Abstract
Heat shock proteins (HSPs) are thought to play a role in the development of cancer and to modulate tumor response to cytotoxic therapy. In this study, we have examined the expression of hsf and HSP genes in normal human prostate epithelial cells and a range of prostate carcinoma cell lines derived from human tumors. We have observed elevated expressions of HSF1, HSP60, and HSP70 in the aggressively malignant cell lines PC-3, DU-145, and CA-HPV-10. Elevated HSP expression in cancer cell lines appeared to be regulated at the post–messenger ribonucleic acid (mRNA) levels, as indicated by gene chip microarray studies, which indicated little difference in heat shock factor (HSF) or HSP mRNA expression between the normal and malignant prostate cell lines. When we compared the expression patterns of constitutive HSP genes between PC-3 prostate carcinoma cells growing as monolayers in vitro and as tumor xenografts growing in nude mice in vivo, we found a marked reduction in expression of a wide spectrum of the HSPs in PC-3 tumors. This decreased HSP expression pattern in tumors may underlie the increased sensitivity to heat shock of PC-3 tumors. However, the induction by heat shock of HSP genes was not markedly altered by growth in the tumor microenvironment, and HSP40, HSP70, and HSP110 were expressed abundantly after stress in each growth condition. Our experiments indicate therefore that HSF and HSP levels are elevated in the more highly malignant prostate carcinoma cells and also show the dominant nature of the heat shock–induced gene expression, leading to abundant HSP induction in vitro or in vivo.
INTRODUCTION
Heat shock proteins (HSPs) were first discovered as a cohort of proteins that is induced en masse by heat shock and other chemical and physical stresses in a wide range of species (Lindquist and Craig 1988; Georgopolis and Welch 1993). The HSPs (Table 1) have been subsequently characterized as molecular chaperones, proteins that have in common the property of modifying the structures and interactions of other proteins (Lindquist and Craig 1988; Beckmann et al 1990; Gething and Sambrook 1992; Georgopolis and Welch 1993; Netzer and Hartl 1998). Molecular chaperone function dictates that the HSP often interact in a stoichiometric, one-on-one manner with their substrates, necessitating high intracellular concentrations of the proteins (Lindquist and Craig 1988; Georgopolis and Welch 1993). As molecules that shift the balance from denatured, aggregated protein conformation toward ordered, functional conformation, HSPs are particularly in demand when the protein structure is disrupted by heat shock, oxidative stress, or other protein-damaging events (Lindquist and Craig 1988; Gething and Sambrook 1992; Georgopolis and Welch 1993). The HSP27, HSP40, HSP70, and HSP110 genes have therefore evolved a highly efficient mechanism for mass synthesis during stress, with powerful transcriptional activation, efficient messenger ribonucleic acid (mRNA) stabilization, and selective mRNA translation (Voellmy 1994). HSP27, HSP70, HSP90, and HSP110 increase to become the dominantly expressed proteins after stress (Hickey and Weber 1982; Landry et al 1982; Li and Werb 1982; Subjeck et al 1982; Henics et al 1999) (Zhao et al 2002). Heat shock factor (HSF) proteins have been shown to interact with the promoters of many HSP genes and ensure prompt transcriptional activation in stress and equally precipitous switch off after recovery (Sorger and Pelham 1988; Wu 1995). The hsf gene family includes HSF1 (hsf1), the molecular coordinator of the heat shock response, as well as 2 less well-characterized genes, hsf2 and hsf4 (Rabindran et al 1991; Schuetz et al 1991) (Nakai et al 1997). In addition to the class of HSPs induced by heat, cells also contain a large number of constitutively expressed HSP homologs, which are also listed in Table 1. The constitutive HSPs are found in a variety of multiprotein complexes containing both HSPs and cofactors (Buchner 1999). These include HSP10-HSP60 complexes that mediate protein folding and HSP70- and HSP90-containing complexes that are involved in both generic protein-folding pathways and in specific association with regulatory proteins within the cell (Netzer and Hartl 1998). HSP90 plays a particularly versatile role in cell regulation, forming complexes with a large number of cellular kinases, transcription factors, and other molecules (Buchner 1999; Grammatikakis et al 2002).
Table 1.
Heat shock protein family genes studied by microchip array analysis

Many tumor types contain high concentrations of HSP of the HSP28, HSP70, and HSP90 families compared with adjacent normal tissues (Ciocca et al 1993; Yano et al 1999; Cornford et al 2000; Strik et al 2000; Ricaniadis et al 2001; Ciocca and Vargas-Roig 2002). We have concentrated here on HSP gene expression in prostate carcinoma. The progression of prostatic epithelial cells to the fully malignant, metastatic phenotype is a complex process and involves the expression of oncogenes as well as escape from androgen-dependent growth and survival (Cornford et al 2000). There is a molecular link between HSP expression and tumor progression in prostate cancer in that HSP56, HSP70, and HSP90 regulate the function of the androgen receptor (AR) (Froesch et al 1998; Grossmann et al 2001). Escape from AR dependence during tumorigenesis may involve altered HSP-AR interactions (Grossmann et al 2001). The role of HSPs in tumor development may also be related to their function in the development of tolerance to stress (Li and Hahn 1981). Thermotolerance is induced in cells preconditioned by mild stress coordinately with the expression of high HSP levels (Landry et al 1982; Li and Werb 1982; Subjeck et al 1982). Elevated HSP expression appears to be a factor in tumor pathogenesis, and, among other mechanisms, this may involve the ability of individual HSPs to block the pathways of apoptosis and permit malignant cells to arise despite the triggering of apoptotic signals during transformation (Volloch and Sherman 1999). De novo HSP expression may also afford protection of cancer cells from treatments such as chemotherapy and hyperthermia by thwarting the proapoptotic influence of these modalities (Gabai et al 1998; Hansen et al 1999; Blagosklonny 2001; Asea et al 2001; Van Molle et al 2002). The mechanisms underlying HSP induction in tumor cells are not known but may reflect the genetic alterations accompanying malignancy or the disordered state of the tumor microenvironment, which would be expected to lead to cellular stress.
Here, we have examined expression of hsf and HSP genes in immortalized normal human prostate epithelial cells and a range of prostate carcinoma cells obtained from human tumors at the mRNA and protein levels. Our aim was to determine whether hsf-HSP expression profiles are conserved in cells that express varying degrees of malignancy, under resting conditions and after heat and ionizing radiation. In addition, we have compared HSP expression profiles of a metastatic human prostate carcinoma cell line growing either in monolayer culture or as a tumor xenograft in nude mice. These studies were prompted by findings in our laboratory that prostate carcinoma cells are considerably more sensitive to heat-induced apoptosis in vivo growing as tumors compared with similar cells growing in tissue culture in vitro. Our studies show that, although the hsf-HSP expression profiles are similar in normal and malignant prostate-derived cells at the mRNA level, expression at the protein level was very different. HSF1 and HSP protein expression was highest in the 3 aggressively metastatic prostate cancer cell lines (PC-3, DU-145, and CA-HPV-10). Although the gene expression patterns of constitutive HSP differ enormously in PC-3 cells in vitro and in xenografts in vivo, stress induction of HSP genes is not markedly altered by exposure to the tumor microenvironment, indicating the hierarchical rank of the stress response that permits it to override other forms of regulation.
MATERIALS AND METHODS
Cell lines and tissue culture
Normal prostate epithelial cell line PZ-HPV-7 and human prostate carcinoma cell lines PC-3, DU-145, LNCap, and CA-HPV-10 were obtained from American Type Culture Collection (ATCC, Manassas, VA, USA). As reported previously, cell line PZ-HPV-7 was derived from normal prostate tissue, whereas CA-HPV-10 was derived from primary prostate cancer tissue and grows in nude mice to form distant metastases. Both cell lines were established by HPV transformation (Weijerman et al 1994). PC-3 was from a human prostatic carcinoma metastatic to bone, and DU-145 was from a prostatic carcinoma metastatic to brain (Stone et al 1978; Kaighn et al 1979). PC-3 is a p53-deficient cell line, and DU-145 is p53 mutant (Carroll et al 1993). PZ-HPV-7 and PZ-HPV-10 cells have inactivated p53 (as well as Rb repression) because of the expression of the HPV-18 proteins E6 and E7 (Wang and Lu 2004). Cell line LNCap was also derived from a metastatic lesion, the supraclavicular lymph node of a patient with prostate carcinoma. It expresses high affinity–specific ARs as well as wild-type p53, and its growth is androgen dependent (Horoszewicz et al 1983). We found that PC-3, DU-145, LNCap, and CA-HPV-10 cells were all able to lead to tumor formation when xenografted into nude mice, whereas PZ-HPV-7 cells did not form tumors. PC-3, DU-145, and CA-HPV-10 tumor growth led to the formation of distant metastases in mice, whereas LNCap cells were weakly metastatic. Cells were cultured at 37°C in cell culture media and serum concentrations recommended by ATCC and treated, respectively, by heat shock (43°C, 1 hour) in circulating water bath followed by 2-hour recovery in an incubator at 37°C, by 10 Gy irradiation with 2-hour recovery, or by combined heat shock and irradiation with 2-hour recovery. Control cells remained untreated and cultured at 37°C.
Growth of PC-3 cells as tumor xenografts and heat shock–radiation treatment
Male nude mice (8- to 10-weeks old) were purchased from Taconic Farms (Germantown, NY, USA) and housed in laminar flow isolation units in the Dana Farber Cancer Institute's vivarium under alternate dark and light cycles. Animals were maintained on food and water ad libitum. PC-3 cells were grown as xenografts in the flanks of male nude mice. Cells (5 × 106) were implanted subcutaneously into a hind leg of a male nude mice. When tumors grew to at least 10 mm in diameter, hyperthermia and radiation treatment were performed. Hyperthermia experiments were performed by submerging the tumor-bearing limb into a circulating water bath controlled so as to produce a 43°C intratumor temperature for 1 hour as measured by a thermocouple probe inserted into the tumor. Some of the tumor-bearing mice were irradiated with γ radiation at 10 Gy in Gammacell 40 (Canada Limited, Radiochemical company, Toronto, ON, Canada). Two hours after hyperthermia or radiation treatment (or both), the mice were killed and tumors were then excised under sterile conditions and stored in liquid nitrogen right away for RNA isolation.
RNA extraction and preparation for hybridization
Total RNA was extracted from cells using TRIZOL reagent (Life Technologies, Rockville, MD, USA). First- and second-strand complementary deoxyribonucleic acid (cDNA) was synthesized by using Superscript Choice System (Life Technologies); double-stranded cDNA was purified by phenol-chloroform extraction and ethanol precipitation. In vitro transcription and labeling of RNA with biotin was performed with the Enzo Transcript Labeling method (ENZO Diagnostics, Farmingdale, NY, USA). Complementary ribonucleic acid (cRNA) was purified on an affinity resin (Rneasy, Qiagen, Valencia, CA, USA). After quantification by spectrophotometric analysis, 20 μg cRNA were fragmented randomly by incubating at 94°C for 35 minutes in 40 mM Tris-acetate pH 8.1, 100 mM potassium acetate, and 30 mM magnesium acetate.
Array hybridization and scanning
GeneChip probe arrays HuGeneFL (Affymetrix Inc., Santa Clara, CA, USA), which contains probes interrogating approximately 5600 full-length human genes from the Unigene (Build 18), GenBank, and TIGR databases were used in hybridization. Array hybridization, washing, staining, and scanning were performed according to the Affymetrix GeneChip Expression Analysis Technical Manual (Affymetrix) as described previously (Wodicka et al 1997).
Data analysis
Array normalization and expression value calculation were performed using the DNA-Chip Analyzer (dChip) (Li and Wong 2001), available freely on request. Invariant set normalization (Li and Wong 2001) at probe cell level was used to make arrays comparable, and the model-based method (Li and Wong 2001) was used for probe selection and expression level calculation (Eisen et al 1998). These expression levels were attached with standard errors as measurement accuracy, which was subsequently used to compute 90% confidence intervals of fold changes (Li and Wong 2001). Lower confidence bound of fold changes from pairwise comparison were taken to be conservative estimates of the real fold changes.
Immunoblot analysis and antibodies
Prostate cells were trypsinized, washed twice with cold phosphate-buffered saline (PBS), and lysed in a modified radioimmunoprecipitation buffer (Tris-HCL [50 mM, pH 7.4], NaCl [150 mM], NP-40 [1%], sodium deoxycholate [0.5%], sodium dodecyl sulfate [SDS, 0.1%] and ethylenediaminetetraacetic acid [5 mM]) containing a protease inhibitor cocktail (Boehringer Mannheim, GmbH, Germany). After centrifugation at 14 000 × g for 10 minutes, the supernatants were used as cell extracts. Cell extracts were then subjected to SDS–polyacrylamide gel electrophoresis and transferred onto nitrocellulose membranes (Schleicher & Schuell, Mannheim, Germany) using wet transfer apparatus (Bio-Rad Laboratories, Richmond, CA, USA) for HSF1, HSF2, Hsp70, Hsp60, Hsp90, and β-actin. Antibodies were obtained from the following sources: polyclonal antibody against HSF1, monoclonal antibodies against Hsp70, Hsp60, and Hsp90 from Stressgen Biotechnologies Corp (Victoria, Canada), polyclonal antibody against HSF2 from Santa Cruz Biotechnologies Corp (Santa Cruz, CA, USA) and monoclonal antibody against β-actin from Sigma (St Louis, MO, USA).
TUNEL staining and determination of the apoptotic index
The ApoDirect™ kit (Phoenix Flow Systems, San Diego, CA, USA) was used for terminal transferase (TUNEL) staining of single cell suspensions. Apoptotic PC-3 cells grown in monolayer culture tend to detach from the dish while undergoing apoptosis. Detached cells were therefore collected at 500 × g for 5 minutes before detachment with trypsin of the monolayer to harvest cells. Cells were washed in PBS, pH 7.4 (PBS), and fixed in 1% w/v paraformaldehyde in PBS. After cell fixation, the free 3′ ends of the apoptotic fragments were labeled using the TdT enzyme, which conjugated the DNA fragments with a flourescein–deoxyuridine triphosphate tag, counterstained with propidium iodide for DNA content and analyzed using a dual-channel flow cytometer (Becton Dickson FACScan, Mountain View, CA, USA). Negative and positive control cells were included with each experiment. The apoptotic index was determined as the number of TUNEL-positive cells (ie, apoptotic cells) divided by the total number of cells in the dish (cells in the media and cells attached in monolayer). We also examined apoptosis in cells in sections of tumors using TUNEL staining of sections from formalin-fixed, paraffin-embedded tumors as described by the manufacturer (Phoenix Flow Systems).
RESULTS
Because HSP expression is positively correlated with a poor prognosis in terms of patient survival and decreased sensitivity of malignant cells to treatment by a range of modalities, we examined HSP expression in a range of prostate carcinoma cell lines of varying degrees of malignancy. For a normal control we used immortalized normal prostatic epithelium (PZ-HPV-7). Malignant cell lines included the hormone-responsive, p53-proficient, poorly metastatic LnCap line as well as the fully metastatic androgen-resistant and p53-inactive cell lines (DU-145, PC-3, and CA-HPV-10). Each cell line was either sham treated, heat shocked at 43°C for 1 hour, irradiated at 10 Gy, or given the combined treatment. Both RNA and cellular protein were then extracted and analyzed by microarray and immunoblot assays.
HSP expression in 5 prostate carcinoma cell lines in vitro
We first analyzed the expression of the 26 HSP genes listed in Table 1 in the 5 prostate-derived cell lines. The genes examined in the study included ubiquitin, HSP10, 4 members of the HSP27 family, 2 HSP40 members, HSP47, HSP60, 11 members of the HSP70 family, 2 HSP90 family members, and HSP105 (also known as HSP110) (Table 1). When we compared the relative expression levels of these genes in untreated prostatic cell lines, we found that HSP gene expression was dominated by HSP90α and HSP90β, ubiquitin, HSP28, HSP60, and HSP70-8 (Fig 1). Although the levels of these mRNA species fluctuated to some degree between cell lines, there was no obvious correlation between expression of any of the HSP mRNAs and the relative malignancy of the cell line (Fig 1). No consistent differences were observed between cell lines, although the CA-HPV-10 cell lines had lower HSP60, HSP70-4, HSP70-8, and HSP90 and HSP105 than the other cell lines (Fig 1). PZ-HPV-7 cells were low in ubiquitin HSP28, HSP70-1B, and HSP90 family, although they were high in HSP70-1A (Fig 1). Western analysis carried out previously indicated that PC-3 cells express HSP70 (HSP72) at the protein level under basal conditions, in contrast to many normal cells (Jones et al 2004). As can be seen in Figure 1, the HSP70-1A and HSP70-1B genes are expressed under these conditions, and the products of these genes may be the ones detected as “HSP72” in Western analysis of proteins extracted from the prostate-derived cell lines (Jones et al 2004). Expression of the strictly HSF1-inducible HSP70B′ gene (Schiller et al 1988) is barely detectable in any of the cell lines under nonstress conditions, suggesting that the level of transcriptional activation of HSF1 is minimal in these unstressed cells and that basal expression of other HSPs is likely due to the activity of additional factors (Fig 1). Additional HSP70 family genes, including HSP70-4 (HSP70RY), HSP70-5 (grp78 or BiP), HSP70-8, and hsc70 (Table 1) are expressed fairly abundantly at 37°C (Fig 1). The exact relative importance of these closely homologous HSP family genes in intracellular chaperone activity and cell regulation is not understood in any comprehensive manner, and it is not clear which of these gene products is detected by the available “anti-HSP70” antibodies (with the exception of the more divergent HSP70-5 and HSP70-8). Some of these issues are reviewed by Tavaria et al (1996).
Fig 1.
Constitutive expression of 27 HSP genes at 37°C in the 5 prostate-derived cell lines growing in tissue culture. Cells were grown to confluence at 37°C and harvested in TRIzol reagent for total RNA isolation before microarray analysis as in Materials and Methods. Expression levels of the HSP genes listed in Table 1 have been normalized by dChip to be comparable with each other. PC, PC-3; DU, DU-145; LN, LNCap; CA, CA-HPV-10; PZ, PZ-HPV-7. Asterisks indicate that some bars for expression levels have to be broken to fit the Y-axis scale. Experiments were repeated twice with reproducible results
When the 5 prostate cell lines were next subjected to heat shock, a different pattern of relative HSP gene expression was observed in cells recovering from the heat, and HSP40-1, HSP70-1A, HSP70-1B, HSP70B, and HSP105 were the most strongly induced genes (Fig 2A). We detected major differences between LnCap and the other cell lines, with HSP induction being relatively decreased. In LnCap cells, HSP induction was low and HSP40-1, HSP47, HSP70-1A, and HSP70-6 were induced at low rates, whereas HSP70B and HSP105 expressions were not detected (Fig 2A). Because the LNCap cell line has been shown to express ARs and to be androgen dependent for growth, compared with the other cells, which have lost AR expression, AR expression could potentially emerge as 1 potential modifying factor in HSP expression in stress (Fig 2B). Ionizing radiation alone or combined with heat had little effect on expression of any of the HSP genes when analyzed at the 2-hour point, and the results have therefore not been included in the analysis.
Fig 2.
(A) Fold expression of 27 HSP family genes assayed 2 hours after heat shock in the 5 prostatic cell lines in vitro. Cells were cultured at 37°C and then treated by heat shock (43°C, 1 hour) followed by 2 hours recovery at 37°C. Control cells remained untreated and cultured in a 37°C incubator. Cells were harvested in TRIzol reagent before gene expression analysis as above. HSP gene expression levels have been normalized by dChip to be comparable with each other. Relative gene expression profiles of the HSP genes after heat were shown as folds of expression levels from their corresponding, non–heat shocked controls. Asterisks indicate that some bars for fold expressions were broken to fit the Y-axis scale. Experiments were repeated twice with reproducible results. (B) Expression of androgen receptor messenger ribonucleic acid (mRNA) in PC-3, DU-145, LNCap, CA-HPV-10, and PZ-HPV-7 cells was determined as above (A). (C) Expression of estrogen receptor-related genes in PC-3, DU-145, LNCap, CA-HPV-10, and PZ-HPV-7 cells was determined as in (A)
Hsf family gene expression in prostatic cell lines
Because many HSPs contain heat shock elements (HSEs), binding sites for HSF activation, we next examined the relative expression of the hsf genes (hsf1, hsf2, and hsf4) in RNA extracted from each of the 5 cell lines (Fig 3). As can be seen, the relative level of the individual hsf mRNA species was fairly similar in each of the 5 cell types (Fig 3). HSF1 was the most abundantly expressed of the hsf genes at the mRNA level and was present at approximately 3 times the level of HSF4 and 4 times the level of HSF2 mRNA (Fig 3). HSF1, HSF2, and HSF4 mRNA levels were not markedly altered by either heat shock or ionizing radiation treatment, although heat shock did cause slight decreases in HSF4 in some cell types.
Fig 3.
Expression profiles of the hsf1, hsf2, and hsf4 genes in prostate carcinoma cells after heat shock, ionizing radiation, or combined heat and radiation. Cells were treated with heat shock (H; 43°C, 1 hour), irradiated with γ radiation at 10 Gy (R), or treated with combined heat shock and irradiation (HR). Cells after heat shock or irradiation treatments (or both) were then allowed to recover at 37°C for 2 hours. Control cells (C), remained untreated at 37°C. Control and treated cells were then harvested in TRIzol reagent. Total ribonucleic acid (RNA) isolation, complementary RNA (cRNA) preparation, array hybridization, washing, staining and scanning were performed as given in Materials and Methods. Gene expression levels have been normalized by dChip to be comparable with each other. Experiments were repeated twice with reproducible results
HSF and HSP expression at the protein level
The mRNA expression studies were puzzling to us especially because most previous work indicated upregulation of HSF and HSP proteins in cancer and that activation of the heat shock gene expression is associated with more aggressive malignant phenotypes. Therefore, we next examined the relative levels of expression of HSF and HSP proteins by immunoblot assay (Fig 4A). In contrast to the microarray studies (Figs 1–3), marked differences were seen in HSP expression at the protein level (Fig 4A). HSF1 levels were highest in the 3 metastatic cell lines PC-3, DU-145, and CA-HPV-10 (Fig 4A). HSF1 was detectable in PZ-HPV-7, but we were not able to detect HSF1 in LnCap cells by immunoblot analysis (Fig 4A). Similar trends were seen in HSF2 expression, which was also undetectable in LnCap (Fig 4A). Curiously, HSF2 levels seemed to be elevated after heat shock in most cell lines in contrast to earlier suggestions (Mathew et al 1998). Because HSF1 and HSF2 collaborate in HSP induction, coordinate changes in their levels may have a striking impact on HSP transcription particularly after heat shock (He et al 2003). The dichotomy between the mRNA and protein expression levels suggests that HSF levels are regulated in these cells at the post-mRNA level (Figs 1–4). Roles for differential translation of hsf mRNA or dynamic regulation of HSF protein turnover (or both) are indicated. HSP protein expression was correlated more or less well with HSF1 levels (Fig 4A). HSP60, in particular was lowest in the PZ-HPV-7 and LnCap cells that had the lowest HSF1 protein levels (Fig 4A). HSP70 levels correlate to some degree with HSF1, and HSP70 expression was highest in PC-3 with elevated HSF1 and lowest in LnCap that express minimal HSF1 (Fig 4B). HSP90 levels were however not closely correlated with HSF1 (Fig 4A). A remarkable finding here was that LnCap cells do express HSP70 abundantly after heat shock despite very low HSF1 and HSF2 protein concentrations (Fig 4A). However, LnCap cells do synthesize HSF1 and HSF2 mRNA, and it is possible that low-level protein expression takes place (Fig 3). It is also possible that other factors may compensate for low HSF1 or that posttranscriptional events such as thermal mRNA stabilization may occur (Henics et al 1999; Zhao et al 2002). The factor STAT1 has, for instance been shown to be involved in HSP70 and HSP90 transcription (Stephanou and Latchman 1999; Madamanchi et al 2001), and HSP mRNAs have been shown to contain 3′destabilization sequences that are inactivated by heat shock (Zhao et al 2002). Despite the undoubted importance of HSF1 in HSP expression, our experiments do suggest that other factors play a role both before and after heat shock.
Fig 4.
(A) Expression of HSF1, HSF2, and molecular chaperone HSPs in prostate carcinoma cells. Cells were grown at 37°C or heat shocked at 43°C for 60 minutes and allowed to recover at 37°C for 6 hours. Controls and heat shock cells were then lysed in modified radioimmunoprecipitation (RIPA) buffer and 10 μg of protein was subjected to immunoblot analysis for HSF1, HSF2, Hsp70, Hsp90, Hsp60, and β-actin. Experiments were repeated twice with reproducible results. (B) HSP70 levels were quantitated by densitometric analysis of the autoradiographs from (A)
Kinetics of HSP, HSF mRNA, and protein expression
We next carried out a kinetic analysis of HSP mRNA expression in 1 of the cell lines, PC-3 cells, recovering from heat shock (Fig 5). We have plotted the expression of the group of HSP genes that were most strongly induced by heat in the experiments in (Fig 5). The most abundantly expressed HSP gene was HSP28, which has a high basal level and is also strongly induced (5-fold) by heat, which effectively dominated HSP mRNA expression in cells recovering from heat shock. For HSP28 and most of the other HSP genes, mRNA levels increased markedly by 3 and 6 hours after treatment and decayed toward baseline values at 24 hours, although a notable exception was the HSP70B gene, which was powerfully induced by 3 hours after heat but decayed rapidly by 6 hours. The levels of the various HSP70 mRNAs after heat was also very high, with HSP70-1A, HSP70-1B, and HSP70B′ being strongly induced (Fig 5). The combined levels of HSP70 mRNA from each of these genes would therefore be predicted to be even more abundant than HSP28 after heating (Fig 5). HSP40-1 and HSP90-1 were also in this abundantly expressed HSP group. HSP60, HSP47, and HSP105 although strongly induced by heat were less abundant than the HSP28, HSP70, or HSP90 genes (Fig 5).
Fig 5.
Kinetic changes in the expression of HSP messenger ribonucleic acid (mRNA) in PC-3 cells after heat shock. Cells were heat shocked at 43°C for 1 hour and lysed immediately after heat or after recovery at 37°C for 3, 6, and 24 hours after heat shock. mRNA was harvested in TRIzol reagent before gene expression analysis as above. Data from HSP genes with the most abundant expression at the 3-, 6-, or 24-hour points after heat shock were used to plot the figure. Experiments were repeated twice with reproducible results
We also examined the levels of HSF mRNA expression in the PC-3 cells during the 24-hour period after stress (Fig 6). There was minimal alteration in HSF2 and HSF4 levels in the 24-hour period, although HSF1 mRNA levels did decline by approximately 25% during this time. These findings are consistent with earlier studies, indicating that HSF regulation by stress occurs at the posttranscriptional level (Wu 1995).
Fig 6.
Kinetic changes in expression of hsf genes in PC-3 cells after heat shock. The kinetic studies were carried out in PC-3 cells treated with heat shock at 43°C for 1 hour before recovery at 37°C. Cells lysed immediately after heat or after recovery at 37°C for 3, 6, and 24 hours after heat shock were harvested in TRIzol reagent and total ribonucleic acid (RNA) was isolated. Complementary RNA (cRNA) preparation, array hybridization, washing, staining and scanning were performed as above. Hsf1, hsf2, and hsf4 expression levels have been normalized by dChip to be comparable with each other. Experiments were repeated twice with reproducible results
We next examined the expression of HSP genes at the protein level by immunoblot assay (Fig 7A). It must be noted that we are measuring aggregate levels of HSP70 and HSP90 proteins here because the available antibodies do not distinguish between all HSP70 gene products or HSP90α and HSP90β. Consistent with the microarray data, levels of HSF1 and HSF2 remain constant after heat shock, although we do observe the decrease in electrophoretic mobility of HSF1 at 0 and 3 hours after heat that is due to heat-induced hyperphosphorylation (Fig 7A). HSP70 and HSP90 accumulate progressively after heat until the 24-hour period, again consistent with previous reports in the literature (Landry et al 1982; Li and Werb 1982; Subjeck et al 1982). Interestingly, because HSP mRNA levels begin to decline at around 6 hours (Fig 5), these experiments suggest that HSP70 and HSP90 proteins may be stabilized at the protein level in heat shocked cells (Fig 7A). In fact, significantly elevated levels of HSP are seen up to 72 hours after heat shock, again consistent with the proteins being very stable in heat shocked cells (Landry et al 1982; Li and Werb 1982; Subjeck et al 1982). Interestingly, despite the increase in HSP60 mRNA after heat shock, we saw no increase in HSP60 protein from 1– 24 hours after heat, again showing disharmony between mRNA and protein levels under a number of conditions (Fig 7A). Because heat shock is envisaged as a modality to be used in combination with ionizing radiation, we examined the effects of combining these 2 modalities on HSP70 expression at the 24-hour point in DU-145 and PC-3 cells (Fig 7B). HSP70 was abundantly induced in both cell lines at the 24-hour point after heat shock, and combination with ionizing radiation did not markedly affect HSP70 expression (Fig 7B). These data are in line with a large body of data in the literature as well as with the mRNA expression data accumulated in our study, which indicate that ionizing radiation is not a powerful inducer of HSP expression, at least under the conditions investigated so far.
Fig. 7.
Kinetic changes in hsf and hsp gene expression at the protein level. (A) Expression of heat shock factor and molecular chaperone proteins in PC-3 cells before and after heat shock. PC-3 cells were grown at 37°C or heat shocked at 43°C for 60 minutes and allowed to recover at 37°C for 0, 3, 6, and 24 hours. Control and heat shock cells were lysed in a modified radioimmunoprecipitation (RIPA) buffer, and 15 μg of protein was subjected to immunoblot analysis for the heat shock factors and chaperone proteins. β-Actin levels were probed as a loading control. (B) HSP70 expression in DU-145 and PC-3 cells either untreated (c) or 24 hours after ionizing radiation alone, heat shock alone, or heat shock plus radiation as in Materials and Methods
HSP expression in prostate carcinoma cells growing as tumors in nude mice in vivo with and without treatment with heat
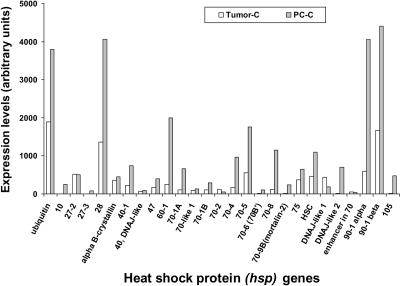
We next went on to examine the expression of HSP genes in PC-3 cells growing as tumor xenografts in the thighs of nude mice. Cells grow as a solid mass in this location, and staining of sections of growing PC-3 tumors with anti-human antibodies indicate that more than 97% of the cells in tumors when at a volume of 0.5 mL are of human origin. We are therefore confident that the majority of mRNAs in our analysis are of PC-3 origin, although a small contamination with infiltrating normal cells of mouse origin is also likely. Our findings are that the relative expression levels of most of the HSPs are strikingly lower in the tumor in vivo before heat shock compared with the same cell line in tissue culture (Fig 8). This is most apparent in the highly expressed genes for ubiquitin, HSP28, HSP60 and HSP70-4, HSP70-8, HSP105, and HSP90 (Fig 8). These interesting changes in HSP expression were not correlated with changes in HSF1, HSF2, or HSF4, which did not undergo marked changes in level when cells were transferred from in vitro growth to growth as tumor xenografts (Fig 9). Changes in basal levels of HSPs are likely the result of posttranslational alteration in HSF1 or other factors in response to the internal milieu of the tumor (Fig 9).
Fig 8.
Comparison of the messenger ribonucleic acid (mRNA) levels of 27 constitutive HSP genes in PC-3 cells growing either in tissue culture or growing as tumor xenografts in vivo in the thighs of nude mice. Ribonucleic acid (RNA) from tissue culture cells was harvested in TRIzol reagent as above, and tumors were excised from sacrificed mice, snap frozen in liquid nitrogen, minced, and prepared for RNA isolation as described in Materials and Methods. Total RNA isolation, complementary RNA (cRNA) preparation, array hybridization, washing, staining, and scanning were performed as described above. Expression levels of the HSP genes have been normalized as above. Experiments were repeated twice with reproducible results
Fig 9.
Hsf messenger ribonucleic acid (mRNA) levels in PC-3 cells growing either in tissue culture or growing as tumor xenografts in vivo in the thighs of nude mice. Tissue culture cells were harvested in TRIzol reagent as above, and tumors were excised from sacrificed mice, snap frozen in liquid nitrogen, minced, and prepared for RNA isolation as described in Materials and Methods. Heat shock and radiation treatments were performed as in Materials and Methods. Total RNA isolation, complementary RNA (cRNA) preparation, array hybridization, washing, staining, and scanning were performed as described above. Expression levels of the HSP genes have been normalized as above. Experiments were repeated twice with reproducible results
However, when cells or tumors were exposed to the same heat shock condition before recovery at 37°C, the pattern of HSP induction was remarkably similar when PC-3 cells were grown either in monolayers or as tumor xenografts in nude mice (Fig 10). Massive induction of HSP40-1, HSP70-1A, HSP70-1B, HSP70B′, and HSP105 was observed in cells growing in vitro and in vivo (Fig 10). The relative increase in HSP28 in this experiment was low because of the high basal level of this protein. Thus, growth in vivo appeared to suppress the constitutive expression of many HSP family members but did not prevent the ability of cells to mount a sturdy heat shock response (Fig 10). Heat shock did not cause any dramatic changes in HSF mRNA levels in tissue culture or in tumors, in line with most data in the literature that point to a posttranslational regulation of HSF by heat shock (Fig 9). Likewise, HSF1 protein levels were similar in PC-3 cells growing in either monolayers or in tumors in vivo and were not markedly changed in cells recovering (24 hours after) heat shock in vitro or in vivo (Fig 11). HSP70 and HSP90 protein levels were reduced in the PC-3 cells growing in tumors consistent with the microarray study (Fig 8). However, both HSP70 and HSP90 levels were induced in PC-3 tumor cells recovering from heat shock (Fig 11), again consistent with the mRNA data (Fig 10).
Fig 10.
Comparison of HSP messenger ribonucleic acid (mRNA) expression after heat shock between PC-3 cells in tissue culture or isogenic PC-3 cells growing as tumor xenografts in vivo in the legs of nude mice. PC-3 cells either growing in vitro or as tumor xenografts were treated with heat shock (43°C, 1 hour) in circulating water bath followed by 2-hour recovery as described in Materials and Methods. Control cells were cultured in a 37°C incubator, and control tumors remained untreated. Tissue culture cells were harvested in TRIzol reagent as above, and tumors were excised from sacrificed mice, snap frozen in liquid nitrogen, minced, and prepared for RNA isolation as described in Materials and Methods. Gene expression levels have been normalized by dChip to be comparable with each other. Relative gene expression profiles of the HSP genes after heat were shown as folds of expression levels from their corresponding, non–heat shocked controls. Experiments were repeated twice with reproducible results
Fig 11.
Expression of HSF1 and HSP proteins in PC-3 cells growing in tissue culture or as xenografts in nude mice. PC-3 cells either growing in vitro or as tumor xenografts were then either maintained under unstressed conditions as controls (c) or treated with heat shock (43°C, 1 hour) in a circulating water bath followed by 24 hours recovery to permit HSP protein expression. Proteins were then extracted, subjected to 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE), and analyzed by immunoblot with antibodies to HSP90, HSP70, and HSF1. Anti-human β-actin was probed as a control for even loading. Experiments were repeated twice with reproducible results
Finally, to examine the relative sensitivity of prostate carcinoma cells to heat shock in tissue culture or in human xenografts, we examined apoptosis by TUNEL staining after a similar dose of heat shock (1 hour at 43°C). As indicated in our earlier studies, heat shock causes a slowly developing apoptosis, reaching approximately 5% of the population by 48 hours after heat (Fig 12). The same dose of heat shock to PC-3 tumors causes a much larger increase in apoptotic rate, which reached approximately 80–90% by 48 hours (Fig 12). Despite the difference in the degree of apoptosis, the time course of apoptosis development was similar during growth in vitro and in the tumors after heat shock (Fig 12).
Fig 12.
Apoptotic index in PC-3 cells exposed to hyperthermia in vitro and in tumors. Cells and tumors in mice were exposed to hyperthermia at 43°C for 60 minutes and apoptotic index determined by TUNEL assay as described in Materials and Methods from 0–96 hours after treatment. Apoptotic index in tumors was assayed by TUNEL analysis of paraffin sections of tumors. Sections were scored “blind” and in duplicate. At least 200 cells were scored in total at each time point. Experiments were repeated twice with consistent results
DISCUSSION
The experiments described here are largely supportive of the notion that HSP gene expression and HSF activity and expression are increased in more advanced stages of cancer (Fig 4). The most striking finding in the study was the elevation of HSF1 and HSP levels in aggressively malignant prostate carcinoma cell lines (Fig 4). It is significant that these changes in HSF and HSP levels would not have been predicted from microarray studies of HSF (Fig 3) and HSP (Fig 1) mRNA levels. The increased HSF levels observed in the metastatic prostate carcinoma cell lines in particular appear to be due to altered regulation of either mRNA translation or protein turnover (or both) (Figs 3 and 4). Although we do not at this stage know the mechanisms involved, 1 candidate could be differential activity of the proteosome in the metastatic cell lines: both HSF1 and HSF2 are targets for proteosomal degradation (Mathew et al 1998). Despite these differences in HSP expression between cells of varying degrees of malignancy under growth conditions, stress caused a major shift in HSP gene expression and activation of HSP40-1, HSP70-1A, HSP70-1B, HSP70-6 (HSP70B), DNA-J2–like, and HSP105 in all cells (Fig 2). Even in LnCap cells with minimal HSF1 and HSF2 expression, heat-inducible HSP70 protein expression was observed (Fig 4). Interestingly, we observed minimal induction of the HSP70B gene in LnCap cells: because the HSP70B promoter is known to be almost exclusively induced by stress through the HSE in its promoter, the findings may suggest that a mechanism for HSP70 induction alternative to HSF1 activation may be operative in LnCap cells (Schiller et al 1988). Increased HSP expression in cancer patients has been shown to signal a poor response to treatment by a number of modalities, suggesting that HSP expression is involved with development of resistance to treatment in addition to being involved in the mechanisms of malignant progression (Ciocca et al 1993, Cornford et al 2000; Yamamoto et al 2001; Ciocca and Vargas-Roig 2002; Mese et al 2002). In addition, subpopulations of LnCap-derived cells, selected for enhanced capacity to metastasize, have been shown to express elevated levels of HSF1, HSP70, and HSP27 compared with nonselected controls (Hoang et al 2000). This may be highly significant because our studies indicate minimal levels of HSF1 and HSP in the poorly metastatic parent LnCap cells (Figs 1 and 4). Previous studies have also indicated that elevated HSP70 expression occurs at an early stage in cellular immortalization from embryonic stem cells (Ravagnan et al 2001). We had to use immortalized prostatic epithelial cells for our normal controls and may have missed a very early change in HSP expression during the immortalization process.
As indicated by the kinetic studies (Figs 5–7), HSPs are activated at a number of regulatory levels by stress in addition to transcriptional activation, and these may include stress-induced mRNA stabilization, differential translation, and protein stabilization (Hickey and Weber 1982; Zhao et al 2002). HSF1 activity and HSP expression appear to be subject to differential regulation by a number of pathways at normal temperatures but are largely independent of such regulation when exposed to heat shock, which overrides constitutive regulation and permits prompt induction of this emergency response.
Growth of PC-3 cells in vivo as tumor xenografts was accompanied by a marked decrease in constitutive HSP expression (Figs 8 and 11). Decreased HSP expression was part of a global switch in gene expression that accompanies the switch of PC-3 cells from growth as monolayers in tissue culture to growth as tumors in vivo (D. Tang and S.K. Calderwood, in preparation). Many reports indicate changes in a wide range of cellular properties as cells grow as tumors, and these properties may reflect the remodeling of gene expression patterns. These changes may reflect adaptation to the chemical nature of the tumor microenvironment and the alterations in cell-cell interaction in growth as a tumor in vivo. Our studies also indicate the remarkable sturdiness of the heat shock response that remains intact in the PC-3 cells growing in vivo despite the global rearrangements in other gene expressions mentioned above (Figs 10 and 11).
The elevation in HSF1 and HSP levels in cancer shown in our studies and in those of others and its association with a poor prognosis and inferior response to therapy suggests the strategy of targeting HSP in cancer therapy. Treatment with HSP70 antisense oligonucleotides, for instance, can cause tumor cell apoptosis on its own and can synergize with heat shock in cell killing (Jones et al 2004). Indeed, it has been shown that antagonizing heat-inducible HSP expression with quercitin, a bioflavonoid drug that inhibits HSF1 activation, or by using antisense oligonucleotides directed against HSP70 mRNA further sensitizes PC-3 cells to heat-induced apoptosis in vitro and leads to tumor regression in vivo (Asea et al 2001, Lepchammer et al 2002; Jones et al 2004) (A. Asea et al, personal communication). The strategy of targeting HSP expression or function in cancer cells may thus be indicated. Such a strategy might prove particularly effective because constitutive HSP expression is reduced in tumors, and this might be related to increased killing of PC-3 tumor cells by heat (Fig 12).
Acknowledgments
We thank the members of the Boston Hyperthermia Program: Irving Kaplan, Mark Hurwitz, Michael Sherman, and Kullervo Hynynen for their moral support and critical input. We thank the Harvard JCRT Foundation for substantive support. This work was supported by NIH grants CA47407, CA31303, CA50642, and CA77465.
REFERENCES
- Asea A, Ara G, Teicher BA, Stevenson MA, Calderwood SK. Effects of the flavonoid drug quercitin on the response of human prostate tumors to hyperthermia in vivo. Int J Hyperthermia. 2001;17:347–356. doi: 10.1080/02656730110053146.0265-6736(2001)017<0347:EOTFDQ>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Baker RT, Board PG. The human ubiquitin gene family: structure of a gene and pseudogenes from the Ub B subfamily. Nucleic Acids Res. 1987;15:443–463. doi: 10.1093/nar/15.2.443.0305-1048(1987)015<0443:THUGFS>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya T, Karnezis AN, Murphy SP, Hoang T, Freeman BC, Phillips B, Morimoto RI. Cloning and subcellular localization of human mitochondrial hsp70. J Biol Chem. 1995;270:1705–1710. doi: 10.1074/jbc.270.4.1705.0021-9258(1995)270<1705:CASLOH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Beckmann RP, Mizzen LA, Welch WJ. Interaction of hsp70 with newly synthesized proteins; implications for protein folding and assembly. Science. 1990;248:850–854. doi: 10.1126/science.2188360.0193-4511(1990)248<0850:IOHWNS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Blagosklonny MV. Re: role of the heat shock response and molecular chaperones in oncogenesis and cell death. J Natl Cancer Inst. 2001;93:239–240. doi: 10.1093/jnci/93.3.239-a.0027-8874(2001)093<0239:RROTHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Bonnycastle LL, Yu CE, Hunt CR, Trask BJ, Clancy KP, Weber JL, Patterson D, Schellenberg GD. Cloning, sequencing, and mapping of the human chromosome 14 heat shock protein gene (HSPA2) Genomics. 1994;23:85–93. doi: 10.1006/geno.1994.1462.0888-7543(1994)023<0085:CSAMOT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Buchner J. Hsp 90 & Co.—a holding for folding. TIBS. 1999;24:136–142. doi: 10.1016/s0968-0004(99)01373-0.0376-5067(1999)024<0136:HCHFF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Carper SW, Rocheleau TA, Storm FK. cDNA sequence of a human heat shock protein HSP27. Nucleic Acids Res. 1990;18:6457. doi: 10.1093/nar/18.21.6457.0305-1048(1990)018<6457:CSOAHH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll AG, Voeller HJ, Sugars L, Gelmann EP. p53 oncogene mutations in three human prostate cancer cell lines. Prostate. 1993;23:123–134. doi: 10.1002/pros.2990230206.0270-4137(1993)023<0123:POMITH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chellaiah A, Davis A, Mohanakumar T. Cloning of a unique human homologue of the Escherichia coli DNAJ heat shock protein. Biochim Biophys Acta. 1993;1174:111–113. doi: 10.1016/0167-4781(93)90103-k.0006-3002(1993)1174<0111:COAUHH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chen JJ, McNealy DJ, Dalal S, Andophy EJ. Isolation, sequence analysis and characterization of a cDNA encoding human chaperonin 10. Biochim Biophys Acta. 1994;1219:189–190. doi: 10.1016/0167-4781(94)90268-2.0006-3002(1994)1219<0189:ISAACO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Clark GM, Tandon AK, Fuqua SA, Welch WJ, McGuire WL. Heat shock protein hsp70 in patients with axillary lymph node-negative breast cancer: prognostic implications. J Natl Cancer Inst. 1993;85:570–574. doi: 10.1093/jnci/85.7.570.0027-8874(1993)085<0570:HSPHIP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Ciocca DR, Vargas-Roig LM. Hsp27 as a prognostic and predictive factor in cancer. Prog Mol Subcell Biol. 2002;28:205–218. doi: 10.1007/978-3-642-56348-5_11.0079-6484(2002)028<0205:HAAPAP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Cornford PA, Dodson AR, and Parsons KF. et al. 2000 Heat shock protein expression independently predicts clinical outcome in prostate cancer. Cancer Res. 60:7099–7105. [PubMed] [Google Scholar]
- Domanico SZ, DeNagel DC, Dahlseid JN, Green JM, Pierce SK. Cloning of the gene encoding peptide-binding protein 74 shows that it is a new member of the heat shock protein 70 family. Mol Cell Biol. 1993;13:3598–3610. doi: 10.1128/mcb.13.6.3598.0270-7306(1993)013<3598:COTGEP>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D. Clustering analysis and display of genome-wide expression patterns. Proc Natl Acad Sci U S A. 1998;95:14863–14868. doi: 10.1073/pnas.95.25.14863.0027-8424(1998)095<14863:CAADOG>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fathallah DM, Cherif D, Dellagi K, Arnaout MA. Molecular cloning of a novel human hsp70 from a B cell line and its assignment to chromosome 5. J Immunol. 1993;151:810–813.0022-1767(1993)151<0810:MCOANH>2.0.CO;2 [PubMed] [Google Scholar]
- Froesch BA, Takayama S, Reed JC. BAG-1L protein enhances androgen receptor function. J Biol Chem. 1998;273:11660–11666. doi: 10.1074/jbc.273.19.11660.0021-9258(1998)273<11660:BPEARF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gabai VL, Meriin AB, Yaglom JA, Volloch VZ, Sherman MY. Role of hsp70 in regulation of stress-kinase JNK: Implications in apoptosis and aging. FEBS Lett. 1998;438:1–4. doi: 10.1016/s0014-5793(98)01242-3.0014-5793(1998)438<0001:ROHIRO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Georgopolis C, Welch WJ. Role of the major heat shock proteins as molecular chaperones. Ann Rev Cell Biol. 1993;9:601–634. doi: 10.1146/annurev.cb.09.110193.003125.0743-4634(1993)009<0601:ROTMHS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Gething MJ, Sambrook J. Protein folding in the cell. Nature. 1992;355:33–45. doi: 10.1038/355033a0.0028-0836(1992)355<0033:PFITC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Grammatikakis N, Vultur A, Ramana CV, Siganou A, Schweinfest CW, Watson DK, Raptis L. The role of Hsp90N, a new member of the Hsp90 family, in signal transduction and neoplastic transformation. J Biol Chem. 2002;277:8312–8320. doi: 10.1074/jbc.M109200200.0021-9258(2002)277<8312:TROHAN>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Grossmann ME, Huang H, Tindall DJ. Androgen receptor signaling in androgen-refractory prostate cancer. JNCI. 2001;93:1687–1697. doi: 10.1093/jnci/93.22.1687.0027-8874(2001)093<1687:ARSIAP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hansen RK, Parra I, Lemieux P, Oesterreich S, Hilsenbeck SG, Fuqua SA. Hsp27 overexpression inhibits doxorubicin-induced apoptosis in human breast cancer cells. Breast Cancer Res Treat. 1999;56:187–196. doi: 10.1023/a:1006207009260.0167-6806(1999)056<0187:HOIDAI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hata M, Okumura K, Seto M, Ohtsuka K. Genomic cloning of a human heat shock protein 40 (Hsp40) gene (HSPF1) and its chromosomal localization to 19p13.2. Genomics. 1996;38:446–449. doi: 10.1006/geno.1996.0653.0888-7543(1996)038<0446:GCOAHH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- He H, Soncin F, and Grammatikakis N. et al. 2003 Elevated expression of heat shock factor 2A stimulates HSF1-induced transcription during stress. J Biol Chem. 278:35465–35475. [DOI] [PubMed] [Google Scholar]
- Henics T, Nagy E, Oh HJ, Csermely P, von Gabain A, Subjeck JR. Mammalian HSP70 and HSP110 proteins bind to RNA motifs involved in mRNA stability. J Biol Chem. 1999;274:17318–17324. doi: 10.1074/jbc.274.24.17318.0021-9258(1999)274<17318:MHAHPB>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hickey E, Weber LA. 1982 Preferential translation of heat shock mRNAs in HeLa cells. In: Heat Shock; from Bacteria to Man, ed Schlesinger M, Ashburner M, Tissieres A. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY, 199–206. [Google Scholar]
- Hoang AT, Huang J, Rudra-Ganguly N, Zheng J, Powell WC, Rabindran SK, Wu C, Roy-Burman P. A novel association between the human heat shock transcription factor 1 (HSF1) and prostate adenocarcinoma. Am J Pathol. 2000;156:857–864. doi: 10.1016/S0002-9440(10)64954-1.0002-9440(2000)156<0857:ANABTH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoe KL, Won M, and Chung KS. et al. 1998 Isolation of a new member of DnaJ-like heat shock protein 40 (Hsp40) from human liver. Biochim Biophys Acta. 1383:4–8. [DOI] [PubMed] [Google Scholar]
- Horoszewicz JS, Leong SS, Kawinski E, Karr JP, Rosenthal H, Chu TM, Mirand EA, Murphy GP. LNCaP model of human prostatic carcinoma. Cancer Res. 1983;43:1809–1818.0008-5472(1983)043<1809:LMOHPC>2.0.CO;2 [PubMed] [Google Scholar]
- Hunt C, Morimoto RI. Conserved features of eukaryotic hsp70 genes revealed by comparison with the nucleotide sequence of human hsp70. Proc Natl Acad Sci U S A. 1985;82:6455–6459. doi: 10.1073/pnas.82.19.6455.0027-8424(1985)082<6455:CFOEHG>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegawa S, Sudo K, Okui K, Nakamura Y. Isolation, characterization and chromosomal assignment of human colligin-2 gene (CBP2) Cytogenet Cell Genet. 1995;71:182–186. doi: 10.1159/000134103.0301-0171(1995)071<0182:ICACAO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Iwaki A, Nagano T, Nakagawa M, Iwaki T, Fukumaki Y. Identification and characterization of the gene encoding a new member of the alpha-crystallin/small hsp family, closely linked to the alpha B-crystallin gene in a head-to-head manner. Genomics. 1997;45:386–394. doi: 10.1006/geno.1997.4956.0888-7543(1997)045<0386:IACOTG>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Iwaki T, Iwaki A, Goldman JE. Alpha B-crystallin in oxidative muscle fibers and its accumulation in ragged-red fibers: a comparative immunohistochemical and histochemical study in human skeletal muscle. Acta Neuropathol (Berl) 1993;85:475–480. doi: 10.1007/BF00230485.0001-6322(1993)085<0475:ABIOMF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Jindal S, Dudani AK, Singh B, Harley CB, Gupta RS. Primary structure of a human mitochondrial protein homologous to the bacterial and plant chaperonins and to the 65-kilodalton mycobacterial antigen. Mol Cell Biol. 1989;9:2279–2283. doi: 10.1128/mcb.9.5.2279.0270-7306(1989)009<2279:PSOAHM>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones EL, Zhau MJ, Stevenson MA, and Calderwood SK. 2004 The 70 kilodalton heat shock protein is an inhibitor of apoptosis in prostate cancer. Int J Hyperthermia, in press. [DOI] [PubMed] [Google Scholar]
- Kaighn ME, Narayan KS, Ohnuki Y, Lechner JF, Jones LW. Establishment and chracterization of a human prostate carcinoma cell line. Invest Urol. 1979;17:16–23.0021-0005(1979)017<0016:EACOAH>2.0.CO;2 [PubMed] [Google Scholar]
- Lam WY, Wing Tsui SK, Law PT, Luk SC, Fung KP, Lee CY, Waye MM. Isolation and characterization of a human heart cDNA encoding a new member of the small heat shock protein family=mHSPL27. Biochim Biophys Acta. 1996;1314:120–124. doi: 10.1016/s0167-4889(96)00121-8.0006-3002(1996)1314<0120:IACOAH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Landry J, Bernier D, Chretien P, Nicole LM, Tanguay RM, Marceau N. Synthesis and degradation of heat shock proteins during the development and decay of thermotolerance. Cancer Research. 1982;42:2457–2461.0008-5472(1982)042<2457:SADOHS>2.0.CO;2 [PubMed] [Google Scholar]
- Lechpammer S, Asea A, Mallick R, Zhong R, Sherman MY, Calderwood SK. Development of an XTT tetrazolium salt-based assay for detection of hyperthermia sensitizers in a high-flux screening programme. Int J Hyperthermia. 2002;18:203–215. doi: 10.1080/02656730110110034.0265-6736(2002)018<0203:DOAXTS>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Leung TK, Rajendran MY, Monfries C, Hall C, Lim L. The human heat shock protein family. Expression of a novel heat-inducible hsp70 (hsp70b') and isolation of its cDNA and genomic DNA. Biochem J. 1990;267:125–132. doi: 10.1042/bj2670125.0264-6021(1990)267<0125:THHSPF>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Wong WH. Model-based analysis of oligonucleotide arrays: Expression index computation and outlier detection. Proc Natl Acad Sci USA. 2001;98:31–36. doi: 10.1073/pnas.011404098.0027-8424(2001)098<0031:MAOOAE>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li GC, Hahn GM. A proposed operational model for thermotolerance. Cancer Research. 1981;40:4501–4508.0008-5472(1981)040<4501:APOMFT>2.0.CO;2 [PubMed] [Google Scholar]
- Li GC, Werb Z. Correlation between the synthesis of heat shock proteins and the development of thermotolerance in Chinese hamster fibroblasts. Proc Natl Acad Sci USA. 1982;79:3218–3222. doi: 10.1073/pnas.79.10.3218.0027-8424(1982)079<3218:CBTSOH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindquist S, Craig EA. The heat shock proteins. Ann Rev Genet. 1988;22:631–637. doi: 10.1146/annurev.ge.22.120188.003215.0066-4197(1988)022<0631:THSP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Madamanchi NR, Li S, Patterson C, Runge MS. Thrombin regulates vascular smooth muscle cell growth and heat shock proteins via the JAK-STAT pathway. J Biol Chem. 2001;276:18915–18924. doi: 10.1074/jbc.M008802200.0021-9258(2001)276<18915:TRVSMC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Mathew A, Mathur SK, Morimoto RI. Heat shock response and degradation: Regulation of hsf2 by the ubiquitin-proteasome pathway. Mol Cell Biol. 1998;18:5091–5098. doi: 10.1128/mcb.18.9.5091.0270-7306(1998)018<5091:HSRADR>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mese H, Sasaki A, Nakayama S, Yoshioka N, Yoshihama Y, Kishimoto K, Matsumura T. Prognostic significance of heat shock protein 27 (HSP27) in patients with oral squamous cell carcinoma. Oncol Rep. 2002;9:341–344.1021-335X(2002)009<0341:PSOHSP>2.0.CO;2 [PubMed] [Google Scholar]
- Milner CM, Campbell RD. Structure and expression of the three MHC-linked HSP70 genes. Immunogenetics. 1990;32:242–251. doi: 10.1007/BF00187095.0093-7711(1990)032<0242:SAEOTT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Nagase T, Seki N, and Ishikawa K. et al. 1996 Prediction of the coding sequences of unidentified human genes. VI. The coding sequences of 80 new genes (KIAA0201-KIAA0280) deduced by analysis of cDNA clones from cell line KG-1 and brain. DNA Res. 3:321–329. 341–354. [DOI] [PubMed] [Google Scholar]
- Nakai A, Tanabe M, Kawazoe Y, Inazawa J, Morimoto RI, Nagata K. HSF4, a new member of the human heat shock factor family which lacks properties of a transcriptional activator. Mol Cell Biol. 1997;17:469–481. doi: 10.1128/mcb.17.1.469.0270-7306(1997)017<0469:HANMOT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Netzer WF, Hartl FU. Protein folding in the cytosol: Chaperonin-dependent and -independent mechanisms. TIBS. 1998;23:68–74. doi: 10.1016/s0968-0004(97)01171-7.0376-5067(1998)023<0068:PFITCC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rabindran SK, Giorgi G, Clos J, Wu C. Molecular cloning and expression of a human heat shock factor, HSF1. Proc Natl Acad Sci USA. 1991;88:6906–6910. doi: 10.1073/pnas.88.16.6906.0027-8424(1991)088<6906:MCAEOA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ravagnan L, Gurbuxani S, and Susin SA. et al. 2001 Heat-shock protein 70 antagonizes apoptosis-inducing factor. Nat Cell Biol. 3:839–843. [DOI] [PubMed] [Google Scholar]
- Rebbe NF, Hickman WS, Ley TJ, Stafford DW, Hickman S. Nucleotide sequence and regulation of a human 90-kDa heat shock protein gene. J Biol Chem. 1989;264:15006–15011.0021-9258(1989)264<15006:NSAROA>2.0.CO;2 [PubMed] [Google Scholar]
- Ricaniadis N, Kataki A, Agnantis N, Androulakis G, Karakousis CP. Long-term prognostic significance of HSP-70, c-myc and HLS-DR expression in patients with malignant melanoma. Eur J Surg Oncol. 2001;27:88–93. doi: 10.1053/ejso.1999.1018.0748-7983(2001)027<0088:LPSOHC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schiller P, Amin J, Ananthan J, Brown ME, Scott WA, Voellmy R. Cis-acting elements involved in the regulated expression of a human HSP70 gene. J Mol Biol. 1988;203:97–105. doi: 10.1016/0022-2836(88)90094-0.0022-2836(1988)203<0097:CEIITR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Schuetz TJ, Gallo GJ, Sheldon L, Tempst P, Kingston RE. Isolation of a cDNA for HSF2; evidence for two heat shock factors in humans. Proc Natl Acad Sci USA. 1991;88:6911–6915. doi: 10.1073/pnas.88.16.6911.0027-8424(1991)088<6911:IOACFE>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song HY, Dunbar JD, Zhang YX, Guo D, Donner DB. Identification of a protein with homology to hsp90 that binds the type 1 tumor necrosis factor receptor. J Biol Chem. 1995;270:3574–3581.0021-9258(1995)270<3574:IOAPWH>2.0.CO;2 [PubMed] [Google Scholar]
- Sorger PK, Pelham HRB. Yeast heat shock factor is an essential DNA-binding protein that exhibits temperature-dependent phosphorylation. Cell. 1988;54:855–864. doi: 10.1016/s0092-8674(88)91219-6.0092-8674(1988)054<0855:YHSFIA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Stephanou A, Latchman DS. Transcriptional regulation of the heat shock protein genes by STAT family transcription factors. Gene Expr. 1999;7:311–319.1052-2166(1999)007<0311:TROTHS>2.0.CO;2 [PMC free article] [PubMed] [Google Scholar]
- Stone KR, Mickey DD, Wunderli H, Mickey GH, Paulson DF. Isolation of a human prostate carcinoma cell line (DU145) Int J Cancer. 1978;21:274–281. doi: 10.1002/ijc.2910210305.0020-7136(1978)021<0274:IOAHPC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Strik HM, Weller M, Frank B, Hermisson M, Deininger MH, Dichgans J, Meyermann R. Heat shock protein expression in human gliomas. Anticancer Res. 2000;20:4457–4462.0250-7005(2000)020<4457:HSPEIH>2.0.CO;2 [PubMed] [Google Scholar]
- Subjeck JR, Sciandra JJ, Johnson RJ. Heat shock proteins and thermotolerance; a comparison of induction kinetics. Br J Radiol. 1982;55:579–584. doi: 10.1259/0007-1285-55-656-579.0007-1285(1982)055<0579:HSPAAC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Tavaria M, Gabriele T, Kola I, Anderson RL. A hitchhiker's guide to the human Hsp70 family. Cell Stress Chaperones. 1996;1:23–28. doi: 10.1379/1466-1268(1996)001<0023:ahsgtt>2.3.co;2.1466-1268(1996)001<0023:AHGTTH>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting J, Lee AS. Human gene encoding the 78,000-dalton glucose regulated protein and its pseudogene: structure, conservation, and regulation. DNA. 1988;7:275–286. doi: 10.1089/dna.1988.7.275. [DOI] [PubMed] [Google Scholar]
- Van Molle W, Wielockx B, Mahieu T, Takada M, Taniguchi T, Sekikawa K, Libert C. HSP70 protects against TNF-induced lethal inflammatory shock. Immunity. 2002;16:685–695. doi: 10.1016/s1074-7613(02)00310-2.1074-7613(2002)016<0685:HPATLI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Voellmy R. Transduction of the stress signal and mechanisms of transcriptional regulation of heat shock/stress protein expression in higher eukaryotes. Crit Rev Eukaryotic Gene Expr. 1994;4:357–401.1045-4403(1994)004<0357:TOTSSA>2.0.CO;2 [PubMed] [Google Scholar]
- Volloch VZ, Sherman MY. Oncogenic potential of Hsp72. Oncogene. 1999;18:3648–3651. doi: 10.1038/sj.onc.1202525.0950-9232(1999)018<3648:OPOH>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wang HL, Lu DW. Detection of human papillomavirus DNA and expression of p16, Rb and p53 proteins in small cell lung carcinomas of the uterine cervix. Am J Surg Pathol. 2004;28:901–908. doi: 10.1097/00000478-200407000-00009.0147-5185(2004)028<0901:DOHPDA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Weijerman PC, Konig JJ, Wong ST, Niesters HG, Peehl DM. Lipofection-mediated immortalization of human prostatic epithelial cells of normal and malignant origin using human papillomavirus type 18 DNA. Cancer Res. 1994;54:5579–5583.0008-5472(1994)054<5579:LIOHPE>2.0.CO;2 [PubMed] [Google Scholar]
- Wodicka L, Dong H, Mittamann M, Ho MH, Lockhart DJ. Genome-wide expression monitoring in Saccharomyces cerevisiae. Nat Biotechnol. 1997;15:1359–1367. doi: 10.1038/nbt1297-1359.1087-0156(1997)015<1359:GEMISC>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Wu C. Heat shock transcription factors: Structure and regulation. Ann Rev Cell Dev Biol. 1995;11:441–469. doi: 10.1146/annurev.cb.11.110195.002301.1081-0706(1995)011<0441:HSTFSA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Okamoto A, Isonishi S, Ochiai K, Ohtake Y. Heat shock protein 27 was up-regulated in cisplatin resistant human ovarian tumor cell line and associated with the cisplatin resistance. Cancer Lett. 2001;168:173–181. doi: 10.1016/s0304-3835(01)00532-8.0304-3835(2001)168<0173:HSPWUI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Yamazaki M, Akaogi K, Miwa T, Imai T, Soeda E, Yokoyama K. Nucleotide sequence of a full-length cDNA for 90 kDa heat-shock protein from human peripheral blood lymphocytes. Nucleic Acids Res. 1989;17:7108. doi: 10.1093/nar/17.17.7108.0305-1048(1989)017<7108:NSOAFC>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano M, Naito Z, Yokoyama M, Shiraki Y, Ishiwata T, Inokuchi M, Asano G. Expression of hsp90 and cyclin D1 in human breast cancer. Cancer Lett. 1999;137:45–51. doi: 10.1016/s0304-3835(98)00338-3.0304-3835(1999)137<0045:EOHACD>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Zhao M, Tang D, Lechpammer S, Hoffman A, Asea A, Stevenson MA, Calderwood SK. Double stranded RNA-dependent protein kinase (pkr) is essential for thermotolerance, accumulation of HSP70 and stabilization of ARE-containing HSP70-mRNA during stress. J Biol Chem. 2002;277:44539–44547. doi: 10.1074/jbc.M208408200.0021-9258(2002)277<44539:DSRPKP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]