Abstract
Acute Hepatopancreatic Necrosis Disease (AHPND), a highly destructive shrimp disease, has inflicted severe setbacks on the shrimp farming industry worldwide. As the use of antibiotics is discouraged due to emerging antibiotic-resistant bacteria and the pollution of ecosystems, there is a pressing demand for novel, sustainable alternatives. Hence, the influence of bovine lactoferrin (bLF) and hen ovotransferrin (OT), two natural antimicrobial proteins, on the growth of three AHPND-causing Vibrio parahaemolyticus (Vp) strains (M0904, TW01 and PV1) was examined. Additionally, we explored their potential to affect selected Vp virulence factors such as biofilm formation, swimming and swarming, cell surface hydrophobicity, and activity of released lipases and caseinases. Lag phases of all bacterial growth curves were significantly prolonged in the presence of bLF or OT (1, 5 and 10 mg/mL), and bLF (5 and 10 mg/mL) completely inhibited growth of all strains. In addition, bLF or OT significantly reduced biofilm formation (all tested bLF and OT concentrations for Vp M0904 and Vp PV1), bacterial swimming motility (0.5 mg/mL bLF and OT for Vp M0904 and Vp TW01; 1 mg/mL bLF and OT for all strains), cell surface hydrophobicity (for all strains, all bLF and OT concentrations tested except for 0.125 mg/mL OT for Vp PV1) and lipase activity (1 mg/mL bLF and OT for all strains and 0.5 mg/mL bLF and OT for Vp PV1). These promising in vitro results suggest that bLF and/or OT might be used as novel agents for combating AHPND and warrant further research to elucidate the underlying mechanisms of action to fully unlock their potential for AHPND disease management.
Keywords: Vibrio parahaemolyticus, AHPND, acute hepatopancreatic necrosis disease, bovine lactoferrin, ovotransferrin, transferrin
1. Introduction
In the last decades, the global increase in demand for seafood has led to the intensification of aquaculture production systems. This intensification has introduced a range of challenges, including the emergence of infectious disease outbreaks. One such disease that has a profound impact on the shrimp farming industry worldwide is Acute Hepatopancreatic Necrosis Disease (AHPND). This disease was identified in China in 2009 for the first time under the name Early Mortality Syndrome (EMS), and it has since spread to other Asian countries such as Thailand, Vietnam and Malaysia, but also Mexico and the United States [1,2,3]. Still, AHPND continues to inflict large mortalities and losses in shrimp farms, where it develops after approximately eight days post stocking, with severe mortalities (up to 100%) occurring within 20 to 30 days [2]. The number one causative agent is the Gram-negative Vibrio (V.) parahaemolyticus. However, only strains harboring the pVA1 plasmid (69 kb) which carries the pirAB toxin genes are able to induce AHPND [4,5]. More recently, other Vibrio species have been identified with a plasmid homologous to pVA1 harboring the pirAB genes, such as V. harveyi [6], V. campbellii [7,8,9], V. owensii [10] and V. punensis [11]. Central to the pathogenicity of AHPND in shrimp is the PirAB binary toxin, yet other factors that contribute to virulence are biofilm formation, flagellar motility, extracellular proteases and lipases, and type III and VI secretion systems [2,12]. Antibiotics have been used and, at times, misused for decades to address bacterial diseases in aquaculture [13], resulting in the pollution of aquatic ecosystems and the emergence of antibiotic-resistant bacteria, including V. parahaemolyticus and other Vibrio species [14,15,16]. Consequently, many countries have implemented prohibitions and restrictions on the use of antibiotics, creating a pressing demand for novel, sustainable alternatives [13,17]. In the present study, we investigated the potential of two such alternatives, bovine lactoferrin (bLF) and hen ovotransferrin (OT), belonging to the family of transferrins (TFs), a class of multifunctional glycoproteins naturally found in both vertebrates and invertebrates. This family has conserved characteristics and all primarily serve to control iron levels in biological fluids [18]. Beyond their iron transport function, TFs have gained significant attention for their involvement in immune defense mechanisms such as antibacterial, -fungal, -viral, -parasitic, antioxidant and anti-inflammatory activities [17,19,20]. Both TFs used in this study, bLF and OT, are easily accessible and produced on an industrial scale. Structurally, they both consist of two homologous lobes (N and C), each able to reversibly bind one Fe3+ cation along with one CO32− [18,21]. In native conditions, they generally contain 15 to 20% iron and are called holo-transferrins, while when they contain less than 5% iron, they are called apo-TF. Both TFs are widely used in various industries, such as food preservation and nutritional supplements, and in numerous medications due to their non-toxic and ecological features [17,22]. Important antibacterial modes of action of the TFs are iron sequestration, rendering iron inaccessible to invading pathogens and interaction with the bacterial cell membrane through binding to lipopolysaccharide (LPS), porins or other outer membrane proteins (OMPs) in Gram-negative bacteria, resulting in perturbed membrane stability and structure [17,18,21]. Furthermore, they are proteolytic enzymes, able to degrade important virulence factors of, for example, enterohemorrhagic and enterotoxigenic Escherichia (E.) coli (EHEC and ETEC) [23,24]; can disrupt the bacterial Type III secretion system (T3SS) [25]; and have been shown to inhibit the attachment and cell entry of obligatory intracellular Chlamydia (C.) [26].
Historically, the antibacterial effects of bLF and OT were first studied using various bacteria of human and mammals including E. coli strains [23,24], Chlamydia spp. [26,27] and Bacillus spp. [28,29]. Regarding Vibrio spp., inhibition of growth by bLF was observed for human pathogenic V. parahaemolyticus strains (non-AHPND) [30] and V. cholerae [31], and bLF was able to work synergistically with antibiotics to inhibit growth in V. fluvialis, V. alginolyticus, V. vulnificus and V. furnissii [31]. Furthermore, treatment of V. cholerae with bLF resulted in severe membrane damage, such as the occurrence of bacterial protrusion and filamentation [31]. Recently, multiple advantages of using TFs have been proposed for fish [17] and crustaceans [32,33]. Studies with TFs or TF derivatives in shrimp are scarce but rendered positive results such as enhanced growth, reduced mortalities, decreased disease outbreaks and stimulated immune responses [32,33]. In this research, we explored the potential of bLF and OT for use in aquaculture against Vibrio parahaemolyticus induced AHPND. In vitro experiments on three different AHPND+ V. parahaemolyticus strains were performed to investigate the effect of bLF and OT on the growth of the bacteria and on selected virulence factors. To our knowledge, this is the first study investigating the in vitro effects of both bLF and OT on AHPND-causing Vibrio parahaemolyticus strains. The findings of this study provide valuable insights into the antimicrobial effects of the transferrins on this significant shrimp pathogen. This holds promise for the potential application of these compounds as bioactive feed additives in shrimp aquaculture. Such applications could significantly influence various aspects of the aquaculture industry, including food security, economic stability, employment and social welfare.
2. Materials and Methods
2.1. Transferrins
Stock solutions of ovotransferrin (OT) (Bioseutica, Zeewolde, The Netherlands) and bovine lactoferrin (bLF) (Ingredia, Arras, France) were prepared in filtered (0.2 µm), autoclaved artificial seawater (FAASW) containing 35 g/L of Instant Ocean synthetic sea salt (Aquarium systems, Sarrebourg, France), and filtered and sterilized (0.22 µm). According to the manufacturers, OT was 4.8% iron saturated, while bLF was 9% iron saturated.
2.2. Bacterial Strains and Growth Conditions
Three different V. parahaemolyticus strains were used in this study: Vp M0904 was isolated in Northwestern Mexico (received from A.C. Mazatlàn unit of Aquaculture), Vp TW01 in Southern Thailand and Vp PV1 in China (both received from Robins McIntosh). All three strains were isolated from AHPND-affected shrimp and were previously confirmed to have the pirAB genes by PCR with PirAB2020 primers (Phiwsaiya et al., 2017). Depending on the experiment, the bacteria were cultured in either Marine Broth (MB) (Carl Roth, Karlsruhe, Germany), Tryptic Soy Broth (TSB) (Carl Roth, Karlsruhe, Germany) with addition of 15 g/L extra NaCl, or Luria–Bertani (LB) 35 medium (10 g/L tryptone (Carl Roth, Karlsruhe, Germany), 5 g/L yeast extract (Carl Roth, Karlsruhe, Germany) and 35 g/L NaCl (Carl Roth, Karlsruhe, Germany) in distilled water). Cultures were grown overnight at 28 °C with shaking at 140 rpm before further use.
2.3. PirAB Production by Vp Test Strains
PirAB production was verified by Western blotting. Briefly, overnight cultures were centrifuged for 15 min at 4000× g (4 °C), and supernatants were filtered (0.22 µm) to produce cell-free supernatant (CFS). Toxins in CFS were concentrated using ultra-centrifugal filters with a molecular weight cut-off of 10 kDa. Samples were treated with 0.01 M β-mercaptoethanol (5 min, 95 °C) and run on a 4–15% Mini-Protean TGX Stain-Free Protein Gel (Bio-Rad, Hercules, CA, USA) (15 min, 130 V; 45 min, 150 V). Western blotting was performed (60 min, 100 V) and PVFD membranes (Bio-Rad, Hercules, CA, USA) were incubated with in-house-produced polyclonal rabbit anti-PirA or polyclonal rabbit anti-PirB antibodies (1:7500) followed by a polyclonal goat anti-rabbit IgG (HRP) antibody (1:10,000; Genscript, Piscataway, NJ, USA). Clarity Western ECL Substrate (Bio-Rad, Hercules, CA, USA) was used for detection.
2.4. Effect of bLF and OT on Bacterial Growth
To test the bacteriostatic/bactericidal activity of OT and bLF on V. parahaemolyticus, the effect on the growth curves of the three different strains was assayed in flat-bottomed 96-well plates (VWR International, Radnor, PA, USA). The strains were first grown overnight in TSB (+1.5% NaCl) and centrifuged, and the pellets were resuspended in fresh broth to obtain four different bacterial concentrations (101, 103, 105 and 107 CFU/mL). All bacterial concentrations were supplemented with 10, 5, 1, 0.1, 0.01, 0.001 and 0 mg/mL of bLF or OT and were tested in triplicate in 96-well plates. Pure broth was used as blanks. Growth of the cultures was monitored for 24 h at 28 °C by hourly absorbance measurements at 550 nm with a spectrophotometer (Infinite® 200 PRO, Tecan, Mechelen, Belgium). Data were analyzed using a modified Gompertz model [34], represented by the following formula:
| Y = Y0 + (YM − Y0) * exp(−exp(K * (lag − X)/(YM − Y0) + 1)) |
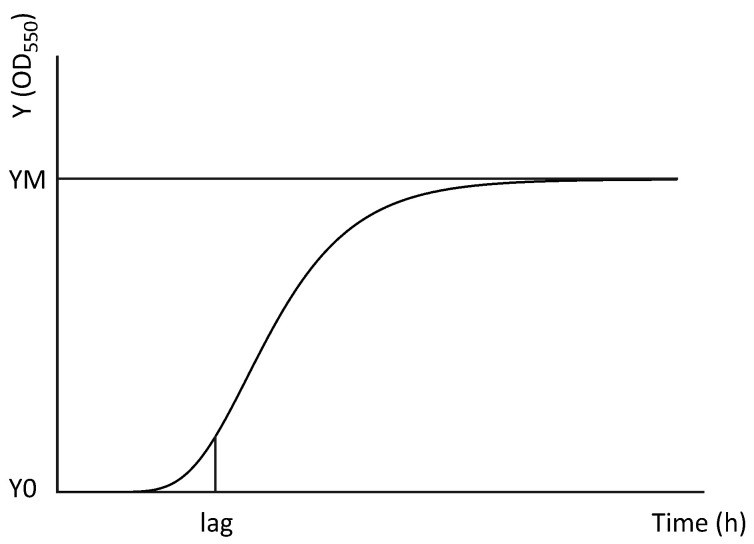
Parameters Y0 and YM are the starting and maximum population, respectively, expressed as a value of optical density (OD) at 550 nm; lag is expressed in h and is indicative of the duration of the lag phase; and K is a parameter expressed as reciprocal time units (h−1). A visual representation can be found in Figure 1.
Figure 1.
Visual representation of the modified Gompertz growth function (Y = Y0 + (YM − Y0) * exp(−exp(K * (lag − X)/(YM − Y0) + 1)). Y0 and YM represent the starting population and maximum population (upper asymptote), respectively, and are both expressed as values of optical density (OD550). Lag (expressed in h) represents the time point when the exponential bacterial growth actually begins.
2.5. Effect of bLF and OT on Bacterial Virulence Factors
The effect of bLF and OT (0, 0.125, 0.250, 0.5 and 1 mg/mL) on bacterial biofilm formation, swimming and swarming motility, cell surface hydrophobicity (CSH) and activity of bacterial lipases and caseinases was investigated. Overnight cultures were grown in LB35 for all experiments except for the biofilm formation assay (MB).
2.5.1. Biofilm Formation
The effect of the two TFs on biofilm formation was assayed in 96-well plates using a crystal violet staining. Overnight cultures were diluted with fresh broth to OD550 0.1 and supplemented with 0, 0.125, 0.250, 0.5 or 1 mg/mL bLF or OT. Four replicates of 200 µL were introduced to the wells of a 96-well plate and incubated for 48 h at 28 °C without shaking. The wells were washed three times with 200 µL PBS to remove the bacterial cell cultures, dried for 30 min and the remaining bacteria were fixated with 200 µL methanol (99%, Thermo Fisher Scientific, Cambridge, UK). After 2 h, the methanol was removed and the plates were left to dry overnight. Fixed cells were stained with 200 µL of 0.1% crystal violet (Carl Roth, Karlsruhe, Germany) for 20 min. Plates were carefully washed with tap water to remove the excess of crystal violet and dried again for 2 h. Finally, 95% ethanol (200 µL/well) (Thermo Fisher Scientific, Cambridge, UK) was added to dissolve the crystal violet and the absorbance was measured at 570 nm with a spectrophotometer.
2.5.2. Swimming and Swarming Motility
For the swimming and swarming motility tests, soft agar plates of LB35 were prepared with 0.2% and 0.6% agar (Biokar Diagnostics, Pantin, France), respectively, comprising the TF concentrations mentioned before. Overnight cultures were diluted to OD550 0.1, and 2 µL was used for the inoculation of five replicate plates per condition. Culture plates were incubated in the upright (swimming) or inverted (swarming) positions for maximum 20 h at 28 °C, after which the diameters of the colonies were measured.
2.5.3. Cell Surface Hydrophobicity (CSH)
Furthermore, the effect of the TFs on CSH was measured with the bacterial adherence to hydrocarbons (BATH) test based on (12). Briefly, 25 µL of overnight culture was brought first in fresh LB35 broth and incubated with the TF test concentrations for 24 h at 28 °C and 140 rpm. Thereafter, cultures were centrifuged at 11,000× g for 15 min, and pellets were washed with PBS and once again centrifuged at 11,000× g for 7 min. The resulting cultures were diluted in PBS to an OD550 value of 0.5 (=A0). A total 4 ml of these suspensions were added to 1 mL of p-xylene (Honeywell, Charlotte, NC, USA), mixed and then left to separate for 30 min at room temperature. Finally, triplicates of the watery phase (200 µL) were added to the wells of the 96-well plates and OD550nm was measured (=Ai). PBS was used as a blank. Hydrophobicity (%) was determined as follows:
| Hydrophobicity (%) = ((A0 − Ai)/A0) * 100 |
| (<20% = not hydrophobic, 20–50% = moderate, >50% = strong) |
2.5.4. Extracellular Enzyme Activity
Lipase activity was measured in quintuplicate on LB35 agar plates (15 g/L agar) comprising 1% Tween80 (Merck, Darmstadt, Germany) and the previously mentioned TF test concentrations. Overnight cultures were diluted to OD550 0.1 and 2 µL was used for the inoculation. Plates were incubated inverted for three days at 28 °C before measurements. The caseinase activity of the supernatants from the three strains was tested as described by [12]. Briefly, overnight cultures were 1:100 diluted in LB35 and incubated for 24 h at 28 °C and 140 rpm with the TF test concentrations. The cultures were then centrifuged for 15 min at 15,000× g and 75 µL of the supernatant was mixed with 125 µL azocasein (2% w/v; Megazyme, Wicklow, Ireland) solution and incubated for 30 min at 37 °C. To end proteolysis, 660 µL trichloroacetic acid (10%) (Merck, Darmstadt, Germany) was added. The solutions were kept at −20 °C for 30 min to precipitate the remaining azocasein and subsequently centrifuged for 10 min at 10,000× g. A total 600 µL of the supernatants was added to 700 µL of NaOH (1 M) (VWR International, Radnor, PA, USA), three replicates of 200 µL were placed in 96-well plates and the OD440 nm was measured. LB35 was used as blank.
2.5.5. Statistical Analysis
Data were analyzed with GraphPad Prism. For the growth curves, statistical analysis of the derived parameters YM and lag was performed by one-way ANOVA with Dunnett multiple comparison testing, where the mean of each condition was compared with the mean of the control. Data of all other tests were also analyzed with one-way ANOVA with Dunnett multiple comparison testing to compare the mean of each condition with the mean of the respective control. For both swimming and swarming motility, the data were first log-transformed.
3. Results
3.1. PirAB Production by Vp Test Strains
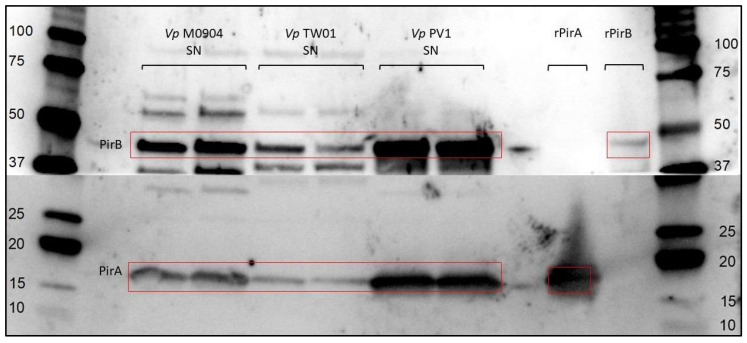
The production of PirA and PirB was successfully demonstrated for all test strains (Figure 2).
Figure 2.
Western blot of concentrated supernatant of Vp M0904, PV1 and TW01. Blot was cut after protein transfer and incubated separately with either anti-PirA or anti-PirB antibodies. Recombinantly produced PirA and PirB (rPirA/B) were used as controls.
3.2. Effect of bLF and OT on Bacterial Growth
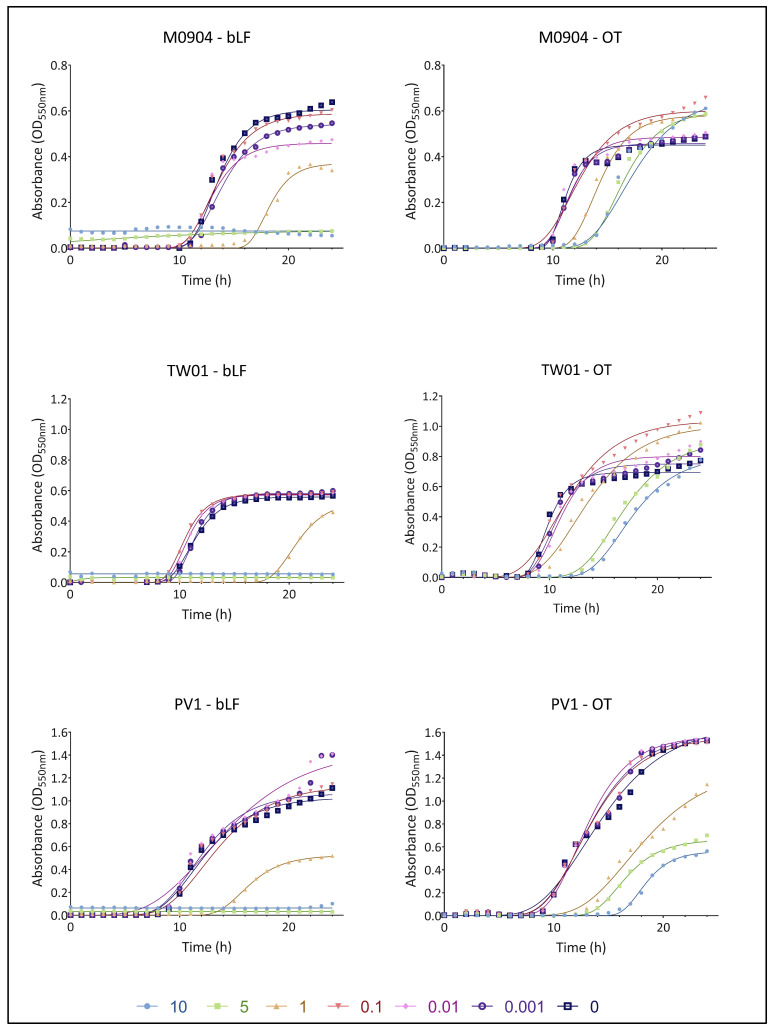
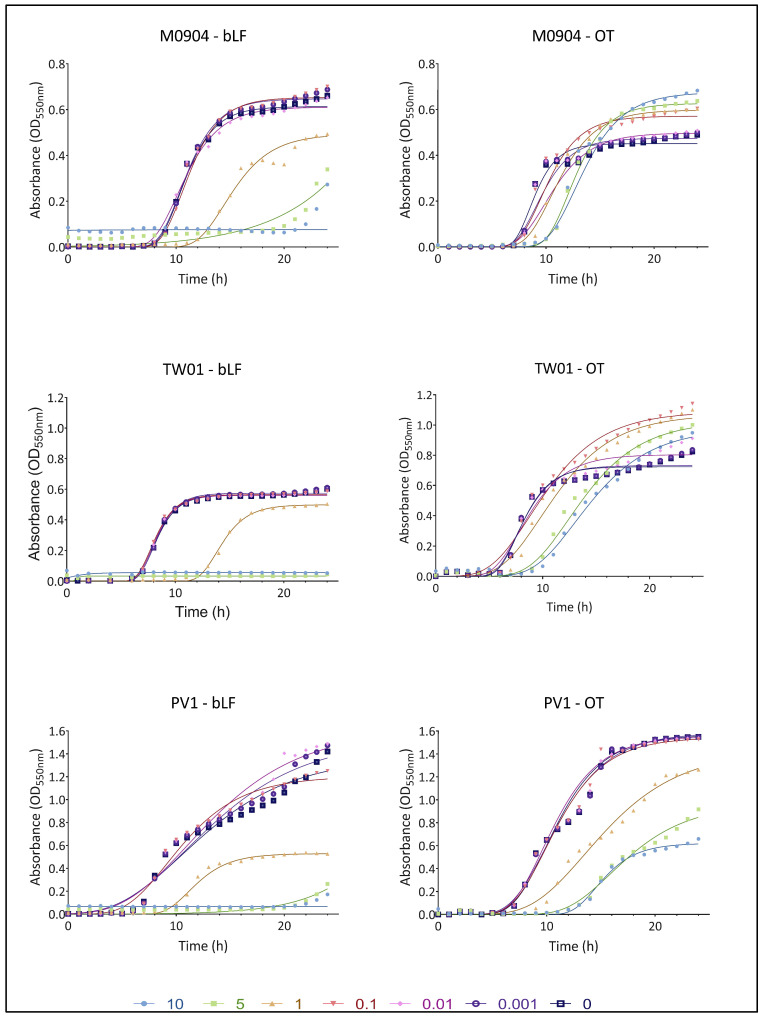
The growth curves for all test strains in the presence or absence of transferrins are shown in Figure 3 (for 101 CFU/mL) and Figure 4 (for 103 CFU/mL). A summary of the parameters YM and lag, derived from non-linear fitting to the Gompertz model, can be found in Table 1.
Figure 3.
Growth curves of Vp M0904, Vp TW01 and Vp PV1, with a starting concentration of 101 CFU/mL, and incubation with bLF and OT at concentrations 0, 0.001, 0.01 0.1, 1, 5 and 10 mg/mL. Individual points represent the mean OD550 (n = 3); curves are the non-linear fitting to the modified Gompertz model.
Figure 4.
Growth curves of Vp M0904, Vp TW01 and Vp PV1, with a starting concentration of 103 CFU/mL, and incubation with bLF and OT at concentrations 0, 0.001, 0.01 0.1, 1, 5 and 10 mg/mL. Individual points represent the mean OD550 (n = 3); curves are the non-linear fitting to the modified Gompertz model.
Table 1.
A summary of the bacterial growth parameters of Vp M0904, Vp TW01 and Vp PV1 with starting populations of 101 and 103 CFU/mL, derived from the modified Gompertz model. YM represents the maximum population, expressed as values of optical density (OD550); Lag represents the length of the lag time in h. Between brackets, significant differences between transferrin treatment and relative control (0 mg/mL) are indicated by asterisks: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001. Non-significant differences are indicated by ‘ns’. (/: modified Gompertz model was not able to calculate growth parameters due to too long lag phase or completely flat curve.)
| Vp M0904 | |||||
|---|---|---|---|---|---|
| Treatment (mg/mL) | bLF | OT | |||
| YM | Lag | YM | Lag | ||
| 101 CFU/mL | 10 | 0.075 (****) | / | 0.656 (****) | 13.375 (****) |
| 5 | 0.080 (****) | / | 0.598 (****) | 13.500 (****) | |
| 1 | 0.370 (****) | 16.382 (****) | 0.580 (****) | 11.757 (****) | |
| 0.1 | 0.588 (ns) | 10.700 (ns) | 0.601 (****) | 9.125 (ns) | |
| 0.01 | 0.458 (****) | 10.546 (*) | 0.485 (ns) | 9.509 (ns) | |
| 0.001 | 0.541 (****) | 11.084 (ns) | 0.457 (ns) | 9.527 (ns) | |
| 0 | 0.605 | 11.142 | 0.450 | 9.625 | |
| 103 CFU/mL | 10 | / | / | 0.675 (****) | 10.176 (****) |
| 5 | / | / | 0.626 (****) | 10.068 (****) | |
| 1 | 0.493 (****) | 11.972 (****) | 0.598 (****) | 8.160 (**) | |
| 0.1 | 0.653 (*) | 8.644 (ns) | 0.571 (****) | 7.481 (ns) | |
| 0.01 | 0.610 (ns) | 7.907 (ns) | 0.498 (***) | 7.478 (ns) | |
| 0.001 | 0.647 (*) | 8.396 (ns) | 0.474 (ns) | 7.213 (ns) | |
| 0 | 0.613 | 8.475 | 0.451 | 7.098 | |
| Vp TW01 | |||||
| Treatment (mg/mL) | bLF | OT | |||
| YM | Lag | YM | Lag | ||
| 101 CFU/mL | 10 | / | / | 0.801 (*) | 13.694 (****) |
| 5 | / | / | 0.908 (***) | 12.802 (****) | |
| 1 | 0.530 (ns) | 18.256 (****) | 1.008 (****) | 9.078 (ns) | |
| 0.1 | 0.576 (ns) | 8.664 (*) | 1.036 (****) | 7.392 (ns) | |
| 0.01 | 0.572 (ns) | 8.977 (ns) | 0.801 (ns) | 8,450 (ns) | |
| 0.001 | 0.581 (ns) | 9.588 (*) | 0.751 (ns) | 8.248 (ns) | |
| 0 | 0.558 | 9.148 | 0.695 | 8.051 | |
| 103 CFU/mL | 10 | / | / | 0.977 (****) | 9.181 (****) |
| 5 | / | / | 1.027 (****) | 8.723 (***) | |
| 1 | 0.498 (****) | 12.184 (****) | 1.070 (****) | 6.106 (ns) | |
| 0.1 | 0.564 (ns) | 6.439 (ns) | 1.088 (****) | 5.123 (ns) | |
| 0.01 | 0.560 (ns) | 6.532 (ns) | 0.804 (ns) | 5.394 (ns) | |
| 0.001 | 0.574 (ns) | 6.643 (ns) | 0.731 (ns) | 5.711 (ns) | |
| 0 | 0.561 | 6.624 | 0.724 | 5.825 | |
| Vp PV1 | |||||
| Treatment (mg/mL) | bLF | OT | |||
| YM | Lag | YM | Lag | ||
| 101 CFU/mL | 10 | / | / | 0.561 (****) | 16.184 (****) |
| 5 | / | / | 0.661 (****) | 13.651 (****) | |
| 1 | 0.523 (****) | 13.489 (****) | 1.290 (**) | 12.527 (****) | |
| 0.1 | 1.137 (ns) | 8.645 (ns) | 1.559 (ns) | 8.977 (ns) | |
| 0.01 | 1.447 (***) | 7.303 (ns) | 1.564 (ns) | 9.411 (ns) | |
| 0.001 | 1.065 (ns) | 8.107 (ns) | 1.586 (ns) | 9.058 (ns) | |
| 0 | 1.029 | 8.191 | 1.668 | 8.613 | |
| 103 CFU/mL | 10 | / | / | 0.621 (****) | 12.428 (****) |
| 5 | / | / | 0.964 (****) | 11.916 (****) | |
| 1 | 0.528 (***) | 9.032 (***) | 1.429 (ns) | 8.674 (***) | |
| 0.1 | 1.204 (ns) | 5.386 (ns) | 1.544 (ns) | 6.848 (ns) | |
| 0.01 | 1.615 (ns) | 5.110 (ns) | 1.554 (ns) | 6.760 (ns) | |
| 0.001 | 1.534 (ns) | 4.845 (ns) | 1.585 (ns) | 6.687 (ns) | |
| 0 | 1.375 | 4.584 | 1.565 | 6.939 | |
For Vp M0904, amounts of 10 and 5 mg/mL bLF were able to completely prevent bacterial growth when starting with 101 CFU/mL. Furthermore, compared with the control, there was a significantly longer lag phase; thus, growth inhibition was observed when using 0.01 and 1 mg/mL bLF and 1, 5 and 10 mg/mL OT. When starting with 103 CFU/mL of Vp M0904, 1, 5 and 10 mg/mL bLF and 5 and 10 mg/mL OT were able to significantly postpone growth. Interestingly, 0.001, 0.01 and 1 mg/mL bLF were able to significantly lower maximal growth (YM) when starting with 101 CFU/mL, while 0.1, 1, 5 and 10 mg/mL OT significantly increased maximal growth. Similar effects were observed when using 103 CFU/mL. For Vp TW01, 10 and 5 mg/mL bLF completely prevented bacterial growth, regardless of whether the cultures were started with 101 or 103 CFU/mL. Moreover, 0.001, 0.1 and 1 mg/mL bLF and 1, 5 and 10 mg/mL OT significantly postponed bacterial growth as shown by the longer lag phase at a starting concentration of 101 CFU/mL. For the 103 CFU/mL cultures, bacterial growth was significantly delayed when using 1 mg/mL bLF and 5 and 10 mg/mL OT. For a starting concentration of 103 CFU/mL in the presence of 1 mg/mL bLF, the maximal bacterial growth rate (YM) was significantly lower compared with the controls. On the other hand, regardless of the starting concentration, maximal bacterial growth values (YM) were significantly higher than those for the controls when using 0.1, 1, 5 and 10 mg/mL OT. For Vp PV1, regardless of the starting concentration, 5 and 10 mg/mL bLF again completely inhibited growth while 1 mg/mL bLF significantly prolonged the lag phase for both bacterial starting concentrations. The same was observed for 1, 5 and 10 mg/mL OT. Regardless of the starting concentration, the maximal bacterial growth rate (YM) was significantly lower when adding 1 mg/mL bLF or 1, 5 and 10 mg/mL OT. However, a significant increase in YM was observed when using 0.01 mg/mL bLF.
The growth curves and parameters for the cultures that were started with 105 and 107 CFU/mL can be found in Supplemental data (Figures S1 and S2, Table S1). In general, similar observations can be made as those for the experiments starting with 101 and 103 CFU/mL. Lag phases were significantly prolonged, with the highest concentrations of transferrins compared with the control when starting with 105 CFU/mL and with differences becoming less pronounced with 107 CFU/mL. Regardless of the starting populations, addition of the highest concentration of OT resulted again in a significantly higher YM for Vp M0904 and TW01, and in a significantly lower YM for Vp PV1. Addition of bLF had a varying effect on YM, resulting in a higher YM for 10 mg/mL in Vp M0904 (105 and 107 CFU/mL) and 0.1, 5 and 10 mg/mL in Vp PV1 (107 CFU/mL); lowering YM at concentrations higher than 1 mg/mL in Vp PV1 (105 CFU/mL); and having no effect on TW01.
3.3. Effect of bLF and OT on Bacterial Virulence Factors
3.3.1. Biofilm Formation
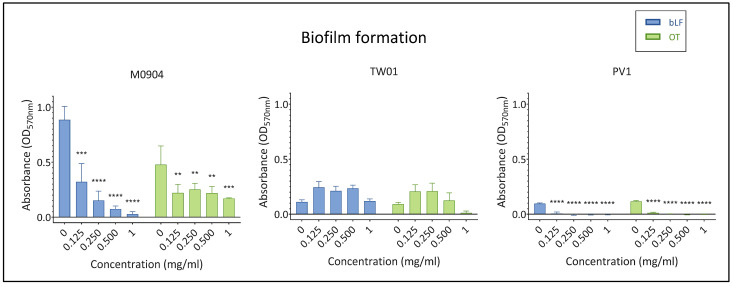
In the absence of transferrins, all test strains were able to create biofilms (Figure 5). However, biofilm production by Vp M0904 was more pronounced (OD570 > 0.4) than that for Vp TW01 and Vp PV1 (both (OD570 = 0.1). All transferrin concentrations significantly inhibited biofilm formation by Vp M0904 or Vp PV1. Transferrins had no effect on biofilm formation by Vp TW01.
Figure 5.
Effect of bLF and OT on biofilm formation. Mean absorbance at OD570nm and 95% confidence interval are shown on the graphs. Significant differences between transferrin treatment and relative control (0 mg/mL) are indicated by asterisks: ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3.3.2. Swimming and Swarming Motility
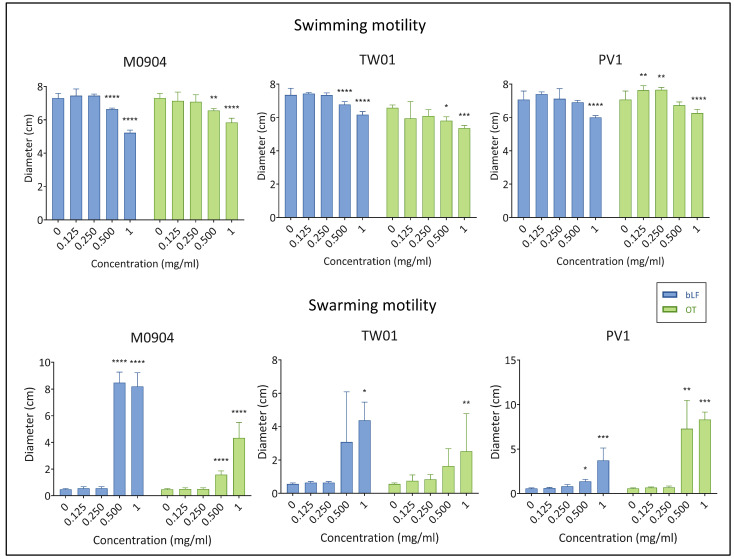
All test strains showed a comparable swimming motility in the absence of transferrins. The swimming motility of Vp M0904 and Vp TW01 was significantly reduced by both bLF and OT at 0.5 and 1.0 mg/mL (Figure 6 and Figure 7). For Vp PV1, this was only the case at 1 mg/mL of both transferrins. Strangely, for Vp PV1, lower concentrations of OT (0.125 and 0.25 mg/mL) significantly increased swimming. Swarming by our test strains was not observed without transferrins or when only low concentrations (<0.5 mg/mL) of transferrins were present in the medium. However, at the highest (1 mg/mL) bLF and OT concentration used, swarming by all test strains significantly increased (Figure 6 and Figure 7). Depending on the strain, 0.5 mg/mL of one or both transferrins also significantly increased swarming.
Figure 6.
Effect of bLF and OT on swimming and swarming motility. Mean diameters of the swimming or swarming colonies and 95% confidence interval are shown on the graphs. Significant differences between transferrin treatment and relative control (0 mg/mL) are indicated by asterisks: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
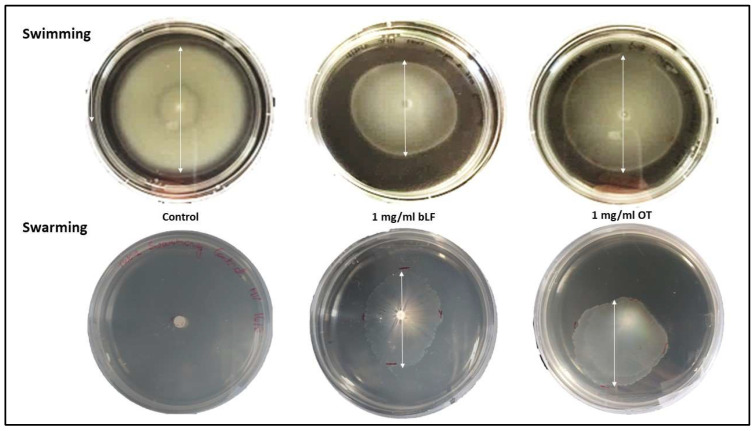
Figure 7.
Pictures of the motility plate assays for Vp TW01 at 0 and 1 mg/mL of bLF or OT. Diameters of the swimming or swarming colony are illustrated by a white arrow. In the presence of transferrins, swimming and swarming decreased or increased, respectively, compared with the controls.
3.3.3. Cell Surface Hydrophobicity (CSH)
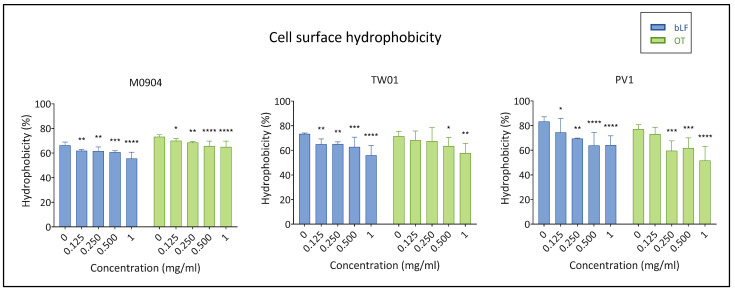
Cell surfaces of all test strains were strongly hydrophobic in the absence of transferrins (CSH > 50%). Bovine LF always significantly lowered CSH for all test strains. All OT concentrations used for Vp M0904 significantly lowered CSH (Figure 8). For Vp PV1 and Vp TW01, CSH was significantly reduced when using ≥0.25 and ≥0.5 mg/mL OT, respectively.
Figure 8.
Effect of bLF and OT on cell surface hydrophobicity (CSH). Mean CSH (%) and 95% confidence interval are shown on the graphs. Significant differences between transferrin treatment and relative control (0 mg/mL) are indicated by asterisks: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
3.3.4. Extracellular Enzyme Activity
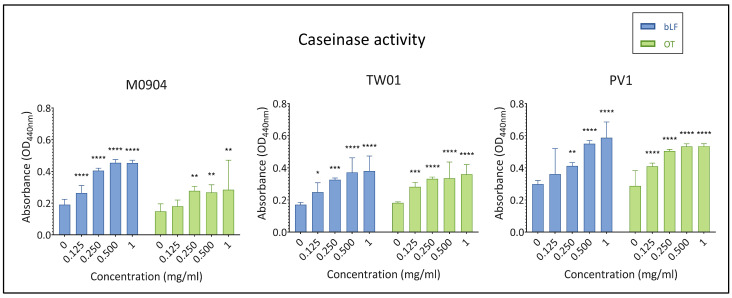
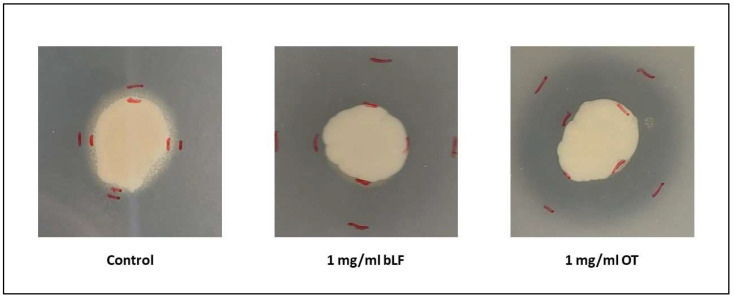
All test strains displayed caseinase activity in the absence of transferrins, and depending on the strain and the transferrin, addition of ≤0.125 or ≤0.250 mg/mL significantly augmented caseinase activity (Figure 9). All test strains displayed lipase activity, albeit minimal, in the absence of transferrins or when low concentrations of transferrins were added to the agar. However, once higher concentrations (1 mg/mL for Vp M0904 and Vp TW01 and 0.5 and 1 mg/mL for Vp PV1) were included in the agar, no precipitation and, thus, no lipase activity was observed anymore (Figure 10).
Figure 9.
Effect of bLF and OT on caseinase activity of the supernatant. Mean absorbance at OD440 nm and 95% confidence interval are shown on the graphs. Significant differences between transferrin treatment and relative control (0 mg/mL) are indicated by asterisks: * p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001.
Figure 10.
Pictures of the lipase activity assay for Vp TW01 at 0 and 1 mg/mL of bLF or OT. A precipitation zone around the colony indicates extracellular lipase activity by the bacteria.
4. Discussion
This is the first report assessing the antibacterial activity of bovine lactoferrin (bLF) and ovotransferrin (OT) on AHPND-causing V. parahaemolyticus strains. Previous research has explored their antimicrobial properties against various pathogens, revealing bacteriostatic, bactericidal and toxin-degrading activities [17,23,30,31]. Specifically, bLF has previously demonstrated inhibitory effects on the growth of various Vibrio species, including human pathogenic (non-AHPND) V. parahaemolyticus [30], V. cholerae, V. fluvialis, V. vulnificus and V. alginolyticus [31]. Additionally, preliminary tests suggest that bLF can positively impact fish and shrimp health, with benefits such as an enhanced growth, reduced mortalities, decreased disease outbreaks and stimulated immune responses [17,32,33], making them a potential antibiotic alternative for use in aquaculture. As a crucial first step, this study investigated the in vitro effects of bLF and OT on AHPND-causing V. parahaemolyticus strains from different geographical regions.
The growth curve assay revealed that both bLF and OT exhibited antibacterial effects on AHPND-positive V. parahaemolyticus strains. The highest concentrations of bLF completely inhibited growth for 24 h in all three strains, indicating a bactericidal effect. Additionally, a bacteriostatic effect was observed for the highest concentrations of OT and some concentrations of bLF since growth was significantly delayed. Intriguingly, a strain-specific effect on the maximal population concentration (YM) was observed for OT, as YM was increased in strains Vp M0904 and TW01 for some OT concentrations, but not in Vp PV1. A difference in effectivity between bLF and OT was also observed in various E. coli strains by Dierick, Van der Weken, Rybarczyk, Vanrompay, Devriendt and Cox [23]. They found that bLF concentrations of 5 mg/mL and higher could inhibit growth, whereas OT had no impact on growth. Furthermore, Leon-Sicairos, Canizalez-Roman, de la Garza, Reyes-Lopez, Zazueta-Beltran, Nazmi, Gomez-Gil and Bolscher [30] observed a concentration-dependent activity of bLF on V. parahaemolyticus strains, with the lowest concentration (10 µM) inhibiting growth and higher concentrations (30 and 40 µM) having no effect on growth anymore. Altogether, the antibacterial effect of transferrins seem to be depending on the transferrin type, bacterial species and strain, and used concentrations. The observed antibacterial effects likely result from the combined effect of iron sequestration by the TFs, and direct damage to the bacterial cell membrane, as previously described [17,18,21]. However, the observation that two out of three strains eventually exhibited increased growth with OT indicates potential adaptation mechanisms. In each context, bacteria must adequately sense, process and invoke functional responses to environmental changes to ensure their survival and proliferation, and they have evolved strategies to do so [35]. Given the essential role of iron for bacterial growth, bacteria have evolved strategies to thrive in low-iron environments. V. parahaemolyticus, for instance, employs siderophores (Vibrioferrin) to scavenge iron from host iron-binding proteins such as TF, LF and ferritin [36]. While prior research has shown vibrioferrin’s ability to capture iron from human TF, but not from human LF [37], its interaction with bLF or OT has not been explored to the best of our knowledge. In addition to siderophores, bacterial outer membranes can directly bind transferrins through receptors [38], although V. parahaemolyticus lacks identified transferrin receptors. It is plausible that the strains tested in our study produce siderophores or TF receptors specific to OT but not bLF, accounting for our observed outcomes. Several genes involved in vibrioferrin production have been identified in the genomes of the three Vp strains (unpublished results). Furthermore, it is important to note that V. parahaemolyticus produces numerous extracellular proteases, which could potentially degrade TFs into smaller peptides or nucleic acids, providing a nutrient source for the pathogen [39]. In this case, the high OT concentrations could provide an extra nutrient source to the bacteria, leading to the higher maximal growth observed for strains Vp M0904 and TW01 in the presence of high OT concentrations.
To further understand the antibacterial mechanisms of bLF and OT, their impact on a set of virulence factors was studied. All concentrations of both bLF and OT inhibited biofilm formation in strains Vp M0904 and PV1, while Vp TW01 remained unaffected. For the control conditions, there was considerable variation in biofilm-formation ability among the three strains, with Vp M0904 consistently producing more biofilm. This variability among other strains of V. parahaemolyticus has been observed in previous studies too [40,41,42]. Notably, a reduction in biofilm formation has been observed in other bacteria following LF treatment, including Pseudomonas aeruginosa, Klebsiella pneumoniae and E. coli, and was mainly attributed to membrane damage [21]. Biofilms play a critical role in shielding microorganisms from environmental fluctuations, antibiotics and disinfectants, making them essential for Vibrio species’ adaptation and survival in aquatic ecosystems like aquaculture [41,42,43]. Consequently, a decrease in biofilm formation may indicate a reduction in bacterial virulence. Furthermore, both tested transferrins affected flagella-mediated swimming and swarming motility, with swimming being inhibited and swarming induced at high transferrin concentrations. These motility processes are pivotal in the pathogenesis of V. parahaemolyticus, facilitating initial attachment and infection by overcoming repulsive forces between bacterial cells and host surfaces [43]. Consequently, inhibiting swimming motility may reduce the bacteria’s infectiousness. Moreover, lower swimming motility has been linked to reduced biofilm formation capacity in V. parahaemolyticus, a correlation validated by our results [12,41,44]. Conversely, the induction of swarming motility is suggested to be a result of the iron depletion caused by the transferrins, as Vibrio species tend to activate swarming in nutrient-deficient environments [41]. Additionally, low-iron conditions have been shown to stimulate both swarming and T3SS regulons in other V. parahaemolyticus strains [45].
Another virulence factor assessed in our study is CSH. Our results demonstrate that all CSH values exceeded 50%, indicating a robust hydrophobicity of the bacterial cell surfaces, consistent with previous research [40,41]. However, the introduction of TFs led to a significant reduction in CSH. Considering the documented binding capabilities of bLF and OT to LPS, porins and other outer membrane proteins, it is plausible that this interaction may be concealing hydrophobic regions of, e.g., LPS [46]. Consequently, cells with a lower CSH will have a higher tendency to remain in aqueous environments rather than attaching to hydrophobic (a)biotic surfaces [46,47]. Therefore, CSH plays a critical role in the initial cell attachment step of biofilm formation [12,43], and the observed decrease in CSH may contribute to the diminished biofilm formation observed in our strains exposed to transferrins.
Lastly, we examined the effect of bLF and OT on extracellular enzyme activity—more specifically, lipases and caseinases. Without transferrins, all three strains showed caseinase and lipase activity, as expected for V. parahaemolyticus strains [40]. However, the presence of the transferrins significantly stimulated caseinase activity, while lipase activity diminished at high transferrin concentrations. These extracellular enzymes play a crucial role in host tissue damage, facilitating nutrient acquisition and tissue penetration by the pathogens [12,39]. Consequently, the reduction in lipase activity could potentially diminish bacterial virulence. Conversely, the induction of caseinase activity might have the opposite effect. Since caseinase production in certain Vibrio species is suggested to be regulated by quorum sensing (QS) [48], further investigation into the impact of transferrins on QS regulators in V. parahaemolyticus is warranted.
5. Conclusions
In summary, our findings collectively underscore the substantial impact of both bLF and OT on AHPND-causing V. parahaemolyticus strains. The introduction of the transferrins resulted in reduced CSH and decreased swimming motility, which can be directly correlated with the observed inhibition of biofilm formation. This inhibition is of particular significance, given the challenges posed by Vibrio species biofilm formation in the context of aquaculture treatment. Moreover, the growth inhibition observed holds promise for future applications. Nevertheless, it is noteworthy that two out of three strains exhibited the ability to recover and even attain higher maximal growth in the presence of OT, suggesting that this compound may be less suitable for combating AHPND-causing V. parahaemolyticus.
In conclusion, our study provides valuable insights into the antibacterial effects of bLF and OT on AHPND-causing V. parahaemolyticus strains, giving new perspectives for their use in shrimp aquaculture. The observed antibacterial effects, coupled with transferrins’ reported immune-stimulating properties in other studies, make them promising candidates for further in vivo research related to their use as antimicrobials in shrimp aquaculture against AHPND.
Acknowledgments
We would like to thank Brigitte Van Moffaert for the provided technical assistance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/microorganisms11122912/s1. Figure S1. Growth curves of Vp M0904, Vp TW01 and Vp PV1, with a starting concentration of 105 CFU/mL, and incubation with bLF and OT at concentrations 0, 0.001, 0.01 0.1, 1, 5 and 10 mg/mL. Individual points represent the mean OD550 (n = 3), curves are the non-linear fitting to the modified Gompertz model. Figure S2. Growth curves of Vp M0904, Vp TW01 and Vp PV1, with a starting concentration of 107 CFU/mL, and incubation with bLF and OT at concentrations 0, 0.001, 0.01, 0.1, 1, 5 and 10 mg/mL. Individual points represent the mean OD550 (n = 3), curves are the non-linear fitting to the modified Gompertz model. Table S1. A summary of the bacterial growth parameters of Vp M0904, Vp TW01 and Vp PV1 with populations 105 and 107 CFU/mL, derived from the modified Gompertz model.
Author Contributions
M.V. (Marieke Vandeputte), writing—original draft preparation; M.V. (Marieke Vandeputte), M.V. (Margaux Verhaeghe) and L.W., methodology; M.V. (Marieke Vandeputte), visualization; M.V. (Marieke Vandeputte), P.B. and D.V., writing—review and editing; P.B. and D.V., funding acquisition. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available in this article and in Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by Ghent University (F2020/IOF-ConcepTT/045).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kumar R., Ng T.H., Wang H.C. Acute hepatopancreatic necrosis disease in penaeid shrimp. Rev. Aquac. 2020;12:1867–1880. doi: 10.1111/raq.12414. [DOI] [Google Scholar]
- 2.Kumar V., Roy S., Behera B.K., Bossier P., Das B.K. Acute hepatopancreatic necrosis disease (Ahpnd): Virulence, pathogenesis and mitigation strategies in Shrimp aquaculture. Toxins. 2021;13:524. doi: 10.3390/toxins13080524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Soto-Rodriguez S.A., Lozano-Olvera R., Montfort G.R.C., Zenteno E., Sánchez-Salgado J.L., Vibanco-Pérez N., Aguilar Rendón K.G. New Insights into the Mechanism of Action of PirAB from Vibrio Parahaemolyticus. Toxins. 2022;14:243. doi: 10.3390/toxins14040243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Han J.E., Tang K.F.J., Tran L.H., Lightner D.V. Photorhabdus insect-related (Pir) toxin-like genes in a plasmid of Vibrio parahaemolyticus, the causative agent of acute hepatopancreatic necrosis disease (AHPND) of shrimp. Dis. Aquat. Org. 2015;113:33–40. doi: 10.3354/dao02830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lee C.T., Chen I.T., Yang Y.T., Ko T.P., Huang Y.T., Huang J.Y., Huang M.F., Lin S.J., Chen C.Y., Lin S.S., et al. The opportunistic marine pathogen Vibrio parahaemolyticus becomes virulent by acquiring a plasmid that expresses a deadly toxin. Proc. Natl. Acad. Sci. USA. 2015;112:10798–10803. doi: 10.1073/pnas.1503129112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Muthukrishnan S., Defoirdt T., Ina-Salwany M.Y., Yusoff F.M., Shariff M., Ismail S.I., Natrah I. Vibrio parahaemolyticus and Vibrio harveyi causing Acute Hepatopancreatic Necrosis Disease (AHPND) in Penaeus vannamei (Boone, 1931) isolated from Malaysian shrimp ponds. Aquaculture. 2019;511:734227. doi: 10.1016/j.aquaculture.2019.734227. [DOI] [Google Scholar]
- 7.Dong X., Bi D., Wang H., Zou P., Xie G., Wan X., Yang Q., Zhu Y., Chen M., Guo C., et al. pirABvp-Bearing Vibrio parahaemolyticus and Vibrio campbellii pathogens isolated from the Same AHPND-affected pond possess highly similar pathogenic plasmids. Front. Microbiol. 2017;8:1859. doi: 10.3389/fmicb.2017.01859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ke H.M., Prachumwat A., Yu C.P., Yang Y.T., Promsri S., Liu K.F., Lo C.F., Lu M.Y.J., Lai M.C., Tsai I.J., et al. Comparative genomics of Vibrio campbellii strains and core species of the Vibrio Harveyi clade. Sci. Rep. 2017;7:srep41394. doi: 10.1038/srep41394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kondo H., Van P.T., Dang L.T., Hirono I. Draft genome sequence of non-Vibrio parahaemolyticus acute hepatopancreatic necrosis disease strain KC13.17.5, isolated from diseased shrimp in Vietnam. Genome Announc. 2015;3:e00978-15. doi: 10.1128/genomeA.00978-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liu L., Xiao J., Zhang M., Zhu W., Xia X., Dai X., Pan Y., Yan S., Wang Y. A Vibrio owensii strain as the causative agent of AHPND in cultured shrimp, Litopenaeus vannamei. J. Invertebr. Pathol. 2018;153:156–164. doi: 10.1016/j.jip.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 11.Restrepo L., Bayot B., Arciniegas S., Bajaña L., Betancourt I., Panchana F., Reyes Muñoz A. PirVP genes causing AHPND identified in a new Vibrio species (Vibrio punensis) within the commensal Orientalis clade. Sci. Rep. 2018;8:13080. doi: 10.1038/s41598-018-30903-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sathiyamoorthi E., Faleye O.S., Lee J.H., Raj V., Lee J. Antibacterial and Antibiofilm Activities of Chloroindoles Against Vibrio parahaemolyticus. Front. Microbiol. 2021;12:714371. doi: 10.3389/fmicb.2021.714371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lulijwa R., Rupia E.J., Alfaro A.C. Antibiotic use in aquaculture, policies and regulation, health and environmental risks: A review of the top 15 major producers. Rev. Aquac. 2019;12:640–663. doi: 10.1111/raq.12344. [DOI] [Google Scholar]
- 14.Elmahdi S., DaSilva L.V., Parveen S. Antibiotic resistance of Vibrio parahaemolyticus and Vibrio vulnificus in various countries: A review. Food Microbiol. 2016;57:128–134. doi: 10.1016/j.fm.2016.02.008. [DOI] [PubMed] [Google Scholar]
- 15.Tan C.W., Rukayadi Y., Hasan H., Thung T.Y., Lee E., Rollon W.D., Hara H., Kayali A.Y., Nishibuchi M., Radu S. Prevalence and antibiotic resistance patterns of Vibrio parahaemolyticus isolated from different types of seafood in Selangor, Malaysia. Saudi J. Biol. Sci. 2020;27:1602–1608. doi: 10.1016/j.sjbs.2020.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stratev D., Fasulkova R., Krumova-Valcheva G. Incidence, virulence genes and antimicrobial resistance of Vibrio parahaemolyticus isolated from seafood. Microb. Pathog. 2023;177:106050. doi: 10.1016/j.micpath.2023.106050. [DOI] [PubMed] [Google Scholar]
- 17.Abdelnour S.A., Ghazanfar S., Abdel-Hamid M., Abdel-Latif H.M.R., Zhang Z., Naiel M.A.E. Therapeutic uses and applications of bovine lactoferrin in aquatic animal medicine: An overview. Vet. Res. Commun. 2023;47:1015–1029. doi: 10.1007/s11259-022-10060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Legros J., Jan S., Bonnassie S., Gautier M., Croguennec T., Pezennec S., Cochet M.F., Nau F., Andrews S.C., Baron F. The role of ovotransferrin in egg-white antimicrobial activity: A review. Foods. 2021;10:823. doi: 10.3390/foods10040823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rosa L., Cutone A., Lepanto M.S., Paesano R., Valenti P. Lactoferrin: A natural glycoprotein involved in iron and inflammatory homeostasis. Int. J. Mol. Sci. 2017;18:1985. doi: 10.3390/ijms18091985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wu J., Acero-Lopez A. Ovotransferrin: Structure, bioactivities, and preparation. Food Res. Int. 2012;46:480–487. doi: 10.1016/j.foodres.2011.07.012. [DOI] [Google Scholar]
- 21.Zarzosa-Moreno D., Avalos-Gómez C., Ramírez-Texcalco L.S., Torres-López E., Ramírez-Mondragón R., Hernández-Ramírez J.O., Serrano-Luna J., de la Garza M. Lactoferrin and its derived peptides: An alternative for combating virulence mechanisms developed by pathogens. Molecules. 2020;25:5763. doi: 10.3390/molecules25245763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rathnapala E.C.N., Ahn D.U., Abeyrathne S. Functional properties of ovotransferrin from chicken egg white and its derived peptides: A review. Food Sci. Biotechnol. 2021;30:619–630. doi: 10.1007/s10068-021-00901-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dierick M., Van der Weken H., Rybarczyk J., Vanrompay D., Devriendt B., Cox E. Porcine and Bovine Forms of Lactoferrin Inhibit Growth of Porcine Enterotoxigenic Escherichia coli and Degrade Its Virulence Factors. Appl. Environ. Microbiol. 2020;86:e00524-20. doi: 10.1128/AEM.00524-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kieckens E., Rybarczyk J., Barth S.A., Menge C., Cox E., Vanrompay D. Effect of lactoferrin on release and bioactivity of Shiga toxins from different Escherichia coli O157:H7 strains. Vet. Microbiol. 2017;202:29–37. doi: 10.1016/j.vetmic.2016.03.013. [DOI] [PubMed] [Google Scholar]
- 25.Ochoa T.J., Noguera-Obenza M., Ebel F., Guzman C.A., Gomez H.F., Cleary T.G. Lactoferrin impairs type III secretory system function in enteropathogenic Escherichia coli. Infect. Immun. 2003;71:5149–5155. doi: 10.1128/IAI.71.9.5149-5155.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Beeckman D.S., Van Droogenbroeck C.M.A.D., De Cock B.J.A., Van Oostveldt P., Vanrompay D.C.G. Effect of ovotransferrin and lactoferrins on Chlamydophila psittaci adhesion and invasion in HD11 chicken macrophages. Vet. Res. 2007;38:729–739. doi: 10.1051/vetres:2007028. [DOI] [PubMed] [Google Scholar]
- 27.Sessa R., Di Pietro M., Filardo S., Bressan A., Rosa L., Cutone A., Frioni A., Berlutti F., Paesano R., Valenti P. Effect of bovine lactoferrin on Chlamydia trachomatis infection and inflammation. Biochem. Cell Biol. 2017;95:34–40. doi: 10.1139/bcb-2016-0049. [DOI] [PubMed] [Google Scholar]
- 28.Niaz B., Saeed F., Ahmed A., Imran M., Maan A.A., Khan M.K.I., Tufail T., Anjum F.M., Hussain S., Suleria H.A.R. Lactoferrin (LF): A natural antimicrobial protein. Int. J. Food Prop. 2019;22:1626–1641. doi: 10.1080/10942912.2019.1666137. [DOI] [Google Scholar]
- 29.Baron F., Jan S., Gonnet F., Pasco M., Jardin J., Giudici B., Gautier M., Guérin-Dubiard C., Nau F. Ovotransferrin plays a major role in the strong bactericidal effect of egg white against the bacillus cereus group. J. Food Prot. 2014;77:955–962. doi: 10.4315/0362-028X.JFP-13-473. [DOI] [PubMed] [Google Scholar]
- 30.Leon-Sicairos N., Canizalez-Roman A., de la Garza M., Reyes-Lopez M., Zazueta-Beltran J., Nazmi K., Gomez-Gil B., Bolscher J.G. Bactericidal effect of lactoferrin and lactoferrin chimera against halophilic Vibrio parahaemolyticus. Biochimie. 2009;91:133–140. doi: 10.1016/j.biochi.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 31.Acosta-Smith E., Viveros-Jiménez K., Canizalez-Román A., Reyes-Lopez M., Bolscher J.G.M., Nazmi K., Flores-Villaseñor H., Alapizco-Castro G., de la Garza M., Martínez-Garcia J.J., et al. Bovine lactoferrin and lactoferrin-derived peptides inhibit the growth of Vibrio cholerae and other Vibrio species. Front. Microbiol. 2018;8:2633. doi: 10.3389/fmicb.2017.02633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chand R.K., Sahoo P.K., Kumari J., Pillai B.R., Mishra B.K. Dietary administration of bovine lactoferrin influences the immune ability of the giant freshwater prawn Macrobrachium rosenbergii (de Man) and its resistance against Aeromonas hydrophila infection and nitrite stress. Fish Shellfish Immunol. 2006;21:119–129. doi: 10.1016/j.fsi.2005.10.010. [DOI] [PubMed] [Google Scholar]
- 33.Zhuang Y., Huang H., Liu X.L., Wang N.A., Zhong G.F. Effect of bovine lactoferricin on the growth performance, digestive capacity, immune responses and disease resistance in Pacific white shrimp, Penaeus vannamei. Fish Shellfish Immunol. 2022;123:282–289. doi: 10.1016/j.fsi.2022.03.012. [DOI] [PubMed] [Google Scholar]
- 34.Tjørve K.M.C., Tjørve E. The use of Gompertz models in growth analyses, and new Gompertz-model approach: An addition to the Unified-Richards family. PLoS ONE. 2017;12:e0178691. doi: 10.1371/journal.pone.0178691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spratt M.R., Lane K. Navigating Environmental Transitions: The Role of Phenotypic Variation in Bacterial Responses; Proceedings of the mBio; Geneva, Switzerland. 28 November–16 December 2022; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.León-Sicairos N., Angulo-Zamudio U.A., de la Garza M., Velázquez-Román J., Flores-Villaseñor H.M., Canizalez-Román A. Strategies of Vibrio parahaemolyticus to acquire nutritional iron during host colonization. Front. Microbiol. 2015;6:702. doi: 10.3389/fmicb.2015.00702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamamoto S., Okujo N., Matsuura S., Fujiwara I., Fujita Y., Al E.T. Siderophore-Mediated Utilization of Iron Bound Transferrin by Vibrio parahaemolyticus. Microbiol. Immunol. 1994;38:687–693. doi: 10.1111/j.1348-0421.1994.tb01843.x. [DOI] [PubMed] [Google Scholar]
- 38.Ekins A., Khan A.G., Shouldice S.R., Schryvers A.B. Lactoferrin receptors in Gram-negative bacteria: Insights into the iron acquisition process. Biometals. 2004;17:235–243. doi: 10.1023/B:BIOM.0000027698.43322.60. [DOI] [PubMed] [Google Scholar]
- 39.Osei-Adjei G., Huang X., Zhang Y. The extracellular proteases produced by Vibrio parahaemolyticus. World J. Microbiol. Biotechnol. 2018;34:68. doi: 10.1007/s11274-018-2453-4. [DOI] [PubMed] [Google Scholar]
- 40.Ashrafudoulla M., Na K.W., Hossain M.I., Mizan M.F.R., Nahar S., Toushik S.H., Roy P.K., Park S.H., Ha S.D. Molecular and pathogenic characterization of Vibrio parahaemolyticus isolated from seafood. Mar. Pollut. Bull. 2021;172:112927. doi: 10.1016/j.marpolbul.2021.112927. [DOI] [PubMed] [Google Scholar]
- 41.Mizan M.F.R., Jahid I.K., Kim M., Lee K.H., Kim T.J., Ha S.D. Variability in biofilm formation correlates with hydrophobicity and quorum sensing among Vibrio parahaemolyticus isolates from food contact surfaces and the distribution of the genes involved in biofilm formation. Biofouling. 2016;32:497–509. doi: 10.1080/08927014.2016.1149571. [DOI] [PubMed] [Google Scholar]
- 42.Ahmed H.A., El Bayomi R.M., Hussein M.A., Khedr M.H.E., Abo Remela E.M., El-Ashram A.M.M. Molecular characterization, antibiotic resistance pattern and biofilm formation of Vibrio parahaemolyticus and V. cholerae isolated from crustaceans and humans. Int. J. Food Microbiol. 2018;274:31–37. doi: 10.1016/j.ijfoodmicro.2018.03.013. [DOI] [PubMed] [Google Scholar]
- 43.Zhu W., Gao J., Liu H., Liu J., Jin T., Qin N., Ren X., Xia X. Antibiofilm effect of sodium butyrate against Vibrio parahaemolyticus. Food Control. 2022;131:108422. doi: 10.1016/j.foodcont.2021.108422. [DOI] [Google Scholar]
- 44.Gu D., Meng H., Li Y., Ge H., Jiao X. A GntR family transcription factor (VPA1701) for swarming motility and colonization of Vibrio parahaemolyticus. Pathogens. 2019;8:235. doi: 10.3390/pathogens8040235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gode-Potratz C.J., Kustusch R.J., Breheny P.J., Weiss D.S., McCarter L.L. Surface sensing in Vibrio parahaemolyticus triggers a programme of gene expression that promotes colonization and virulence. Mol. Microbiol. 2011;79:240–263. doi: 10.1111/j.1365-2958.2010.07445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Danchik C., Casadevall A. Role of Cell Surface Hydrophobicity in the Pathogenesis of Medically-Significant Fungi. Front. Cell. Infect. Microbiol. 2021;10:594973. doi: 10.3389/fcimb.2020.594973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Krasowska A., Sigler K. How microorganisms use hydrophobicity and what does this mean for human needs? Front. Cell. Infect. Microbiol. 2014;4:112. doi: 10.3389/fcimb.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Packiavathy I.A.S.V., Sasikumar P., Pandian S.K., Veera Ravi A. Prevention of quorum-sensing-mediated biofilm development and virulence factors production in Vibrio spp. by curcumin. Appl. Microbiol. Biotechnol. 2013;97:10177–10187. doi: 10.1007/s00253-013-4704-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data presented in this study are available in this article and in Supplementary Materials.