Abstract
Biotransformation of ursonic acid (1) by two fungal strains Aspergillus ochraceus CGMCC 3.5324 and Aspergillus oryzae CGMCC 3.407 yielded thirteen new compounds (4, 5, 7–10, and 13–19), along with five recognized ones. The structural details of new compounds were determined through spectroscopic examination (NMR, IR, and HR-MS) and X-ray crystallography. Various modifications, including hydroxylation, epoxidation, lactonization, oxygen introduction, and transmethylation, were identified on the ursane core. Additionally, the anti-neuroinflammatory efficacy of these derivatives was assessed on BV-2 cells affected by lipopolysaccharides. It was observed that certain methoxylated and epoxylated derivatives (10, 16, and 19) showcased enhanced suppressive capabilities, boasting IC50 values of 8.2, 6.9, and 5.3 μM. Such ursonic acid derivatives might emerge as potential primary molecules in addressing neurodegenerative diseases.
Keywords: ursonic acid, biotransformation, anti-neuroinflammatory, Aspergillus ochraceus, Aspergillus oryzae
1. Introduction
Neurodegenerative diseases (NDDs), including Alzheimer’s disease, Parkinson’s disease, Huntington’s disease, and multiple sclerosis, are an important global healthy problem due to an increase in the aging population [1]. It brings a huge burden to patients and social sanitary systems all over the world. However, the pathologies of these diseases are not fully understood and some factors, such as genetic factors, oxidative stress, neuroinflammation, and environmental factors, are believed to have played a role in the development of NDDs. In clinics, there is no effective cure for these diseases [2,3]. Therefore, it is a very challenging task to find innovative potential drugs for NDDs.
Ursonic acid (UNA, 1), an ursane-type compound with a five-ring triterpene structure, is commonly found in a variety of plants frequently used in traditional remedies [4,5,6]. It exhibits an array of biological properties, encompassing anti-inflammatory, anti-cancer, growth-inhibitory, and anti-protozoan effects [7,8,9]. Notably, being a primary oxo-derivative of ursolic acid (ULA), UNA is a crucial chemical precursor for developing potential drug candidates. However, its medicinal potential and the mechanisms driving its effects remain relatively underexplored [10].
Various semisynthetic derivatives of UNA have been chemically crafted, showcasing enhanced absorption in the digestive tract and amplified medicinal properties [11,12,13]. Yet, these chemical methods have predominantly targeted only the activated substituents at C-3 and C-28 of the molecular framework. The potential for a wider range of structural variations of UNA is restrained due to its dearth of functional units. However, this limitation is difficult to overcome by conventional chemical synthesis.
Utilizing biotransformation emerges as a strategic method to attain structural variation, especially when dealing with intricate natural compounds [14,15,16,17]. The significant improvements in instruments and experimental techniques have enabled the biotransformation process to be carried out in NMR tubes and in situ monitoring using NMR spectroscopy [18,19]. Biotransformation offers a solution for targeting specific molecular sites that traditional chemical procedures may find challenging. In particular, microbial transformation stands out for its ability to provide precise and location-specific alterations in the structure of triterpenoids, thanks to its inherent stereo- and region-selective catalytic potential [20,21,22,23]. Even though numerous microbial adaptations of ULA have been explored to yield derivatives with augmented solubility and therapeutic traits, such endeavors with UNA are not as prevalent [24]. Hence, leveraging biotransformation to craft UNA derivatives is of immense significance.
Anti-neuroinflammatory effects of triterpenoids from medicinal plants are well reported [25]. Nitric oxide (NO) is a molecule which is highly linked with immunity and inflammation [26]. The anti-neuroinflammatory activity of some ULA derivatives has been evaluated with lipopolysaccharide (LPS)-induced BV-2 microglia [27]. As ongoing research to find triterpenoid derivatives with anti-neuroinflammatory activity, in this study, we identified 13 previously undescribed derivatives of UNA derived from the biotransformation processes of two fungal varieties: Aspergillus ochraceus CGMCC 3.5324 and Aspergillus oryzae CGMCC 3.407. Additionally, we evaluated the anti-neuroinflammatory properties of these biotransformation derivatives to inhibit LPS-induced NO production in BV-2 cells.
2. Results
2.1. Biotransformation of Ursonic Acid
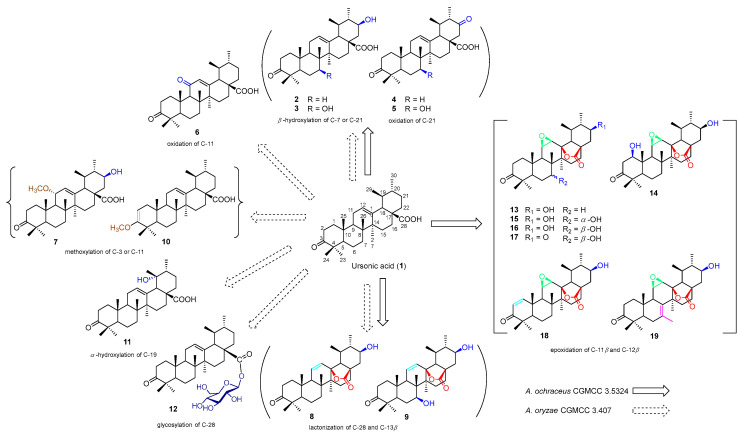
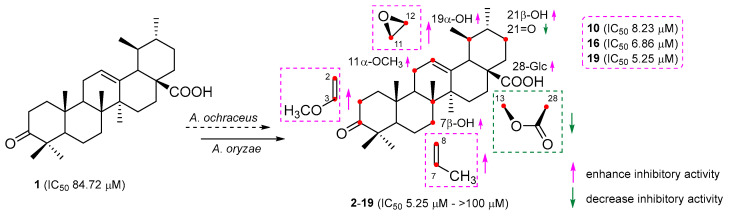
We introduced 2.0 g of UNA to A. ochraceus cultures and a lesser quantity of 1.2 g to A. oryzae cultures. Following a week-long incubation period, both cultures were merged and subsequently sieved. We proceeded to extract the filtrates using ethyl acetate in three separate stages. After concentrating these extracts, we differentiated them through multiple rounds of column chromatography and semi-preparative high-performance liquid chromatography (HPLC). This process yielded a total of 18 distinct compounds (2–19). Specifically, A. ochraceus produced 13 of these derivatives (2–5, 8, 9, and 13–19), while A. oryzae was responsible for the remaining 11 (2–12) (Figure 1).
Figure 1.
Biotransformation of ursonic acid (1) by A. ochraceus and A. oryzae.
To ascertain the molecular structures of these derivatives, we undertook a thorough examination utilizing diverse spectral studies and X-ray crystallography. Remarkably, among these, 13 (4, 5, 7–10, and 13–19) were identified as novel compounds, as shown in Figure 1. Detailed 1H and 13C NMR data pertaining to these compounds are presented in Table 1, Table 2, Table 3 and Table 4. Furthermore, all relevant spectra can be found in the Supplementary Materials, labeled as Figures S1–S104. For compounds already known in the scientific literature, we determined their identities through spectral comparison. These were recognized as 3-oxo-21β-hydroxy-12-en-urs-28-oic acid (2) [28], 3-oxo-7β,21β-dihydroxy-12-en-28-oic acid (3) [29], 3,11-dioxo-12-en-urs-28-oic acid (6) [30], 3-oxo-19α-hydroxy-12-en-urs-28-oic acid (11), and ursonic acid -28-O-β-d-glucopyranosyl ester (12) [31] by juxtaposing their spectroscopic data with previously documented findings.
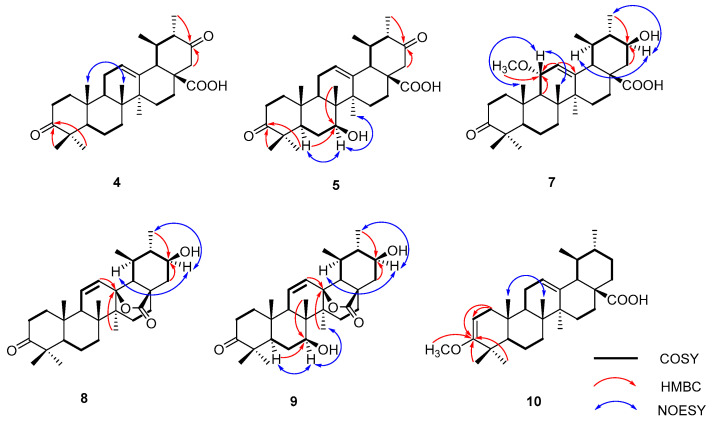
Compound 4, based on HR-ESI-MS measurements, displayed a molecular structure C30H44O4, as shown by the [M − H]− ion at m/z 467.3174 (calcd. for C30H43O4, 467.3161). This structure was 14 amu heavier than UNA. The 13C NMR spectrum notably exhibited a fresh carbonyl signal at δC 209.8 ppm. In the HMBC spectrum, connections between H-22 (δH 2.57 and 2.35) and C-21 (δC 209.8) became evident (Figure 2). Also, correlations of 30-CH3 (δH 1.01) with the carbonyl signal at δC 209.8 were noticed. These data led to the identification of the carbonyl group at C-21, thus finalizing compound 4 as 3,21-dioxo-urs-12-en-28-oic acid.
Figure 2.
Key 1H-1H COSY, HMBC, and NOESY correlations for compounds 4, 5, and 7–10.
For compound 5, its HR-ESI-MS data revealed a molecular formula of C30H44O5, as reflected by the [M − H]− ion at m/z 483.3126 (calcd. for C30H43O5, 483.3112). This was 30 amu heavier than UNA. The 1H NMR spectrum showed a new signal at δH 3.89, indicating the presence of a hydroxyl group. The 13C NMR spectrum distinguished itself with an oxygenated methine at δC 73.4 and a carbonyl signal at δC 210.4 ppm. HMBC correlations between 26-CH3 (δH 0.81) and the oxygenated methine at δC 73.4 suggested the attachment of the hydroxyl group to C-7 (Figure 2). The NOESY data further linked H-7 (δH 3.87) and 27-CH3 (δH 1.08), affirming the β-orientation of the 7-OH group. The HMBC spectrum showcased H-22 (δH 2.56 and 2.37) correlations with C-21 (δC 210.4). The linkage of 30-CH3 (δH 0.99) with the carbonyl signal at δC 210.4 confirmed the carbonyl’s placement at C-21. Hence, compound 5 was determined to be 3,21-dioxo-7β-hydroxy-urs-12-en-28-oic acid.
Table 1.
The 1H- and 13C-NMR data of compounds 4, 5, and 7 (400 and 100 MHz, respectively, in CDCl3)
| Position | 4 | 5 | 7 a | |||
|---|---|---|---|---|---|---|
| δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | |
| 1 | 39.3 | 1.39, 1.83 m | 39.1 | 1.35, 1.86 m | 40.4 | 1.67, 2.22 m |
| 2 | 34.1 | 2.30, 2.48 m | 34.0 | 2.34, 2.48 m | 34.4 | 2.39, 2.53 m |
| 3 | 217.7 | 217.0 | 217.9 | |||
| 4 | 47.4 | 47.7 | 47.6 | |||
| 5 | 55.2 | 1.23 m | 52.5 | 1.37 m | 55.3 | 1.36 m |
| 6 | 19.5 | 1.24, 1.41 m | 26.6 | 1.55, 1.82 m | 19.6 | 1.32, 1.47 m |
| 7 | 32.3 | 1.29, 1.42 m | 73.4 | 3.89 dd (9.7, 6.0) | 33.1 | 1.31, 1.46 m |
| 8 | 39.5 | 45.2 | 42.3 | |||
| 9 | 46.7 | 1.53 m | 47.0 | 1.44 m | 51.3 | 1.74 d (9.1) |
| 10 | 36.7 | 36.8 | 37.7 | |||
| 11 | 23.6 | 1.53, 1.94 m | 23.7 | 1.93, 2.04 m | 76.5 | 3.84 d (8.6) |
| 12 | 127.2 | 5.35 d (3.8) | 127.5 | 5.42 dd (4.6, 2.7) | 125.7 | 5.52 m |
| 13 | 137.1 | 136.6 | 141.6 | |||
| 14 | 41.9 | 43.4 | 42.3 | |||
| 15 | 29.7 | 1.14 d (6.0), 1.18 m | 31.6 | 1.49, 1.92 m | 28.1 | 1.15, 1.77 m |
| 16 | 27.9 | 1.06, 1.80 m | 30.4 | 1.37, 1.54 m | 25.2 | 1.77, 1.88 m |
| 17 | 51.2 | 51.1 | 48.4 | |||
| 18 | 52.4 | 2.62 m | 51.1 | 2.08 m | 51.8 | 2.29 d (11.4) |
| 19 | 41.5 | 1.72 m | 41.7 | 1.74 m | 37.7 | 1.46 m |
| 20 | 51.1 | 2.08 m | 53.1 | 2.63 d (11.4) | 46.6 | 0.99 m |
| 21 | 209.8 | 210.4 | 71.1 | 3.44 m | ||
| 22 | 50.5 | 2.35, 2.57 m | 50.3 | 2.37 m, 2.56 d (12.9) | 44.5 | 1.58, 2.09 m |
| 23 | 26.6 | 1.02 s | 26.6 | 1.04 s | 26.7 | 1.09 s |
| 24 | 21.5 | 0.96 s | 21.5 | 0.98 s | 21.5 | 1.04 s |
| 25 | 15.3 | 0.99 s | 12.4 | 1.00 s | 15.7 | 1.13 s |
| 26 | 17.0 | 0.76 s | 9.7 | 0.81 s | 18.5 | 0.86 s |
| 27 | 23.8 | 0.99 s | 23.4 | 1.08 s | 22.7 | 1.15 s |
| 28 | 180.0 | 179.1 | 175.7 | |||
| 29 | 18.4 | 0.95 d (5.7) | 18.4 | 0.95 d (6.4) | 17.2 | 0.99 d (6.5) |
| 30 | 12.5 | 1.01 d (3.7) | 15.4 | 0.99 d (5.7) | 16.5 | 1.09 d (6.3) |
| -OCH3 | 54.7 | 3.29 s | ||||
a H and C were measured at 600 and 150 MHz, respectively.
Compound 7, as determined by its HR-ESI-MS data, had a molecular formula of C31H48O5. This was supported by the [M − H]− ion at m/z 499.3433 (calcd. for C31H47O5, 499.3423), indicating that it was 46 amu heavier than UNA. In the 1H NMR spectrum, two prominent signals at δH 3.84 and 3.44 emerged. Additionally, the 13C NMR and DEPT 135 spectra displayed new methine carbon signals at δC 76.5 and 71.1. The oxygenated methine signal at δC 71.1 was mapped to C-21, drawn from HMBC correlations between C-21 (δC 71.1) and 30-CH3 (δH 1.09) (Figure 2). The shift of C-30 to δC 16.5 was attributed to the γ-gauche effect. The 1H-1H COSY and HSQC spectra depicted a connectivity of H-9 (δH 1.74) → H-11 (δH 3.84) → H-12 (δH 5.52) within the C ring. This traced the oxygenated methine signal at δC 76.5 to C-11. A methoxyl singlet in the 1H NMR spectrum was evident at δH 3.29, with its carbon counterpart at δC 54.7 in the HSQC spectrum. In the HMBC spectrum, a clear connection between C-11 (δC 76.5) and the methoxyl singlet (δH 3.29) was observed. The 11-OCH3 group’s α-orientation was confirmed through NOESY correlations of H-11 (δH 3.84) and 26-CH3 (δH 0.86). Hence, compound 7 was finalized as 3-oxo-11α-methoxy-21β-hydroxy-urs-12-en-28-oic acid.
Compound 8’s molecular formula was inferred to be C30H44O4 based on HR-ESI-MS data ([M − H2O + H]+ m/z 451.3216, calcd. for C30H43O3 451.3212), suggesting a 14 amu difference when contrasted with UNA. An extra low-field proton was observed at δH 3.39 in the 1H NMR reading for 8, with the corresponding carbon reading at δC 71.8 evident in the HSQC reading. The HMBC revealed clear connections between the distinct 30-CH3 (δH 1.01) and the new oxygenated methine reading at δC 71.8 (Figure 2). NOESY correlations between H-21 (δH 3.39) and H-19 (δH 1.82) confirmed the β-orientation of the 21-OH group. In the 1H NMR reading, two vinyl protons appeared at δH 5.92 and 5.51, linking with two sp2 methine readings at δC 133.3 and 128.8 in the HSQC reading. This double bond deviated from the one found in UNA. The 1H-1H COSY connections between H-11 (δH 5.92) and H-9 (δH 1.98) indicated a double-bond shift from C-12 (13) to C-11 (12). This was further verified by long-range connections of H-11 with C-8 (δC 42.0) and C-13 (δC 89.5) and H-12 with C-18 (δC 59.9) in the HMBC spectrum. Additionally, the oxygen-rich quaternary carbon at δC 89.5 was linked to C-13 due to its connections with H-11 and 27-CH3 (δH 1.08). The chemical positioning of C-18 also changed (downshifting from about δC 52.7 to δC 59.9). Analyzing the NMR findings, two potential compositions for this substance were proposed. The first had free 13-OH and 28-COOH. The second suggested a lactone bond between 13-OH and 28-COOH. With a molecular weight of 468, it adhered to the latter configuration. Consequently, compound 8 was identified as 3-oxo-21β-hydroxy-urs-11-en-13β,28β-lactone.
Compound 9’s molecular formula was inferred to be C30H44O5 from its HR-ESI-MS results ([M + COOH]− m/z 529.3171, calcd. for C31H45O7 529.3165), a 16 amu increment from metabolite 8, pointing to an extra oxygen atom. In the 1H NMR reading, two more low-field protons, δH 3.87 and 3.39, were present. The 13C NMR, DEPT 135, and HSQC results displayed two fresh oxygenated methine signals at δC 72.6 and 71.7. Contrasted with 8’s NMR findings, the 13C NMR readings were largely aligned, excluding the B ring. HMBC correlations between the distinct 26-CH3 (δH 1.07) and the new oxygenated methine signal at δC 72.6 were evident (Figure 2). Furthermore, NOESY correlations between H-7 (δH 3.87) and 27-CH3 (δH 1.15) confirmed the β-orientation of the 7-OH group. Thus, compound 9 was identified as 3-oxo-7β,21β-dihydroxy-urs-11-en-13β,28β-lactone.
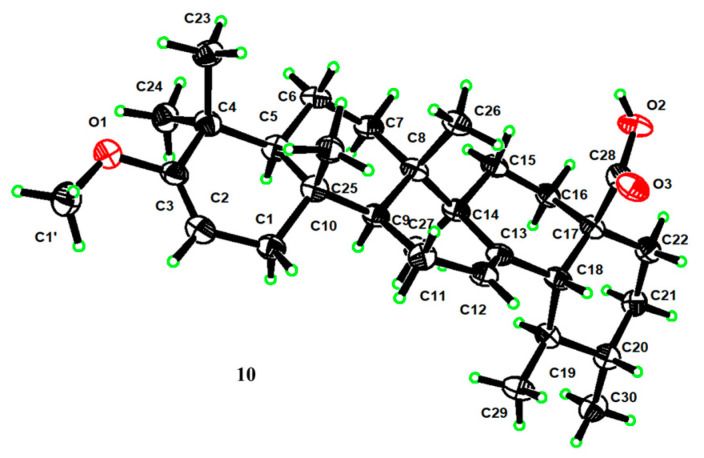
For compound 10, its molecular formula was concluded to be C31H48O3 based on HR-ESI-MS, showing a [M − H]− ion at m/z 467.3534 (calcd. for C31H47O3, 467.3525), a 14 amu increase from UNA. The 1H NMR reading for compound 10 displayed an extra vinyl proton at δH 4.39 and an oxygenated methine at δH 3.47. Their respective carbon signals at δC 89.6 and δC 54.2 appeared in the HSQC reading. The 13C NMR and DEPT 135 findings disclosed a new seasonal double-bond carbon signal at δC 160.6, and when compared to UNA’s NMR readings, metabolite 10’s keto carbonyl signal at C-3 vanished. The carbon signal at δC 89.6 and the new seasonal double-bond carbon signal at δC 160.6 were likely paired due to the HMBC’s vinyl proton (δH 4.39) connections with the seasonal double-bond carbon signal at δC 160.6 (Figure 2). Moreover, the HMBC connections between 23-CH3 (δH 1.04), 24-CH3 (δH 0.93), and the new seasonal double-bond carbon signal (δC 160.6) hinted at the double bond’s positioning at C-2 and C-3. Additionally, the oxygenated methine group should connect to C-3 based on the HMBC correlation of C-3 (δC 160.6) with the oxygenated methine signal at δH 3.47. The 2D structure of compound 10 was further endorsed by suitable crystal X-ray crystallography [Cu Ka; Flack parameter: −0.4(5); CCDC: 2266165] (Figure 3). Sadly, ideal crystals were not acquired, making observation of absolute configurations impossible. Therefore, compound 10 was pinpointed as 3-methoxy-urs-2,12-dien-28-oic acid.
Figure 3.
X-ray ORTEP drawing of compound 10.
Table 2.
The 1H- and 13C-NMR data of compounds 8–10 (400 and 100 MHz, respectively, in CDCl3).
| Position | 8 | 9 | 10 | |||
|---|---|---|---|---|---|---|
| δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | |
| 1 | 39.0 | 1.38, 2.02 m | 38.5 | 1.34, 2.00 m | 39.7 | 1.70, 2.00 m |
| 2 | 33.9 | 2.36, 2.57 m | 33.7 | 2.37, 2.55 m | 89.6 | 4.39 dd (6.7, 1.8) |
| 3 | 216.8 | 216.1 | 160.6 | |||
| 4 | 47.6 | 47.2 | 37.1 | |||
| 5 | 54.6 | 1.27 m | 51.8 | 1.89 m | 53.0 | 1.12 m |
| 6 | 18.8 | 1.49, 1.62 m | 29.8 | 1.52, 1.64 m | 19.4 | 1.37, 1.48 m |
| 7 | 30.5 | 1.22, 1.38 m | 72.6 | 3.87 dd (9.9, 5.5) | 32.4 | 1.37,1.51 m |
| 8 | 42.0 | 46.8 | 39.4 | |||
| 9 | 52.4 | 1.98 m | 52.3 | 1.36 m | 46.1 | 1.54 m |
| 10 | 36.1 | 36.0 | 35.9 | |||
| 11 | 133.3 | 5.92 dd (10.3, 1.6) | 132.1 | 5.85 d (10.4) | 23.3 | 1.91, 1.97 m |
| 12 | 128.8 | 5.51 dd (10.3, 3.2) | 129.5 | 5.53 dd (10.3, 3.1) | 126.1 | 5.27 t (3.6) |
| 13 | 89.5 | 89.4 | 137.7 | |||
| 14 | 41.6 | 42.9 | 42.0 | |||
| 15 | 25.6 | 1.17, 1.68 m | 29.4 | 1.18, 1.91 m | 28.0 | 1.11, 1.86 m |
| 16 | 23.7 | 1.50 m, 1.92 td (13.0, 5.7) | 23.8 | 1.48, 1.92 m | 24.1 | 1.67, 2.00 m |
| 17 | 45.6 | 45.5 | 48.1 | |||
| 18 | 59.9 | 1.64 m | 60.1 | 1.64 m | 52.7 | 2.19 dd (11.5, 1.7) |
| 19 | 36.0 | 1.82 m | 36.0 | 1.84 m | 39.1 | 1.34 m |
| 20 | 47.8 | 0.78 m | 47.8 | 0.80 m | 38.8 | 1.01 m |
| 21 | 71.8 | 3.39 m | 71.7 | 3.39 m | 30.7 | 1.34, 1.51 m |
| 22 | 40.3 | 1.40 m, 2.09 dd (12.8, 4.5) | 40.2 | 1.37 m, 2.09 dd (12.8, 4.5) | 36.8 | 1.67, 1.71 m |
| 23 | 26.0 | 1.03 s | 26.0 | 1.04 s | 28.6 | 1.04 s |
| 24 | 20.8 | 0.98 s | 20.8 | 0.98 s | 20.0 | 0.93 s |
| 25 | 17.8 | 0.99 s | 17.1 | 0.98 s | 15.4 | 0.95 s |
| 26 | 18.6 | 1.03 s | 13.7 | 1.07 s | 17.0 | 0.80 s |
| 27 | 16.0 | 1.08 s | 16.3 | 1.15 s | 23.5 | 1.08 s |
| 28 | 178.5 | 178.5 | 183.7 | |||
| 29 | 17.3 | 0.97 d (6.0) | 17.8 | 0.97 d (5.8) | 17.0 | 0.86 d (6.4) |
| 30 | 14.4 | 1.01 d (6.1) | 14.4 | 1.01 d (6.4) | 21.2 | 0.94 d (5.4) |
| -OCH3 | 54.2 | 3.47 s | ||||
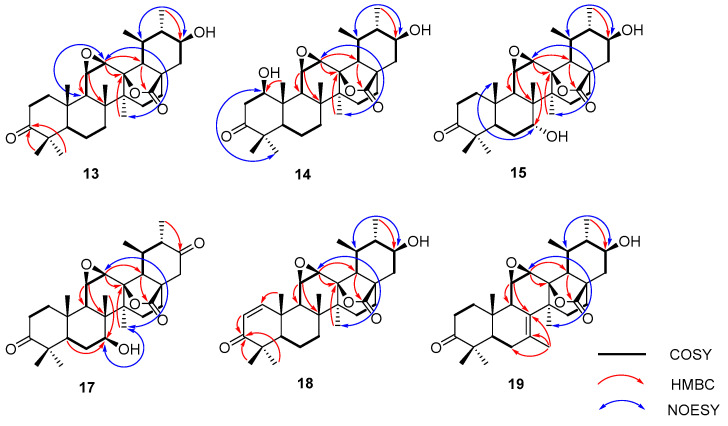
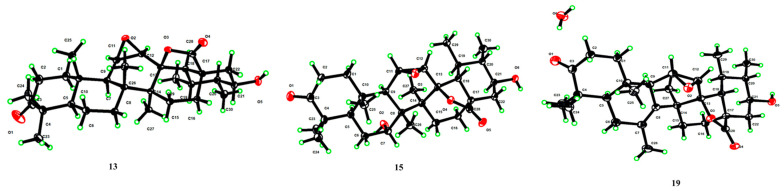
Compound 13’s molecular formula was deduced as C30H44O5 from its HR-ESI-MS readings (m/z 529.3173 [M + COOH]−, computed for C31H45O7 at 529.3165). When juxtaposed with UNA (1) in the 1H NMR analysis, three distinct low-field protons surfaced at δH 3.16, 3.41, and 3.49. The 13C NMR and DEPT 135 analyses further identified three unique downfield methine carbon resonances at δC 51.3, 52.0, and 71.5. Key correlations were seen in the HMBC analysis, notably the 30-CH3 resonance (δH 1.13) associated with C-19 (δC 36.3), C-20 (δC 47.5), and an unfamiliar oxygenated methine resonance at δC 71.5 (Figure 4). Both the 1H-1H COSY and HSQC analyses showcased a spin system stretching from H-18 (δH 1.99) to H-22 (δH 1.51 and 2.21) in the E ring. These insights suggested the addition of a hydroxyl unit to C-21. The NOESY analysis underscored correlations between H-21 (δH 3.49) and H-19 (δH 1.97), indicating that the 21-OH unit possessed a β-orientation. Two proton resonances emerged in the 1H NMR reading at δH 3.41 and 3.16, linked to epoxide protons on C-11 and C-12. The 13C NMR reading aligned with the traits of the epoxy-γ-lactone component, evidenced by distinct peaks for C-11, C-12, and C-13 [32]. The HMBC reading (Figure 4) and other NMR analyses exposed a spin system in the C ring extending from H-9 (δH 1.62) to H-12 (δH 3.16). The NOESY study also identified associations between H-12 (δH 3.16) and 27-CH3 (δH 1.09), pinpointing a β-orientation for the C-11(12) epoxy unit (Figure 4). Our 1D and 2D NMR analyses conclusively disclosed the spatial arrangements across various ring junctions and specific orientations of functional groups. A conclusive configuration for 13 was substantiated through X-ray crystallographic evaluation [Cu Ka; Flack metric: 0.08(8); CCDC: 2256928] as (5R, 8R, 9R, 10S, 11R, 12R, 13S, 14S, 17R, 18R, 19S, 20S, 21S) (Figure 5). As a result, the identity of compound 13 was established as 3-oxo-21β-hydroxyl-11β,12β-epoxyl-urs-13β,28β-lactone.
Figure 4.
Key 1H-1H COSY, HMBC, and NOESY correlations for compounds 13–18 and 17–19.
Figure 5.
X-ray ORTEP drawing of compounds 13, 15, and 19.
Table 3.
The 1H- and 13C-NMR data of compounds 13–15 (400 and 100 MHz, respectively, in CDCl3).
| Position | 13 | 14 a | 15 b | |||
|---|---|---|---|---|---|---|
| δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | |
| 1 | 39.6 | 1.62, 2.26 m | 78.8 | 4.25 dd (7.2, 6.6) | 39.1 | 1.67, 2.18 m |
| 2 | 33.9 | 2.48, 2.71 m | 45.8 | 2.99 dd (14.8, 6.6) 3.20 dd (14.8, 7.2) |
33.6 | 2.41, 2.62 m |
| 3 | 216.6 | 214.1 | 218.4 | |||
| 4 | 47.7 | 47.5 | 46.9 | |||
| 5 | 55.1 | 1.36 m | 51.1 | 1.40 dd (12.6, 3.1) | 46.3 | 2.02 m |
| 6 | 19.0 | 1.56, 1.72 m | 19.1 | 1.52, 1.71 m | 28.8 | 1.32, 2.05 m |
| 7 | 32.5 | 1.14, 1.39 m | 32.3 | 1.03, 1.36 m | 72.5 | 3.35 m |
| 8 | 40.1 | 40.7 | 42.5 | |||
| 9 | 49.2 | 1.62 m | 50.2 | 1.98 br.s | 44.9 | 2.14 m |
| 10 | 37.3 | 43.9 | 37.3 | |||
| 11 | 52.0 | 3.41 d (3.8) | 54.8 | 4.78 d (3.8) | 53.0 | 3.41 m |
| 12 | 51.3 | 3.16 d (3.8) | 52.1 | 3.28 d (3.8) | 51.4 | 3.13 d (3.8) |
| 13 | 88.8 | 89.2 | 90.2 | |||
| 14 | 41.9 | 42.2 | 42.6 | |||
| 15 | 25.5 | 1.20, 1.75 m | 26.1 | 1.16, 1.79 m | 24.4 | 1.60, 2.08 m |
| 16 | 23.4 | 1.58, 1.97 m | 23.9 | 1.55, 2.12 m | 22.9 | 1.38, 2.08 m |
| 17 | 46.3 | 46.6 | 46.2 | |||
| 18 | 60.8 | 1.99 m | 60.0 | 2.00 d (11.2) | 60.9 | 1.85 dd (11.6, 1.4) |
| 19 | 36.3 | 1.97 m | 36.7 | 2.09 m | 36.7 | 1.98 m |
| 20 | 47.5 | 0.97 m | 48.4 | 1.12 m | 47.2 | 0.81 m |
| 21 | 71.5 | 3.49 m | 70.7 | 3.76 m | 70.4 | 3.32 ddd (11.6, 10.0, 4.6) |
| 22 | 40.2 | 1.51, 2.21 m | 41.6 | 1.92, 2.56 m | 40.0 | 1.32, 1.98 m |
| 23 | 26.0 | 1.11 s | 26.8 | 1.16 s | 24.9 | 0.96 s |
| 24 | 20.8 | 1.09 s | 20.6 | 1.14 s | 20.0 | 0.95 s |
| 25 | 17.6 | 1.32 s | 14.1 | 1.55 s | 16.7 | 1.18 s |
| 26 | 19.3 | 1.31 s | 19.8 | 1.59 s | 20.2 | 1.10 s |
| 27 | 15.9 | 1.09 s | 16.0 | 1.19 s | 16.7 | 1.45 s |
| 28 | 177.7 | 178.5 | 179.6 | |||
| 29 | 18.6 | 1.20 d (5.0) | 18.6 | 1.13 d (6.2) | 17.7 | 1.11 d (5.7) |
| 30 | 14.6 | 1.13 d (6.3) | 15.0 | 1.33 d (6.4) | 13.5 | 0.99 d (6.4) |
a H and C were measured at 600 and 150 MHz, respectively, in Pyridine-d5. b H and C were measured in CD3OD.
The molecular composition of compound 14 was confirmed as C30H44O6, supported by HR-ESI-MS readings (m/z 545.3156 [M + COOH]−, estimated for C31H45O8 at 545.3154), marking an increase of 16 amu from compound 13. In the 1H NMR analysis, a distinct resonance at δH 4.25 (1H, dd, J = 7.2, 6.6 Hz) was seen, along with a corresponding oxygenated methine resonance at δC 78.8 in the 13C NMR reading. Hence, compound 14 was inferred to be a hydroxyl derivative of 13. Within the HMBC analysis, crucial associations were observed between the 25-CH3 resonance (δH 1.55) and multiple carbon resonances, including the newly observed δC 78.8 (Figure 4). The NOESY study revealed correlations between H-1 (δH 4.25) and both H-5 (δH 1.40) and H-9 (δH 1.98), suggesting a β-orientation for the 1-OH group (Figure 4). Using analyses mirroring the NMR data of 13, the epoxy-γ-lactone component was ascribed to positions C-11 through C-13 and C-28. The NOESY study further highlighted associations between H-12 (δH 3.28) and 27-CH3 (δH 1.19), pinpointing a β-orientation for the C-11(12) epoxy segment (Figure 4). Consequently, compound 14’s identity was resolved as 3-oxo-1β,21β-dihydroxyl-11β,12β- epoxyl-urs-13β,28β-lactone.
For compound 15, its molecular configuration was discerned as C30H44O6 from the HR-ESI-MS measurements (m/z 545.3130 [M + COOH]−, estimated for C31H45O8 at 545.3114). The 1H NMR analysis exhibited four distinct low-field proton resonances, and the 13C NMR displayed four unique downfield carbon resonances. Key associations in the HMBC were seen between the resonance of C-7 (δC 72.5) and 26-CH3 (δH 1.10) (Figure 4). NOESY associations of H-7 (δH 3.35) with both 25-CH3 (δH 1.18) and 26-CH3 (δH 1.10) proposed an α-orientation for the 7-OH segment (Figure 4). The oxygenated methine resonance at δC 70.4 was ascribed to C-21 based on HMBC associations with various resonances (Figure 4). Moreover, the NOESY study demonstrated links between H-21 (δH 3.32) and H-19 (δH 1.98), suggesting a β-orientation for the 21-OH segment (Figure 4). Employing assessments akin to the NMR data of 13, the epoxy-γ-lactone segment was positioned at C-11 through C-13 and C-28. Subsequent NOESY associations between H-12 (δH 3.13) and 27-CH3 (δH 1.45) indicated a β-orientation for the C-11(12) epoxy fragment (Figure 4). After thorough validation using X-ray crystallography [Cu Ka; Flack value: 0.04(6); CCDC: 2256926] (Figure 5), compound 15’s structure was firmly established as 3-oxo-7α,21β-dihydroxyl-11β,12β-epoxyl-urs-13β,28β-lactone.
Compound 16 displayed an [M + COOH]− at m/z 545.3123 (calcd. for C31H45O8, 545.3114) signifying a molecular composition of C30H44O6. Compound 16 possessed two more hydroxylation sites at C-7 and C-21. One hydroxyl placement was determined at C-7 through the HMBC connections of 26-CH3 (δH 1.28) to C-7 (δC 73.7). Also, the NOESY interaction of H-7 (δH 3.79) with 27-CH3 (δH 1.20) indicated the β-orientation of the 7-OH. A second hydroxyl spot was discerned at C-21 due to the HMBC interactions of the distinctive 30-CH3 resonance (δH 1.08) with C-19 (δC 37.6), C-20 (δC 48.6), and a freshly oxygenated methine signal at δC 71.9. The upfield shift of C-30 to δC 14.7, attributed to the γ-gauche effect, substantiated the hydroxylation’s position at C-21. Moreover, the NOESY connections of H-21 (δH 3.42) to H-19 (δH 2.08) highlighted that the 21-OH group had a β-orientation. Relative to the NMR spectra of 13, the epoxy-γ-lactone segment was attributed to C-11, C-12, C-13, and C-28. Hence, compound 16 was pinpointed as 3-oxo-7β,21β-dihydroxyl-11β,12β-epoxyl-urs-13β,28β-lactone.
For compound 17, the molecular composition was delineated as C30H42O6 by HR-ESI-MS, noting an [M + COOH]− ion at m/z 543.2968 (calcd. for C31H43O8, 543.2958), showing a 2 amu mass reduction in comparison to 16, pointing to a dehydrogenated variant of compound 16. The 13C NMR reading of compound 17 displayed an oxymethine at δC 73.0 and an extra carbonyl frequency at δC 208.4, implying that compound 17 emerged as a carbonylated variant of 16. The carbonyl cluster was linked to C-21 owing to the HMBC interaction between C-21 (δC 208.4) and 30-CH3 (δH 1.09). Moreover, the NOESY interplay of H-7 (δH 3.78) with 27-CH3 (δH 1.14) indicated the β-orientation of the 7-OH. Consequently, compound 17 was pinpointed as 3,21-dioxo-7β-hydroxyl-11β,12β-epoxyl-urs-13β,28β-lactone.
Compound 18 displayed an [M + COOH]− at m/z 527.2993 (calcd. for C31H43O7, 527.3001), representing a molecular composition of C30H42O5, which was 18 amu less than compound 14. In the 1H NMR spectrum, two distinctive signals emerged at δH 7.62 (1H, d, J = 10.3 Hz) and δH 5.89 (1H, d, J = 10.3 Hz), with associated olefin carbon signals at δC 160.5 and δC 126.1 in the 13C NMR spectrum. Therefore, compound 18 appeared to be a desiccated variant of compound 14. The HMBC spectrum revealed links of the distinct 25-CH3 resonance (δH 1.41) with C-5 (δC 54.5), C-9 (δC 45.1), C-10 (δC 41.4), and a fresh olefin carbon frequency at δC 160.5 (Figure 4). Furthermore, the carbonyl carbon signal at C-3 moved upfield to δC 207.3 due to the π → π conjugate effect, suggesting the presence of a double bond between C-1 and C-2. In the HMBC spectrum, connections of the signature 30-CH3 resonance (δH 1.09) with C-19 (δC 37.4), C-20 (δC 48.6), and a novel oxygenated methine signal at δC 71.8 were noted (Figure 4). Moreover, the NOESY interactions of H-21 (δH 3.42) with H-19 (δH 2.05) underscored that the 21-OH group had a β-orientation (Figure 4). By comparing with the NMR spectra of 13, the epoxy-γ-lactone segment was pinpointed at C-11, C-12, C-13, and C-28. The NOESY interactions of H-12 (δH 3.28) with 27-CH3 (δH 1.14) revealed the β-orientation of the C-11(12) epoxy group. Thus, compound 18 was identified as 3-oxo-21β-hydroxyl-11β,12β-epoxyl-urs-1-ene-13β,28β-lactone.
The molecular structure of compound 19 was deduced as C30H42O5, as evidenced by the HR-ESI-MS displaying an [M + COOH]− ion at m/z 527.3017 (calcd. for C31H43O7, 527.3017). Three additional low-field proton signals were spotted at δH 3.09, 3.30, and 3.42 in the 1H NMR spectrum. Furthermore, in the 13C NMR and DEPT 135 spectra, three more downfield methine carbon signals at δC 52.5, 52.6, and 71.7, along with two double-bond quaternary carbon signals at δC 128.5 and 129.7, were identified. The HMBC spectrum showcased correlations of the signature 30-CH3 resonance (δH 1.05) with C-19 (δC 35.3), C-20 (δC 47.5), and a new oxygenated methine signal at δC 71.7 (Figure 4). The NOESY interactions of H-21 (δH 3.42) with H-19 (δH 1.88) highlighted the β-orientation of the 21-OH group (Figure 4). The HMBC links of C-8 (δC 128.5) and C-7 (δC 129.7) with a methyl signal at δH 1.82 and of H-11 (δH 3.30) with C-8 (δC 128.5) and C-9 (δC 49.5) proposed a double bond at C-7(8) with the methyl group positioned at C-7. Using an analysis analogous to the NMR spectra of 13, the epoxy-γ-lactone portion was mapped at C-11, C-12, C-13, and C-28. Additionally, the NOESY interactions of H-12 (δH 3.09) with 27-CH3 (δH 1.18) signified a β-orientation for the C-11(12) epoxy group. A subsequent X-ray crystallographic assessment [Cu Ka; Flack parameter: −0.09(10); CCDC: 2256927] (Figure 5) corroborated both the structure and definitive configuration of 19. Hence, compound 19’s structure was established as (5R, 9R, 10S, 11R, 12R, 13S, 14S, 17R, 18R, 19S, 20S, 21S) 3-oxo-21β-hydroxyl-7-methyl-11β,12β-epoxyl-7-ene-26-norurs-13β,28β-lactone.
Table 4.
The 1H- and 13C-NMR data of compounds 16–19 (500 and 125 MHz, respectively, in CD3OD).
| Position | 16 | 17 a | 18 | 19 a | ||||
|---|---|---|---|---|---|---|---|---|
| δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | δ C | δH (J in Hz) | |
| 1 | 40.0 | 1.70, 2.19 m | 39.2 | 1.58, 2.23 m | 160.5 | 7.62 d (10.3) | 39.4 | 1.54, 2.27 m |
| 2 | 34.8 | 2.51, 2.67 m | 33.7 | 2.49, 2.69 m | 126.1 | 5.89 d (10.3) | 34.4 | 2.24 m 2.75 td (14.1, 5.5) |
| 3 | 219.2 | 215.6 | 207.3 | 216.1 | ||||
| 4 | 48.3 | 47.3 | 46.1 | 47.1 | ||||
| 5 | 53.3 | 1.60 m | 52.7 | 1.45 m | 54.5 | 1.76 m | 50.2 | 1.36 dd (12.5, 4.1) |
| 6 | 30.8 | 1.62, 1.77 m | 30.3 | 1.46, 1.70 m | 19.5 | 1.63, 1.76 m | 32.5 | 1.58, 2.61 m |
| 7 | 73.7 | 3.79 dd (10.7, 4.3) | 73.0 | 3.78 t (7.6) | 33.6 | 1.17, 1.50 m | 129.7 | |
| 8 | 46.7 | 45.4 | 42.3 | 128.5 | ||||
| 9 | 49.1 | 1.60 m | 48.1 | 1.46 m | 45.1 | 1.98 m | 49.5 | 2.37 m |
| 10 | 38.4 | 37.2 | 41.4 | 36.6 | ||||
| 11 | 53.9 | 3.44 m | 52.3 | 3.43 m | 52.8 | 3.75 d (3.8) | 52.6 | 3.30 dd (3.8, 1.5) |
| 12 | 53.0 | 3.19 d (3.9) | 51.4 | 3.24 d (3.8) | 52.8 | 3.28 d (3.8) | 52.5 | 3.09 d (3.8) |
| 13 | 91.3 | 88.7 | 90.9 | 87.8 | ||||
| 14 | 44.4 | 43.1 | 43.3 | 43.4 | ||||
| 15 | 30.4 | 1.29, 1.88 m | 29.2 | 1.70, 1.96 m | 26.7 | 1.28, 1.64 m | 35.5 | 1.70, 2.14 m |
| 16 | 24.6 | 1.41, 2.08 m | 23.9 | 1.58, 1.70 m | 24.4 | 1.43 m 2.14 td (13.3, 6.0) |
23.7 | 1.50, 1.88 m |
| 17 | 47.6 | 48.4 | 47.7 | 46.6 | ||||
| 18 | 62.4 | 1.99 d (11.7) | 60.6 | 2.49 m | 62.0 | 2.04 m | 59.5 | 1.91 m |
| 19 | 37.6 | 2.08 m | 38.3 | 2.28 m | 37.4 | 2.05 m | 35.3 | 1.88 m |
| 20 | 48.6 | 0.92 m | 51.0 | 2.04 m | 48.6 | 0.92 d | 47.5 | 0.90 m |
| 21 | 71.9 | 3.42 m | 208.4 | - | 71.8 | 3.42 m | 71.7 | 3.42 m |
| 22 | 41.1 | 1.41, 2.06 m | 47.0 | 2.58, 2.61 m | 41.1 | 1.44, 2.06 m | 39.8 | 1.44, 2.13 m |
| 23 | 26.6 | 1.10 s | 26.0 | 1.11 s | 27.6 | 1.15 s | 25.3 | 1.01 s |
| 24 | 21.2 | 1.07 s | 20.8 | 1.08 s | 21.8 | 1.11 s | 22.3 | 1.07 s |
| 25 | 18.1 | 1.25 s | 17.4 | 1.30 s | 21.5 | 1.41 s | 15.6 | 1.19 s |
| 26 | 14.9 | 1.28 s | 13.9 | 1.36 s | 20.4 | 1.32 s | 23.2 | 1.82 s |
| 27 | 16.4 | 1.20 s | 16.4 | 1.14 s | 16.4 | 1.14 s | 24.3 | 1.18 s |
| 28 | 181.0 | 176.3 | 180.7 | 177.7 | ||||
| 29 | 18.9 | 1.19 d (6.3) | 19.6 | 1.29 d (6.3) | 18.9 | 1.21 d (5.6) | 18.4 | 1.11 d (5.8) |
| 30 | 14.7 | 1.08 d (6.6) | 11.2 | 1.09 d (6.5) | 14.8 | 1.09 d (6.4) | 14.6 | 1.05 d (6.4) |
a H and C were measured at 400 and 100 MHz, respectively, in CDCl3.
2.2. Anti-Neuroinflammatory Activities
To assess the potential capabilities of all modified products in counteracting neuroinflammation, we measured their suppressive effects on NO generation within LPS-triggered BV-2 cells using the Griess method. Table 5 illustrates the findings. The majority of these modified substances exhibited stronger suppression capabilities on NO generation compared to the base compound, UNA. Specifically, compounds 7, 10, 13, 16, 18, and 19 had notable suppression outcomes with IC50 values of 11.58, 8.23, 15.19, 6.86, 17.42, and 5.25 µM, respectively, surpassing the base compound’s IC50 at 84.72 µM. In addition, compounds 2, 3, 12, 14, and 15 exhibited intermediate suppression results with IC50 values of 38.17, 20.93, 31.05, 22.57, and 36.64 µM, respectively. Conversely, compounds 4, 8, and 9 did not showcase any suppression capabilities towards NO generation.
Table 5.
Inhibitory effects of transformed products on NO production induced by LPS in BV-2 cells (mean ± SD, n = 3).
| Compounds | IC50 (μM) | Cell Viability (%) | Compounds | IC50 (μM) | Cell Viability (%) |
|---|---|---|---|---|---|
| L-NMMA a | 28.25 ± 2.97 | 100.51 ± 4.36 | 10 | 8.23 ± 2.61 | 104.41 ± 4.05 |
| Ursonic acid (1) | 84.72 ± 3.22 | 98.84 ± 3.61 | 11 | 42.48 ± 3.70 | 101.29 ± 3.42 |
| 2 | 38.17 ± 4.09 | 101.25 ± 2.45 | 12 | 31.05 ± 3.98 | 98.23 ± 4.37 |
| 3 | 20.93 ± 2.13 | 100.33 ± 3.92 | 13 | 15.19 ± 3.07 | 102.64 ± 3.59 |
| 4 | >100 | 103.62 ± 3.18 | 14 | 22.57 ± 3.44 | 104.05 ± 4.92 |
| 5 | 52.81 ± 3.34 | 100.56 ± 4.11 | 15 | 36.64 ± 2.33 | 101.78 ± 3.18 |
| 6 | 50.24 ± 3.16 | 104.79 ± 4.23 | 16 | 6.86 ± 3.52 | 97.82 ± 4.75 |
| 7 | 11.58 ± 2.01 | 99.17 ± 3.72 | 17 | 40.79 ± 3.26 | 100.37 ± 3.54 |
| 8 | >100 | 102.48 ± 3.54 | 18 | 17.42 ± 2.72 | 103.24 ± 3.26 |
| 9 | >100 | 103.95 ± 3.86 | 19 | 5.25 ± 3.19 | 102.83 ± 3.03 |
a L-NMMA as a positive control.
3. Discussion
Earlier research on triterpenes reveals that microbial modifications possess a heightened catalytic propensity, resulting in a variety of hydroxylated and carbonylated byproducts [33]. A. ochraceus is prevalently found in the environment, commonly in soil and decaying plant matter. Historically, A. ochraceus has been employed as a biocatalyst in the hydroxylation processes of steroids, triterpenes, flavonoids, and coumarins [34,35,36,37]. In our present investigation, we discerned that A. ochraceus primarily initiated hydroxylation, oxidation, lactonization, and epoxidation reactions on UNA.
A. ochraceus exhibited the ability to concurrently initiate hydroxylation, lactonization, and epoxidation processes on UNA. In our current study, we isolated seven unique compounds (13–19) that simultaneously possessed the 21β-hydroxyl group, 11β,12β-epoxyl group, and 13β,28β-lactone. Notably, A. ochraceus exhibited a preference for initiating the 11β,12β-epoxidation, yielding compounds 13–19, which are unveiled here for the first time. Furthermore, A. ochraceus triggered a transmethylation process, resulting in the formation of the distinctive ursane structure 19. This process showcased an atypical biocatalytic transformation.
A. oryzae is predominantly identified in specific regions within China and Japan and is integral to the fermentation process of certain edibles. Its utility as a biocatalyst in the hydroxylation, oxidation, and lactonization of isoflavones, triterpenes, and sterols is well-documented [38,39,40,41]. In earlier findings, we have ascertained that A. oryzae can facilitate hydroxylation, acetylation, and epoxidation processes on cycloastragenol, a distinct cycloartane-type triterpene [42]. In this study, A. oryzae predominantly initiated 7β,21β-hydroxylation (2, 3, and 6–9), 21-oxidation (4 and 5), and 13β,28β-lactonization (8 and 9) reactions on UNA. Intriguingly, A. oryzae also showcased its ability to drive a methoxylation process either at C-3 or C-11, resulting in compounds 7 and 10.
The position and arrangement of hydroxyl and epoxyl groups on the UNA structure can influence their capacity to inhibit NO activity. Compound 2, which had a hydroxyl group at C-21β, demonstrated stronger inhibitory effects on NO generation compared to UNA itself. This finding indicated that introducing a hydroxyl group at C-21β could amplify the compound’s inhibitory effect on NO production. On the contrary, compound 4, containing a carbonyl group at C-21, presented a notably reduced inhibition compared to compound 2, indicating the detrimental effect of carbonylation at C-21. Moreover, compounds 3 and 5, which had a hydroxyl group at C-7β, presented more potent inhibitory effects than that of compounds 2 and 4, respectively. These indicated that hydroxylation at C-7β could enhance the NO inhibitory activity. In a parallel fashion, the inhibitory impact on NO production by compounds 13–19, which possessed an epoxyl group at C-11β and C-12β, surpassed that of compounds 8 and 9. This finding pointed to the conclusion that epoxidation at C-11β and C-12β could significantly augment inhibitory effects on NO production. Meanwhile, compounds 8 and 9, carrying a lactone group at C-13β and C-28, did not exhibit any inhibitory activities, insinuating that having a lactone group at these positions could be detrimental to inhibiting NO production. Compounds 10, 16, and 19 showcased the strongest inhibitory potential, with IC50 values of 8.23, 6.86, and 5.25 µM, respectively (Figure 6). Such results underlined the promise of these compounds as primary candidates for addressing neuronal injuries.
Figure 6.
Preliminary structure–activity relationship of biotransformation products.
The biotransformation of UNA by A. ochraceus CGMCC 3.5324 and A. oryzae CGMCC 3.407 produced 18 derivatives, of which 13 were novel compounds (4, 5, 7–10, and 13–19). The principal reaction types observed were region-selective hydroxylation, epoxidation, lactonization, carbonylation, and transmethylation. Notably, A. ochraceus demonstrated the capability to concurrently catalyze the epoxidation at C-11(12) and the lactonization at C-13(28). Additionally, the epoxidation and lactonization reactions were stereo-selective at C-11β, C-12β, and C-13β positions. Achieving such specific reactions through conventional chemical synthesis is challenging. On the other hand, A. oryzae facilitated hydroxylation, oxidation, and lactonization reactions and uniquely catalyzed the methoxylation reaction, resulting in two distinct products. Some of these biotransformed derivatives exhibited significant inhibitory effects on NO production, positioning them as potential anti-neuroinflammatory agents. This research underscored the potential of biotransformation for the structural diversification of UNA, enabling the discovery of valuable derivatives. With the distinct biocatalytic capabilities of the fungi studied, a combination of microbial transformation and chemical semi-synthesis could be leveraged to produce an even broader array of UNA derivatives and analogs.
4. Materials and Methods
4.1. General
NMR spectra were recorded using Bruker AV-400, DRX-500, and AV-600 spectrometers. X-ray crystallographic analysis was conducted on a Bruker APEX-II CCD detector, utilizing graphite-monochromated Cu Kα radiation (λ = 1.54178 Å) from Bruker Biospin, Rheinstetten, Germany. Optical rotations were determined with a JASCO P-1020 digital polarimeter. Melting points, taken with an XT4A apparatus (Dianguang Corp., Shanghai, China), were uncorrected. IR spectra were obtained using a Nicolet 5700 FT-IR microscope spectrometer (Thermo Fisher Scientific Inc., Waltham, MA, USA). HR-ESI-MS spectral data were sourced from an Agilent 6540 UHD Q-TOF mass spectrometer. Reversed-phase preparative HPLC was conducted on a Shimadzu LC-20A instrument equipped with an SPD-20A UV detector and using YMC-Pack ODS-A (5 μm, 10.0 × 250 mm) columns.
4.2. Microorganism and Substance
UNA (1) was procured from Push Bio-technology Co., Ltd., Chengdu, China. Its authenticity was confirmed by comparing its physical and spectroscopic data with previously reported values. The purity was verified to be above 98% through UV-HPLC analysis. All solvents used were of AR grade, sourced from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. The strains A. ochraceus CGMCC 3.5324 and A. oryzae CGMCC 3.407 were acquired from the China General Microbiological Culture Collection Center (CGMCC). They were maintained on potato slants solidified with agar and stored at 4 °C. The BV-2 cell line was sourced from the Cell Bank of the Chinese Academy of Sciences.
4.3. Biotransformation Procedures
Biotransformation experiments were conducted in 1000 mL flasks, each containing 400 mL of liquid potato medium. These flasks were incubated on a rotary shaker at 26 °C with a shaking speed of 160 rpm. After a 24 h pre-culture period, 20 mg of the substrate dissolved in 2 mL of ethanol was added to each flask. Fermentation then proceeded for 7 days. In total, 2.0 g of UNA was used for A. ochraceus and 1.2 g for A. oryzae. After the 7-day incubation, the cultures from each flask were combined and filtered. The resulting filtrates underwent extraction with ethyl acetate (EtOAc) three times. The organic layers were then gathered, and after solvent evaporation, residues weighing 3.4 g and 2.3 g were obtained for A. ochraceus and A. oryzae, respectively.
4.4. Extraction and Isolation
A. ochraceus CGMCC 3.5324: The residual material, weighing 3.4 g, was subjected to silica gel column chromatography using a gradient elution of dichloromethane (CH2Cl2) to methanol (CH3OH) ranging from 100:1 to 1:1 (v/v). This separation yielded five primary fractions (Fr.1–Fr.5). These fractions were further purified using semi-preparative HPLC to isolate the pure compounds. Fr.1 produced compounds 4 (23.6 mg), 13 (24.5 mg), 18 (18.6 mg), and 19 (32.1 mg). Compound 2 (58.5 mg) was isolated from Fr.2. Fr.3 gave rise to compounds 14 (14.5 mg), 15 (28.8 mg), 3 (20.9 mg), and 5 (15.7 mg). Fr.4 produced compounds 16 (13.9 mg) and 17 (25.2 mg). Lastly, compounds 8 (23.6 mg), 9 (19.5 mg), and 1 (93.8 mg) were obtained from Fr.5.
A. oryzae CGMCC 3.407: The crude extract, with a mass of 2.3 g, underwent chromatographic separation on an ODS-C18 open column using a gradient elution of methanol (CH3OH) to water (H2O) with the following ratios: 20:80, 40:60, 60:40, 80:20, 90:10, and 100:0 (v/v). This resulted in five primary fractions (Fr.1–Fr.4). Each fraction was then further purified by semi-preparative HPLC to obtain the pure compounds. Specifically, Fr.1 yielded compounds 2 (20.5 mg), 6 (16.1 mg), 7 (13.6 mg), and 12 (15.4 mg). Fr.2 produced compounds 3 (22.1 mg), 4 (17.4 mg), 5 (23.8 mg), and 11 (19.2 mg). Fr.3 gave rise to compound 10 (18.5 mg), while Fr.4 produced compounds 8 (12.5 mg), 9 (24.4 mg), and 1 (78.2 mg).
4.5. Compound Characterization
Characterization of 3,21-dioxo-urs-12-en-28-oic acid (4), White powder; mp 232–225 °C; [α: +28.6° (c = 0.1, MeOH); IR (KBr): νmax 3607, 2956, 1768, 1724, 1705, 1384, 1235, 1052 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) (for data, see Table 1); HR-ESI-MS: m/z 467.3174 [M − H]− (calcd. for C30H43O4, 467.3161).
Characterization of 3,21-dioxo-7β-hydroxy-urs-12-en-28-oic acid (5), White powder; mp 251–254 °C; [α: +36.8° (c = 0.1, MeOH); IR (KBr): νmax 3657, 2934, 1761, 1726, 1702, 1367, 1245, 1063 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) (for data, see Table 1); HR-ESI-MS: m/z 483.3126 [M − H]− (calcd. for C30H43O5, 483.3112).
Characterization of 3-oxo-11α-methoxy-21β-hydroxy-urs-12-en-28-oic acid (7), White powder; mp 268–271 °C; [α: +18.9° (c = 0.1, MeOH); IR (KBr): νmax 3667, 2965, 1763, 1711, 1376, 1239, 1042 cm−1; 1H NMR (CDCl3, 600 MHz) and 13C NMR (CDCl3, 150 MHz) (for data, see Table 1); HR-ESI-MS: m/z 499.3433 [M − H]− (calcd. for C31H47O5, 499.3423).
Characterization of 3-oxo-21β-hydroxy-urs-11-en-13β,28β-lactone (8), White powder; mp 245–247 °C; [α: +30.2° (c = 0.1, MeOH); IR (KBr): νmax 3673, 2954, 1742, 1703, 1388, 1264, 1039 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) (for data, see Table 2); HR-ESI-MS: m/z 451.3216 [M − H2O+H]+ (calcd. for C30H43O3, 451.3212).
Characterization of 3-oxo-21β-hydroxy-urs-11-en-13β,28β-lactone (9), White powder; mp 261–265 °C; [α: +58.5° (c = 0.1, MeOH); IR (KBr): νmax 3685, 2972, 1751, 1712, 1371, 1255, 1046 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) (for data, see Table 2); HR-ESI-MS: m/z 529.3171 [M + COOH]− (calcd. for C31H45O7, 529.3165).
Characterization of 3-methoxy-urs-2,12-dien-28-oic acid (10), White powder; mp 284–288 °C; [α: +31.7° (c = 0.1, MeOH); IR (KBr): νmax 3631, 2955, 1719, 1383, 1264, 1052 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) (for data, see Table 2); HR-ESI-MS: m/z 467.3525 [M − H]− (calcd. for C31H47O3, 467.3525).
Characterization of 3-oxo-21β-hydroxy-11β,12β-epoxyl-urs-13β,28β-lactone (13), White powder; mp 257–263 °C; [α: +47.1° (c = 0.1, MeOH); IR (KBr): νmax 3612, 2943, 1765, 1718, 1377, 1226, 1048 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) (for data, see Table 3); HR-ESI-MS: m/z 529.3173 [M + COOH]− (calcd. for C31H45O7, 529.3165).
Characterization of 3-oxo-1β,21β-dihydroxyl-11β,12β-epoxyl-urs-13β,28β-lactone (14), White powder; mp 278–280 °C; [α: +72.3° (c = 0.1, MeOH); IR (KBr): νmax 3568, 3527, 2961, 1768, 1722, 1362, 1239, 1054 cm−1; 1H NMR (Pyridine-d5, 600 MHz) and 13C NMR (Pyridine-d5, 150 MHz) (for data, see Table 3); HR-ESI-MS: m/z 545.3156 [M + COOH]− (calcd. for C31H45O8, 545.3114).
Characterization of 3-oxo-7α,21β-dihydroxyl-11β,12β-epoxyl-urs-13β,28β-lactone (15), White powder; mp 270–274 °C; [α: +24.5° (c = 0.1, MeOH); IR (KBr): νmax 3685, 3618, 2956, 1767, 1717, 1322, 1247, 1053 cm−1; 1H NMR (CD3OD, 400 MHz) and 13C NMR (CD3OD, 100 MHz) (for data, see Table 3); HR-ESI-MS: m/z 545.3130 [M + COOH]− (calcd. for C31H45O8, 545.3114).
Characterization of 3-oxo-7β,21β-dihydroxyl-11β,12β-epoxyl-urs-13β,28β-lactone (16), White powder; mp 268–273 °C; [α: +83.1° (c = 0.1, MeOH); IR (KBr): νmax 3647, 3586, 2959, 1765, 1713, 1351, 1232, 1055 cm−1; 1H NMR (CD3OD, 500 MHz) and 13C NMR (CD3OD, 125 MHz) (for data, see Table 4); HR-ESI-MS: m/z 545.3123 [M + COOH]− (calcd. for C31H45O8, 545.3114).
Characterization of 3,21-dioxo-7β-hydroxyl-11β,12β-epoxyl-urs-13β,28β-lactone (17), White powder; mp 247–249 °C; [α: +20.3° (c = 0.1, MeOH); IR (KBr): νmax 3674, 2956, 1765, 1721, 1714, 1366, 1267, 1068 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) (for data, see Table 4); HR-ESI-MS: m/z 543.2968 [M + COOH]− (calcd. for C31H43O8, 543.2958).
Characterization of 3-oxo-21β-hydroxyl-11β,12β-epoxyl-urs-1-ene-13β,28β-lactone (18), White powder; mp 242–245 °C; [α: +38.6° (c = 0.1, MeOH); IR (KBr): νmax 3622, 3031, 2955, 1765, 1689, 1332, 1267, 1091 cm−1; 1H NMR (CD3OD, 500 MHz) and 13C NMR (CD3OD, 125 MHz) (for data, see Table 4); HR-ESI-MS: m/z 527.2993 [M + COOH]− (calcd. for C31H43O7, 527.3009).
Characterization of 3-oxo-21β-hydroxyl-7-methyl-11β,12β-epoxyl-7-ene-26-norurs-13β,28β-lactone (19), White powder; mp 282–284 °C; [α: −35.7° (c = 0.1, MeOH). IR (KBr): νmax 3598, 2971, 1767, 1716, 1380, 1233, 1048 cm−1; 1H NMR (CDCl3, 400 MHz) and 13C NMR (CDCl3, 100 MHz) (for data, see Table 4); HR-ESI-MS: m/z 527.3017 [M + COOH]− (calcd. for C31H43O7, 527.3009).
4.6. Anti-Neuroinflammatory Activities
NO production was assessed indirectly using the Griess reaction by measuring nitrite concentration in a culture medium. BV-2 cells were cultured in Dulbecco’s Modified Eagle Medium, supplemented with 10% FBS, 100 units/mL of penicillin, and 100 μg/mL of streptomycin. These cells were seeded in 96-well plates at a density of 8 × 104 cells/well and incubated for 24 h. Post incubation, they were exposed to 100 ng/mL of LPS alongside different compound concentrations for 48 h. A mixture of 100 μL culture supernatant and Griess reagent was left at room temperature for 10 min, with absorbance later read at 570 nm. L-NMMA served as the positive control. Cell viability was determined using the MTT assay, and experiments were conducted in triplicate. The IC50 for NO production inhibition was computed using GraphPad Prism 7.00 software.
4.7. X-ray Crystallographic Analyses
Colorless needle crystals of compound 10 were obtained using an acetone–H2O mixture (3:1). Similarly, compounds 13 and 19 were derived from a MeOH–H2O mixture (9:1), and compound 15 from an acetone–H2O mixture (8:2). A suitable crystal was chosen and analyzed on a Bruker APEX-II CCD diffractometer, with the crystal maintained at 173.0 K during data collection. The structure was deciphered using the ShelXT structure solution program within Olex2, employing Intrinsic Phasing. Refinement was performed with the ShelXL package using least squares minimization.
Deposition numbers for compounds 10, 13, 15, and 19 in the Cambridge Crystallographic Data Centre (CCDC) are 2266165 and 2256926-2256928, respectively.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/molecules28247943/s1.
Author Contributions
Conceptualization, G.-T.C., W.-L.W. and B.L.; formal analysis, A.-D.W. and B.-Y.F.; investigation, Y.Y.; methodology, Y.-N.W., D.S. and J.Y.; resources, M.Y. and J.-L.L. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The NMR data for compounds 4, 5, 7–10, and 13–19 have been deposited to the Harvard Dataverse at https://doi.org/10.7910/DVN/TYF1KG.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Natural Science Foundation of China (32100317, 32200314), Natural Science Project of Nantong Science and Technology Board (MS12022026), Traditional Chinese Medicine Science and Technology Development Project of Jiangsu Province (MS2021073), and the Large Instrument Open Fund of Nantong University (KFJN2262).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Romano R., Bucci C. Antisense therapy: A potential breakthrough in the treatment of neurodegenerative diseases. Neural Regen. Res. 2024;19:1027–1035. doi: 10.4103/1673-5374.385285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arjunan A., Sah D.K., Woo M., Song J. Identification of the molecular mechanism of insulin-like growth factor-1 (IGF-1): A promising therapeutic target for neurodegenerative diseases associated with metabolic syndrome. Cell Biosci. 2023;13:16. doi: 10.1186/s13578-023-00966-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ding W.B., Lin H.Y., Hong X., Ji D.L., Wu F. Poloxamer 188-mediated anti-inflammatory effect rescues cognitive deficits in paraquat and maneb-induced mouse model of Parkinson’s disease. Toxicology. 2020;436:152437. doi: 10.1016/j.tox.2020.152437. [DOI] [PubMed] [Google Scholar]
- 4.Guo S., Duan J.A., Qian D., Tang Y.P., Wu D.W., Su S.L., Wang H.Q., Zhao Y.N. Content variations of triterpenic acid, nucleoside, nucleobase, and sugar in jujube (Ziziphus jujuba) fruit during ripening. Food Chem. 2015;167:468–474. doi: 10.1016/j.foodchem.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 5.Mahjoub F., Akhavan R.K., Yousef M., Mohebbi M., Salari R. Pistacia atlantica Desf. A review of its traditional uses, phytochemicals and pharmacology. J. Med. Life. 2018;11:180–186. doi: 10.25122/jml-2017-0055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Son J., Lee S.Y. Therapeutic potential of ursonic acid: Comparison with ursolic acid. Biomolecules. 2020;10:1505. doi: 10.3390/biom10111505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Capela R., Moreira R., Lopes F. An overview of drug resistance in Protozoal diseases. Int. J. Mol. Sci. 2019;20:5748. doi: 10.3390/ijms20225748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hevener E., Verstak T.A., Lutat K.E., Riggsbee D.L., Mooney J.W. Recent developments in topoisomerase targeted cancer chemotherapy. Acta Pharm. Sin. B. 2018;8:844–861. doi: 10.1016/j.apsb.2018.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Son J., Lee S.Y. Ursonic acid exerts inhibitory effects on matrix metalloproteinases via ERK signaling pathway. Chem.-Biol. Interact. 2020;315:108910. doi: 10.1016/j.cbi.2019.108910. [DOI] [PubMed] [Google Scholar]
- 10.Furtado N.A.J.C., Pirson K., Edelberg H., Miranda L.M., Loira-Pastoriza C., Preat V., Larondelle Y., Andre C.M. Pentacyclic triterpene bioavailability: An overview of in vitro and in vivo studies. Molecules. 2017;22:400. doi: 10.3390/molecules22030400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Borkova L., Frydrych I., Jakubcova N., Adamek R., Liskova B., Gurska S., Medvedíkova M., Hajduch M. Synthesis and biological evaluation of triterpenoid thiazoles derived from betulonic acid, dihydrobetulonic acid, and ursonic acid. Eur. J. Med. Chem. 2020;185:111806. doi: 10.1016/j.ejmech.2019.111806. [DOI] [PubMed] [Google Scholar]
- 12.Mlala S., Oyedeji A.O., Gondwe M., Oyedeji O.O. Ursolic acid and its derivatives as bioactive agents. Molecules. 2019;24:2751. doi: 10.3390/molecules24152751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu P.P., Zhang B.J., Cui X.P., Yang Y., Jiang Z.Y., Zhou Z.H., Zhong Y.Y., Mai Y.Y., Ouyang Z., Chen H.S., et al. Synthesis and biological evaluation of novel ursolic acid analogues as potential α-glucosidase inhibitors. Sci. Rep. 2017;7:45578. doi: 10.1038/srep45578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diao M.X., Li C., Li J.X., Lu J., Xie N.Z. Probing the biotransformation process of sclareol by resting cells of Hyphozyma roseonigra. Food Chem. 2022;70:10563–10570. doi: 10.1021/acs.jafc.2c04651. [DOI] [PubMed] [Google Scholar]
- 15.Liu J.H., Xin Y., Qiu Z.D., Zhang Q., He T.Z., Qiu Y., Wang W.N. Cordyceps sinensis-mediated biotransformation of notoginsenoside R1 into 25-OH-20(S/R)-R2 with elevated cardioprotective effect against DOX-induced cell injury. RSC Adv. 2022;12:12938–12946. doi: 10.1039/D2RA01470J. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo J., Mobley R., Woodfne S., Drijfhout F., Horrocks P., Ren X.D., Li W.W. Biotransformation of artemisinin to a novel derivative via ring rearrangement by Aspergillus niger. Microb. Biotechnol. 2022;106:2433–2444. doi: 10.1007/s00253-022-11888-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hudlicky T., Reed J.W. Applications of biotransformations and biocatalysis to complexity generation in organic synthesis. Chem. Soc. Rev. 2009;38:2981–2982. doi: 10.1039/b901172m. [DOI] [PubMed] [Google Scholar]
- 18.Gerothanassis I.P. Ligand-observed in-tube NMR in natural products research: A review on enzymatic biotransformations, protein-ligand interactions, and in-cell NMR spectroscopy. Arab. J. Chem. 2023;16:104536. doi: 10.1016/j.arabjc.2022.104536. [DOI] [Google Scholar]
- 19.Tsagogiannis E., Vandera E., Primikyri A., Asimakoula S., Tzakos A.G., Gerothanassis I.P., Koukkou A.-I. Characterization of protocatechuate 4,5-dioxygenase from Pseudarthrobacter phenanthrenivorans Sphe3 and in situ reaction monitoring in the NMR tube. Int. J. Mol. Sci. 2021;22:9647. doi: 10.3390/ijms22179647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Siddiqui M., Atia T.W., Choudhary M.I., Atta U.R. Biotransformation studies on bioactive compounds: 25 years of interesting research at the ICCBS. Chem. Synth. 2023;3:25. doi: 10.20517/cs.2023.02. [DOI] [Google Scholar]
- 21.Lu Y.J., Tang Y.F., Wu Y.N., Zhang X.Y., Yi Y., Wang W.L., Wang A.D., Yang M., Fan B.Y., Chen G.T. Microbial transformation of betulonic acid by Circinella muscae CGMCC 3.2695 and anti-neuroinflammatory activity of the products. Phytochemistry. 2022;204:113431. doi: 10.1016/j.phytochem.2022.113431. [DOI] [PubMed] [Google Scholar]
- 22.Song K.N., Lu Y.J., Chu C.J., Wu Y.N., Huang H.L., Fan B.Y., Chen G.T. Biotransformation of betulonic acid by the fungus Rhizopus arrhizus CGMCC 3.868 and antineuroinflammatory activity of the biotransformation products. J. Nat. Prod. 2021;84:2664–2674. doi: 10.1021/acs.jnatprod.1c00480. [DOI] [PubMed] [Google Scholar]
- 23.Yu H., Chen Y.H., Cheng Z., Li H.J., Bian H.H., Yang X.C., Lv J., Liu W., Su L., Sun P. Anti-inflammatory oleanane-type triterpenoids produced by Nonomuraea sp. MYH522 through microbial transformation. J. Agric. Food Chem. 2023;71:3777–3789. doi: 10.1021/acs.jafc.2c09062. [DOI] [PubMed] [Google Scholar]
- 24.Xu S.H., Zhang C., Wang W.W., Yu B.Y., Zhang J. Site-selective biotransformation of ursane triterpenes by Streptomyces griseus ATCC 13273. RSC Adv. 2017;7:20754–20759. doi: 10.1039/C7RA01811H. [DOI] [Google Scholar]
- 25.Yadav V.R., Prasad S., Sung B., Kannappan R., Aggarwal B.B. Targeting inflammatory pathways by triterpenoids for prevention and treatment of cancer. Toxins. 2010;2:2428–2466. doi: 10.3390/toxins2102428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Coleman J.W. Nitric oxide in immunity and inflammation. Int. Immunopharmacol. 2001;1:1397–1406. doi: 10.1016/S1567-5769(01)00086-8. [DOI] [PubMed] [Google Scholar]
- 27.Zhang C., Xu S.H., Ma B.L., Wang W.W., Yu B.Y., Zhang J. New derivatives of ursolic acid through the biotransformation by Bacillus megaterium CGMCC 1.1741 as inhibitors on nitric oxide production. Bioorg. Med. Chem. Lett. 2017;27:2575–2578. doi: 10.1016/j.bmcl.2017.03.076. [DOI] [PubMed] [Google Scholar]
- 28.Tu W.C., Luo R.H., Yuan E., Sakah K.J., Yang Q.Y., Xiao W.L., Zheng Y.T., Liu M.F. Triterpene constituents from the fruits of Cyclocarya paliurus and their anti-HIV-1IIIB activity. Nat. Prod. Res. 2023;37:1787–1796. doi: 10.1080/14786419.2022.2120874. [DOI] [PubMed] [Google Scholar]
- 29.Chu C.J., Song K.N., Zhang Y.Z., Yang M., Fan B.Y., Huang H.J., Chen G.T. Biotransformation of ursolic acid by Circinella muscae and their anti-neuroinflammatory activities of metabolites. Nat. Prod. Res. 2022;36:2777–2782. doi: 10.1080/14786419.2021.1925893. [DOI] [PubMed] [Google Scholar]
- 30.Leal A.S., Wang R., Salvador J.A.R., Jing Y. Synthesis of novel ursolic acid heterocyclic derivatives with improved abilities of antiproliferation and induction of p53, p21waf1 and NOXA in pancreatic cancer cells. Bioorg. Med. Chem. 2012;20:5774–5786. doi: 10.1016/j.bmc.2012.08.010. [DOI] [PubMed] [Google Scholar]
- 31.Cheng D.L., Cao X.P. Pomolic acid derivatives from the root of Sanguisorba officinalis. Phytochemistry. 1992;31:1317–1320. doi: 10.1016/0031-9422(92)80499-5. [DOI] [Google Scholar]
- 32.Ikuta A., Morikawa A. Triterpenes from Stauntonia hexaphylla callus tissues. J. Nat. Prod. 1992;55:1230–1233. doi: 10.1021/np50087a008. [DOI] [Google Scholar]
- 33.Shah S.A.A., Tan H.L., Sultan S., Faridz M.A.B.M., Shah M.A.B.M., Nurfazilah S., Hussain M. Microbial-catalyzed biotransformation of multifunctional triterpenoids derived from phytonutrients. Int. J. Mol. Sci. 2014;15:12027–12060. doi: 10.3390/ijms150712027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Edyta K.S., Tomasz J. Microbial transformations of 7-methoxyflavanone. Molecules. 2012;17:14810–14820. doi: 10.3390/molecules171214810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Letizia C.M., Benedetta G., Immacolata S., Valerio D., Diego R., Andrea P., Roberto L., Pinheiro S.O.R., Francesco M. Development of a high-yielding bioprocess for 11α-hydroxylation of canrenone under conditions of oxygen-enriched air supply. Steroids. 2016;116:1–4. doi: 10.1016/j.steroids.2016.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Rong S., Tang X., Guan S., Zhang B., Li Q., Cai B., Huang J. Effects of impeller geometry on the 11α-hydroxylation of canrenone in Rushton turbine-stirred tanks. J. Microbiol. Biotechnol. 2021;31:890–901. doi: 10.4014/jmb.2104.04002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tomasz T., Agnieszka B., Jaroslaw P., Ewa H. Transformation of xanthohumol by Aspergillus ochraceus. J. Basic Microb. 2014;54:66–71. doi: 10.1002/jobm.201200320. [DOI] [PubMed] [Google Scholar]
- 38.Converti A., Gandolfi R., Zilli M., Molinari F., Binaghi L., Perego P., Borghi M. Synthesis of ethyl phenylacetate by lyophilized mycelium of Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2005;67:637–640. doi: 10.1007/s00253-004-1830-0. [DOI] [PubMed] [Google Scholar]
- 39.Dong Y.S., Teng H., Qi S.S., Liu L., Wang H., Zhao Y.K., Xiu Z.L. Pathways and kinetics analysis of biotransformation of Dioscorea zingiberensis by Aspergillus oryzae. Biochem. Eng. J. 2010;52:123–130. doi: 10.1016/j.bej.2010.07.011. [DOI] [Google Scholar]
- 40.Sikander A., Wajeeha N. Biotransformation of L-tyrosine to dopamine by a calcium alginate immobilized mutant strain of Aspergillus oryzae. Appl. Biochem. Biotechnol. 2016;179:1435–1444. doi: 10.1007/s12010-016-2075-y. [DOI] [PubMed] [Google Scholar]
- 41.Wang H., Liu L., Guo Y.X., Dong Y.S., Zhang D.J., Xiu Z.L. Biotransformation of piceid in Polygonum cuspidatum to resveratrol by Aspergillus oryzae. Appl. Microbiol. Biotechnol. 2007;75:763–768. doi: 10.1007/s00253-007-0874-3. [DOI] [PubMed] [Google Scholar]
- 42.Chen C., Ni Y.H., Jiang B.C., Song Y., Xu B.H., Fan B.Y., Huang H.L., Chen G.T. Anti-aging derivatives of cycloastragenol produced by biotransformation. Nat. Prod. Res. 2021;35:2685–2690. doi: 10.1080/14786419.2019.1662011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The NMR data for compounds 4, 5, 7–10, and 13–19 have been deposited to the Harvard Dataverse at https://doi.org/10.7910/DVN/TYF1KG.