Abstract
A detailed transcription map of the prolate-headed lactococcal phage c2 has been constructed. Transcription of about one-third of the genome, encoding 22 open reading frames, began within the first 2 min of infection and produced at least 12 overlapping transcripts that persisted until lysis occurred at 30 min after initiation of infection. The remaining two-thirds of the genome, encoding 17 open reading frames, was divergently transcribed, beginning between 4 and 6 min after initiation of infection, and resulted in at least 18 overlapping transcripts that persisted until lysis. Five very strong, simultaneously active, and probably unregulated early promoters and a single positively regulated late promoter were identified. The late promoter had an extended −10 sequence, had a significant basal level of activity in the uninduced state, and was induced to high activity by a phage gene product. The complex overlapping pattern of transcripts resulted from the action of the multiple early promoters, inefficient termination of transcription, and (possibly) processing of a late precursor transcript(s). Phage proteins were not required for these processes, and the host RNA polymerase was probably used for both early and late transcription.
Lactococci are used extensively as starter cultures in the production of fermented dairy products. These bacteria are often exposed to phages during the manufacturing process, which may result in the lysis of susceptible strains. Lactococcal phages are classified into 12 species (25). Most lactococcal phage species conform to one of two morphotypes: B1, a small isometric head and a long noncontractile tail; and B2, a prolate head and a long noncontractile tail. Nearly all of the small isometric phages fall into two species groups designated 936 and P335, represented by type phages P008 and P335, respectively (25). The prolate phages form one highly homologous species group, c2, represented by type phage c6A (25). All members of a species share extensive DNA homology and usually have similar structural protein profiles.
The manufacturing environment provides a high degree of selective pressure for lactococcal strains that have acquired resistance to phage attack. Many naturally occurring phage resistance mechanisms have been identified, and most stages of the phage life cycle are susceptible to one or another of these mechanisms (15). Phage resistance mechanisms are grouped into the following four main categories, based on the life-cycle stage they affect: adsorption inhibition, DNA penetration blocking, DNA restriction/modification systems, and abortive infection (Abi) mechanisms. The first three affect the earliest stages of phage infection. Abi mechanisms act at a stage after phage gene expression has been initiated, when it is usually too late to prevent the death of the host cell but still sufficient to prevent the production of viable progeny phage, and their effectiveness is usually species specific. Twelve Abi mechanisms have been identified, designated AbiA, -B, -C, -D, -D1, -E, -F, -G, -H, -I, -J, and -K (10, 13, 14, 15, 40, 50, 59). While they are all effective against 936 species of phage, only AbiD1, AbiD, and AbiF, which form a homology group (14), are fully effective against prolate phages as well. Other mechanisms may be partially effective against prolate phages, and the resistance might be improved by increasing the copy number of the mechanism, as shown for AbiA (11). Some progress has been made in identifying the phage target or mode of action of Abi mechanisms active against 936 and P335 species phages (2, 13, 15, 44). At least one mechanism promotes phage mRNA degradation (44). However, very little is known about how Abi mechanisms act against prolate phages. Molecular knowledge of the prolate phage life cycle would aid our understanding of these resistance mechanisms.
“Novel” resistance mechanisms have been designed to supplement natural phage resistance mechanisms. The phage genome itself has received attention as a potential source of novel phage resistance mechanisms. A cloned copy of the origin of replication from phages φ31 and φ50 (P335 species) was shown to confer phage resistance to the host cell when introduced on a plasmid, presumably by competing with the infecting phage for essential replication factors (21, 41). Antisense mRNA mechanisms directed against an open reading frame (ORF) of unknown function from phage φ7–9 (P335 species) are effective at inhibiting phage propagation (28). Antisense constructs directed against two other ORFs of φ7–9 (29) and the major capsid protein of phage F4-1 (8), a small isometric phage of unknown species, have a smaller inhibitory effect on phage propagation. However, the above strategies are not effective against prolate phages. The cloned origin of phage c2 replication did not inhibit phage c2 propagation, and antisense mechanisms directed against prolate phage c2 genes produced no inhibitory effect (47). No other novel phage resistance mechanisms against prolate phages have been reported. Development of novel resistance mechanisms against prolate phages requires more detailed information on the molecular mechanisms of phage replication. However, little information is available on the molecular biology of the prolate lactococcal phages. Data on the transcription of prolate phages might identify targets for the design of novel resistance mechanisms, such as transcription repressors or activators, and the targets of Abi mechanisms that affect prolate phage transcription.
We report here a detailed transcription map of phage c2, the identification of five simultaneously active and probably unregulated early promoters, a single positively regulated late promoter, evidence for inefficient termination of transcription, and the potential for the host-encoded processing of late transcripts. The transcription control of phage c2 was not tightly regulated and phage proteins were required only for upregulating the late promoter activity.
MATERIALS AND METHODS
Strains, media, and culture conditions.
Bacteriophage c2 (46) was propagated in Lactococcus lactis subsp. lactis MG1363 (16). Preparation of phage stocks and concentration and purification of phages by CsCl gradient centrifugation were carried out as previously described (24).
DNA methods.
Small-scale preparation of plasmids from Escherichia coli was as described by Holmes and Quigley (22) or He et al. (18). Large-scale plasmid isolation from E. coli was done by a modification of the method of Ish-Horowicz and Burke (23). Plasmid DNA was isolated from lactococci as described by O’Sullivan and Klaenhammer (42). Bacteriophage c2 DNA was isolated as described by Jarvis (24). Restriction endonuclease digests, agarose gel electrophoresis, and DNA ligations were done by standard procedures described by Sambrook et al. (53) according to the recommendations of the enzyme suppliers. Electrotransformation of L. lactis and E. coli was as described by Wells et al. (65).
Isolation of RNA.
Phage c2-infected MG1363 cells were prepared as described by Beresford et al. (1) with some modifications as follows. Batch cultures (1 liter) of MG1363 were prepared by inoculating M17G prewarmed to 30°C with 15 ml of an overnight culture followed by incubation at 30°C until the optical density at 600 nm was 0.1. CaCl2 was added to a final concentration of 5 mM, and incubation continued for 5 min. The cells were pelleted by centrifugation at 22 to 27°C for 2 min at 16,000 × g, resuspended in 50 ml of prewarmed (30°C) M17G containing 5 mM CaCl2, and incubated at 30°C. The sample(s) (2.0 ml) was removed, and the cells were pelleted by centrifugation at 16,000 × g for 16 s at 22 to 27°C. The supernatant was removed by aspiration, and the cell pellet was frozen in a liquid nitrogen or ethanol-dry ice bath. Phage c2 was added to the remaining culture from a high-titer phage preparation to a multiplicity of infection of 10, and the time was recorded as the start of infection. At the indicated time intervals, 2-ml samples of c2-infected cells were harvested and frozen as described above. Frozen cells were stored at −80°C. Synchronous infection was indicated by clearing of the culture and the inability to pellet cells by centrifugation 30 min after initiation of infection.
RNA was prepared from frozen cell pellets as described by Magni et al. (35) with modifications: frozen cell pellets were resuspended in 1.6 ml of 20 mM sodium acetate (pH 5.5)–1 mM EDTA–400 μl of macaloid clay suspension (53)–200 μl of 10% sodium dodecyl sulfate. Resuspended cells were transferred into 2 ml of water-saturated phenol-chloroform (1:1) and vortexed repeatedly during 10 min of incubation at 70°C. The phases were separated by centrifugation, and the aqueous layer was extracted twice with 2 ml of water-saturated phenol prewarmed at 70°C and once with 2 ml of chloroform. RNA was precipitated with a 0.1 volume of 3 M sodium acetate (pH 7.0) and 2.5 volumes of 100% ethanol. Precipitated RNA was recovered by centrifugation, washed with 75% ethanol, and resuspended in 100 μl TE (10 mM Tris; 1 mM EDTA, pH 7.5). The RNA was extracted twice with buffer-saturated phenol (pH >7.4 [53]), once with phenol-chloroform (1:1, buffer-saturated), and once with chloroform. RNA was precipitated with sodium acetate and ethanol as described above and resuspended in 100 μl of TE. RNA (50 μl) was incubated with DNase I (10 μl of 100 mM MgCl2, 10 mM dithiothreitol; 29 μl of TE [pH 7.5], 10 μl of DNase I [100 U, RNase free], 1 μl of pancreatic RNase inhibitor [10 U]) for 25 min at 22 to 27°C. A 0.1 volume of 200 mM EDTA (pH 8.0) was added, and the RNA was extracted once with phenol-chloroform and once with chloroform and then precipitated with sodium acetate and ethanol as described above. The RNA was resuspended in TE (pH 7.5), and the concentration and purity were determined by the absorbances at 260, 270, and 280 nm (58). Procedures to minimize RNase contamination of materials were used (53).
Northern blots and hybridizations.
RNA samples were denatured with formaldehyde and formamide (53) and analyzed by electrophoresis through 1 or 1.5% agarose gels. Gels and running buffer contained 0.22 M formaldehyde, 0.04 M MOPS (morpholinepropanesulfonic acid), 10 mM sodium acetate, and 1 mM EDTA (60). After electrophoresis, gels were soaked in 10× SSC (53) for 30 min with three changes of solution and then transferred to Hybond N+ membranes (Amersham) by capillary transfer with 10× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate). RNA was fixed to the membrane by UV irradiation according to the manufacturer’s instructions. RNA transfer was visualized by methylene blue staining of blots (20). Hybridizations were done in Rapid-hyb buffer (Amersham) according to the manufacturer’s instructions and the following conditions: 68°C for 2.5 h (DNA probes), 42°C for 1 h (oligonucleotide probes), or 70°C for 2.5 h (RNA probes). The high-stringency washing of the membranes was done in 0.2× SSC–0.1% sodium dodecyl sulfate at the hybridization temperature. The probes are described in Tables 1 and 2. Probes were labeled with 32P by using the RadPrime system (DNA; random primer; Life Technologies, Gaithersburg, Md.) or the RiboProbe system (RNA; Promega, Madison, Wis.), or they were end-labeled with T4 polynucleotide kinase (oligonucleotides [53]) according to the manufacturer’s instructions.
TABLE 1.
Main early transcripts of phage c2 detected by Northern hybridization
| Probe (coordinates)a | Hybridization of transcript size (knt [SD])b:
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 5.2 (0.18) | 4.1 (0.13) | 2.3 (0.13) | 1.8 (0.07) | 1.4 (0.09) | 1.1 (0.06) | 0.70 (0.07) | 0.26–0.36 (0.02) | |
| EP1 (6902–6926) | + | + | + | |||||
| A (6813–7236) | + | + | + | |||||
| B (6525–7011) | + | + | + | + | + | |||
| C (5359–7011) | + | + | + | + | + | |||
| EP2a (6553–6577) | + | + | + | + | ||||
| EP2b (6480–6504) | + | + | + | + | ||||
| D (5359–6025) | + | + | + | + | ||||
| E (4504–5002) | + | |||||||
| F (4421–4749) | + | |||||||
| G (3428–4348) | + | + | ||||||
| H (2922–4050) | + | + | ||||||
| EP6 (2361–2385) | + | + | + | |||||
| I (1732–2536) | + | + | + | |||||
| J (230–1059) | + | + | + | |||||
| K (230–423) | + | + | + | |||||
EP1 through EP6 were single-stranded oligonucleotides; F was a single-stranded RNA; all other probes were double-stranded DNA.
Nucleotide positions are according to GenBank sequence accession number L48605. The average transcript size is given. “+” indicates hybridization. Weak transcripts of 7.3 and 6.8 knt and other weak transcripts are not listed.
TABLE 2.
Main late transcripts of phage c2 detected by Northern hybridization
| Probe (coordinates)a | Hybridization of transcript size (knt [SD])b:
|
|||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10.2 (0.44) | 9.2 (0.24) | 8.3 (0.25) | 7.9 (0.25) | 6.9 (0.13) | 5.2 (0.17) | 5.1 (0.14) | 4.5 (0.11) | 4.5 (0.11) | 3.9 (0.07) | 3.8c (0.10) | 3.6 (0.17) | 2.0 (0.04) | 0.65 (0.07) | 0.43 (0.04) | 0.2 | |
| LP1 (7360–7336) | + | + | + | |||||||||||||
| L (7230–7630) | + | + | + | |||||||||||||
| M (8014–7990) | + | + | + | |||||||||||||
| N (9420–10870) | + | + | + | |||||||||||||
| O (11784–10792) | ± | + | + | + | ||||||||||||
| P (12045–12751) | + | + | + | + | ± | |||||||||||
| Q (13358–13810) | + | + | + | |||||||||||||
| R (13129–15256) | + | + | + | ± | + | + | ||||||||||
| S (15385–15361) | + | + | + | ± | + | + | ||||||||||
| T (16298–16917) | + | + | ± | + | + | |||||||||||
| U (18645–19375) | + | + | + | + | + | + | + | + | ||||||||
| V (21908–22169) | + | + | + | + | + | + | + | + | + | + | + | |||||
| W (21154–22169) | + | + | + | + | + | + | + | + | + | + | + | |||||
LP1, M, and S were single-stranded oligonucleotides; O was a single-stranded RNA; and all other probes were double-stranded DNA.
Nucleotide positions are according to GenBank sequence accession number L48605. The average transcript size is given. “+” indicates strong hybridization; “±” indicates weak hybridization. A large transcript corresponding to the entire late region and other weak transcripts are not listed.
Two overlapping transcripts of 3.8 knt were detected.
Primer extension analysis.
The 5′ ends of RNA transcripts were determined by using the avian myeloblastosis virus reverse transcriptase primer extension system (Promega) according to the manufacturer’s instructions. The synthetic oligonucleotides used were as follows: EP1 (5′ position 6902, TTCAGTGACATCACACAGGGCTACC, 3′ position 6926), EP2a (5′ position 6480, GCTAAAATTGTAATCAATAACCTCC, 3′ position 6504), EP2b (5′ position 6553, AGAACCAAACTCCATAAAGTGAACC, 3′ position 6577), EP3 (5′ position 6317, GAACCATTTGTTCCCAGTCTTTAGC, 3′ position 6341), EP4 (5′ position 5135, TTTAATTCTTTCAGCGTCTCGGACC, 3′ position 5159), EP5 (5′ position 4117, CTCTAATACATTCAACGGCAGTACG, 3′ position 4141), EP6 (5′ position 2361, ATGGGTATGCTTTGAATGATAGGAC, 3′ position 2385), LP1 (5′ position 7384, GGCTATAAGAAGTCACGACC, 3′ position 7365), LP1b (5′ position 7360, TGTTAACGCCGTAATCAGTTTTGTC, 3′ position 7336), LP2 (5′ position 7990, CCTACAACCCTCAAACTCTTTAATC, 3′ position 7966), and LP3 (5′ position 11660, TGAATACAACCCCATACCCTAGACC, 3′ position 11636).
The sequencing reactions were performed by the dideoxy method with the f-mol DNA Cycle Sequencing System (Promega) according to the manufacturer’s protocols for direct incorporation of [α-35S]dATP or primers end labeled with [γ-32P]ATP.
β-Galactosidase assays.
Cell lysates were prepared from duplicate cultures grown to mid-log phase and disrupted by using a Constant Systems cell disrupter (Warwick, United Kingdom) according to the manufacturer’s instructions. β-Galactosidase activity was assayed in triplicate samples of freshly prepared cell lysates with the Stratagene (La Jolla, Calif.) β-galactosidase assay system according to the manufacturer’s instructions. The Bio-Rad (Hercules, Calif.) protein assay system was used to determine the protein concentration according to the manufacturer’s instructions.
RESULTS
Phage c2 transcription map.
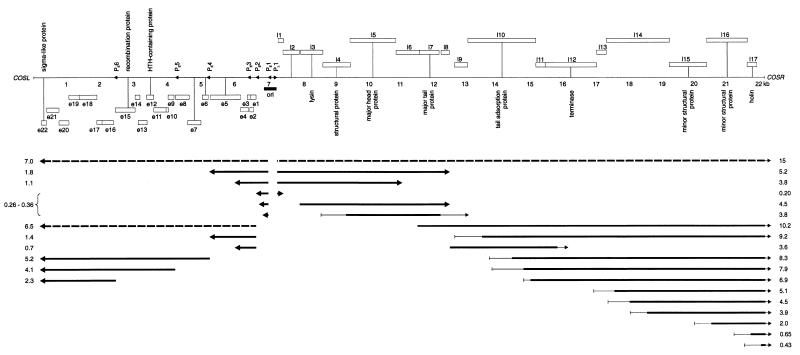
Beresford et al. (1) constructed a low-resolution transcription map that showed that phage c2 early gene transcription occurs in the left third of the genome within the first 5 min of infection and that late gene transcription occurs in the right two-thirds of the genome 10 min after infection is initiated. Sequence analysis of the genome suggested that the early and late regions should be transcribed from several putative early promoters and at least one divergent late promoter (34). RNA was isolated from phage c2-infected L. lactis MG1363 at various time points until cell lysis occurred at 30 min and was then analyzed by Northern hybridization. Tables 1 and 2 and Fig. 1 summarize the results of 58 hybridizations with 28 different DNA, RNA, or oligonucleotide probes to four separate RNA preparations. In addition to the listed transcripts, weakly hybridizing bands of various sizes were often visible on autoradiograms. RNA preparations treated with RNase A did not produce any hybridization bands, and single-stranded RNA and DNA probes confirmed the location and the divergent transcription pattern predicted by sequence analysis.
FIG. 1.
Transcript map of phage c2. The numbers directly beneath the genome indicate the distance from cosL (in kilobases). Open boxes below or above the genome indicate leftward (e1 to e22)- or rightward (l1 to l17)-reading ORFs in the early and late regions, respectively. The different box heights indicate the three different reading frames. Putative gene functions are indicated. Arrowheads indicate the leftward-directed early promoters (PE1 to PE6) and the rightward-directed late promoter (PL1). An origin of replication (ori) region is indicated by the solid bar. Solid arrows, position and direction of transcripts; broken arrows, weak transcripts not shown in Tables 1 and 2; thin lines, transcript start or end point occurs within region. Transcript sizes are indicated at the edges of the panel. The upper panel was previously published (34).
Early transcription.
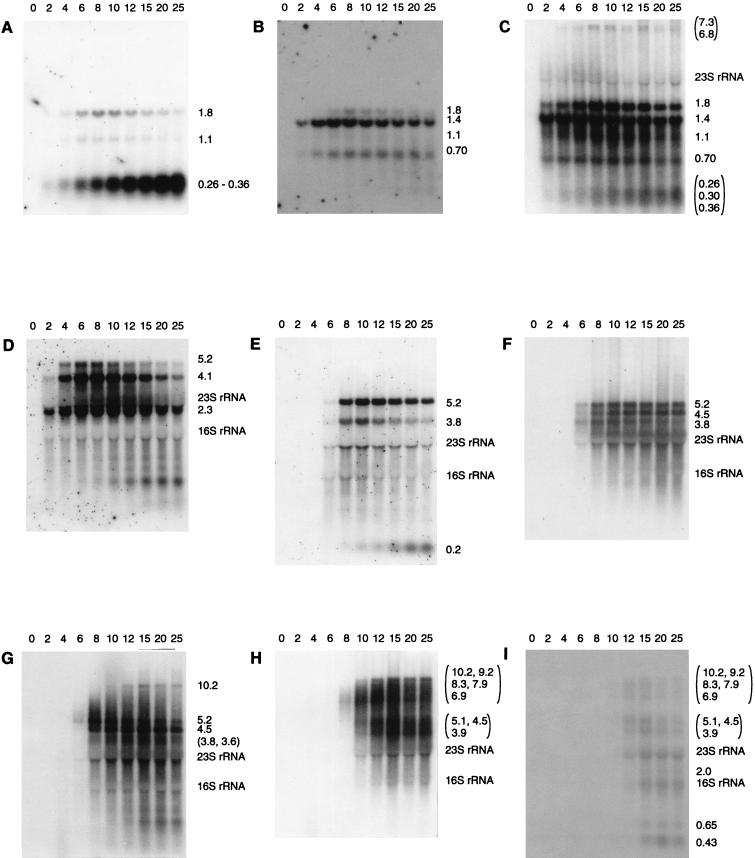
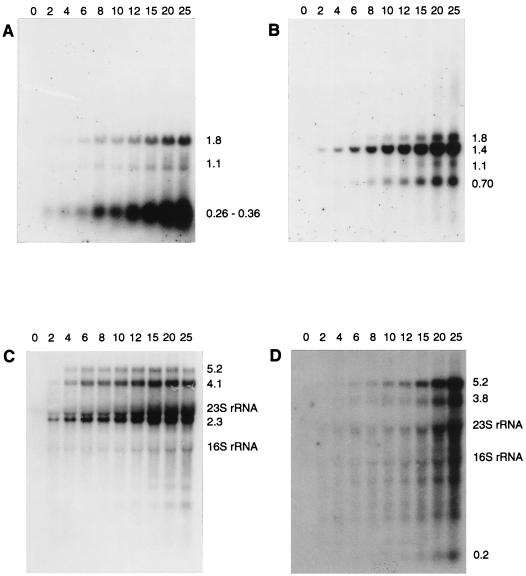
The mapped locations of the early transcripts corresponded to the predicted start sites of the early promoters identified by sequence analysis (34), except that no transcripts were seen that corresponded to transcription initiation at PE3. Transcripts of genes located throughout the early region were generally visible within the first 2 min of infection (Fig. 2A), reached maximum levels between 4 and 6 min, and then gradually decreased in abundance at later time points. Small amounts of two large transcripts of ca. 6.8 and 7.3 kilonucleotides (knt) that corresponded to the region encoding all early ORFs were seen (Fig. 2C). A broad band of 0.26 to 0.36 knt that mapped to PE1 appeared to consist of three separate transcripts of 0.26, 0.30 and 0.36 knt (Fig. 2C). These transcripts corresponded to a noncoding region of the genome. Two longer transcripts (1.80 and 1.09 knt) which would encode several early ORFs at their 3′ end were also mapped to PE1, but they were poorly expressed compared to the smaller, noncoding products (Fig. 2A).
FIG. 2.
Northern blot analysis of early and late transcripts. RNA was extracted at various times after infection, indicated above the panels (minutes) and hybridized to the following probes: A, EP1; B, EP2b; C, probe C; D, EP6; E, LP1b; F, probe N; G, probe P; H, probe U; and I, probe V. The sizes of transcripts and the positions of the rRNA are indicated to the right of each panel. For blots probed with oligonucleotides (panels A, B, D, and E), 4 μg of total RNA per lane was used. For blots probed with double-stranded DNA (panels C, F, G, H, and I), 1.5 μg of total RNA was used.
An approximate estimate of the relative abundance of the early transcripts was determined from Northern hybridizations. Band intensities in an individual hybridization experiment were compared between transcripts that had an equivalent length of sequence hybridizing to the probe. The three main transcripts corresponding to map coordinates 0 to 5 kb (2.3 knt from PE6, 4.1 knt from PE5, and 5.2 knt from PE4) were all more abundant than transcripts corresponding to the remainder of the early region (map coordinates, 5 to 7 kb). Transcripts from PE2 (1.4 and 0.70 knt) were more abundant than the 1.8- and 1.1-knt transcripts from PE1. Therefore, the region from e17 to e22, inclusive, contained the most highly transcribed ORFs, while ORFs e1 through e5, inclusive, were the least transcribed. These data suggest that the relative order of promoter strength is as follows: PE6, PE5, and PE4 > PE2 > PE1, with PE3 having negligible activity. However, factors other than promoter activity, such as transcript stability, might influence the observed transcript abundance.
The 0.26- to 0.36-knt bands from PE1 increased in intensity throughout the infection cycle and were very strong at cell lysis, in contrast to the general pattern of the early transcripts and the other transcripts from PE1, where abundance declined late in infection. The large quantity of the 0.26- to 0.36-knt bands was not consistent with their production by cleavage of the other, much-less-abundant transcripts arising from PE1. This shows that PE1 must have remained at least partially active throughout infection and that the late decline in the 1.8- and 1.1-knt transcripts from PE1, and perhaps the general decline in the early transcripts from the other promoters, was not due to complete inactivation of the early promoters by, for example, a general repressor of host promoters or a modification of the RNA polymerase.
Late transcription.
A distinct middle expression period was not observed. Late transcripts of 5.2 and 3.8 knt (Fig. 2E) were mapped to PL1, a putative promoter located at the 5′ end of the late region (34). These transcripts were the earliest late transcripts to appear, becoming visible at 6 min postinfection and reaching high levels at 8 min. The next transcripts to be detected were a 3.6-knt band, a second 3.8-knt band, and a 4.5-knt band (Fig. 2F and G). These transcripts all initiated downstream of PL1, terminated within the central third of the late region (Fig. 1), and appeared 2 to 4 min later than the earliest late transcripts (8 to 10 min postinfection). All of the transcripts that contained the 3′ end of the late region reached significant levels at 12 to 15 min postinfection, 4 to 7 min later than the earliest late transcripts. This pattern is in sharp contrast to early transcription, where transcripts corresponding to both the 5′ and the 3′ ends of the early region were visible at the earliest time point. Transcripts corresponding to the 3′ end of the late region yielded broad, indistinct bands that appeared to consist of multiple transcripts with different 5′ ends and a common 3′ end (Fig. 2H and I), perhaps caused by multiple start or processing sites. A very weak band that corresponded to the entire 14-knt late region was sometimes observed at late time points. A band of ca. 0.20 knt was also mapped to PL1. Some late transcripts also appeared to decline in abundance at very late time points.
The relative amounts of the late transcripts were estimated by the same strategy as described for the early region. The strongest bands were those that corresponded to the 5′ end of the late region (map coordinates 7.2 to 12 kb), with the following order of abundance: 5.2 and 4.5 knt > 0.20 knt > 3.8 knt > all other late transcripts. Therefore, the region l3 to l7, inclusive, contains the most highly transcribed late ORFs. There were also many different transcripts corresponding to the 3′ end of the late region, in particular the region corresponding to the holin (l17), that collectively might make up a large amount of mRNA.
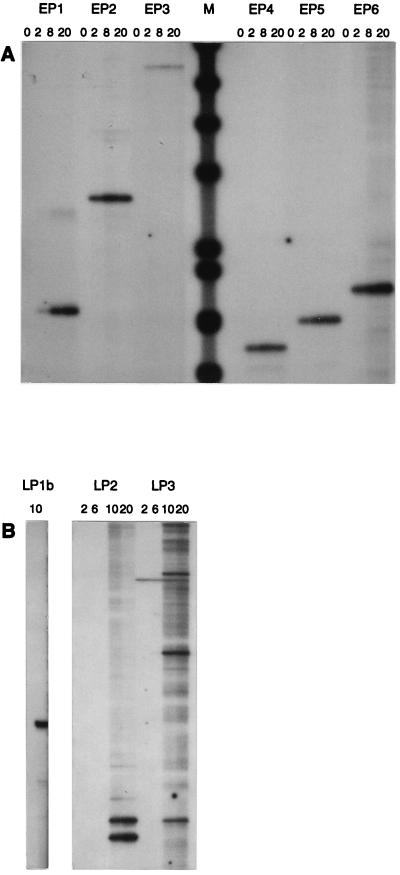
Primer extension and sequence of promoters.
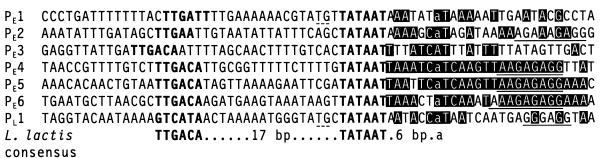
Primer extension experiments with RNA extracted at various time points confirmed that all of the putative early promoters except PE3 were active and that there was a single start site that did not change throughout infection (Fig. 3A). Each transcription start site was precisely mapped to the following positions by comparing the primer extension product with a sequencing ladder generated with the same primer (not shown): PE1, 7023; PE2, 6662; PE4, 5238; PE5, 4233; and PE6, 2487. The −10 and −35 promoter hexamers were identified by comparison to the consensus sequences recognized by the L. lactis ς39 transcription factor (Fig. 4). PE4, PE5, and PE6 all had perfect consensus −10 and −35 sequences separated by the consensus spacing of 17 bp. In addition, they all showed remarkable conservation of sequence between the −10 sequence and the first 19 nt of the predicted transcripts. PE2 had minor variations in the −35 sequence and less conservation of sequence downstream from the −10 sequence. PE1 had minor deviations from the consensus in the −35 sequence, less conservation of sequence downstream from the −10 sequence, and a nonconsensus spacing of 16 bp between the −10 and −35 sequences. PE3 had perfect −10 and −35 sequences, but they were separated by 20 bp; there was also less conservation of sequence downstream from the −10 sequence and, unlike the other promoters, it was located within an ORF. The similarity of the promoters to the consensus promoter structure closely correlated with the estimated promoter activities: PE4, PE5, and PE6 > PE2 > PE1, and PE3 was inactive.
FIG. 3.
Primer extension analysis of early (A) and late (B) transcripts. Time points (minutes) and oligonucleotides are indicated above the panels. Size markers (M) were φX174 fragments (AMV Primer Extension System; Promega).
FIG. 4.
Comparison of promoter sequences. Boldface type, −10 and −35 hexamers; broken underline, TG doublet of extended −10 sequence; solid underline, Shine-Dalgarno sequence; shaded, nucleotides downstream from −10 sequence conserved between PE4, PE5 and/or PE6.
PL1 was identified by sequence analysis because it had a perfect −10 sequence but no recognizable −35 sequence and was appropriately located immediately upstream of the first late ORF (34). Primer extension experiments confirmed that PL1 was active late in infection, that there was a single start site (Fig. 3B) at map position 7222, and that the amount of late mRNA was below the detectable level when template RNA was isolated from cells within the first 5 min of infection (not shown). No other late promoter sequences were identified by sequence analysis. However, by targeting primer extension reactions to the predicted ends of some late transcripts, mapping 5′ ends to the following positions was possible: 7882 and 7875, upstream of the lysin gene (l3), which could correspond to the 4.5-knt band; and 11346, 11450, and 11545, upstream of the major tail shaft protein gene (l7), which might correspond to the 10.2-knt band (Fig. 3B). Examination of the sequences surrounding these putative transcript start sites did not reveal promoter consensus sequences or conserved sequences that could indicate a nonconsensus promoter structure. However, small regions of sequence with the potential to form a stem-loop were found adjacent (5′) to the mapped start sites upstream of l7 as follows: 11346 (GAAAATGATTTCAc, start site in lowercase, single- and double-underlined sequences can form a stem-loop), 11430 (CCCTTATAAGgG), 11545 (ACCGTTTGCGGt), but these were not found adjacent to the start sites upstream of l3. No other sequence similarities were obvious. Stem-loop structures are important for the endonucleolytic cleavage of mRNA transcripts by RNase III (12, 37, 43) and RNase E (9), although the secondary structures involved are generally larger than those noted above.
Intrinsic terminator sequences.
A putative intrinsic (rho-independent) terminator (19) was previously identified at the end of the early region by sequence analysis (34). The early transcripts from PE4, PE5, and PE6 were consistent with termination at this sequence. Putative terminator-like sequences were identified at the map coordinates corresponding to the 3′ ends of the following early transcripts: 1.8 and 1.4 knt, 1.1 and 0.70 knt, and 0.30 knt. A terminator sequence was not observed at the predicted 3′ end of the 0.36-knt transcript. However, this position corresponded to the site of transcription initiation from PE2, and perhaps interference between RNA polymerase molecules transcribing the nascent 0.36-knt transcript and those initiating at PE2 caused the termination of transcription. A strongly predicted terminator was not found at the 3′ end of the late region, after l17. However, some very unusual sequence features are present in this region, with several direct and inverted repeats, an extensive trinucleotide repeat, and extraordinarily A/T-rich regions (33). Whether these unusual sequences could cause termination of transcription is unknown. Terminator-like sequences were identified at positions corresponding to the end of one of the 3.8-knt and the 4.5- and 5.2-knt late transcripts.
The overlapping transcript pattern observed for phage c2 might be explained, in part, by inefficient termination at the 3′ ends of the 0.26- to 0.36-knt, 1.1-knt, and 0.70-knt early transcripts and the 3.8-, 5.2-, and 4.5-knt late transcripts, since significant read-through of transcription, as well as termination, was observed at these positions. The putative terminators at each of the corresponding positions had relatively poor similarity to the consensus intrinsic terminator structure. In contrast, termination at the ends of the 2.3-, 4.1-, 5.2-, 1.4-, and 1.8-knt early transcripts was relatively strong, and the corresponding putative terminator sequences were also more strongly predicted. Even small deviations from the consensus terminator structure have been shown to decrease the efficiency of termination (4).
A phage protein(s) activates the late promoter but is not required for termination or processing of transcripts.
The involvement of a phage-encoded protein in transcription was investigated by examining transcription in the absence of phage protein synthesis. Chloramphenicol has been shown to inhibit phage protein synthesis in L. lactis at a concentration of 20 μg/ml (45). Chloramphenicol at concentrations of 20 μg/ml (added 1 min before infection), 30 μg/ml, and 50 μg/ml (added 5 min before infection) prevented cell lysis. No significant difference in effect on transcription between the different chloramphenicol concentrations was seen. The sizes of both early and late transcripts were unchanged by chloramphenicol treatment, showing that the transcription termination sites and processing of transcripts, if present, were not dependent on phage proteins. Early gene transcription was slightly delayed by chloramphenicol, as observed for other phages (45), and the transcripts accumulated throughout infection rather than decreasing at later time points. Chloramphenicol greatly reduced late transcript levels (Fig. 5). However, they slowly accumulated throughout infection and reached very high concentrations at time points taken after untreated cells have normally lysed. Steady-state transcript levels were never reached in the presence of chloramphenicol, even at 70 min postinfection. This slow, steady increase in late transcript abundance contrasts with the rapid rise to a maximum level, followed by a slight decline, observed during a normal infection. These data are consistent with a positively regulated late promoter that is active at a reduced level in the absence of phage proteins but has increased activity in the presence of a phage early gene product(s).
FIG. 5.
Effect of chloramphenicol on transcription. RNA was extracted from cells infected with phage c2 in the presence of chloramphenicol at 30 (B) or 50 (A, C, and D) μg/ml and analyzed by Northern hybridization to the following probes: A, EP1; B, EP2b; C, EP6; and D, LP1b. Four micrograms of total RNA per lane was used. Timepoints, transcript sizes, and rRNA amounts are as indicated for Fig. 2.
There are several possible explanations as to why early transcripts and at least some late transcripts decreased late in infection in the absence of chloramphenicol, whereas in the presence of chloramphenicol they progressively increased throughout infection. Competition between early and late promoters for the transcription apparatus and/or a rate-limiting decrease in other required components may cause a general decrease in the transcription rate after late gene expression is induced during a normal infection. A phage-induced decrease in transcript stability might occur later in infection. Packaging of the DNA into the phage heads might cause a reduction in the amount of DNA available to act as a template for transcription late in infection.
The late promoter has a high basal level of activity.
To test the basal level of activity of the uninduced late promoter, it was cloned into a promoter screening vector and assayed for the expression of a β-galactosidase reporter gene. A 74-bp sequence (map coordinates 7159 to 7232) that corresponded to the region from 51 bp upstream of the PL1 −10 sequence to 10 bp downstream of the PL1 transcription start site was amplified by PCR and directionally cloned, through restriction sites incorporated in the amplification primers, into the EcoRI and BglII sites of pKS1 (54) to create pKS20. pKS1 is a promoter-screening vector derived from pTREX (64) that contains a multiple cloning site for the insertion of a promoter, coupled via a translation initiation region to the E. coli lacZ reporter gene. The β-galactosidase specific activity obtained for lysates from cells containing pKS20 (1,088.6 U/mg, standard deviation = 57.56 U/mg) was as high as the activity observed when PL1 was substituted for the strong constitutive lactococcal promoter P1 (1,098.15 U/mg, standard deviation = 43.06 U/mg). A promoter-less vector negative control gave a specific activity of 88.85 U/mg (standard deviation = 98.92 U/mg). These data show that PL1 had a high basal level of activity. While it is possible that the PL1 activity observed for pKS20 does not truly reflect the in vivo activity of the uninduced PL1, the data are consistent with the delayed accumulation to high levels of late transcripts in chloramphenicol-treated cells. The Northern hybridization data showed that PL1 activity was induced to very high levels during infection, presumably by a phage early gene product. The early promoters were much stronger than the uninduced PL1 because early transcripts were visible well before late transcripts during both a normal infection and in chloramphenicol-treated cells.
DISCUSSION
Double-stranded DNA bacteriophages usually regulate the timing of gene expression into two or three phases by a variety of mechanisms that act primarily, but not exclusively, on transcription. Promoter recognition, transcription activity, transcription termination, and the processing of transcripts may be modulated by the following: covalent modification of the host RNA polymerase, proteins that bind to the host RNA polymerase core (e.g., sigma factors or inactivating proteins), DNA-binding activators or repressors, phage-encoded RNA polymerase, control of the efficiency of transcription termination, transcript cleavage or degradation by RNase, and antisense RNA (reviewed in reference 5). Some phages inject an RNA polymerase or a transcription regulatory protein at the time of infection. In addition, the entry of the DNA of some phages occurs directionally and in stages, which influences the timing of gene expression. Most phages employ several mechanisms to produce an often complex and overlapping temporal pattern of transcripts. However, much of what we understand of phage biology has been gained through the study of the phages of gram-negative bacteria and a few Bacillus phages. Relatively little is known about the molecular biology of phages from other gram-positive hosts, such as the lactococci.
We have constructed a detailed transcription map of lactococcal phage c2. Early transcription was driven by five early promoters that were simultaneously active at the start of infection. The early promoters were highly active in the absence of phage protein synthesis, and they all had a near-perfect L. lactis ς39 consensus promoter structure, suggesting that the host RNA polymerase was used for phage c2 early transcription. Sequence analysis of phage c2 and the related phage bIL67 produced no evidence for a phage-encoded RNA polymerase (34, 55). In addition, DNA from phage c2 and the closely related phage c6A can be used to transfect host cells by electroporation (48), suggesting that injection of an RNA polymerase or a regulatory protein and/or a stepwise controlled entry of the phage DNA is not necessary for phage replication. A single late promoter (PL1) was identified upstream of the first late ORF. The transcriptional activity of PL1 was greatly enhanced by an early-expressed protein about 4 min after early gene transcription was detected. Sensitivity to chloramphenicol suggests that an antisense RNA mechanism does not control late transcription independently of a regulatory protein. Transcriptional activity from at least one, and potentially all, of the early promoters persisted throughout the phage life cycle, showing that at least some RNA polymerase molecules remain able to recognize consensus promoters throughout the infection. A simple explanation is that a DNA-binding activator protein enables efficient transcription from PL1 by the unmodified host RNA polymerase. Two potential transcription regulation proteins were identified by sequence analysis of the c2 genome sequence (34). One protein, gpe12, contains a helix-turn-helix motif (3) and the other, gpe22, has some similarity to two conserved sigma factor domains (34), although the protein is too small (56 amino acids) to function as a sigma factor per se. These features suggest that one or both proteins might bind DNA in a sequence-specific manner and, therefore, are candidates for transcription regulators.
The transcription pattern of both the early and late regions was complex with many overlapping transcripts. In the early region, this was caused by the action of multiple promoters combined with inefficient termination of transcription. In the late region, multiple promoters were not identified and it is possible that transcripts were initiated at a single late promoter (PL1) and were processed into smaller overlapping transcripts. If the rate of RNA synthesis in L. lactis is similar to that in E. coli (30 to 60 nt per min [reviewed in reference 30]), it would take 4 to 8 min for a full-length transcript corresponding to the entire late region to be synthesized. This corresponds well to the 4- to 7-min delay in the appearance of transcripts which start downstream from PL1. These data are consistent with the downstream transcripts being produced by the action of an RNase on a larger precursor molecule initiated from PL1. Sequentially activated late promoters are less likely because the appearance of the downstream late transcripts occurred in the absence of protein synthesis and was concurrent with PL1 activity and because the sizes were not affected by chloramphenicol treatment. Similarly, insensitivity to chloramphenicol shows that termination sites and cleavage sites were not determined by phage proteins.
The early promoters were much stronger than the uninduced late promoter and, therefore, much stronger than the strong constitutive lactococcal promoter P1 (62). We were unable to clone the early promoter PE6. This might be due to its high transcriptional activity, as shown for strong phage T5 promoters (17). The phage T5 promoters are exceptionally strong, can outcompete all other promoters present in a plasmid, and have sequence features similar to those observed for the phage c2 early promoters PE4, PE5, and PE6, i.e., perfect consensus structure and strong conservation downstream of the start nucleotide (36). Therefore, the phage c2 early promoters could be among the strongest lactococcal promoters identified to date and might be a mechanism that allows the phage to subvert the host cell metabolism rapidly. Multiple simultaneously active early promoters with high affinity for the host RNA polymerase might allow phage c2 to outcompete the many host promoters for the host transcription apparatus. Fewer late promoters would be required because the phage genome will have replicated and prolate lactococcal phages degrade the host DNA late in infection (49). A genome organization of multiple early promoters and few late promoters is found in many other lytic phages of gram-positive hosts, such as the Bacillus phages abbe, PZA, Nf, SPP1, and SPO1 (38, 39, 52, 56, 57) and the Lactococcus phages studied to date (936 species: sk1 [7], bIL41 [45], and bIL66 [2]). In contrast, we could find few examples of lytic phages from gram-negative hosts that have a similar organization.
Several small early transcripts were identified that corresponded to the noncoding region between PE1 and PE2. Unlike the other transcripts, they steadily increased in intensity throughout infection. PE1 is located within a region of DNA that functions as an origin of DNA replication (63). It is possible that the production of the small transcripts from PE1 is required for DNA replication. DNA replication that is dependent on transcription of promoters within the origin of replication has been well documented for phages λ and T4 (30).
PL1 did not have a recognizable −35 sequence but had an extended −10 sequence (yntnTGyTATAAT; uppercase letters, strongly preferred; lowercase letters, preferred; y, pyrimidine possibly preferred; n, probably unspecified [27, 31]) and still had very high activity in the absence of its phage-encoded activator protein(s). A middle promoter that is positively regulated by a phage-encoded protein and lacks a −35 sequence has been extensively characterized from the lactococcal phage φ31 (61). However, unlike PL1, the φ31 middle promoter was inactive in the absence of phage proteins and the −10 sequence did not conform to the typical extended structure. Transcription from an extended promoter in the absence of a −35 sequence has been demonstrated in E. coli (27, 31), Streptococcus pneumoniae (51), and recently in L. lactis (32). The present work shows that an extended promoter without a −35 sequence can function as a very strong promoter in L. lactis.
Differences in phage protein synthesis rates are often determined at the translation level rather than by the synthesis of multiple RNA species, and one mechanism involves differential ribosome loading (6). However, phage c2 has a large number of transcripts, which could allow greater control of protein synthesis at the transcription level. Weakly expressed genes might be grouped together and efficiently translated from a small amount of mRNA. Products required in greater amounts could be grouped on parts of the genome that generate more RNA transcripts. Consistent with this hypothesis, most phage c2 genes have Shine-Dalgarno sequences with good calculated binding energies to the 3′ end of L. lactis 16S rRNA (26 of 37 genes have Shine-Dalgarno sequence binding energies of <−14.0 kcal/mol, with 1 kcal = 4.184 kJ [34]) and could be efficiently translated. The genes for the most abundant structural proteins present in the completed phage particle (gpl4, gpl5, and gpl7 [34]) are all grouped in the left third of the late region and are represented on the most abundant late transcripts. In contrast, the least abundant structural proteins (gpl10, gpl15, and gpl16 [34]) are encoded on the weaker transcripts arising from the right two-thirds of the late region. In addition, the lysis genes, holin (l17) and lysin (l3), are at opposite ends of the late region, which seems consistent with their predicted timing of expression. The holin determines the timing of cell lysis and is required to allow the lysin to pass through the cell membrane and gain access to and hydrolyze the cell wall substrate (66). The transcripts encoding the lysin reach high levels at 8 min postinfection, while transcripts encoding the holin are delayed until 15 min. This delay in transcription, perhaps combined with a lower efficiency of translation of the holin, might contribute to the 10- to 15-min delay between the observed intracellular appearance of the lysin (26) and cell lysis.
The information on phage c2 transcription is important to the understanding of natural phage resistance mechanisms and to the design of novel phage resistance mechanisms. Abi resistance mechanisms can now be screened for effects on phage c2 transcription, and the target of the mechanism can be identified. Novel resistance mechanisms which use antisense mRNA directed against several phage c2 early and late genes, including the major tail protein, produce no inhibitory effect (47). In contrast, antisense mRNA directed against several genes of the P335-species phage φ7–9 inhibits phage propagation (28, 29). Phage c2 transcription was characterized by very strong promoters, rapid mRNA production, and many overlapping transcripts. These features might account for the apparent resistance of phage c2 to antisense effects. Very strong synthesis of antisense mRNA might be required to produce a resistance phenotype. The transcription pattern of other prolate phages can now be compared to identify common features in transcription control across the species, which might be useful targets for the design of novel resistance mechanisms. Our results suggest that phage c2, and potentially all prolate phages, may not provide easy targets for developing novel resistance methods. Phage c2 appears to have a very simple system controlling gene expression that is heavily dependent on host proteins and, therefore, targets for novel phage resistance mechanisms may prove difficult to identify.
ACKNOWLEDGMENTS
The research was funded in part by the New Zealand Foundation for Research, Science, and Technology. Collaborative work between the New Zealand Dairy Research Institute and the University of Cambridge was assisted by a Higher Education Links Award from the British Council.
REFERENCES
- 1.Beresford T P J, Ward L J H, Jarvis A W. Temporally regulated transcriptional expression of the genomes of lactococcal bacteriophages c2 and sk1. Appl Environ Microbiol. 1993;59:3708–3712. doi: 10.1128/aem.59.11.3708-3712.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bidnenko E, Ehrlich D, Chopin M C. Phage operon involved in sensitivity to the Lactococcus lactis abortive infection mechanism AbiD1. J Bacteriol. 1995;177:3824–3829. doi: 10.1128/jb.177.13.3824-3829.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brennan R G, Matthews B W. The helix-turn-helix DNA binding motif. J Biol Chem. 1989;264:1903–1906. [PubMed] [Google Scholar]
- 4.Brennan S M, Geiduschek E P. Regions specifying transcriptional termination and pausing in the bacteriophage SP01 terminal repeat. Nucleic Acids Res. 1983;11:4157–4175. doi: 10.1093/nar/11.12.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calendar R. The bacteriophages. 1 and 2. New York, N.Y: Plenum Press; 1988. [Google Scholar]
- 6.Casjens S, Hendrix R. Control mechanisms in dsDNA bacteriophage assembly. In: Calendar R, editor. The bacteriophages. Vol. 1. New York, N.Y: Plenum Press; 1988. pp. 15–91. [Google Scholar]
- 7.Chandry P S, Davidson B E, Hillier A J. Temporal transcription map of the Lactococcus lactis bacteriophage sk1. Microbiology. 1994;140:2251–2261. doi: 10.1099/13500872-140-9-2251. [DOI] [PubMed] [Google Scholar]
- 8.Chung D K, Chung S K, Batt C A. Antisense RNA directed against the major capsid protein of Lactococcus lactis subsp. cremoris bacteriophage 4-1 confers partial resistance to the host. Appl Microbiol Biotechnol. 1992;37:79–83. doi: 10.1007/BF00174207. [DOI] [PubMed] [Google Scholar]
- 9.Cohen S N, McDowall K J. RNase E—still a wonderfully mysterious enzyme. Mol Microbiol. 1997;23:1099–1106. doi: 10.1111/j.1365-2958.1997.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 10.Deng Y M, Harvey M L, Liu C Q, Dunn N W. A novel plasmid-encoded phage abortive infection system from Lactococcus lactis biovar. diacetylactis. FEMS Microbiol Lett. 1997;146:149–154. doi: 10.1111/j.1574-6968.1997.tb10185.x. [DOI] [PubMed] [Google Scholar]
- 11.Dinsmore P K, Klaenhammer T R. Phenotypic consequences of altering the copy number of abiA, a gene responsible for aborting bacteriophage infections in Lactococcus lactis. Appl Environ Microbiol. 1994;60:1129–1136. doi: 10.1128/aem.60.4.1129-1136.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunn J J, Studier F W. Complete nucleotide sequence of bacteriophage T7 DNA and the locations of T7 genetic elements. J Mol Biol. 1983;166:477–535. doi: 10.1016/s0022-2836(83)80282-4. [DOI] [PubMed] [Google Scholar]
- 13.Emond E, Holler B J, Boucher I, Vandenbergh P A, Vedamuthu E R, Kondo J K, Moineau S. Phenotypic and genetic characterization of the bacteriophage abortive infection mechanism AbiK from Lactococcus lactis. Appl Environ Microbiol. 1997;63:1274–1283. doi: 10.1128/aem.63.4.1274-1283.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Garvey P, Fitzgerald G F, Hill C. Cloning and DNA sequence analysis of two abortive infection phage resistance determinants from the lactococcal plasmid pNP40. Appl Environ Microbiol. 1995;61:4321–4328. doi: 10.1128/aem.61.12.4321-4328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garvey P, Van Sinderen D, Twomey D P, Hill C, Fitzgerald G F. Molecular genetics of bacteriophage and natural phage defense systems in the genus Lactococcus. Int Dairy J. 1995;5:905–947. [Google Scholar]
- 16.Gasson M J. Plasmid complements of Streptococcus lactis NCDO 712 and other lactic streptococci after protoplast-induced curing. J Bacteriol. 1983;154:1–9. doi: 10.1128/jb.154.1.1-9.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gentz R, Langner A, Chang A C, Cohen S N, Bujard H. Cloning and analysis of strong promoters is made possible by the downstream placement of a RNA termination signal. Proc Natl Acad Sci USA. 1981;78:4936–4940. doi: 10.1073/pnas.78.8.4936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.He M, Wilde A, Kaderbhai M A. A simple single-step procedure for small-scale preparation of Escherichia coli plasmids. Nucleic Acids Res. 1990;18:1660. doi: 10.1093/nar/18.6.1660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Henkin T M. Control of transcription termination in prokaryotes. Annu Rev Genet. 1996;30:35–57. doi: 10.1146/annurev.genet.30.1.35. [DOI] [PubMed] [Google Scholar]
- 20.Herrin D L, Schmidt G W. Rapid, reversible staining of Northern blots prior to hybridization. BioTechniques. 1988;6:196–197. [PubMed] [Google Scholar]
- 21.Hill C, Miller L A, Klaenhammer T R. Cloning, expression, and sequence determination of a bacteriophage fragment encoding bacteriophage resistance in Lactococcus lactis. J Bacteriol. 1990;172:6419–6426. doi: 10.1128/jb.172.11.6419-6426.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Holmes D S, Quigley M. A rapid boiling method for the preparation of bacterial plasmids. Anal Biochem. 1981;114:193–197. doi: 10.1016/0003-2697(81)90473-5. [DOI] [PubMed] [Google Scholar]
- 23.Ish-Horowicz D, Burke J F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981;9:2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jarvis A W. Differentiation of lactic streptococcal phages into phage species by DNA-DNA homology. Appl Environ Microbiol. 1984;47:343–349. doi: 10.1128/aem.47.2.343-349.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jarvis A W, Fitzgerald G F, Mata M, Mercenier A, Neve H, Powell I B, Ronda C, Saxelin M, Teuber M. Species and type phages of lactococcal bacteriophages. Intervirology. 1991;32:2–9. doi: 10.1159/000150179. [DOI] [PubMed] [Google Scholar]
- 26.Jarvis A W, Lubbers M W, Beresford T P, Ward L J, Waterfield N R, Collins L J, Jarvis B D. Molecular biology of lactococcal bacteriophage c2. Dev Biol Stand. 1995;85:561–567. [PubMed] [Google Scholar]
- 27.Keilty S, Rosenberg M. Constitutive function of a positively regulated promoter reveals new sequences essential for activity. J Biol Chem. 1987;262:6389–6395. [PubMed] [Google Scholar]
- 28.Kim S G, Batt C A. Antisense mRNA-mediated bacteriophage resistance in Lactococcus lactis. Appl Environ Microbiol. 1991;57:1109–1113. doi: 10.1128/aem.57.4.1109-1113.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim S G, Bor Y C, Batt C A. Bacteriophage resistance in Lactococcus lactis ssp. lactis using antisense ribonucleic acid. J Dairy Sci. 1992;75:1761–1767. doi: 10.3168/jds.S0022-0302(92)77935-1. [DOI] [PubMed] [Google Scholar]
- 30.Kornberg A, Baker T A. DNA replication. 2nd ed. New York, N.Y: W. H. Freeman & Company; 1992. [Google Scholar]
- 31.Kumar A, Malloch R A, Fujita N, Smillie D A, Ishihama A, Hayward R S. The minus 35-recognition region of Escherichia coli sigma 70 is inessential for initiation of transcription at an “extended minus 10” promoter. J Mol Biol. 1993;232:406–418. doi: 10.1006/jmbi.1993.1400. [DOI] [PubMed] [Google Scholar]
- 32.Liu C-Q, Harvey M L, Dunn N W. Cloning of a gene encoding nisin resistance from Lactococcus lactis subsp. lactis M189 which is transcribed from an extended −10 promoter. J Gen Appl Microbiol. 1997;43:67–73. doi: 10.2323/jgam.43.67. [DOI] [PubMed] [Google Scholar]
- 33.Lubbers M W, Ward L J, Beresford T P, Jarvis B D, Jarvis A W. Sequencing and analysis of the cos region of the lactococcal bacteriophage c2. Mol Gen Genet. 1994;245:160–166. doi: 10.1007/BF00283263. [DOI] [PubMed] [Google Scholar]
- 34.Lubbers M W, Waterfield N R, Beresford T P J, Le Page R W F, Jarvis A W. Sequencing and analysis of the prolate-headed lactococcal bacteriophage c2 genome and identification of the structural genes. Appl Environ Microbiol. 1995;61:4348–4356. doi: 10.1128/aem.61.12.4348-4356.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Magni C, Marini P, de Mendoza D. Extraction of RNA from gram-positive bacteria. BioTechniques. 1995;19:880–884. [PubMed] [Google Scholar]
- 36.McCorquodale D J, Warner H R. Bacteriophage T5 and related phages. In: Calendar R, editor. The bacteriophages. Vol. 1. New York, N.Y: Plenum Press; 1988. pp. 439–475. [Google Scholar]
- 37.Mitra S, Bechhofer D H. Substrate specificity of an RNase III-like activity from Bacillus subtilis. J Biol Chem. 1994;269:31450–31456. [PubMed] [Google Scholar]
- 38.Montenegro M A, Trautner T A. In vivo transcription of Bacillus subtilis bacteriophage SPP1. Mol Gen Genet. 1981;181:512–517. doi: 10.1007/BF00428744. [DOI] [PubMed] [Google Scholar]
- 39.Nuez B, Salas M. Bacteriophage Nf DNA region controlling late transcription: structural and functional homology with bacteriophage φ29. Nucleic Acids Res. 1993;21:2861–2865. doi: 10.1093/nar/21.12.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O’Connor L, Coffey A, Daly C, Fitzgerald G F. AbiG, a genotypically novel abortive infection mechanism encoded by plasmid pCI750 of Lactococcus lactis subsp. cremoris UC653. Appl Environ Microbiol. 1996;62:3075–3082. doi: 10.1128/aem.62.9.3075-3082.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.O’Sullivan D J, Hill C, Klaenhammer T R. Effect of increasing the copy number of bacteriophage origins of replication, in trans, on incoming-phage proliferation. Appl Environ Microbiol. 1993;59:2449–2456. doi: 10.1128/aem.59.8.2449-2456.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.O’Sullivan D J, Klaenhammer T R. Rapid mini-prep isolation of high quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl Environ Microbiol. 1993;59:2730–2733. doi: 10.1128/aem.59.8.2730-2733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Panganiban A T, Whiteley H R. Bacillus subtilis RNase III cleavage sites in phage SP82 early mRNA. Cell. 1983;33:907–913. doi: 10.1016/0092-8674(83)90033-8. [DOI] [PubMed] [Google Scholar]
- 44.Parreira R, Ehrlich S D, Chopin M C. Dramatic decay of phage transcripts in lactococcal cells carrying the abortive infection determinant AbiB. Mol Microbiol. 1996;19:221–230. doi: 10.1046/j.1365-2958.1996.371896.x. [DOI] [PubMed] [Google Scholar]
- 45.Parreira R, Valyasevi R, Lerayer A L S, Ehrlich S D, Chopin M C. Gene organization and transcription of a late-expressed region of a Lactococcus lactis phage. J Bacteriol. 1996;178:6158–6165. doi: 10.1128/jb.178.21.6158-6165.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pillidge C J, Jarvis A W. DNA restriction maps and classification of the lactococcal bacteriophages c2 and sk1. N Z J Dairy Sci Technol. 1988;23:411–416. [Google Scholar]
- 47.Polzin, K. M., L. J. Collins, M. W. Lubbers, and A. W. Jarvis. Unpublished results.
- 48.Powell I B, Achen M G, Hillier A J, Davidson B E. A simple and rapid method for genetic transformation of lactic streptococci by electroporation. Appl Environ Microbiol. 1988;54:655–660. doi: 10.1128/aem.54.3.655-660.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Powell I B, Tulloch D L, Hillier A J, Davidson B E. Phage DNA synthesis and host DNA degradation in the life cycle of Lactococcus lactis bacteriophage c6A. J Gen Microbiol. 1992;138:945–950. doi: 10.1099/00221287-138-5-945. [DOI] [PubMed] [Google Scholar]
- 50.Prevots F, Daloyau M, Bonin O, Dumont X, Tolou S. Cloning and sequencing of the novel abortive infection gene abiH of Lactococcus lactis ssp. lactis biovar. diacetylactis S94. FEMS Microbiol Lett. 1996;142:295–299. doi: 10.1111/j.1574-6968.1996.tb08446.x. [DOI] [PubMed] [Google Scholar]
- 51.Sabelnikov A G, Greenberg B, Lacks S A. An extended −10 promoter alone directs transcription of the DpnII operon of Streptococcus pneumoniae. J Mol Biol. 1995;250:144–155. doi: 10.1006/jmbi.1995.0366. [DOI] [PubMed] [Google Scholar]
- 52.Salas M. Phages with protein attached to the DNA ends. In: Calendar R, editor. The bacteriophages. Vol. 1. New York, N.Y: Plenum Press; 1988. pp. 169–191. [Google Scholar]
- 53.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 54.Schofield, K. M. Unpublished results.
- 55.Schouler C, Ehrlich S D, Chopin M C. Sequence and organization of the lactococcal prolate-headed bIL67 phage genome. Microbiology. 1994;140:3061–3069. doi: 10.1099/13500872-140-11-3061. [DOI] [PubMed] [Google Scholar]
- 56.Stewart C. Bacteriophage SPO1. In: Calendar R, editor. The bacteriophages. Vol. 1. New York, N.Y: Plenum Press; 1988. pp. 477–515. [Google Scholar]
- 57.Stuber D, Morelli G, Bujard H, Montenegro M A, Trautner T A. Promoter sites in the genome of Bacillus subtilis phage SPP1. Mol Gen Genet. 1981;181:518–521. doi: 10.1007/BF00428745. [DOI] [PubMed] [Google Scholar]
- 58.Stulnig T M, Amberger A. Exposing contaminating phenol in nucleic acid preparations. BioTechniques. 1994;16:402–404. [PubMed] [Google Scholar]
- 59.Su P, Harvey M, Im H J, Dunn N W. Isolation, cloning and characterisation of the abiI gene from Lactococcus lactis subsp. lactis M138 encoding abortive phage infection. J Biotechnol. 1997;54:95–104. doi: 10.1016/s0168-1656(97)01692-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsang S S, Yin X, Guzzo-Arkuran C, Jones V S, Davison A J. Loss of resolution in gel electrophoresis of RNA: a problem associated with the presence of formaldehyde gradients. BioTechniques. 1993;14:380–381. [PubMed] [Google Scholar]
- 61.Walker S A, Klaenhammer T R. Molecular characterization of a phage-inducible middle promoter and its transcriptional activator from the lactococcal bacteriophage φ31. J Bacteriol. 1998;180:921–931. doi: 10.1128/jb.180.4.921-931.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Waterfield N R, Le Page R W, Wilson P W, Wells J M. The isolation of lactococcal promoters and their use in investigating bacterial luciferase synthesis in Lactococcus lactis. Gene. 1995;165:9–15. doi: 10.1016/0378-1119(95)00484-n. [DOI] [PubMed] [Google Scholar]
- 63.Waterfield N R, Lubbers M W, Polzin K M, Le Page R W, Jarvis A W. An origin of DNA replication from Lactococcus lactis bacteriophage c2. Appl Environ Microbiol. 1996;62:1452–1453. doi: 10.1128/aem.62.4.1452-1453.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Wells J M, Schofield K M. Cloning and expression vectors for lactococci. In: Bozoglu T F, Ray B, editors. Lactic acid bacteria: current advances in metabolism, and applications. NATO ASI series. H98. Heidelberg, Germany: Springer Verlag; 1996. pp. 37–62. [Google Scholar]
- 65.Wells J M, Wilson P W, Le Page R W. Improved cloning vectors and transformation procedure for Lactococcus lactis. J Appl Bacteriol. 1993;74:629–636. doi: 10.1111/j.1365-2672.1993.tb05195.x. [DOI] [PubMed] [Google Scholar]
- 66.Young R. Bacteriophage lysis: mechanism and regulation. Microbiol Rev. 1992;56:430–481. doi: 10.1128/mr.56.3.430-481.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]