K.S., Kim S.S., Ha J., Kang I., et al. Cyclophilin B is involved in p300-mediated degradation of CHOP in tumor cell adaptation to hypoxia. Cell Death Differ. 2014;21:438–450. doi: 10.1038/cdd.2013.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhang H., Fan Q., Xie H., Lu L., Tao R., Wang F., Xi R., Hu J., Chen Q., Shen W., et al. Elevated Serum Cyclophilin B Levels Are Associated with the Prevalence and Severity of Metabolic Syndrome. Front. Endocrinol. 2017;8:360. doi: 10.3389/fendo.2017.00360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Tang Q.Q., Lane M.D. Role of C/EBP homologous protein (CHOP-10) in the programmed activation of CCAAT/enhancer-binding protein-beta during adipogenesis. Proc. Natl. Acad. Sci. USA. 2000;97:12446–12450. doi: 10.1073/pnas.220425597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Fu S., Watkins S.M., Hotamisligil G.S. The role of endoplasmic reticulum in hepatic lipid homeostasis and stress signaling. Cell Metab. 2012;15:623–634. doi: 10.1016/j.cmet.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 273.Han J., Kaufman R.J. The role of ER stress in lipid metabolism and lipotoxicity. J. Lipid Res. 2016;57:1329–1338. doi: 10.1194/jlr.R067595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Zhou H., Liu R. ER stress and hepatic lipid metabolism. Front Genet. 2014;5:112. doi: 10.3389/fgene.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Basseri S., Austin R.C. Endoplasmic reticulum stress and lipid metabolism: Mechanisms and therapeutic potential. Biochem. Res. Int. 2012;2012:841362. doi: 10.1155/2012/841362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Chen X., Goodman J.M. The collaborative work of droplet assembly. Biochim. Biophys. Acta Mol. Cell Biol. Lipids. 2017;1862(Pt B):1205–1211. doi: 10.1016/j.bbalip.2017.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Olzmann J.A., Carvalho P. Dynamics and functions of lipid droplets. Nat. Rev. Mol. Cell Biol. 2019;20:137–155. doi: 10.1038/s41580-018-0085-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Miura S., Gan J.W., Brzostowski J., Parisi M.J., Schultz C.J., Londos C., Oliver B., Kimmel A.R. Functional conservation for lipid storage droplet association among Perilipin, ADRP, and TIP47 (PAT)-related proteins in mammals, Drosophila, and Dictyostelium. J. Biol. Chem. 2002;277:32253–32257. doi: 10.1074/jbc.M204410200. [DOI] [PubMed] [Google Scholar]
- 279.Al-Mansoori L., Al-Jaber H., Prince M.S., Elrayess M.A. Role of Inflammatory Cytokines, Growth Factors and Adipokines in Adipogenesis and Insulin Resistance. Inflammation. 2022;45:31–44. doi: 10.1007/s10753-021-01559-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Makki K., Froguel P., Wolowczuk I. Adipose tissue in obesity-related inflammation and insulin resistance: Cells, cytokines, and chemokines. ISRN Inflamm. 2013;2013:139239. doi: 10.1155/2013/139239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 281.Roszer T. Metabolic impact of adipose tissue macrophages in the early postnatal life. J. Leukoc. Biol. 2022;112:1515–1524. doi: 10.1002/JLB.3MR0722-201R. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.Weisberg S.P., McCann D., Desai M., Rosenbaum M., Leibel R.L., Ferrante A.W., Jr. Obesity is associated with macrophage accumulation in adipose tissue. J. Clin. Invest. 2003;112:1796–1808. doi: 10.1172/JCI200319246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Cinti S., Mitchell G., Barbatelli G., Murano I., Ceresi E., Faloia E., Wang S., Fortier M., Greenberg A.S., Obin M.S. Adipocyte death defines macrophage localization and function in adipose tissue of obese mice and humans. J. Lipid Res. 2005;46:2347–2355. doi: 10.1194/jlr.M500294-JLR200. [DOI] [PubMed] [Google Scholar]
- 284.Jia Q., Morgan-Bathke M.E., Jensen M.D. Adipose tissue macrophage burden, systemic inflammation, and insulin resistance. Am. J. Physiol. Endocrinol. Metab. 2020;319:E254–E264. doi: 10.1152/ajpendo.00109.2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Martos-Rus C., Katz-Greenberg G., Lin Z., Serrano E., Whitaker-Menezes D., Domingo-Vidal M., Roche M., Ramaswamy K., Hooper D.C., Falkner B., et al. Macrophage and adipocyte interaction as a source of inflammation in kidney disease. Sci. Rep. 2021;11:2974. doi: 10.1038/s41598-021-82685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Lopez-Ortega O., Moreno-Corona N.C., Cruz-Holguin V.J., Garcia-Gonzalez L.D., Helguera-Repetto A.C., Romero-Valdovinos M., Arevalo-Romero H., Cedillo-Barron L., Leon-Juarez M. The Immune Response in Adipocytes and Their Susceptibility to Infection: A Possible Relationship with Infectobesity. Int. J. Mol. Sci. 2022;23:6154. doi: 10.3390/ijms23116154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 287.Nance S.A., Muir L., Lumeng C. Adipose tissue macrophages: Regulators of adipose tissue immunometabolism during obesity. Mol. Metab. 2022;66:101642. doi: 10.1016/j.molmet.2022.101642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Fischer-Posovszky P., Wang Q.A., Asterholm I.W., Rutkowski J.M., Scherer P.E. Targeted deletion of adipocytes by apoptosis leads to adipose tissue recruitment of alternatively activated M2 macrophages. Endocrinology. 2011;152:3074–3081. doi: 10.1210/en.2011-1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Faria S.S., Correa L.H., Heyn G.S., de Sant’Ana L.P., Almeida R.D.N., Magalhaes K.G. Obesity and Breast Cancer: The Role of Crown-Like Structures in Breast Adipose Tissue in Tumor Progression, Prognosis, and Therapy. J. Breast Cancer. 2020;23:233–245. doi: 10.4048/jbc.2020.23.e35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 290.Fuster J.J., Ouchi N., Gokce N., Walsh K. Obesity-Induced Changes in Adipose Tissue Microenvironment and Their Impact on Cardiovascular Disease. Circ. Res. 2016;118:1786–1807. doi: 10.1161/CIRCRESAHA.115.306885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Yao J., Wu D., Qiu Y. Adipose tissue macrophage in obesity-associated metabolic diseases. Front. Immunol. 2022;13:977485. doi: 10.3389/fimmu.2022.977485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Ou M.Y., Zhang H., Tan P.C., Zhou S.B., Li Q.F. Adipose tissue aging: Mechanisms and therapeutic implications. Cell Death Dis. 2022;13:300. doi: 10.1038/s41419-022-04752-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 293.Zuo L., Prather E.R., Stetskiv M., Garrison D.E., Meade J.R., Peace T.I., Zhou T. Inflammaging and Oxidative Stress in Human Diseases: From Molecular Mechanisms to Novel Treatments. Int. J. Mol. Sci. 2019;20:4472. doi: 10.3390/ijms20184472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Wang T., Sharma A.K., Wolfrum C. Novel insights into adipose tissue heterogeneity. Rev. Endocr. Metab. Disord. 2022;23:5–12. doi: 10.1007/s11154-021-09703-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 295.Ruiz-Ojeda F.J., Mendez-Gutierrez A., Aguilera C.M., Plaza-Diaz J. Extracellular Matrix Remodeling of Adipose Tissue in Obesity and Metabolic Diseases. Int. J. Mol. Sci. 2019;20:4888. doi: 10.3390/ijms20194888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 296.Ghosh A.K., Garg S.K., Mau T., O’Brien M., Liu J., Yung R. Elevated Endoplasmic Reticulum Stress Response Contributes to Adipose Tissue Inflammation in Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2015;70:1320–1329. doi: 10.1093/gerona/glu186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 297.Weinstock A., Brown E.J., Garabedian M.L., Pena S., Sharma M., Lafaille J., Moore K.J., Fisher E.A. Single-Cell RNA Sequencing of Visceral Adipose Tissue Leukocytes Reveals that Caloric Restriction Following Obesity Promotes the Accumulation of a Distinct Macrophage Population with Features of Phagocytic Cells. Immunometabolism. 2019;1:e190008. doi: 10.20900/immunometab20190008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 298.Auger C., Kajimura S. Adipose Tissue Remodeling in Pathophysiology. Annu. Rev. Pathol. 2023;18:71–93. doi: 10.1146/annurev-pathol-042220-023633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 299.Tchkonia T., Pirtskhalava T., Thomou T., Cartwright M.J., Wise B., Karagiannides I., Shpilman A., Lash T.L., Becherer J.D., Kirkland J.L. Increased TNFalpha and CCAAT/enhancer-binding protein homologous protein with aging predispose preadipocytes to resist adipogenesis. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1810–E1819. doi: 10.1152/ajpendo.00295.2007. [DOI] [PubMed] [Google Scholar]
- 300.Chung K.W., Chung H.Y. The Effects of Calorie Restriction on Autophagy: Role on Aging Intervention. Nutrients. 2019;11:2923. doi: 10.3390/nu11122923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Cui J., Bai X.Y., Shi S., Cui S., Hong Q., Cai G., Chen X. Age-related changes in the function of autophagy in rat kidneys. Age. 2012;34:329–339. doi: 10.1007/s11357-011-9237-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 302.Melendez A., Talloczy Z., Seaman M., Eskelinen E.L., Hall D.H., Levine B. Autophagy genes are essential for dauer development and life-span extension in C. elegans. Science. 2003;301:1387–1391. doi: 10.1126/science.1087782. [DOI] [PubMed] [Google Scholar]
- 303.Sorrenti V., Davinelli S., Scapagnini G., Willcox B.J., Allsopp R.C., Willcox D.C. Astaxanthin as a Putative Geroprotector: Molecular Basis and Focus on Brain Aging. Mar. Drugs. 2020;18:351. doi: 10.3390/md18070351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Gao L., Liu X., Luo X., Lou X., Li P., Li X., Liu X. Antiaging effects of dietary supplements and natural products. Front. Pharmacol. 2023;14:1192714. doi: 10.3389/fphar.2023.1192714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Aman Y., Schmauck-Medina T., Hansen M., Morimoto R.I., Simon A.K., Bjedov I., Palikaras K., Simonsen A., Johansen T., Tavernarakis N., et al. Autophagy in healthy aging and disease. Nat. Aging. 2021;1:634–650. doi: 10.1038/s43587-021-00098-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Wang M., Miller R.A. Fibroblasts from long-lived mutant mice exhibit increased autophagy and lower TOR activity after nutrient deprivation or oxidative stress. Aging Cell. 2012;11:668–674. doi: 10.1111/j.1474-9726.2012.00833.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 307.Kitada M., Koya D. Autophagy in metabolic disease and ageing. Nat. Rev. Endocrinol. 2021;17:647–661. doi: 10.1038/s41574-021-00551-9. [DOI] [PubMed] [Google Scholar]
- 308.Mau T., Yung R. Adipose tissue inflammation in aging. Exp. Gerontol. 2018;105:27–31. doi: 10.1016/j.exger.2017.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 309.Ghosh A.K., Mau T., O’Brien M., Garg S., Yung R. Impaired autophagy activity is linked to elevated ER-stress and inflammation in aging adipose tissue. Aging. 2016;8:2525–2537. doi: 10.18632/aging.101083. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.