Abstract
In situ expression of 2 multidrug resistance genes, mdr49 and mdr65, of Drosophila melanogaster was examined in wild-type third instar larval tissues under physiological conditions and after heat shock or colchicine feeding. Expression of these 2 genes was also examined in tumorous tissues of lethal (2) giant larvae l(2)gl4 mutant larvae. These 2 mdr genes show similar constitutive expression in different larval tissues under physiological conditions. However, they are induced differentially by endogenous (tumorous growth) and exogenous stresses (colchcine feeding or heat shock): whereas heat shock and colchicine feeding induce mdr49, tumorous condition is accompanied by enhanced expression of mdr49 and mdr65 genes.
INTRODUCTION
A major impediment to most drug therapies is overexpression of the multidrug resistance genes, which makes cells or organisms resistant to a wide range of drugs (Beck 1990; Roninson 1992; Ling 1993, 1997). One of the most extensively studied mechanisms responsible for multidrug resistance phenotype is the overexpression of drug efflux pump proteins, the P-glycoproteins (PGP). PGPs are transmembrane glycoproteins of about 170 kDa belonging to a super family of adenosine triphosphate– binding cassette transporters (Juliano and Ling 1976). These proteins function in multidrug resistance by acting as drug efflux pumps to maintain the intracellular concentrations of drug below the cytotoxic levels. The PGP-coding multidrug resistance genes (mdr) have been identified in a wide range of species. In human and rodents, multiple mdr genes exist. The normal physiological function of PGPs in the absence of cytotoxic drugs is still not known because most of these genes were implicated in the development of multidrug-resistant phenotype. mdr1 knockout mice are viable and fertile as long as they are not challenged with any drugs (Borst and Schinkel 1997). Expression of PGP on the secretory surfaces in a number of tissues including adrenal gland, kidney, liver, intestinal tract, uterine epithelium, etc, suggests a role either in transporting substances across the cell membrane or decreasing absorption from the surroundings (Thiebault et al 1987). Expression in the capillary endothelial cells of the brain, nerves, testis, and placenta suggests a role in keeping the toxins out of the system (Arceci et al 1988). In human and mouse, the expression of mdr1 gene appears to be affected also by heat shock, heavy metals, differentiation-inducing agents, chemotherapeutics, hormones, and ultraviolet light (see Sukhai and Piquette-Miller 2000 for review).
Homology search in Drosophila led to identification of 3 mdr genes, named according to their chromosomal locations as mdr49, mdr50, and mdr65 (Wu et al 1991; Gerrard et al 1993). The Drosophila mdr homologues share approximately 50% identity to mammalian homologues and 53% homology among themselves at the nucleotide level (Wu et al 1991; Gerrard et al 1993). The mdr49 deletion mutants showed increased sensitivity to dietary colchicine, which did not affect expression of the mdr65 gene (Wu et al 1991). It has also been shown that the mdr65 gene is responsible for the alpha amanitin resistance found in a population of Drosophila melanogaster (Begun and Whitely 2000). In this study, expression of mdr49 and mdr65 in different tissues of late third instar larvae of D melanogaster was examined by in situ hybridization using gene specific riboprobes. The results show that although there are no tissue-specific differences in expression of these 2 genes under physiological conditions, heat shock induces only mdr49 in all tissues, whereas colchicine feeding enhances mdr49 expression in larval gut and brain tissues. On the other hand, malignant tumors in lethal (2) giant larvae l(2)gl4 homozygous larvae show enhanced levels of transcripts of both mdr49 as well as mdr65 genes.
RESULTS AND DISCUSSION
Expression of Drosophila mdr49 and mdr65 genes under physiological conditions and during stress
RNA-RNA in situ hybridizations were carried out to study the expression of mdr49 and mdr65 genes in different larval tissues under normal conditions and after heat shock. The study revealed a basal level of expression of mdr49 (Fig 1A,F,K) as well as mdr65 RNA (Fig 1A′,F′,K′) in the control larval tissues such as salivary glands, brain, and wing imaginal discs. The mdr transcripts were present only in the cytoplasm with little signal in the nucleus. The expression patterns of mdr49 and mdr65 transcripts in the different tissues were found to be similar with little tissue-specific differences.
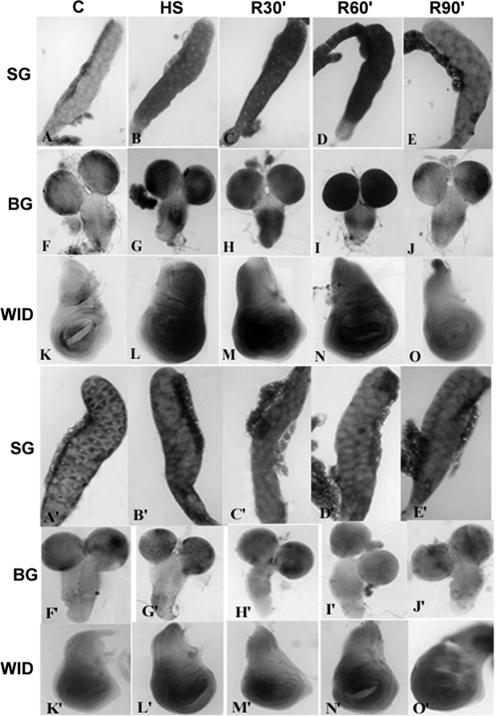
Fig 1.
Expression of mdr49 (A to O) and mdr65 (A′ to O′) genes in wild-type late third instar larval tissues in control (column C) tissues (SG = salivary glands; BG = brain ganglia; WID = wing imaginal discs), after heat shock at 37°C 40 minutes (column HS), or after 30 minutes (column R30′), 60 minutes (column R60′), and 90 minutes (column R90′) recovery (at 22°C ± 1) from heat shock. The larvae were quickly dissected in Poels salt solution, PSS (Lakhotia and Tapadia 1998), and the tissues were fixed and processed for RNA-RNA in situ hybridization following Prasanth et al 2000. The mdr49 and mdr65 clones in pUC18 vector (received from Dr James Croop) were digested with EcoRI, and the 0.7 Kb and 1.7 Kb EcoRI fragments, corresponding to the 3′ ends of mdr49 and mdr65, respectively, were subcloned in pBS KS+ vector and named pBSmdr49 and pBSmdr65, respectively. For obtaining antisense dig-labeled riboprobes, the pBSmdr49 was digested with HindIII, whereas the pBSmdr65 was digested with XhoI. The linearized fragments were used for in vitro transcription using digoxigenin-labeled uridine triphosphate (Transcription Kit, Roche, Germany) and T3 and T7 ribonucleic acid (RNA) polymerase for pBSmdr49 and pBSmdr65, respectively. After in vitro transcription, the pBSmdr65 probe was chopped by incubating it with 2× carbonate buffer at 65°C for 15 minutes, and the reaction was stopped with sodium acetate and acetic acid (Lehmann and Tautz 1994). This reduced the size of pBSmdr65 probe to facilitate penetration in the tissues. Antisense riboprobes were hybridized to cellular RNA, and hybridization signal was detected using anti-DIG-alkaline phosphatase as described earlier (Prasanth et al 2000; Rajendra et al 2001). All the images in this and Figures 3 and 4 were collected on a Nikon E800 microscope equipped with the Nikon digital camera DXM1200
It is known that promoters of human multidrug resistance genes harbor stress-responsive elements, which respond to heat shock, heavy metals, and cytostatic drugs (Chin et al 1990; Kioka et al 1992). To study expression of Drosophila mdr genes under heat stress, larvae were heat shocked at 37°C for 40 minutes and were either immediately dissected and different tissues were fixed or were allowed to recover from heat shock for different time periods at room temperature before tissue fixation. A 40-minute heat shock caused a significant increase in the expression of mdr49 in different larval tissues (Fig 1B,G,L) when compared with control tissues (Fig 1A,F,K). Interestingly, levels of the mdr49 transcripts continued to remain increased even after 30 minutes (Fig 1C,H,M) or 60 minutes recovery (Fig 1D,I,N) from heat shock. However, the expression returned to basal non–heat shock levels after 90 minutes recovery (Fig 1E,J,O). The levels of mdr65 transcripts, on the other hand, remained comparable with the control tissues (Fig 1A′,F′,K′), after heat shock (Fig 1B′,G′,L′), or after recovery from heat shock for 30 minutes (Fig 1C′,H′,M′), 60 minutes (Fig 1D′,I′,N′), or 90 minutes (Fig 1E′,J′,O′).
The induction of the heat shock genes in eukaryotes by heat and other forms of stress are mediated by the heat shock transcription factor (HSF) (Fernandes et al 1995). HSF has been shown to be 1 of the major regulators of human mdr1 gene expression (Kim et al 1997); it has been further shown that the expression was reduced when HSF was antagonized using quercitin (Kim et al 1998). In order to examine the involvement of HSF in the upregulation of mdr49 gene after heat shock, polytene chromosomes were immunostained with anti-HSF antibody. HSF was indeed found to be present at mdr49 gene locus on polytene chromosomes from heat shocked salivary glands (Fig 2A) suggesting that the increase in expression is most likely mediated via the HSF. However, HSF was not present at the 65A region, where mdr65 gene is located, either in control or in heat shocked salivary glands (Fig 2A′).
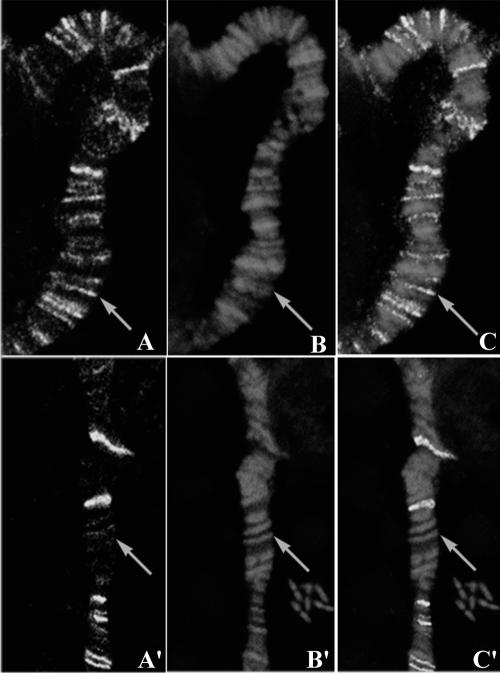
Fig 2.
Heat shock factor (HSF) localization on salivary gland polytene chromosomes. Salivary glands were dissected from wild-type late third instar larvae, heat shocked for 30 minutes at 37°C. Squash preparations were prepared following Dangli and Bautz (1983) and processed for immunostaining using the anti-HSF antibody (1:50 dilution, kind gift from Dr Carl Wu) as described earlier (Prasanth et al 2000). The signal was detected using Alexa Fluor 488 anti-mouse antibody (1:200 dilution, Molecular Probes Inc). Polytene chromosomes were counter stained with propidium iodide (PI). Preparations were examined under BIO-RAD Radiance 2000 confocal microscope using 60× oil immersion objective. A and A′ show the antibody localization on 2R and 3L chromosomes, respectively; B, B′ are same chromosome regions counterstained with PI. C and C′ show the merged images. Arrow points to the 49E band in A, B, C and 65A band in A′, B′, C′
Response of mdr49 and mdr65 to colchicine
One of the substrates transported by PGP is colchicine, and it has been widely used in isolating drug-resistant cell lines. Expression of mdr49 and mdr65 in response to dietary colchicine was examined to assess the potential role of these genes against colchicines toxicity. Drosophila larvae were fed on food containing nontoxic concentration (10 μM) of colchicine (Wu et al 1991), and the levels of mdr49 and mdr65 transcripts in larval tissues were examined by RNA-RNA in situ hybridization with the mdr49 and mdr65 riboprobes, respectively. Larvae fed on colchicine food showed increased expression of mdr49 in brain (Fig 3B) and gut (Fig 3F) compared with those not fed on colchicine (Fig 3A,E). The mdr65 riboprobe did not reveal any difference in expression of mdr65 gene between the colchicine-fed larval brain and gut (Fig 3D,H, respectively) and control larval brain and gut (Fig 3C,G, respectively). Imaginal discs and salivary glands did not show enhanced expression under these conditions with either of the probes (not shown).
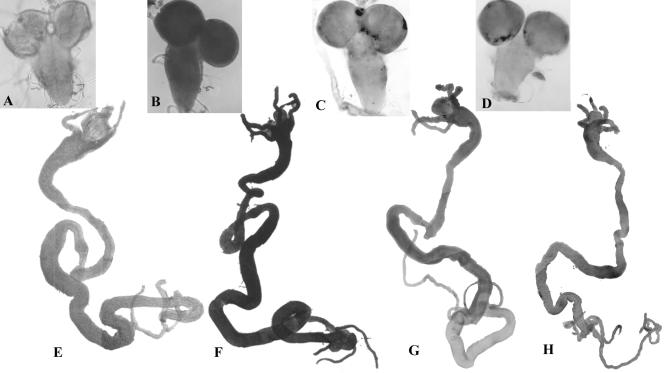
Fig 3.
Expression of mdr49 gene is enhanced upon colchicine feeding. Wild-type flies were allowed to lay eggs on food containing 10 M colchicine, and the larvae were grown on the same food. This concentration although not lethal slows the development. Whole organ RNA-RNA in situ was carried out using antisense riboprobe. There was an elevated level of mdr49 transcripts in brain (B) and gut (F) of colchicine-fed larvae when compared with control brain (A) and gut (E). Mdr65 riboprobe did not show any enhanced expression in brain (D) and gut (H) of colchicine-fed larvae when compared with control brain (C) and gut (G)
Overexpression of mdr genes in Drosophila l(2)gl4 mutant
The mdr genes have been shown to overexpress in mammalian tumor cells without any previous exposure to drugs (Chin et al 1992). It is postulated that the pathways leading to the tumor formation may somehow also trigger the mdr expression (Lee et al 1994). To assess whether the Drosophila mdr genes are also upregulated in response to tumor progression, larval tissues from wild-type and tumor suppressor mutant, lethal(2) giant larvae (l(2)gl4), were immunostained with anti-Mdr antibody. The l(2)gl4 mutation results in malignant transformation of late larval imaginal discs and larval brain (Mechler et al 1985). Compared with wild type (Fig 4A,G), the l(2)gl4 tumorous brain and imaginal discs (Fig 4B,H) showed enhanced levels of Mdr protein. RNA-RNA in situ hybridization with mdr49 and mdr65 riboprobes showed that, unlike heat shock and colchicine feeding, both mdr49 (Fig 4D,J) and mdr65 genes (Fig 4F,L) were overexpressed in l(2)gl4 larval tissues when compared with the expression of mdr49 (Fig 4C,I) and mdr65 (Fig 4E,K) genes in wild-type tissues.
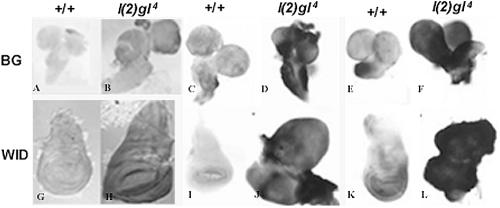
Fig 4.
Expression of P-glycoprotein (PGP) in wild-type and tumorous tissues of l(2)gl4 larvae. Immunostaining with PGP antibody (A,B,G,H) shows that compared with the wild-type (+/+) brain (A) and wing imaginal disc (G), the tumorous brain (B) and wing imaginal disc (H) from l(2)gl4 mutant larvae show enhanced expression of PGP. RNA-RNA in situ hybridization with mdr49 (C,D,I,J) or mdr65 (E,F,K,L) riboprobe to brain (BG) and wing imaginal disc (WID) of +/+ and l(2)gl4 larval tissues reveals enhanced expression of both mdr49 (D,J) and mdr65 (F,L) transcript levels in l(2)gl4 larval tissues. For whole organ immunostaining of the larval tissues, the PGP antibody (kind gift from Dr J. Croop) was used at 1:200 dilution and its binding was chromogenically detected using horseradish peroxidase–labeled anti-rabbit antibody as described earlier (Prasanth et al 2000)
Results of this study show that unlike in mouse, which shows tissue-specific expression of different mdr genes (Croop et al 1989; Hsu et al 1989), the mdr49 and mdr65 genes of Drosophila do not show any tissue specificity in their developmental expression. It needs to be examined further whether the third mdr gene (mdr50, Gerrard et al 1993) of D melanogaster shows a tissue-specific expression.
It is significant that nonphysiological conditions, such as heat shock, colchicine feeding, or tumorous development, evoke differential induction of the 2 mdr genes analyzed in this study. It has been shown in human cell lines and tissues that mdr genes use alternative promoters under different stimuli (Ueda et al 1997). Similarly distinct nuclear protein binding sites in the promoter of murine mdr gene have also been identified (Yu et al 1993). Analysis of promoter regions of the mdr genes of Drosophila may reflect differences in the regulation of these genes.
Thus, the Drosophila Mdr proteins have physiological roles as transporters for various endogenous compounds and also protect the organism against cytotoxic compounds and environmental insults. Because these functions are similar to those of the vertebrate Mdr protein, Drosophila provides a genetically amenable system to elucidate the complex regulation of mdr genes.
Acknowledgments
This work was supported by grant from Council for Scientific and Industrial Research to M.G.T. We thank the Department of Science and Technology, Government of India, for the Confocal Microscope facility. We would also like to thank Prof J. Croop for providing the mdr49, mdr65 clones and the P-glycoprotein antibody and for his valuable suggestions. We also thank Prof Carl Wu for providing HSF antibodies.
REFERENCES
- Arceci R, Croop J, Horwitz S, Housman D. The gene encoding multidrug resistance is induced and expressed at high levels during pregnancy in the secretory epithelium of the uterus. Proc Natl Acad Sci U S A. 1988;84:4350–4354. doi: 10.1073/pnas.85.12.4350.0027-8424(1988)084<4350:TGEMRI>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck WT. 1990 Mechanisms of multidrug resistance in human tumor cells. The roles of P-glycoprotein, DNA topoisomerase II, and other factors. Cancer Treat Rev. 17(Suppl A). 11–20. [DOI] [PubMed] [Google Scholar]
- Begun DJ, Whitely P. Genetics of α-amanitin resistance in a natural population of Drosophila melanogaster. Heredity. 2000;85:184–190. doi: 10.1046/j.1365-2540.2000.00729.x.0018-067X(2000)085<0184:GOARIA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Borst P, Schinkel AH. Genetic dissection of the function of mammalian P-glycoprotein. Trends Genet. 1997;13:217–222. doi: 10.1016/S0168-9525(97)01112-8.0168-9525(1997)013<0217:GDOTFO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Chin KV, Tanaka S, Darlington G, Pastan I, Gottesmann MM. Heat shock and arsenite increase expression of the multidrug resistance (MDR1) gene in human renal carcinoma cells. J Biol Chem. 1990;265:221–226.0021-9258(1990)265<0221:HSAAIE>2.0.CO;2 [PubMed] [Google Scholar]
- Chin KV, Ueda K, Pastan I, Gottesmann MM. Modulation of activity of the promoter of the human MDR1 gene by Ras and p53. Science. 1992;255:643–645. doi: 10.1126/science.1346476.0193-4511(1992)255<0643:MOAOTP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Croop JM, Raymond M, Haber D, Devault A, Areeci RJ, Gros P, Housman DE. The three mouse multidrug resistance (mdr) genes are expressed in a tissue specific manner in normal mouse tissues. Mol Cell Biol. 1989;9:1346–1350. doi: 10.1128/mcb.9.3.1346.0270-7306(1989)009<1346:TTMMRM>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangli A, Bautz EKF. Differential distribution of nonhistone proteins from polytene chromosomes of Drosophila melanogaster after heat shock. Chromosoma. 1983;88:201–207. doi: 10.1007/BF00285621.0009-5915(1983)088<0201:DDONPF>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Fernandes M, Xiao H, Lis JT. Binding of heat shock factor to and transcriptional activation of heat shock genes in Drosophila. Nucleic Acids Res. 1995;23:4799–4804. doi: 10.1093/nar/23.23.4799.0305-1048(1995)023<4799:BOHSFT>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard R, Stewart C, Dean M. Analysis of mdr50: a Drosophila P-glycoprotein/multidrug resistance gene homologues. Genomics. 1993;17:83–88. doi: 10.1006/geno.1993.1286.0888-7543(1993)017<0083:AOMADM>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Hsu SIH, Lothstein L, Horwitz SB. Differential overexpression of three mdr gene family members in multidrug-resistance J774.2 mouse cells. J Biol Chem. 1989;264:12053–12062.0021-9258(1989)264<12053:DOOTMG>2.0.CO;2 [PubMed] [Google Scholar]
- Juliano RL, Ling V. A surface glycoprotein modulating drug permeabiligy in Chinese hamster ovary cell mutants. Biochem Biophys Acta. 1976;455:152–162. doi: 10.1016/0005-2736(76)90160-7.0006-3002(1976)455<0152:ASGMDP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kim SH, Hur WY, Kang CD, Lim YS, Kim DW, Chung BS. Involvement of heat shock factor in regulating transcriptional activation of MDR1 gene in multidrug resistance cells. Cancer Lett. 1997;115:9–14. doi: 10.1016/s0304-3835(97)04725-3.0304-3835(1997)115<0009:IOHSFI>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kim SH, Yeo GS, Lim YS, Kang CD, Kim CM, Chung B. Suppression of MDT via inhibition of heat shock factor by quercitin I MDR cells. Exp Mol Med. 1998;30:87–92. doi: 10.1038/emm.1998.13.1226-3613(1998)030<0087:SOMVIO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Kioka N, Yamano Y, Komano T, Ueda K. Heat shock responsive elements in the induction of the multidrug resistance gene (MDR1) FEBS Lett. 1992;301:37–40. doi: 10.1016/0014-5793(92)80205-u.0014-5793(1992)301<0037:HSREIT>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lakhotia SC, Tapadia MG. Genetic mapping of the amide response element/s of the hsrω locus of Drosophila melanogaster. Chromosoma. 1998;107:127–135. doi: 10.1007/s004120050288.0009-5915(1998)107<0127:GMOTAR>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lee CH, Bradley G, Ling V. Expression of P-glycoprotein in normal and malignant rat liver cells. Cold Spring Harbor Symp Quant Biol. 1994;59:607–615. doi: 10.1101/sqb.1994.059.01.069.0091-7451(1994)059<0607:EOPINA>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Lehmann R, Tautz D. 1994 In situ hybridiztion to RNA. In: Drosophila melanogaster: Practical Uses in Cell and Molecular Biology. ed Goldstein LSB, Fyrberg EA. 576–597. [DOI] [PubMed] [Google Scholar]
- Ling V. 1993 P- glycoprotein mediated multidrug resistance to cancer chemotherapy. Adv Oncol 3–9. [Google Scholar]
- Ling V. 1997 Multidrug resistance: molecular mechanisms and clinical relevance. Cancer Chemother Pharmacol. 40(Suppl). S3–S8. [DOI] [PubMed] [Google Scholar]
- Mechler BM, McGinnis W, Gehring WJ. Molecular cloning of lethat(2)giant larvae, a recessive oncogene of Drosophila melanogaster. EMBO J. 1985;4:1551–1557. doi: 10.1002/j.1460-2075.1985.tb03816.x.0261-4189(1985)004<1551:MCOLLA>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prasanth KV, Rajendra TK, Lal AK, Lakhotia SC. Omega speckles—a novel class of nuclear speckles containing hnRNPs associated with non-coding hsr-omega RNA in Drosophila. J Cell Sci. 2000;113:3485–3497. doi: 10.1242/jcs.113.19.3485.0021-9533(2000)113<3485:OSNCON>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Rajendra TK, Prasanth KV, Lakhotia SC. Male sterility associated with overexpression of the non-coding hsrw gene in cyst cells of testis of Drosophila melanogaster. J Genet. 2001;80:939–949. doi: 10.1007/BF02728335.0022-1333(2001)080<0939:MSAWOO>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Roninson IB. The role of the MDR1 (P-glycoprotein) gene in multidrug resistance in vitro and in vivo. Biochem Pharmacol. 1992;43:95–102. doi: 10.1016/0006-2952(92)90666-7.0006-2952(1992)043<0095:TROTMP>2.0.CO;2 [DOI] [PubMed] [Google Scholar]
- Sukhai M, Piquette-Miller M. Regulation of the multidrug resistance genes by stress signals. J Pharm Pharm Sci. 2000;3:268–280.1482-1826(2000)003<0268:ROTMRG>2.0.CO;2 [PubMed] [Google Scholar]
- Thiebault T, Tsuruo T, Hamada H, Gottesman M, Pastan I, Willingham H. Cellular localization of the multidrug resistance gene product P-glycoprotein in normal tissues. Proc Natl Acad Sci U S A. 1987;84:7735–7738. doi: 10.1073/pnas.84.21.7735.0027-8424(1987)084<7735:CLOTMR>2.0.CO;2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K, Pastan I, Gottesman MM. Isolation and sequence of the promoter region of the human MDR (PGP) gene. J Biol Chem. 1987;262:17432–17436.0021-9258(1987)262<17432:IASOTP>2.0.CO;2 [PubMed] [Google Scholar]
- Wu C-T, Budding M, Griffin MS, and Croop JM. 1991 Isolation and characterization of Drosophila multidrug resistance gene homologus. Mol Cell Biol 3940–3948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu L, Cohen D, Piekarz RL, Horwitz SB. Three distince nuclear protein binding sites in the promoter of the murine mdr1b gene. J Biol Chem. 1993;268:7520–7526.0021-9258(1993)268<7520:TDNPBS>2.0.CO;2 [PubMed] [Google Scholar]