Abstract
Although cannabidiol and tetrahydrocannabinol in Cannabis species exert their pharmacological effects via the endocannabinoid system, it is believed that other phytochemicals, particularly terpenes, can modulate therapeutic outcomes through the entourage effect. Therefore, to gain a better understanding of the pharmacological effects of Cannabis, obtaining information on phytochemical compositions, including mono-, di-, and sesqui-terpenes in Cannabis species is essential. Applying a sophisticated analytical method is indispensable. In this study, headspace-gas chromatography/mass spectrometry (HS-GC/MS) was employed to identify major terpenes in the leaves and inflorescences of hybrid Cannabis species. The incubation time and temperature conditions for HS-GC/MS were optimized. This method was successfully applied to the leaves (n = 9) and inflorescences (n = 7) of hybrid Cannabis species. A total of 26 terpenes in Cannabis species were detected, and six major components, such as α-pinene (9.8–2270 μg/g), β-pinene (2.6–930 μg/g), myrcene (0.7–17,400 μg/g), limonene (1.3–300 μg/g), β-caryophyllene (60–3300 μg/g), and α-humulene (40–870 μg/g), were quantified. Each sample showed different terpene compositions, but six major terpenes among all the terpenes detected were consistently found in both the leaves and inflorescences of hybrid Cannabis species. In this study, the six major terpenes’ potential in hybrid Cannabis species was evaluated as biomarkers to distinguish hybrid Cannabis species samples. This study contributes to a better understanding of the entourage effect of Cannabis-based botanical drugs.
Keywords: hybrid Cannabis species, terpenes, leaf, inflorescence, headspace, gas chromatography, mass spectrometry, statistical analysis
1. Introduction
Cannabis species contain various bioactive phytochemicals, categorized as cannabinoids and non-cannabinoids [1], used for food, medicine, and even ornamental plants [2]. Their unique pharmacological effects have generated increased interest in many areas, including academia, industry, and the government. Among the bioactive phytochemicals in Cannabis, tetrahydrocannabinol (THC) and cannabidiol (CBD) have psychoactive effects by binding to endocannabinoid receptors [3] and therapeutic effects for epilepsy, pain, and drug addiction [4]. Although both THC and CBD are known to be the most potent bioactive compounds, other phytochemicals in Cannabis species also exhibit characteristic effects on the human body [5]. In particular, terpenes reportedly contribute to the entourage effect, which can modulate the unique psychoactive effects of cannabinoids such as THC and CBD [6]. Therefore, to better understand the entourage effect, identifying and quantifying not only cannabinoids but also terpenes in Cannabis species is crucial.
To enhance their psychoactive and therapeutic effects, crossbreeding different Cannabis species has become an industrial and commercial preference rather than cultivating original Cannabis species. Consequently, in the Cannabis industry and market, finding landrace Cannabis that is not hybrid Cannabis is challenging [7]. Hybrid Cannabis species are known to have distinct effects and are promoted and sold based on their unique entourage effects. Although cannabinoid contents (THC and CBD) in individual hybrid Cannabis species have been presented, providing the terpene contents in these strains to understand the characteristic entourage effect of individual hybrid Cannabis is essential.
Numerous analytical methods have been developed to determine terpenes in Cannabis plants [8,9,10,11,12]. Although high-performance liquid chromatography (HPLC) has been used to determine various bioactive phytochemicals in Cannabis species [13,14,15], gas chromatography (GC) has been widely employed to analyze terpenes contributing to the fragrance and flavor of products [16]. In particular, flame ionization detection (FID) realizes a simple and easy operation method combined with GC, while mass spectrometry (MS) provides reliable qualification and quantification results [17]. To analyze volatile terpenes in Cannabis plants, a delicate sample preparation method should be performed to extract volatile terpenes without significant losses. Solid-phase microextraction (SPME) has been widely used to extract terpenes from natural products [18,19] since it is one of the representative methods to extract volatile compounds from various matrices. Nonetheless, optimization procedures for SPME conditions (such as temperature, solvents, and fibers) should precede sample application and be accompanied by intensive labor and time [20]. A headspace (HS)-SPME method may serve as an alternate simple sample preparation method with automated operation and no solvent usage [21]. Another excellent alternative might be an automated HS method, allowing for direct extraction of volatile compounds from various matrices without needing fibers and solvents [22]. Consequently, automated HS methods have been widely employed to extract bioactive volatile phytochemicals from plant samples [11,23,24].
In this study, we developed an HS-GC/MS method to determine volatile terpenes in the leaves (n = 9) and inflorescences (n = 7) of hybrid Cannabis. Twenty-six terpenes in hybrid Cannabis samples were detected and identified using this method. The developed HS-GC/MS method was optimized and validated to quantify six major and abundant terpenes, including α- and β-pinene, myrcene, limonene, β-caryophyllene, and α-humulene, in the leaves and inflorescences of hybrid Cannabis species. Since individual Cannabis samples exhibited characteristic distributions for terpenes, even in leaves or inflorescences, they could not be categorized into similar distributions. In conclusion, this study provides characteristic terpene distributions and quantification results for six major terpenes in the leaves and inflorescences of hybrid Cannabis. Furthermore, this study helps to better understand the characteristic entourage effect of terpenes in individual hybrid Cannabis.
2. Results and Discussion
2.1. Optimization of HS-GC/MS Conditions
Although GC/FID provides several advantages, such as ease, simplicity, and low cost, GC/MS has been the ‘gold standard’ for identifying and quantifying volatile phytochemicals in plant samples due to its indispensable sensitivity and selectivity [25]. Moreover, when combined with an automated HS system, a GC/MS method reduces the need for labor, minimizes processing time, and decreases the use of harmful solvents. Therefore, in this study, we employed an HS-GC/MS method to identify and quantify terpenes in the leaves and inflorescences of hybrid Cannabis.
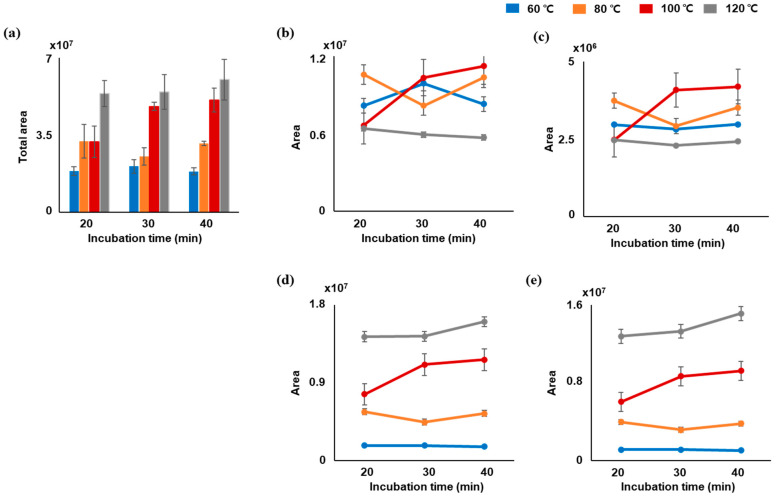
To efficiently extract terpenes from the leaf and inflorescence samples, the HS conditions were optimized in terms of incubation time and temperature using a standard solution of representative terpenes, including α-pinene, myrcene, β-caryophyllene, and α-humulene. As shown in Figure 1, the targeted terpenes were more affected by temperature than by incubation time. In particular, the most effective total area for terpene extraction was at 120 °C. However, it should be noted that terpenes with high volatility, such as α-pinene and myrcene, exhibited lower levels than other investigated temperatures. This finding might be attributed to the degradation of terpenes caused by high temperatures [26]. Although the incubation time parameter had a lesser influence on terpene extraction efficiency, an incubation time of 30 min at 100 °C was shown to be effective.
Figure 1.
Influence of incubation time and headspace temperature on terpene extraction efficiency in leaf samples of Victory: (a) total peak area of terpenes and individual peak area of terpenes such as (b) α-pinene, (c) myrcene, (d) β-caryophyllene, and (e) α-humulene.
Furthermore, we preliminarily investigated total ion chromatograms of representative hybrid Cannabis leaf samples at varying incubation temperatures, where incubation was performed for 30 min. As shown in Figure S1, peak areas for all terpenes in leaf samples increased until 100 °C, while highly volatile terpenes (early eluted) were degraded at 120 °C. Moreover, signal noise, resulting from matrix influences, increased at 120 °C. Therefore, we selected 100 °C and 30 min as the optimal incubation temperature and time, respectively.
2.2. Investigation of Terpenes in the Leaves and Inflorescences
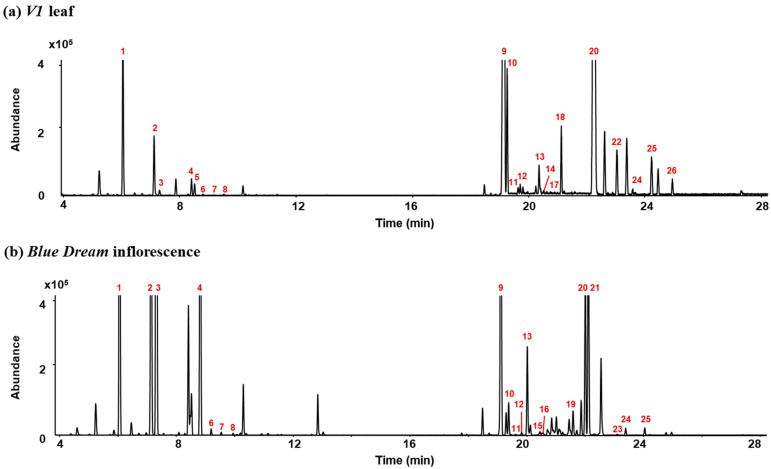
The optimized HS-GC/MS method was preliminarily applied to collected leaf and inflorescence samples of hybrid Cannabis. As shown in Figure 2, a total of 26 terpenes were detected in leaf and inflorescence samples using HS-GC/MS. The detected terpenes were identified based on the National Institute of Standards and Technology (NIST) database and their mass spectral patterns. To further confirm the identified terpenes, the Kovats index (KI) was calculated using an alkane standard solution (C8–C20) and compared to reference KI [27]. The KI and characteristic ions for the 26 terpenes in hybrid Cannabis are summarized in Table 1.
Figure 2.
Total ion chromatograms for (a) V1 leaf and (b) Blue Dream inflorescence samples of hybrid Cannabis. (Peak identities are as follows: 1. α-Pinene, 2. β-Pinene, 3. Myrcene, 4. Limonene, 5. Eucalyptol, 6. E-β-Ocimene, 7. γ-Terpinene, 8. Z-Sabinene hydrate, 9. β-Caryophyllene, 10. α-Bergamotene, 11. α-Guaiene, 12. E-β-Farnesene, 13. α-Humulene, 14. Alloaromadrene, 15. β-Selinene, 16. α-Selinene, 17. Z,E-α-Farnesene, 18. β-Bisabolene, 19. β-sesquiphellandrene, 20. E-α-Bisabolene, 21. Selina-3,7(11)-diene, 22. Caryophyllene oxide, 23. Guaiol, 24. γ-Eudesmol, 25. Bulnesol, 26. α-Bisabolol).
Table 1.
Retention times, the Kovats index (KI), and characteristic ions of 26 terpenes in hybrid Cannabis species.
| Elution Order | Compound Name | M.W | RT (Min) |
KI calc. | KI Ref | Characteristic Ions m/z
(Relative Abundance%) |
|---|---|---|---|---|---|---|
| 1 | α-Pinene | 136 | 6.06 | 935 | 936 | 136 (10), 121 (15), 105 (10), 93 (100), 91 (40), 79 (25), 77 (30) |
| 2 | β-Pinene | 136 | 7.13 | 981 | 978 | 136 (10), 121 (15), 93 (100), 91 (25), 79 (20), 77 (20), 69 (25) |
| 3 | Myrcene | 136 | 7.32 | 989 | 989 | 136 (5), 121 (5), 93 (100), 91 (25), 79 (15), 77 (15), 69 (70), 41 (75) |
| 4 | Limonene | 136 | 8.42 | 1031 | 1030 | 136 (25), 121 (25), 107 (25), 93 (75), 79 (35), 68 (100), 67 (70) |
| 5 | Eucalyptol | 154 | 8.53 | 1035 | 1032 | 154 (70), 139 (60), 125 (15), 111 (80), 93 (60), 81 (90), 71 (70), 55 (40), 43 (100) |
| 6 | E-β-Ocimene | 136 | 8.82 | 1046 | 1048 | 136 (5), 121 (20), 105 (20), 93 (100), 91 (45), 80 (35), 79 (40) |
| 7 | γ-Terpinene | 136 | 9.19 | 1060 | 1060 | 136 (40), 119 (50), 105 (15), 93 (100), 77 (35), 91 (60) |
| 8 | Z-Sabinene hydrate | 154 | 9.53 | 1072 | 1067 | 154 (5), 136 (25), 121 (25), 111 (15), 93 (100), 77 (35), 43 (25) |
| 9 | β-Caryophyllene | 204 | 19.13 | 1426 | 1420 | 204 (10), 189 (25), 175 (15), 161 (45), 147 (30), 133 (95), 120 (45), 105 (60), 93 (100), 79 (75) |
| 10 | trans-α-Bergamotene | 204 | 19.37 | 1435 | 1435 | 204 (5), 189 (5), 161 (5), 119 (100), 107 (30), 93 (95), 79 (25), 69 (35) |
| 11 | α-Guaiene | 204 | 19.45 | 1438 | 1440 | 204 (55), 189 (35), 161 (25), 147 (90), 133 (65), 119 (45), 105 (100), 93 (75), 79 (60), |
| 12 | E-β-Farnesene | 204 | 19.81 | 1453 | 1456 | 204 (5), 189 (5), 161 (15), 133 (30), 120 (25), 107 (10), 93 (65), 79 (25), 69 (100) |
| 13 | α-Humulene | 204 | 20.02 | 1461 | 1453 | 204 (10), 189 (5), 161 (5), 147 (20), 121 (40), 107 (15), 93 (100), 80 (30), 67 (10) |
| 14 | Alloaromadrene | 204 | 20.12 | 1465 | 1460 | 204 (45), 189 (35), 175 (10), 161 (100), 147 (50), 133 (70), 119 (60), 105 (90), 91 (100) |
| 15 | β-Selinene | 204 | 20.85 | 1494 | 1486 | 204 (70), 189 (60), 175 (30), 161 (65), 147 (50), 133 (50), 121 (60), 105 (100), 93 (90) |
| 16 | α-Selinene | 204 | 21.01 | 1500 | 1493 | 204 (50), 189 (100),175 (30), 161 (35), 133 (50), 121 (25), 107 (55), 93 (55) |
| 17 | Z,E-α-Farnesene | 204 | 21.10 | 1504 | 1504 | 204 (5), 161 (10),135 (10), 123 (35), 119 (50), 107 (50), 93 (100), 79 (45), 69 (50) |
| 18 | β-Bisabolene | 204 | 21.23 | 1509 | 1508 | 204 (20), 189 (5), 161 (20), 133 (10), 119 (25), 109 (30), 93 (85), 79 (35), 69 (100) |
| 19 | β-sesquiphellandrene | 204 | 21.62 | 1526 | 1524 | 204 (30), 189 (5), 161 (60), 133 (40), 120 (30), 109 (30), 93 (70), 69 (100) |
| 20 | E-α-Bisabolene | 204 | 22.00 | 1542 | 1540 | 204 (20), 189 (5), 161 (5), 147 (5), 136 (10), 119 (30), 109 (25), 93 (100), 78 (25) |
| 21 | Selina-3,7(11)-diene | 204 | 22.11 | 1546 | 1541 | 204 (55), 189 (25), 161 (100), 133 (20), 122 (60), 107 (50), 91 (30), 81 (20) |
| 22 | Caryophyllene oxide | 222 | 23.10 | 1587 | 1581 | 205 (10), 202 (20), 187 (40), 161 (35), 149 (30), 133 (45), 119 (40), 105 (65), 91 (100), 79 (85) |
| 23 | Guaiol | 222 | 23.38 | 1599 | 1597 | 222 (5), 204 (25), 189 (25), 161 (100), 147 (20), 133 (25), 119 (25), 105 (60), 91 (50) |
| 24 | γ-Eudesmol | 222 | 24.04 | 1629 | 1631 | 222 (5), 204 (60), 189 (100), 161 (80), 147 (25), 133 (60), 119 (20), 105 (45), 91 (50) |
| 25 | Bulnesol | 222 | 24.96 | 1669 | 1666 | 222 (5), 204 (30), 189 (35), 161 (55), 147 (25), 135 (75), 119 (45), 107 (100), 93 (85), |
| 26 | α-Bisabolol | 222 | 25.38 | 1688 | 1683 | 204 (30), 189 (5), 161 (20), 135 (10), 119 (90), 109 (95), 93 (85), 79 (40), 69 (100) |
To investigate the individual terpene compositions of leaf and inflorescence samples in hybrid Cannabis species, the relative abundance of all peaks were calculated based on the total ion chromatograms’ area under the curve. As shown in Table 2, several terpenes, including α- and β-pinenes, myrcene, limonene, β-caryophyllene, bergamotene, and α-humulene, were presented in all leaf samples of hybrid Cannabis. However, the guaiol terpene was not detected in all leaves, and bulnesol was only detected in the Blue Dream variety. The overall terpene compositions in inflorescence samples are shown in Table 3. As shown in Table 3, most terpenes were detected in all inflorescences of hybrid Cannabis species, except for the bulnesol terpene, which was only detected in Blue Dream, similar to the leaf samples. As shown in Table 2 and Table 3, even though some leaf and inflorescence samples originated from the same hybrid Cannabis species, including White Widow, individual terpene compositions substantially differed between leaves and inflorescences. To investigate the relationship between plant organs and/or hybrid Cannabis species, all hybrid Cannabis plants should be grown under uniform growing conditions since spatial differences, organs, and locations can influence individual terpene accumulation [28]. Among the identified 26 terpenes, six monoterpenes (including α- and β-pinenes, myrcene, limonene, β-caryophyllene, and α-humulene) were commonly detected in both the leaves and inflorescences of hybrid Cannabis species. These six terpenes were well-known as predominant terpenes [29] and may contribute to the “entourage effect” of cannabinoids [30].
Table 2.
Relative abundances of 26 terpenes in leaves of hybrid Cannabis.
| Elution Order | Compound Name | Relative Abundance (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Cherry Blossom | V1 | V4 | White Widow | Chung Sam | Blue Dream | Bubble Gum | Purple | Victory | ||
| 1 | α-Pinene | 28 ± 8 | 50 ± 30 | 40 ± 20 | 2.29 ± 0.04 | 4.8 ± 0.5 | 20 ± 10 | 5 ± 1 | 38 ± 5 | 17 ± 1 |
| 2 | β-Pinene | 6.6 ± 0.3 | 15 ± 3 | 13 ± 5 | 1.75 ± 0.09 | 2.0 ± 0.5 | 7 ± 5 | 7 ± 1 | 14 ± 2 | 6.5 ± 0.8 |
| 3 | Myrcene | 6.5 ± 0.2 | 1.14 ± 0.02 | 2.5 ± 0.2 | 4.41 ± 0.06 | 0.08 ± 0.03 | 10 ± 5 | 9 ± 2 | 17 ± 2 | 14 ± 2 |
| 4 | Limonene | 5 ± 1 | 2.5 ± 0.6 | 6.3 ± 0.2 | 6.5 ± 0.1 | 1.9 ± 0.6 | 4 ± 2 | 19 ± 3 | 3.4 ± 0.4 | 3.6 ± 0.6 |
| 5 | Eucalyptol | 0.04± 0.04 | 1.2 ± 0.9 | 1.3 ± 0.5 | 20 ± 5 | ND | 3 ± 2 | ND | ND | 0.68 ± 0.02 |
| 6 | E-β-Ocimene | 0.6 ± 0.3 | 0 ± 1 | 0.47 ± 0.08 | ND | ND | 1.9 ± 0.6 | ND | ND | ND |
| 7 | γ-Terpinene | ND | 0.1 ± 0.2 | 0.05 ± 0.07 | 0.40 ± 0.09 | ND | 0.6 ± 0.1 | ND | 0.02 ± 0.01 | 0.13 ± 0.03 |
| 8 | Z-Sabinene hydrate | ND | 0 ± 1 | 0.3 ± 0.5 | 0 ± 2 | ND | 1.0 ± 0.8 | ND | 0.08 ± 0.05 | 0.4 ± 0.2 |
| 9 | β-Caryophyllene | 21 ± 3 | 10 ± 20 | 10 ± 30 | 22 ± 5 | 42 ± 5 | 20 ± 10 | 13 ± 3 | 7 ± 3 | 18 ± 6 |
| 10 | trans-α-Bergamotene | 4 ± 7 | 5 ± 9 | 4 ± 9 | 1.6 ± 0.5 | 6.3 ± 0.7 | 3.7 ± 0.3 | 5 ± 3 | 2.6 ± 0.4 | 5 ± 2 |
| 11 | α-Guaiene | 0.2 ± 0.3 | <0.01 | ND | 0.31 ± 0.01 | ND | ND | ND | ND | ND |
| 12 | E-β-Farnesene | 2 ± 4 | 1 ± 2 | 0.3 ± 0.7 | 0.9 ± 0.1 | ND | 0.62 ± 0.08 | 0.5 ± 0.5 | ND | 2 ± 1 |
| 13 | α-Humulene | 16 ± 27 | 10 ± 20 | 10 ± 20 | 16 ± 4 | 33 ± 4 | 12.0 ± 0.3 | 18 ± 8 | 7.0 ± 0.2 | 15 ± 2 |
| 14 | Alloaromadrene | 0.2 ± 0.3 | 0 ± 1 | 0 ± 1 | 0.4 ± 0.2 | ND | 0.3 ± 0.3 | ND | 0.17 ± 0.09 | 1.0 ± 0.3 |
| 15 | β-Selinene | 0.3 ± 0.5 | ND | ND | 1.0 ± 0.4 | ND | 0.22 ± 0.03 | 0.57 ± 0.06 | 0.1 ± 0.1 | 0.7 ± 0.8 |
| 16 | α-Selinene | 0.3 ± 0.1 | ND | ND | 1.2 ± 0.3 | ND | ND | ND | 0.23 ± 0.01 | 0.90 ± 0.07 |
| 17 | Z,E-α-Farnesene | 4 ± 7 | 0.12 ± 0.07 | 0.13 ± 0.05 | ND | ND | ND | 1.4 ± 0.8 | 2 ± 1 | ND |
| 18 | β-Bisabolene | 4 ± 7 | 0.3 ± 0.8 | 0.3 ± 0.8 | 3.0 ± 0.9 | 3.4 ± 0.4 | 1.2 ± 0.6 | ND | 0.4 ± 0.1 | 3.8 ± 0.8 |
| 19 | β-sesquiphellandrene | ND | ND | <0.01 | ND | <0.01 | ND | ND | ND | 0.30 ± 0.07 |
| 20 | E-α-Bisabolene | ND | 1 ± 2 | 1 ± 2 | ND | 1.1 ± 0.1 | ND | 9 ± 6 | ND | 8 ± 2 |
| 21 | Selina-3,7(11)-diene | ND | ND | ND | 17 ± 3 | 0.6 ± 0.4 | 5 ± 3 | 9 ± 3 | 6.6 ± 0.2 | 2.7 ± 0.1 |
| 22 | Caryophyllene oxide | 0.43 ± 0.09 | 1 ± 3 | 2 ± 6 | 0.1 ± 0.1 | 4 ± 1 | ND | ND | <0.01 | 0.02 ± 0.01 |
| 23 | Guaiol | ND | ND | ND | ND | ND | ND | ND | ND | ND |
| 24 | γ-Eudesmol | ND | 0 ± 2 | 0 ± 1 | ND | ND | 0.2 ± 0.1 | ND | ND | ND |
| 25 | Bulnesol | ND | 0.1 ± 0.3 | ND | ND | ND | ND | ND | ND | ND |
| 26 | α-Bisabolol | 1 ± 3 | 0.1 ± 0.4 | ND | 0.8 ± 0.7 | 1 ± 1 | 0.2 ± 0.1 | ND | 0.09 ± 0.01 | 0.5 ± 0.9 |
ND means “not detected”.
Table 3.
Relative abundance of 26 terpenes in the inflorescences of hybrid Cannabis.
| Elution Order | Compound Name | Relative Abundance (%) | ||||||
|---|---|---|---|---|---|---|---|---|
| Cherry Blossom | V1 | V4 | White Widow | Chung Sam | Blue Dream | Bubble Gum | ||
| 1 | α-Pinene | 29 ± 8 | 18 ± 7 | 12 ± 4 | 3 ± 1 | 22.8 ± 0.5 | 20 ± 10 | 6 ± 1 |
| 2 | β-Pinene | 10 ± 10 | 10 ± 6 | 8 ± 4 | 5 ± 3 | 3.8 ± 0.4 | 9 ± 3 | 10 ± 2 |
| 3 | Myrcene | 40 ± 30 | 50 ± 20 | 2 ± 1 | 40 ± 10 | 0.13 ± 0.02 | 41 ± 5 | 16 ± 2 |
| 4 | Limonene | 6 ± 3 | 6.6 ± 0.9 | 10 ± 6 | 13 ± 9 | 0.2 ± 0.2 | 2.3 ± 0.6 | 35 ± 1 |
| 5 | Eucalyptol | ND | 0.06 ± 0.08 | ND | 0.5 ± 0.4 | ND | ND | <0.01 |
| 6 | E-β-Ocimene | 5 ± 2 | 9.9 ± 0.5 | 0.9 ± 0.7 | 3 ± 1 | ND | 9 ± 2 | ND |
| 7 | γ-Terpinene | 0.0 ± 0.1 | 0.07 ± 0.03 | 1.1 ± 0.8 | 0.19 ± 0.02 | 0.06 ± 0.03 | 0.17 ± 0.01 | 0.07 ± 0.05 |
| 8 | Z-Sabinene hydrate | 0.02 ± 0.01 | 0.08 ± 0.01 | 0.2 ± 0.2 | 0.11 ± 0.06 | <0.01 | 0.09 ± 0.01 | ND |
| 9 | β-Caryophyllene | 3 ± 1 | 0.7 ± 0.5 | 20 ± 10 | 14.0 ± 0.9 | 31 ± 4 | 6 ± 4 | 10.2 ± 0.3 |
| 10 | trans-α-Bergamotene | 0.9 ± 0.4 | 0.09 ± 0.06 | 6.4 ± 0.9 | 0.4 ± 0.2 | 8 ± 5 | 0.66 ± 0.03 | 0.47 ± 0.03 |
| 11 | α-Guaiene | 0.21 ± 0.09 | 0.19 ± 0.03 | 0.0 ± 0.2 | 2.4 ± 0.3 | 0.9 ± 0.1 | <0.01 | <0.01 |
| 12 | E-β-Farnesene | 1.3 ± 0.6 | 0.05 ± 0.03 | 0.77 ± 0.09 | 0.5 ± 0.3 | 0.22 ± 0.04 | 0.08 ± 0.01 | 0.12 ± 0.09 |
| 13 | α-Humulene | 2.1 ± 0.9 | 0.5 ± 0.5 | 21 ± 2 | 11 ± 2 | 24 ± 4 | 4 ± 2 | 7.4 ± 0.1 |
| 14 | Alloaromadrene | 0.13 ± 0.05 | <0.01 | 0.4 ± 0.2 | 0.11 ± 0.04 | ND | 0.09 ± 0.01 | ND |
| 15 | β-Selinene | 0.04 ± 0.02 | 0.03 ± 0.01 | 0.3 ± 0.2 | 0.8 ± 0.2 | 1 ± 1 | 0.20 ± 0.03 | 0.75 ± 0.07 |
| 16 | α-Selinene | 0.04 ± 0.01 | 0.04 ± 0.09 | 0.29 ± 0.06 | 1 ± 1 | 1.5 ± 0.2 | 0.22 ± 0.02 | 0.9 ± 0.4 |
| 17 | Z,E-α-Farnesene | 0.38 ± 0.09 | 0.2 ± 0.3 | 2.6 ± 0.3 | 2.0 ± 0.6 | 1.40 ± 0.06 | 0.13 ± 0.03 | 0.7 ± 0.1 |
| 18 | β-Bisabolene | 0.5 ± 0.2 | 0.02 ± 0.01 | 2.9 ± 0.4 | 0 ± 1 | 0.3 ± 0.3 | 0.06 ± 0.02 | 0.08 ± 0.05 |
| 19 | β-sesquiphellandrene | 0.09 ± 0.04 | ND | ND | 0.1 ± 0.1 | ND | 0.05 ± 0.03 | ND |
| 20 | E-α-Bisabolene | 0.6 ± 0.3 | 0.0 ± 0.2 | 3.9 ± 0.5 | 2 ± 2 | 4 ± 3 | 1.7 ± 0.5 | ND |
| 21 | Selina-3,7(11)-diene | ND | ND | 0.3 ± 0.1 | 3 ± 2 | 0.1 ± 0.5 | 1.5 ± 0.3 | 12 ± 1 |
| 22 | Caryophyllene oxide | <0.01 | 0.0 ± 0.1 | 0.7 ± 0.4 | 0.06 ± 0.01 | 0.2 ± 0.2 | ND | ND |
| 23 | Guaiol | 0.03 ± 0.02 | 0.1 ± 0.2 | 0.3 ± 0.1 | ND | ND | 0.12 ± 0.08 | ND |
| 24 | γ-Eudesmol | 0.05 ± 0.03 | 0.05 ± 0.01 | 0.5 ± 0.3 | ND | ND | 0.2 ± 0.2 | ND |
| 25 | Bulnesol | ND | ND | ND | ND | ND | 0.07 ± 0.05 | ND |
| 26 | α-Bisabolol | ND | ND | 0.4 ± 0.7 | 0.15 ± 0.06 | 0.03 ± 0.01 | ND | ND |
ND means “not detected”.
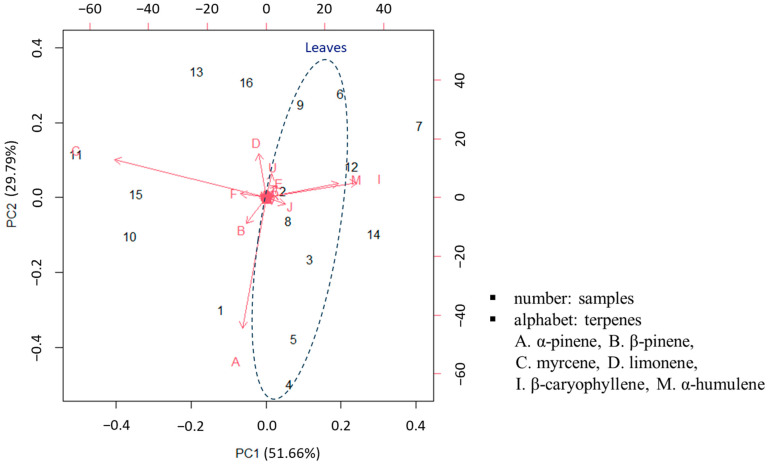
Based on the relative abundance of peak areas for terpenes, hierarchical cluster analysis (HCA) was performed to cluster organs (leaves and inflorescences) in hybrid Cannabis (Figure S2). Most leaf and inflorescence samples of hybrid Cannabis species could not be clustered by individual strains except for Bubble Gum. However, we speculated that the terpene compositions may be related to their organ types (leaves and inflorescences). Furthermore, principal component analysis (PCA) was also performed on the data set without scaling to find major terpenes, which can distinguish individual leaf and inflorescence samples of hybrid Cannabis. Based on PCA results, 51.66% and 29.79% of the variance was explained by PC1 and PC2, respectively. As shown in Figure 3, leaf samples could be grouped. Furthermore, samples from the leaves and inflorescences of White Widow and Blue Dream could be separated since individual leaves or inflorescences had characteristic terpene compositions, respectively. Six terpenes (α- and β-pinenes, myrcene, limonene, β-caryophyllene, and α-humulene) out of 26 terpenes have greater potential to identify individual leaves and inflorescence samples of hybrid Cannabis than other terpenes. These six terpenes would likely contribute to variance explanations for PC1 and PC2 since they were consistently and predominantly present in all leaves and inflorescences of hybrid Cannabis. Although several characteristic terpenes (such as guaiol found in Blue Dream inflorescences) may also be potential markers, they cannot separate all leaf and inflorescence samples of hybrid Cannabis species. Therefore, in this study, we quantified the six major and abundant terpenes as biomarkers using HS-GC/MS.
Figure 3.
Principal component analysis (PCA) of hybrid Cannabis using relative abundances of peak areas for 26 terpenes. (Marks were identified as follows: 1. Cherry Blossom leaf, 2. V1 leaf, 3. V4 leaf, 4. White Widow leaf, 5. Chung Sam leaf, 6. Blue Dream leaf, 7. Bubble Gum leaf, 8. Purple leaf, 9. Victory leaf, 10. Cherry Blossom inflorescence, 11. V1 inflorescence, 12. V4 inflorescence, 13. White Widow inflorescence, 14. Chung Sam inflorescence, 15. Blue Dream inflorescence, 16. Bubble Gum inflorescence, A. α-Pinene, B. β-Pinene, C. Myrcene, D. Limonene, E. Eucalyptol, F. E-β-Ocimene, G. γ-Terpinene, H. Z-Sabinene hydrate I. β-Caryophyllene, J. α-Bergamotene, K. α-Guaiene, L. β-Farnesene (E), M. α-Humulene, N. Alloaromadrene, O. β-Selinene, P. α-Selinene, Q. Z,E-α-Farnesene, R. β-Bisabolene, S. β-sesquiphellandrene, T. E-α-Bisabolene, U. Selina-3,7(11)-diene, V. Caryophyllene oxide, W. Guaiol, X. γ-Eudesmol, Y. Bulnesol, Z. α-Bisabolol).
To quantify the six major terpenes in the leaves and inflorescences of hybrid Cannabis species, commercially available authentic terpene standards were employed. The HS-GC/MS method was validated in terms of quantification limits, calibration range, linearity, precision, and accuracy. Table S1 summarizes the overall data and validation results for quantifying six major terpenes using the HS-GC/MS method.
2.3. Quantification of the Six Major Terpenes in the Leaves and Inflorescences of Hybrid Cannabis Species
In this study, the validated HS-GC/MS method was employed to determine six major terpenes in the leaves (n = 9) and inflorescences (n = 7) of hybrid Cannabis species. As depicted in Table 4, quantified terpenes were found to be highly presented in the inflorescence sample of Cherry Blossom compared to other hybrid Cannabis. Furthermore, the quantification results for most terpenes in inflorescences were higher than in leaves. In the inflorescences of White Widow, Chung Sam, and V4, β-caryophyllene content was the most abundant. The biochemical diversity of terpenes in Cannabis makes it challenging to predict the pharmacological “entourage effect” of Cannabis. Therefore, the quantification results of the six terpenes with characteristic bioactive effects may help demonstrate their distinctive therapeutic outcomes and the “entourage effect” of individual leaf and inflorescence samples of hybrid Cannabis. For example, since α-pinene has antioxidative and anti-inflammatory effects [31,32,33], the inflorescence of Cherry Blossom may be more effective at relieving pain when combined with cannabidiol in Cannabis [34]. Furthermore, due to the analgesic and anti-cancer effects of β-caryophyllene [35], several hybrid Cannabis, including inflorescences of White Widow, Chung Sam, V4, and Cherry Blossom and leaves of Cherry Blossom and Victory, may be more suitable for cancer patients. Since both myrcene and limonene are flavor and fragrance chemicals, the inflorescences of Cherry Blossom, V1, White Widow, and Bubble Gum may be widely preferred by Cannabis users for their potential to inhibit Cannabis use disorders, including vomiting and nausea. Table 4 summarizes the overall calculated quantification results for the six terpenes in all leaves and inflorescences of hybrid Cannabis.
Table 4.
Quantification results for six major terpenes in the leaf and inflorescence samples of hybrid Cannabis.
| Strains | Organ | Concentrations (μg/g) | |||||
|---|---|---|---|---|---|---|---|
| α-Pinene | β-Pinene | Myrcene | Limonene | β-Caryophyllene | α-Humulene | ||
| Cherry Blossom | leaf | 144 ± 8 | 32.9 ± 0.5 | 187 ± 7 | 14 ± 1 | 1200 ± 600 | 300 ± 100 |
| inflorescence | 2270 ± 70 | 930 ± 20 | 17,400 ± 300 | 260 ± 20 | 2000 ± 100 | 500 ± 30 | |
| V1 | leaf | 100 ± 10 | 80 ± 60 | 10 ± 10 | 8 ± 6 | 220 ± 20 | 66 ± 4 |
| inflorescence | 500 ± 100 | 290 ± 60 | 4600 ± 400 | 115 ± 9 | 200 ± 10 | 40 ± 30 | |
| V4 | leaf | 84 ± 5 | 27 ± 3 | 20 ± 10 | 8 ± 2 | 240 ± 60 | 70 ± 20 |
| inflorescence | 93 ± 1 | 60 ± 30 | 50 ± 50 | 47 ± 4 | 2500 ± 300 | 690 ± 80 | |
| White Widow | leaf | 9.8 ± 0.6 | 6.2 ± 0.4 | 79 ± 7 | 13 ± 1 | 950 ± 10 | 236 ± 5 |
| inflorescence | 64 ± 3 | 110 ± 10 | 2000 ± 1000 | 150 ± 30 | 3300 ± 400 | 870 ± 80 | |
| Chung Sam | leaf | 6.9 ± 0.3 | 2.6 ± 0.2 | 0.7 ± 0.2 | 1.6 ± 0.2 | 263 ± 4 | 73 ± 1 |
| inflorescence | 183 ± 5 | 30.1 ± 0.3 | 6.1 ± 0.1 | 1.3 ± 0.2 | 2900 ± 600 | 800 ± 100 | |
| Blue Dream | leaf | 56.5 ± 0.7 | 18.6 ± 0.6 | 200 ± 100 | 6.1 ± 0.1 | 205 ± 4 | 51 ± 4 |
| inflorescence | 200 ± 300 | 116 ± 3 | 1400 ± 900 | 19 ± 1 | 400 ± 200 | 100 ± 60 | |
| Bubble Gum | leaf | 9.79 ± 0.02 | 10.6 ± 0.2 | 75 ± 1 | 16.2 ± 0.6 | 60 ± 40 | 48 ± 2 |
| inflorescence | 93.17 ± 0.03 | 142 ± 1 | 1310 ± 30 | 300 ± 20 | 500 ± 100 | 130 ± 40 | |
| Purple | leaf | 450 ± 10 | 200 ± 50 | 1190 ± 60 | 34 ± 6 | 400 ± 70 | 130 ± 30 |
| Victory | leaf | 332 ± 3 | 122 ± 4 | 1550 ± 60 | 42 ± 3 | 1100 ± 200 | 300 ± 70 |
3. Experimental
3.1. Chemicals and Materials
Analytical grade methanol (MeOH) and ethyl acetate (EA) were purchased from J. T. Baker (Phillipsburg, NJ, USA). Deionized water (DW) was obtained using a Milli-Q purification system (Millipore Co., Bedford, MA, USA). Analytical grade standards of α- and β-pinene, myrcene, limonene, eucalyptol, β-caryophyllene, α-humulene, and alkane standard solutions (C8–C20) were purchased from Sigma–Aldrich (St. Louis, MO, USA). For internal standards, nonane and tetradecane were also purchased from Sigma–Aldrich (St. Louis, MO, USA). Leaves (n = 9) and inflorescences (n = 7) of hybrid Cannabis species were provided by Nongboo Mind (Seoul, Republic of Korea). All Cannabis samples were hybrid Cannabis species (combinations of indica and sativa) strictly supervised by the Korean Government. Therefore, only a limited number of Cannabis samples were allowed to be investigated in this study. All collected samples were sealed and stored in a freezer at −20 °C until analysis.
3.2. Sample Preparation
Fresh leaves and inflorescences from hybrid Cannabis species were prepared by removing superficial moisture with natural drying at room temperature, chopped using scissors, and weighed at 50 mg using an analytical balance (Mettler Toledo, Columbus, OH, USA). Weighed leaf and inflorescence samples were transferred into 10 mL headspace vials.
3.3. HS-GC/MS Conditions
To optimize headspace conditions, the incubation temperature (60–120 °C) and time (20–40 min) were tested. The sample was incubated at 100 °C for 30 min. The syringe temperature, fill speed, and injection speed of the automated headspace were 120 °C, 100 μL/s, and 500 μL/s, respectively. GC-MS analysis was performed by an Agilent 6890N gas chromatograph combined with an Agilent-5973 mass spectrometer equipped with electron ionization (EI) and a quadrupole analyzer. Separation was achieved using an Agilent Technologies DB-5MS column (30 m × 0.25 mm i.d., film thickness of 0.25 µm, J&W Scientific, Folsom, CA, USA). The sample (0.5 mL) in the automated headspace was automatically injected into the injection port heated at 250 °C in split mode (10:1). As a carrier gas, helium (purity: 99.999%) was set at a flow rate of 1 mL/min. The oven temperature was programmed to hold at 60 °C for 1 min, ramp up to 200 °C at a rate of 5 °C/min, and then increase to 250 °C at a rate of 10 °C/min. The temperature of the interface, ion source, and quadrupole was set at 250 °C, 230 °C, and 150 °C, respectively, and the EI energy was set at 70 eV. The mass spectra were acquired in scan mode in the range m/z 40–250 since no substance was detected above m/z 250 in all Cannabis samples in preliminary experiments.
3.4. Qualitative and Quantitative Analysis
For qualitative analysis, the individual detection result was compared with the retention time, mass spectral pattern, database of NIST, mass spectra of authentic standards, GC elution order, and KI values based on previous reports. KI was calculated using a C8–C20 n-alkane solution and applied to a temperature-programming analysis [36]. Calculated KI values were investigated according to the following equation:
| (1) |
where n is the number of n-alkane carbon atoms eluting before the compound x; tn and tn+1 are the retention times that elute before and after compound x. The relative abundance of terpenes was individually investigated for each leaf or inflorescence sample of different hybrid Cannabis varieties. Quantitative analysis was performed on α- and β-pinene, myrcene, limonene, β-caryophyllene, and α-humulene, known as major terpenes in Cannabis. To investigate terpenes and select biomarkers, statistical analyses (such as PCA and HCA) were performed using R 4.1.3 (R Core Team, Vienna, Austria).
3.5. Method Validation
For reliable validation, a standard mixture solution of major volatile components in Cannabis such as α- and β-pinene, myrcene, limonene, β-caryophyllene, and α-humulene was dissolved in methanol at a concentration of 500 μg/mL using nonane and tetradecane as internal standards. For linearity, standard solutions were prepared at 1–250 μg/mL for α- and β-pinene, limonene, β-caryophyllene, and α-humulene, and 1–500 μg/mL for myrcene. Calibration curves were constructed by comparing the peak area ratios of individual compounds to their internal standards versus their concentrations in μg/mL. LOQ was evaluated as the concentration of a standard mixture with a signal-to-noise ratio (S/N) > 10. To obtain repeatability and reproducibility, intra- and inter-day precision were estimated by analyzing triplicates of Cannabis extract. To determine accuracy, Cannabis samples were analyzed by spiking the standard solution at three different concentrations (5, 10, and 20 μg/mL). The accuracy data was obtained by calculating differences before and after spiking the standard solution to match the sample matrices.
| (2) |
4. Conclusions
In this study, we introduced the automated HS-GC/MS method to simply and easily detect 26 terpenes and quantify six major terpenes, namely α- and β-pinenes, myrcene, limonene, β-caryophyllene, and α-humulene, in the leaves (n = 9), and inflorescences (n = 7) of hybrid Cannabis species without intensive time and labor. To enhance terpene extraction efficiency from leaf and inflorescence samples, the HS incubation time and temperature were optimized at 30 min and 100 °C, respectively. Using the established HS-GC/MS method, 26 terpenes were identified based on EI mass spectral patterns and retention indices. Furthermore, based on the PCA results, we investigated components for identifying individual hybrid Cannabis samples and suggested six major terpenes as potential biomarkers. These six terpenes were consistently and predominantly present in all Cannabis samples. Furthermore, we quantified six major terpenes in both leaves and inflorescences of hybrid Cannabis using HS-GC/MS and tried to demonstrate the “entourage effect” specific to individual Cannabis based on quantification results for six terpenes. The HS-GC/MS method used in this study directly detected 26 terpenes and quantified six major terpenes in the leaves and inflorescences of hybrid Cannabis. Our research contributes to a better understanding of terpenes’ “entourage effect” in Cannabis.
Acknowledgments
All leaves and inflorescences of hybrid Cannabis species were kindly provided by Nongboo Mind (Seoul, Republic of Korea).
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28248082/s1, Figure S1: Total ion chromatograms of terpenes in leaves of Victory at different incubation temperature of (a) 60 °C, (b) 80 °C, (c) 100 °C, and (d) 120 °C; Figure S2: Cluster dendrogram of leaves and inflorescences of hybrid Cannabis using relative abundances of peak areas for 26 terpenes; Table S1: Analytical characteristics of the HS-GC/MS method for 6 major terpenes.
Author Contributions
Conceptualization, W.L. and J.H.; Methodology, J.H.; Validation, S.L. and E.J.K.; Investigation, S.L., E.K., S.J.O. and W.L.; Resources, M.C. and C.M.K.; Data curation, E.J.K.; Writing—original draft, W.L.; Writing—review & editing, J.H.; Supervision, J.H. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data are available from a publicly accessible repository.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT) (RS-2023-00223559) as well as the Ministry of Agriculture, Food and Rural Affairs of Republic of Korea (PJ017015 (RS2022RD010270)).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Brenneisen R. Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents. In: ElSohly M.A., editor. Marijuana and the Cannabinoids. Humana; Totowa, NJ, USA: 2007. pp. 17–49. [Google Scholar]
- 2.Hesami M., Pepe M., Baiton A., Salami S.A., Jones A.M.P. New Insight into Ornamental Applications of Cannabis: Perspectives and Challenges. Plants. 2022;11:2383. doi: 10.3390/plants11182383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mechoulam R., Parker L.A. The Endocannabinoid System and the Brain. Annu. Rev. Psychol. 2013;64:21–47. doi: 10.1146/annurev-psych-113011-143739. [DOI] [PubMed] [Google Scholar]
- 4.Russo E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin D., Dai K., Xie Z., Chen J. Secondary Metabolites Profiled in Cannabis Inflorescences, Leaves, Stem Barks, and Roots for Medicinal Purposes. Sci. Rep. 2020;10:3309. doi: 10.1038/s41598-020-60172-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferber S.G., Namdar D., Hen-Shoval D., Eger G., Koltai H., Shoval G., Shbiro L., Weller A. The “Entourage Effect”: Terpenes Coupled with Cannabinoids for the Treatment of Mood Disorders and Anxiety Disorders. Curr. Neuropharmacol. 2020;18:87–96. doi: 10.2174/1570159X17666190903103923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clarke B.A.R.C., Watson D.P. Cannabis and Natural Cannabis Medicines. In: ElSohly M.A., editor. Marijuana and the Cannabinoids. Humana; Totowa, NJ, USA: 2007. pp. 1–15. [Google Scholar]
- 8.Micalizzi G., Vento F., Alibrando F., Donnarumma D., Dugo P., Mondello L. Cannabis sativa L.: A comprehensive review on the analytical methodologies for cannabinoids and terpenes characterization. J. Chromatogr. A. 2021;1637:461864. doi: 10.1016/j.chroma.2020.461864. [DOI] [PubMed] [Google Scholar]
- 9.Shapira A., Berman P., Futoran K., Guberman O., Meiri D. Tandem Mass Spectrometric Quantification of 93 Terpenoids in Cannabis Using Static Headspace Injections. Anal. Chem. 2019;91:11425–11432. doi: 10.1021/acs.analchem.9b02844. [DOI] [PubMed] [Google Scholar]
- 10.Sexton M., Shelton K., Haley P., West M. Evaluation of Cannabinoid and Terpenoid Content: Cannabis Flower Compared to Supercritical CO2 Concentrate. Planta Medica. 2018;84:234–241. doi: 10.1055/s-0043-119361. [DOI] [PubMed] [Google Scholar]
- 11.Pavlovic R., Panseri S., Giupponi L., Leoni V., Citti C., Cattaneo C., Cavaletto M., Giorgi A. Phytochemical and Ecological Analysis of Two Varieties of Hemp (Cannabis sativa L.) Grown in a Mountain Environment of Italian Alps. Front. Plant Sci. 2019;10:1265. doi: 10.3389/fpls.2019.01265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pellati F., Brighenti V., Sperlea J., Marchetti L., Bertelli D., Benvenuti S. New Methods for the Comprehensive Analysis of Bioactive Compounds in Cannabis sativa L. (hemp) Molecules. 2018;23:2639. doi: 10.3390/molecules23102639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.De Backer B., Debrus B., Lebrun P., Theunis L., Dubois N., Decock L., Verstraete A., Hubert P., Charlier C. Innovative development and validation of an HPLC/DAD method for the qualitative and quantitative determination of major cannabinoids in cannabis plant material. J. Chromatogr. B. 2009;877:4115–4124. doi: 10.1016/j.jchromb.2009.11.004. [DOI] [PubMed] [Google Scholar]
- 14.Križman M. A simplified approach for isocratic HPLC analysis of cannabinoids by fine tuning chromatographic selectivity. Eur. Food Res. Technol. 2020;246:315–322. doi: 10.1007/s00217-019-03344-7. [DOI] [Google Scholar]
- 15.Mostafaei Dehnavi M., Ebadi A., Peirovi A., Taylor G., Salami S.A. THC and CBD Fingerprinting of an Elite Cannabis Collection from Iran: Quantifying diversity to underpin Future Cannabis Breeding. Plants. 2022;11:129. doi: 10.3390/plants11010129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nguyen T.-D., Riordan-Short S., Dang T.-T.T., O’Brien R., Noestheden M. Quantitation of Select Terpenes/Terpenoids and Nicotine Using Gas Chromatography–Mass Spectrometry with High-Temperature Headspace Sampling. ACS Omega. 2020;5:5565–5573. doi: 10.1021/acsomega.0c00384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Béres T., Černochová L., Zeljković S.Ć., Benická S., Gucký T., Berčák M., Tarkowski P. Intralaboratory comparison of analytical methods for quantification of major phytocannabinoids. Anal. Bioanal. Chem. 2019;411:3069–3079. doi: 10.1007/s00216-019-01760-y. [DOI] [PubMed] [Google Scholar]
- 18.Correia B., Ahmada S.M., Quinta A. Determination of phytocannabinoids in cannabis samples by ultrasound-assisted solid-liquid extraction and high-performance liquid chromatography with diode array detector analysis. J. Chromatogr. A. 2023;1705:464191. doi: 10.1016/j.chroma.2023.464191. [DOI] [PubMed] [Google Scholar]
- 19.Rocha E.D., Silva V.E.A., Pereira F.C.S., Jean V.M., Souza F.L.C., Baratto L.C., Vieira A.C.M., Carvalho V.M. Qualitative terpene profiling of Cannabis varieties cultivated for medical purposes. Pharmacognosy. 2020;71:e01192019. doi: 10.1590/2175-7860202071040. [DOI] [Google Scholar]
- 20.Arnoldi S., Roda G., Casagni E., DEI CAS M.V., Faré F., Rusconi C.M., Visconti G.L., Gambaro V. Characterization of the Volatile Components of Cannabis Preparations by Solid-Phase Microextraction Coupled to Headspace-Gas Chromatography with Mass Detector (SPME-HSGC/MS) J. Chromatogr. Sep. Tech. 2017;8:1000350. doi: 10.4172/2157-7064.1000350. [DOI] [Google Scholar]
- 21.Lancioni C., Castells C., Candal R., Tascon M. Headspace solid-phase microextraction: Fundamentals and recent advances. Adv. Sample Prep. 2022;3:100035. doi: 10.1016/j.sampre.2022.100035. [DOI] [Google Scholar]
- 22.Lachenmeier D.W., Kroener L., Musshoff F., Madea B. Determination of cannabinoids in hemp food products by use of headspace solid-phase microextraction and gas chromatography–mass spectrometry. Anal. Bioanal. Chem. 2004;378:183–189. doi: 10.1007/s00216-003-2268-4. [DOI] [PubMed] [Google Scholar]
- 23.Virgiliou C., Zisi C., Kontogiannopoulos K.N., Nakas A., Iakovakis A., Varsamis V., Gika H.G., Assimopoulou A.N. Headspace gas chromatography-mass spectrometry in the analysis of lavender’s essential oil: Optimization by response surface methodology. J. Chromatogr. B. 2021;1179:122852. doi: 10.1016/j.jchromb.2021.122852. [DOI] [PubMed] [Google Scholar]
- 24.Calvi L., Pentimalli D., Panseri S., Giupponi L., Gelmini F., Beretta G., Vitali D., Bruno M., Zilio E., Pavlovic R., et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018;150:208–219. doi: 10.1016/j.jpba.2017.11.073. [DOI] [PubMed] [Google Scholar]
- 25.Slosse A., Van Durme F., Eliaerts J., Samyn N., Mangelings D., Heyden Y.V. Analytical strategies for herbal Cannabis samples in forensic applications: A comprehensive review. WIREs Forensic Sci. 2023;5:e1479. doi: 10.1002/wfs2.1479. [DOI] [Google Scholar]
- 26.Yang Y., Kayan B., Bozer N., Pate B., Baker C., Gizir A.M. Terpene degradation and extraction from basic and oregano leaves using subcritical water. J. Chromatogr. A. 2007;1152:262–267. doi: 10.1016/j.chroma.2006.11.037. [DOI] [PubMed] [Google Scholar]
- 27.Boumaraf M., Mekkiou R., Benyahia S., Chalchat J.-C., Chalard P., Benayache F., Benayache S. Essential Oil Composition of Pulicaria undulata (L.) DC. (Asteraceae) Growing in Algeria. Int. J. Pharmacogn. Phytochem. Res. 2016;8:746–749. [Google Scholar]
- 28.Bernstein N., Gorelick J., Koch S. Interplay between chemistry and morphology in medical cannabis (Cannabis sativa L.) Ind. Crop. Prod. 2019;129:185–194. doi: 10.1016/j.indcrop.2018.11.039. [DOI] [Google Scholar]
- 29.Drevinskas T., Maruška A., Telksnys L., Hjerten S., Stankevičius M., Lelešius R., Mickienė R., Karpovaitė A., Šalomskas A., Tiso N., et al. Chromatographic Data Segmentation Method: A Hybrid Analytical Approach for the Investigation of Antiviral Substances in Medicinal Plant Extracts. Anal. Chem. 2019;91:1080–1088. doi: 10.1021/acs.analchem.8b04595. [DOI] [PubMed] [Google Scholar]
- 30.Santiago M., Sachdev S., Arnold J.C., McGregor I.S., Connor M. Absence of Entourage: Terpenoids Commonly Found in Cannabis sativa Do Not Modulate the Functional Activity of Δ9-THC at Human CB1 and CB2 Receptors. Cannabis Cannabinoid Res. 2019;4:165–176. doi: 10.1089/can.2019.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouzenna H., Hfaiedh N., Giroux-Metgesa M.-A., Elfeki A., Talarmin H. Potential protective effects of alpha-pinene against cytotoxicity caused by aspirin in the IEC-6 cells. Biomed. Pharmacother. 2017;93:961–968. doi: 10.1016/j.biopha.2017.06.031. [DOI] [PubMed] [Google Scholar]
- 32.Karthikeyana R., Kanimozhi G., Prasad N.R., Agilan B., Ganesan M., Srithar G. Alpha pinene modulates UVA-induced oxidative stress, DNA damage and apoptosis in human skin epidermal keratinocytes. Life Sci. 2018;212:150–158. doi: 10.1016/j.lfs.2018.10.004. [DOI] [PubMed] [Google Scholar]
- 33.Kim D.-S., Lee H.-J., Jeon Y.-D., Han Y.-H., Kee J.-Y., Kim H.-J., Shin H.-J., Kang J.W., Lee B.S., Kim S.-H., et al. Alpha-Pinene Exhibits Anti-Inflammatory Activity Through the Suppression of MAPKs and the NF-kB Pathway in Mouse Peritoneal Macrophages. Amer. J. Chin. Med. 2015;43:731–742. doi: 10.1142/S0192415X15500457. [DOI] [PubMed] [Google Scholar]
- 34.Silva-Cardoso G.K., Lazarini-Lopes W., Hallak J.E., Crippa J.A., Zuardi A.W., Garcia-Cairasco N., Leite-Panissi C.R.A. Cannabidiol effectively reverses mechanical and thermal allodynia, hyperalgesia, and anxious behaviors in a neuropathic pain model: Possible role of CB1 and TRPV1 receptors. Neuropharmacology. 2021;197:108712. doi: 10.1016/j.neuropharm.2021.108712. [DOI] [PubMed] [Google Scholar]
- 35.Fidyt K., Fiedorowicz A., Strządała L., Szumny A. β-caryophyllene and β-caryophyllene oxide-natural compounds of anticancer and analgesic properties. Cancer Med. 2016;5:3007–3017. doi: 10.1002/cam4.816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Den Dool H., Kratz P.D. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A. 1963;11:463. doi: 10.1016/S0021-9673(01)80947-X. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data are available from a publicly accessible repository.