Abstract
Two methionine biosynthetic genes in Pseudomonas syringae pv. syringae, metX and metW, were isolated, sequenced, and evaluated for their roles in methionine biosynthesis and bacterial fitness on leaf surfaces. The metXW locus was isolated on a 1.8-kb DNA fragment that was required for both methionine prototrophy and wild-type epiphytic fitness. Sequence analysis identified two consecutive open reading frames (ORFs), and in vitro transcription-translation experiments provided strong evidence that the ORFs encode proteins with the predicted molecular masses of 39 and 22.5 kDa. The predicted amino acid sequence of MetX (39 kDa) showed homology to several known and putative homoserine O-acetyltransferases. This enzyme is the first enzyme in the methionine biosynthetic pathway of fungi, gram-negative bacteria of the genus Leptospira, and several gram-positive bacterial genera. Both metX and metW were required for methionine biosynthesis, and transcription from both genes was not repressed by methionine. MetW (22.5 kDa) did not show significant homology to any known protein, including prokaryotic and eukaryotic methionine biosynthetic enzymes. Several classes of methionine auxotrophs, including metX and metW mutants, exhibit reduced fitness on leaf surfaces, indicating a requirement for methionine prototrophy in wild-type epiphytic fitness. This requirement is enhanced under environmentally stressful conditions, suggesting a role for methionine prototrophy in bacterial stress tolerance.
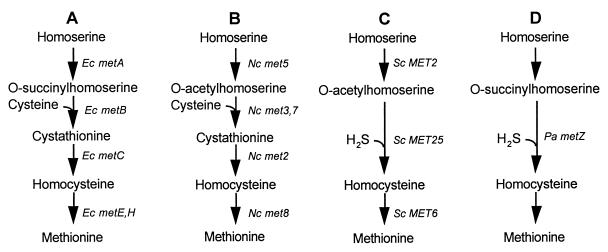
The methionine biosynthetic pathway and its regulation differ among various groups of organisms, and these differences may be interesting from an evolutionary perspective. Major differences among organisms are found in the nature of the acylhomoserine intermediate formed in the first step of the pathway and in the method of assimilation of the sulfur atom (Fig. 1). Interestingly, the gram-positive bacterial species that have been examined (Bacillus, Brevibacterium, Corynebacterium, and Arthrobacter) show greater similarity to fungi than to enteric bacteria both in forming O-acetylhomoserine and in assimilating sulfur primarily by direct sulfhydrylation (42). Differences have also been identified in the regulation of methionine biosynthesis. Most notably, methionine represses the synthesis of the biosynthetic enzymes in enteric bacteria (36) and the yeast Saccharomyces cerevisiae (2) but not in the filamentous fungus Ascobolus immersus (18) or in the spirochete Leptospira meyeri (6).
FIG. 1.
Methionine biosynthetic pathways in different species. (A) E. coli (Ec) (41), (B) N. crassa (Nc) (41), (C) S. cerevisiae (Sc) (41), and Brevibacterium flavum (38), and (D) P. aeruginosa (Pa) (15) pathways are shown. Note that N. crassa can also convert O-acetylhomoserine to homocysteine via direct sulfhydrylation (27) and S. cerevisiae can perform the conversion through transsulfuration (45), but these conversions are not quantitatively important (41).
Foglino et al. (15) demonstrated that the methionine biosynthetic pathway of Pseudomonas aeruginosa differs from that in all other microorganisms examined. P. aeruginosa is the only organism currently known to employ O-succinylhomoserine as a substrate for direct sulfhydrylation (15); most organisms use either an O-succinylhomoserine-transsulfuration combination (Fig. 1A) or O-acetylhomoserine-direct sulfhydrylation (Fig. 1C). In at least some respects, including direct sulfhydrylation in the methionine biosynthetic pathway and enzyme similarities in the threonine synthetic pathway (8), Pseudomonas species are more similar to fungi and gram-positive bacteria than to other gram-negative bacteria. Our understanding of the relatedness between Pseudomonas species and these other organisms should be improved with further studies on the genetics and regulation of metabolic pathways such as that of methionine biosynthesis.
The ability to synthesize methionine has been found to be important in many plant-microbe interactions. For example, methionine prototrophy has been found to be a requirement for virulence, pathogenicity, and/or an ability to induce a hypersensitive response in plants by Pseudomonas syringae pv. phaseolicola, Pseudomonas syringae pv. tabaci, Ralstonia solanacearum, Agrobacterium tumefaciens, and various Corynebacterium species (1, 9, 12, 16, 26, 34, 47). It has also been found that methionine can be converted to the phytotoxin 3-methylthiopropionic acid by Xanthomonas campestris pv. manihotis (13) as well as to the phytohormone ethylene, such as by plant pathogens of the genera Pantoea, Pseudomonas, and Xanthomonas (49). Also, sulfur-containing amino acids, primarily methionine, were found to be required for the in vitro induction of genes involved in pathogenicity and the hypersensitive response (hrp genes) in Xanthomonas campestris pv. campestris (46).
In studies aimed at identifying loci in P. syringae that are required for bacterial growth and survival on leaves, we identified a methionine auxotroph that exhibited particularly poor epiphytic fitness. Although the nutrient environment on a leaf surface is poorly understood, several studies have demonstrated the presence of methionine in leaf exudates (37, 51). A derivative of the P. syringae fitness-reduced methionine auxotroph called mutant 42 showed abundant growth on moist leaves of two different plant species, indicating that methionine concentrations were not limiting for its growth under these conditions (3). Interestingly, of 5,300 transposon mutants that were screened for reduced epiphytic fitness, mutant 42 was among the four mutants that exhibited the poorest survival on leaves under environmentally stressful conditions (3, 4, 33). Methionine biosynthesis therefore appeared to be required for bacterial tolerance to environmentally stressful conditions. In this study, we characterized the locus that was altered in mutant 42 and explored the role of this locus in methionine biosynthesis in P. syringae as well as the role of methionine biosynthesis in epiphytic fitness.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids
| Strain or plasmid | Relevant characteristics | Reference or source |
|---|---|---|
| Strains | ||
| E. coli | ||
| DH5α | F−recA1 φ80 ΔlacZ ΔM15 λ− endA1 supE44 recA1 gyrA | Bethesda Research Laboratories |
| SF800 | NalrpolA(Ts) thy | W. Paranchych |
| P. syringae pv. syringae | ||
| B728a | Rifr MetX+ MetW+ ice nucleation+ bean pathogen | S. S. Hirano |
| 990 | Bean pathogen, isolated from soybean | M. Hendson |
| Mutant 42 | Rifr Kmr Met− epiphytic fitness− Tn5 mutant of B728a | Lindow et al. (33) |
| MX7 | Rifr Kmr methionine auxotroph, marker-exchange mutant of B728a | This study |
| LK2 | Rifr Kmr B728a deletion mutant for inaB | This study |
| SPC9, SPC17, SPC82, SPC89 | Rifr Kmr SprmetX::Tn3-Spice marker-exchange mutants of LK2 | This study |
| SPC52, SPC102 | Rifr Kmr SprmetW::Tn3-Spice marker-exchange mutants of LK2 | This study |
| P. syringae pv. phaseolicola NPS3121 | Bean pathogen, race 2, Rifr | Lindgren et al. (30) |
| P. syringae pv. glycinia PsgO | Soybean pathogen, race 0, Rifr | B. Staskawicz |
| P. syringae pv. tabaci DT113 | Tobacco pathogen | M. Hendson |
| P. syringae pv. aptata PA145 | Sugar beet pathogen | M. Hendson |
| P. syringae pv. lachrymans PL116 | Cucumber pathogen | M. Hendson |
| P. syringae pv. savastanoi 649 | Olive and oleander pathogen | M. Hendson |
| P. syringae pv. pisi C30 | Pea pathogen | M. Hendson |
| P. syringae pv. tomato PT23 | Tomato pathogen | Bender and Cooksey (5) |
| Pseudomonas syringae pv. maculicola Psm145 | Isolated from cauliflower | R. C. Campbell |
| P. aeruginosa Pa1 | Clinical isolate | M. Schroth |
| P. cichorii H64 | Pathogen, isolated from lettuce | M. Hendson |
| P. viridiflava F62L | Broad-host-range pathogen | Hendson et al. (24) |
| R. solanacearum UW25 | Pathogen, isolated from tomato | L. Sequeira |
| X. campestris pv. campestris B28 | Pathogen of Brassica spp. | M. Hendson |
| X. campestris pv. phaseoli B1 | Bean pathogen | M. Hendson |
| Xanthomonas campestris pv. vesicatoria B280 | Pathogen of pepper and tomato | M. Hendson |
| Pantoea agglomerans 299R | Epiphyte, isolated on pear | S. Lindow |
| Plasmids | ||
| pBluescript II KS+ | Apr, cloning vector | Stratagene Co. |
| pCM2 | Tcr, pLAFR3 cosmid containing the metX and metW genes from B728a | This study |
| pLAFR3 | TcrincP-I rlxRK2+lacZα cos+ | Staskawicz et al. (48) |
| pLK2 | Kmr, containing a 1.0-kb SalI deletion within the coding region of iceB | G. Cirvilleri |
| pRK2013 | Kmrtra+ mob+ | Ditta et al. (11) |
| pSPC#103–pSPC#202 | Kmr Spr Srr, pVGA7::Tn3-Spice derivatives | This study |
| pGAN1 | Apr, contains the 1.2-kb PvuII-PstI fragment from pVGA7 in pBluescript II KS+ | This study |
| pVSP61 | KmrlacZα, pVS1 replicon | W. Tucker |
| pVGA7 | Kmr, with 6.9-kb HindIII fragment from pCM2 cloned into pVSP61 | This study |
| pVGA7Ω | Kmr Spr Srr, pVGA7 containing a 2-kb Ω cassette (Spr Srr) inserted into the BamHI site | This study |
| pVGA4Ω | Kmr Spr Srr, pVSP61 containing a Spr Srr Ω cassette and a 4-kb EcoRI fragment from pCM2, which contains metX and a truncated metW | This study |
| pVΩ7Δ | Kmr, pVGA7Ω containing a 0.62-kb in-frame SfiI-ClaI deletion in metX | This study |
| pT7-5 | Apr, protein expression vector, ColE1 ori, φ10 promoter of phage T7 | S. Tabor |
| pT7-6 | Apr, protein expression vector, ColE1 ori, φ10 promoter of phage T7 polylinker oppositely oriented from pT7-5 | S. Tabor |
| pT7P1 | Apr, contains the 2.6-kb NruI-XhoI fragment from pVGA7 in pT7-5, transcription from φ10 promoter oriented NruI→XhoI | This study |
| pT7P2 | Apr, contains the 2.0-kb NruI-SmaI fragment from pVGA7 in pT7-6, transcription from φ10 promoter oriented NruI→SmaI | This study |
| pT7P3 | Apr, contains the 1.8-kb NruI-EcoRI fragment from pVGA7 in pT7-6, transcription from φ10 promoter oriented NruI→EcoRI | This study |
| pT7P4 | Apr, contains the 2.1-kb PvuII-XhoI fragment from pVGA7 in pT7-5, transcription from φ10 promoter oriented PvuII→XhoI | This study |
| pT7P5 | Apr, contains the 1.3-kb PvuII-EcoRI fragment from pVGA7 in pT7-6, transcription from φ10 promoter oriented PvuII→EcoRI | This study |
Media and growth conditions.
P. syringae strains were grown in King’s medium B (KB) (28) or in M9 medium, a defined mineral salts medium supplemented with either 0.4% glucose or another carbon source (43). When necessary, 0.3 mM l-methionine was added to the media. Auxotrophy in P. syringae mutants was evaluated by adding 20 μl of washed cells of an overnight culture to 2 ml of M9 amended with methionine pathway intermediates as described previously (10). Escherichia coli strains were grown in Luria-Bertani medium at 37°C (35). The following concentrations of antibiotics (Sigma) were used for selection: ampicillin (Ap), 50 μg/ml; chloramphenicol (Cm), 20 μg/ml; kanamycin (Km), 15 μg/ml; naladixic acid (Nal), 20 μg/ml; rifampin (Rif), 100 μg/ml; spectinomycin (Sp), 20 μg/ml; and tetracycline (Tc), 15 μg/ml.
Inoculation of plants.
For plant inoculations, cells from 24-h cultures were harvested from a KB agar plate amended with Rif, washed in sterile 10 mM phosphate buffer (pH 7.0), and adjusted to the appropriate concentration by dilution after estimating cell density with a Spectronic 20 (Bausch and Lomb) spectrophotometer. Inoculation of Phaseolus vulgaris L. plants was performed as described previously (31). Plants were incubated for 24 h under moist conditions (3, 33), were allowed to dry for 1 h at 21°C at ambient humidity (about 60% relative humidity), and then were placed in a growth chamber at 28°C, 40% relative humidity and constant light (107,000 lux). For each bacterial strain tested, 10 to 20 pots were inoculated, and at various times during the incubation, 20 primary leaves were collected (1 to 2 leaves per pot). The bacteria on each leaf were removed and quantified as described previously (31).
Recombinant DNA techniques.
Digestion of DNA with restriction endonucleases, preparation of transformation-competent E. coli, and construction of a P. syringae B728a genomic library were performed as described by Sambrook et al. (43). Large- and small-scale isolations of recombinant plasmids from E. coli were performed by alkaline lysis (25). Total genomic DNA was prepared as described previously (43) and purified by CsCl buoyant-density centrifugation. Plasmids were transferred from E. coli to P. syringae by triparental matings with the conjugative helper plasmid pRK2013 (48). Insertions of Tn3-Spice into the methionine biosynthetic locus on the plasmids pCM2 and pVGA7 were selected by antibiotic resistance in a polA-deficient E. coli SF800 (30). The positions and orientations of the transposons were determined by restriction mapping.
To probe chromosomal DNA for sequences homologous to the P. syringae methionine biosynthetic locus, a 1.2-kb PvuII-PstI restriction fragment internal to the locus was labeled with digoxigenin-dUTP from the Genius Nonradioactive DNA Labeling and Detection kit (Boehringer Mannheim) according to the manufacturer’s specifications. Hybridizations were performed at moderate stringency, specifically in a solution consisting of 50% (vol/vol) formamide, 5× SSC (1× SSC is 0.15 M NaCl plus 0.015 M sodium citrate), 0.1% (wt/vol) N-lauroylsarcosine, 0.2% (wt/vol) sodium dodecyl sulfate, and 1% (wt/vol) blocking reagent (Boehringer Mannheim) at 39°C. Immunological detection of the labeled DNA was performed after a 12-h hybridization.
Nucleotide sequencing with double stranded DNA templates was performed by the dideoxy chain termination method (44) with 35S-dATP (Amersham Co.) and the Sequenase 2.0 kit (United States Biochemicals) according to manufacturer’s instructions with the following modification. A 0.5-μl (8.5-U) volume of terminal deoxynucleotidyl transferase (United States Biochemicals) was added to a 3.5-μl mixture of all four deoxynucleoside triphosphates, each at 1 mM in 1× Sequenase buffer. After the dideoxy termination reaction was complete, 1 μl of this solution was added to individual A, C, G, and T reaction tubes with a 30 min incubation at 37°C. Addition of the terminal deoxynucleotidyl transferase to elongate prematurely terminated DNA strands significantly limited artifact banding (14).
DNA sequence analysis.
Software used in sequence analysis was provided by the University of Wisconsin’s Genetics Computer Group, Inc. Peptide sequences from putative open reading frames (ORFs) in the methionine biosynthetic locus were compared to the nucleotide sequence data library in GenBank and EMBL by the TFastA and BLAST programs. Candidate peptide sequences were analyzed with BESTFIT and PILEUP software. The nonrandomness of codon usage for putative ORFs was determined by the CODONPREFERENCE and TESTCODE programs.
Measurement of ice nucleation activity.
The level of expression of Tn3-Spice inaZ fusions at −9°C was quantified by a droplet freezing technique (32). An ice nucleation-deficient strain of P. syringae B728a, designated LK2, was the recipient of Tn3-Spice-containing plasmids. For in vitro expression of merodiploids or marker-exchange haploids, 100 μl of an overnight culture was transferred to 2 ml of fresh medium and placed on a rotary shaker maintained at 24°C for 24 h. Serial dilutions were made, and for each dilution, 40 10-μl droplets were placed on a paraffin-coated aluminum foil surface held at −9°C. The fraction of the 40 droplets that froze was determined, and the ice nuclei were quantified with the equation of Vali (50). The ice nucleation activity was normalized for the cell density, which was determined by dilution plating onto a KB agar plate amended with Rif. Measurements of gene expression in plant-associated bacteria were determined immediately following bacterial recovery from leaf surfaces by sonication (22).
Expression of plasmid-encoded proteins.
A Prokaryotic DNA-Directed Translation kit (Amersham Co.) was used for the identification of peptide products from the methionine biosynthetic locus. Plasmids pT7-5 and pT7-6 (provided by Stan Tabor) containing a ColE1 origin of replication, an ampicillin resistance gene, a polylinker region, and the phage T7 promoter φ10 were used to express proteins encoded by the P. syringae methionine biosynthetic locus. In plasmid pT7-6, the polylinker region is oriented in the opposite direction of that in pT7-5. Plasmid-encoded polypeptides were radiolabeled with l-[35S]methionine (Amersham Co.) and E. coli S-30 extract for 30 min at 37°C. When required, 50 U of T7 RNA polymerase (New England Biolabs) was added to the reaction mixture to drive transcription from the T7 promoter φ10. The reaction products were visualized by autoradiography after separation on a 12 or 15% sodium dodecyl sulfate-polyacrylamide gel.
RESULTS
Isolation and characterization of methionine auxotrophs.
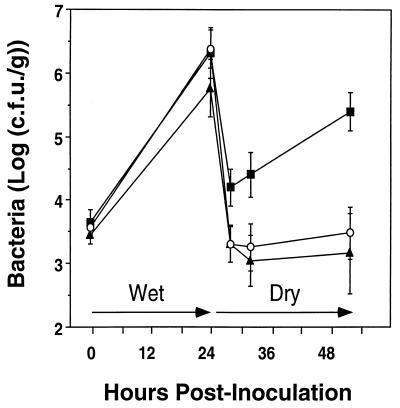
To confirm that the transposon insertion in P. syringae mutant 42 was causal to its reduced epiphytic fitness and methionine auxotrophy, the Tn5-containing region of mutant 42 was introduced into the genome of the parental strain B728a by transplacement (21) to form the marker-exchange mutant MX7. Insertion of Tn5 into the expected region was confirmed by Southern hybridization. Growth and survival of strain MX7 in conducive and stressful environments on bean leaves were nearly identical to those of mutant 42 (Fig. 2). In addition to a similar growth rate in KB and methionine-supplemented minimal medium, MX7 was similar to mutant 42 in its ability to grow on M9 medium supplemented with either l-methionine, cystathionine, or d,l-homocysteine and in its inability to grow on M9 medium supplemented with d,l-homoserine, O-succinylhomoserine, or l-cysteine (50 to 100 μg/ml each).
FIG. 2.
Population sizes of P. syringae pv. syringae B728a (■) and Tn5 mutant derivatives mutant 42 (▴) and MX7 (○) on bean plants exposed to wet and dry conditions. Each point represents the mean ± standard error of the mean of 20 leaf samples.
In a screen of B728a mutants constructed by random Tn5 insertion, nine auxotrophs were identified that exhibited growth on methionine. Based on the sizes of the EcoRI- and KpnI-digested genomic fragments that hybridized to a labeled Tn5 probe, these nine auxotrophs represented at least three distinct classes of mutants. The three class I mutants, which showed hybridization patterns identical to that of MX7, and the three class II mutants grew on the same pathway intermediates as MX7, while the three class III mutants grew on cysteine as well as on these intermediates. Thus, the class I and class II mutants represent at least two distinct loci required for methionine biosynthesis in P. syringae, whereas the class III mutants are probably cysteine auxotrophs that can grow on methionine due to a reverse transsulfuration pathway in which methionine is converted to cysteine (19). All of these mutants exhibited reductions in epiphytic fitness that were similar to the fitness reductions in mutants 42 and MX7 (data not shown). These results indicate that the reduced fitness of the methionine auxotrophs did not result from the loss of a secondary function of any single methionine biosynthetic locus, but rather from a direct requirement for methionine prototrophy for optimum epiphytic fitness.
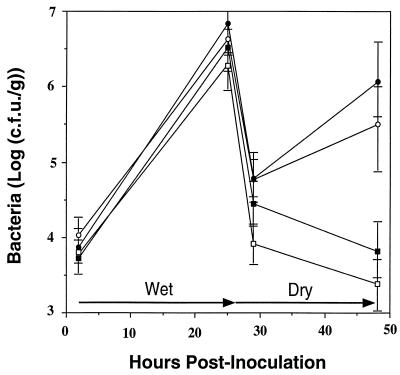
To determine if a methionine limitation contributed to the reduced fitness of the methionine auxotrophs, 30 mM l-methionine was applied to plants 4 h after inoculation with either B728a or MX7 (Fig. 3). Both B728a and MX7 established moderately larger population sizes in the presence of exogenous methionine than in its absence during the 24-h moist incubation period. However, the proportion of the population of B728a or MX7 that survived exposure of leaves to stressful conditions was not altered by the presence of methionine, and MX7 was not improved in its ability to grow on dry leaves in the presence of methionine.
FIG. 3.
Influence of methionine on the growth and survival of P. syringae pv. syringae B728a and MX7 on bean plants exposed to wet and dry conditions. Within 4 h of inoculation, 30 mM l-methionine or sterile H2O was sprayed onto plants. Symbols: B728a with H2O (○), B728a with methionine (•), MX7 with H2O (□), and MX7 with methionine (■).
Cloning and localization of a P. syringae methionine biosynthetic locus.
A plasmid that contained the sequence flanking the Tn5 insertion in MX7 was used to probe a genomic library of P. syringae B728a. Three hybridizing cosmid clones, which were found to contain 6.5 kb of common sequence, were able to restore methionine prototrophy to MX7. A 6.9-kb HindIII fragment from the complementing cosmid pCM2 was subcloned into the broad-host-range vector pVSP61, and the resulting plasmid, designated pVGA7, also restored methionine prototrophy to MX7.
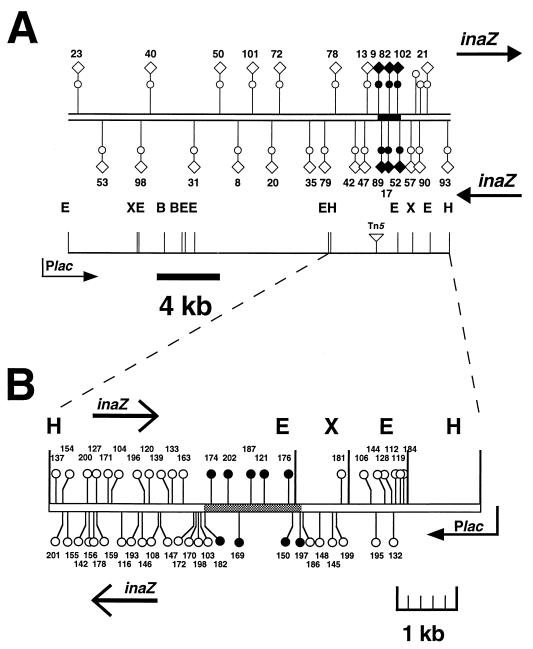
Saturation mutagenesis with the transposon Tn3-Spice was performed on both pCM2, to determine if it contained linked genes required for methionine prototrophy, and pVGA7, to localize the methionine locus interrupted in MX7. The locations and orientations of representative Tn3-Spice insertions are depicted in Fig. 4. The 48 insertions that mapped to the 6.9-kb insert in pVGA7 and the 25 insertions that mapped to the 22-kb insert in pCM2 did not display any strong site or orientation bias. The insertion-containing plasmids were introduced into MX7, and the resulting transconjugants were examined for their growth in the absence of methionine. A total of 15 insertions were identified that prevented restoration of methionine prototrophy by pCM2 and pVGA7. These insertions mapped to a contiguous 1.8-kb region common to both plasmids (Fig. 4). Tn3-Spice insertions spanning 22 kb of pCM2 were incorporated into the chromosome of wild-type strain B728a by marker-exchange mutagenesis; the six insertions that were located in the 1.8-kb region created methionine auxotrophs while the 19 insertions outside of this region were prototrophic.
FIG. 4.
Location and orientation of Tn3-Spice insertions in pCM2 (A) and pVGA7 (B). Closed circles represent insertions that prevented restoration of methionine prototrophy after the plasmids were mobilized into MX7, while open circles represent those plasmid-borne insertions that had no effect on prototrophy. Closed diamonds represent insertions that prevented restoration of methionine prototrophy after introduction into the B728a chromosome by marker-exchange mutagenesis, while open diamonds represent those insertions that had no effect on prototrophy after chromosomal introduction. The location of the metXW locus is relative to selected Tn3-Spice insertion points in pVGA7 and comparison with the nucleotide sequence of this locus (shaded bar in panel B). Insertions in which the ice nucleation reporter gene inaZ is oriented from left to right are placed above the line and oppositely oriented insertions are placed below. The restriction sites of BamHI (B), EcoRI (E), HindIII (H), and XhoI (X) are indicated.
Hybridization of the P. syringae methionine biosynthetic locus to genomic DNA of various bacterial species.
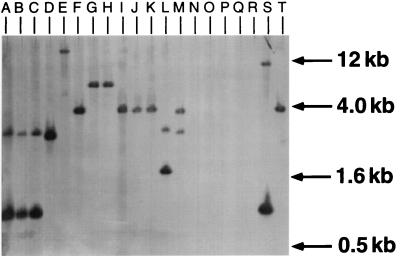
A 1.2-kb PvuII-PstI restriction fragment located entirely within the 1.8-kb methionine biosynthetic locus was used to probe genomic DNA from other bacterial strains. Southern hybridization of EcoRI-restricted genomic DNA revealed that all Pseudomonas strains, including various pathovars and subspecies of P. syringae as well as Pseudomonas viridiflava, Pseudomonas cichorii, and the closely related R. solanacearum, contained sequences homologous to the internal methionine probe (Fig. 5). Homology was also detected in P. aeruginosa, with hybridization of the probe to a 10-kb restriction fragment (data not shown). Pseudomonas syringae pv. syringae B728a, Pseudomonas syringae pv. aptata, Pseudomonas syringae pv. lachrymans, and Pseudomonas syringae pv. pisi exhibited hybridizing fragments that were similar in size (4.0 kb). None of the Xanthomonas, Pantoea agglomerans, or E. coli strains tested exhibited hybridization to the internal methionine fragment (Fig. 5).
FIG. 5.
Hybridization of a 1.2-kb PvuII-PstI restriction fragment internal to the P. syringae methionine biosynthesis locus to EcoRI-digested genomic DNA of the following. Lanes: A, P. syringae pv. phaseolicola (NPS3121); B, Pseudomonas syringae pv. glycinia; C, P. syringae pv. tabaci; D, Pseudomonas syringae subsp. savastanoi; E, R. solanacearum; F, P. syringae pv. syringae (B728a control); G, Pseudomonas syringae pv. tomato; H, Pseudomonas syringae pv. maculicola; I, P. syringae pv. lachrymans; J, P. syringae pv. pisi; K, P. syringae pv. aptata; L, P. viridiflava; M, P. cichorii; N, Xanthomonas campestris pv. vesicatoria; O, X. campestris pv. campestris; P, Xanthomonas campestris pv. phaseoli; Q, Pantoea agglomerans; R, E. coli; S, P. syringae pv. phaseolicola (4324); T, P. syringae pv. syringae (990).
Nucleotide sequence of the methionine biosynthetic locus.
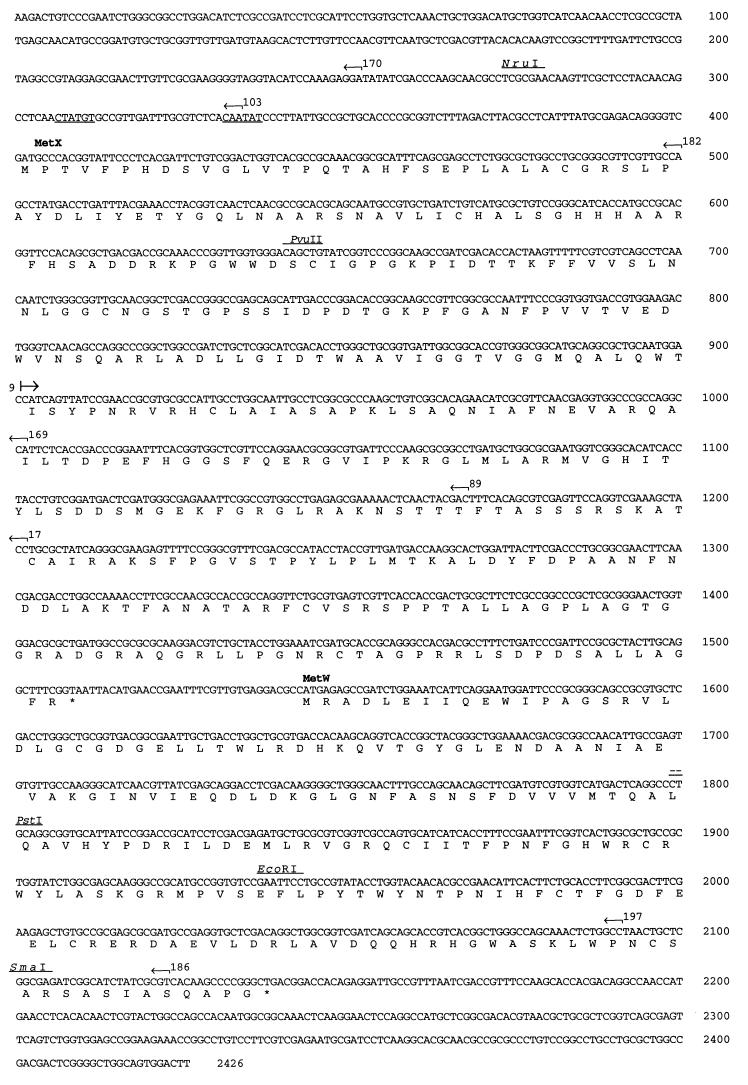
A 2,426-bp region of pVGA7, which contained the entire 1.8-kb region required for methionine prototrophy, was sequenced and was found to contain two potential ORFs (Fig. 6). metX (bp 402 to 1509) is predicted to encode a protein of 369 amino acids with a molecular mass of 39.3 kDa, while metW (bp 1544 to 2138) is located 35 bp downstream of metX and is predicted to encode a protein of 198 amino acid residues with a molecular mass of 22.5 kDa. A potential promoter sequence that weakly matches the P. aeruginosa ς70 consensus sequence, specifically −35 (YSTTGR) and −10 (YRTAAT) (41), is present upstream of metX (Fig. 6). Both ORFs exhibit the codon usage preference characteristic of P. aeruginosa throughout their respective reading frames and exhibit the nucleotide periodicity characteristic of P. aeruginosa over their entire lengths, indicating a high likelihood that they represent Pseudomonas protein-coding regions (data not shown).
FIG. 6.
Nucleotide and deduced amino acid sequences of a 2,426-bp region of pVGA7 containing a 1.8-kb region required for methionine prototrophy. The positions of two ORFs are labeled, and a potential ς70 promoter sequence is underlined upstream from metX at nucleotide positions 307 to 312 and 332 to 337. The insertion points and orientations of various Tn3-Spice insertions, as well as selected restriction sites, are indicated.
The insertion sites of nine Tn3-Spice mutants were sequenced to compare the borders of the methionine locus as determined by saturation transposon mutagenesis with the location of the two ORFs (Fig. 6). Of the insertions tested that prevented restoration of methionine prototrophy following plasmid introduction, five (SPC9, SPC17, SPC89, SPC169, and SPC182; Fig. 4) localized to metX and one (SPC197; Fig. 4) localized to metW. The three insertions that were sequenced that have no effect on methionine prototrophy (SPC103, SPC170, and SPC186; Fig. 4) were located outside of both ORFs.
T7 promoter-directed protein expression.
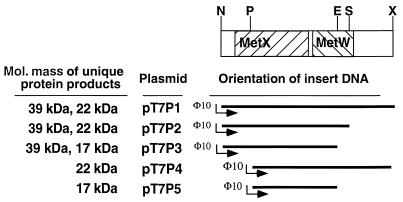
Protein expression from the putative metX and metW regions were examined by using T7 promoter fusions in an E. coli S30 transcription-translation system (Fig. 7). Fusions that included a complete metX sequence and the putative upstream ribosome binding site (plasmids pT7P1, pT7P2, and pT7P3) produced a protein of the predicted size, 39 kDa. When transcription was initiated at the PvuII restriction site that was downstream from the putative metX ribosome binding site (plasmids pT7P4 and pT7P5), a 39-kDa protein was not produced. Fusions that included a complete metW sequence (plasmids pT7P1 and pT7P4) produced a protein of the predicted size, 22 kDa. When the fusion included a MetW that was missing the terminal two codons (pT7P2), the protein product did not differ visibly from the nontruncated metW; however, when it was missing the terminal 67 codons due to truncation at the EcoRI restriction site (pT7P3 and pT7P5), a 17-kDa protein was produced.
FIG. 7.
Protein expression from metX and metW. An E. coli S30 transcription-translation system was used to express proteins from the T7 promoter fusions constructed with the fragments indicated by the restriction map shown at the top. The direction of transcription is from left to right, and radiolabeled protein products were visualized on a denaturing polyacrylamide gel. Restriction enzyme sites are as follows: N, NruI; P, PvuII; E, EcoRI; S, SmaI; X, XhoI.
Roles of metX and metW in methionine prototrophy and epiphytic fitness.
The Tn5 insertion in MX7 was localized to metX by restriction enzyme analysis. Since metX and metW may be in an operon, thus allowing an insertion in metX to exert a polar effect on metW, several constructs were designed to test for a separate role of each ORF in methionine prototrophy and epiphytic fitness (Table 2). The strain SPC102 contains a transposon insertion in metW in the B728a derivative designated LK2 (described below). The plasmid pVGA7Ω contains metX and metW and was constructed by introducing into pVGA7 an Ω cassette that encodes streptomycin and spectinomycin resistance (14a). The plasmid pVGA4Ω contains metX and a truncated metW and was constructed by introducing a 4-kb EcoRI fragment from pCM2 into pVSP61Ω. The latter is a pVSP61 derivative that contains the Ω cassette. The plasmid pVΩ7Δ contains a deletion in metX that allows for the transcription and translation of metW. The 0.62-kb in-frame SfiI-ClaI deletion in this plasmid was confirmed by sequence analysis. As shown in Table 2, the behavior of MX7 containing each of these plasmid constructs demonstrates clearly that both metX and metW are required for methionine prototrophy and for bacterial survival on dry leaves.
TABLE 2.
Roles of metX and metW in methionine prototrophy and epiphytic fitnessa
| Strain | MetX | MetW | Methionine prototrophy | Survival on dry leaves |
|---|---|---|---|---|
| B728a | + | + | + | + |
| MX7 | − | − | − | − |
| SPC102 | + | − | − | − |
| MX7(pVGA7) | + | + | + | + |
| MX7(pVGA4) | + | − | − | − |
| MX7(pVΩ7Δ) | − | + | − | − |
Strains were grown in minimal medium without methionine or on plants for 24 h in wet conditions followed by 24 h without free moisture (40% relative humidity, 28°C, 106 lux).
Sequence analysis.
The deduced amino acid sequences of MetX and MetW were compared with the sequences of known bacterial methionine biosynthetic genes, proteins, and translated nucleic acid sequences in the GenBank Release 103 and EMBL Release 51 databases. The deduced amino acid sequence of MetX exhibited a high degree of homology to several homoserine O-acetyltransferases. Homoserine O-acetyltransferase has been shown to be the first enzyme in the methionine biosynthetic pathway in several organisms (41). The optimal alignment of the MetX protein with several of these sequences is shown in Fig. 8. The greatest similarities were with the homoserine O-acetyltransferase of L. meyeri (54% identity over 224 residues), Haemophilus influenzae (47% identity over 233 residues) and Schizosaccharomyces pombe (40% identity over 227 residues). Other similarities were to the homoserine O-acetyltransferase of Saccharomyces carlsbergensis (MET2 gene product; 37% identity over 170 residues [23]), S. cerevisiae (MET2 gene product; 36% identity over 170 residues [2]), and A. immersus (met2 gene product; 39% identity over 69 residues [18]), as well as to the deacetylcephalosporin C O-acetyltransferase of Acremonium chrysogenum (cefG gene product; 35% identity over 223 residues [20].) In contrast to the strong homology that MetX showed to the above homoserine O-acetyltransferases, as evidenced by quality scores of greater than 250 in alignments with the University of Wisconsin’s Genetics Computer Group BESTFIT program, MetX showed no homology in alignments with the homoserine O-succinyltransferases encoded by E. coli and Salmonella typhimurium. The deduced amino acid sequence of MetW showed no significant homology to any known protein. However, strong homology (59 identical and 34 conserved residues of 139 total) was observed with an uncharacterized and incomplete ORF adjacent to the metYX operon of L. meyeri. This ORF is oriented immediately upstream from the metYX operon (involved in the first two steps of methionine biosynthesis) and transcribed in the opposite orientation (6).
FIG. 8.
Optimal alignment (PILEUP) of the deduced amino acid sequence of MetX from P. syringae (P. syri) to the homoserine O-acetyltransferases of L. meyeri (L. meye) (accession no. Y10744), H. influenzae (H. infl) (accession no. L42023), and Schizosaccharomyces pombe (S. pomb) (accession no. Z69909). Residues identical in three or all of the sequences are shown in blackened areas.
Transcriptional analysis of the P. syringae methionine biosynthetic locus.
Transcriptional analysis of the methionine biosynthetic locus was performed with the promoterless ice nucleation gene inaZ contained in Tn3-Spice as a reporter of transcription (29). To eliminate background ice nucleation activity, an inaB (ice nucleation gene) deletion mutant of B728a, designated LK2, was constructed. Transcriptional activity was examined in two types of constructs: merodiploid strains of LK2 containing Tn3-Spice insertions in the cosmid pCM2 and LK2 marker-exchange derivatives containing chromosomal fusions. The ice nucleation activities exhibited by LK2 prototrophic merodiploids containing Tn3-Spice insertions within metX (pSPC9 and pSPC13; Fig. 4) or metW (pSPC102; Fig. 4) were very similar in a rich medium or M9 minimal salts medium after 24 h with or without 0.3 mM l-methionine, indicating that methionine did not influence transcription of either gene. Similarly, none of the genomic Tn3-Spice fusions of LK2 appeared to be regulated by methionine or induced on bean leaves (Table 3). Regulation of metX and metW transcription appeared to be constitutive in culture, i.e., they were not influenced by growth on moist bean leaves, growth on moist leaves followed by drying of the leaf surface, nutrient status or pH of the growth medium, heat shock, or the presence of ς54, an alternative sigma factor present in P. syringae (data not shown). Tn3-spice insertions oriented in the direction of transcription of metX (strains SPC9 and SPC82) and metW (strain SPC102) expressed a moderate level of transcription compared with levels reported for other P. syringae genes (29). By contrast, Tn3-spice insertions oriented opposite the direction of transcription of metX (strain SPC17) and metW (strain SPC52) expressed at least a 500-fold-lower ice nucleation activity.
TABLE 3.
Ice nucleation activity of chromosomal Tn3-Spice fusions in P. syringae LK2
| Straina | Log ice nuclei/cell
|
|||
|---|---|---|---|---|
| KBb | M9 plus methioninec | Wet bean leavesd | Dry bean leavese | |
| LK2 | NDf | ND | <−5 | <−5 |
| SPC9 | −4.47 Kg (M)h | −4.09 K (M) | −3.55 J (M) | −3.80 JK (M) |
| SPC17 | −7.24 J (O) | −7.13 J (O) | <−5 | −4.76 J (O) |
| SPC52 | −7.18 J (P) | −7.04 J (P) | —i | — |
| SPC82 | −4.30 K (S) | −3.96 K (S) | −4.13 J (S) | −3.63 JK (S) |
| SPC102 | −4.53 K (T) | −4.11 K (T) | — | — |
The SPC strains are LK2 marker-exchange derivatives in which the number corresponds to the insertion designation shown in Fig. 2.
Grown for 24 h on KB agar plates.
Grown for 24 h on M9 minimal salts medium amended with 0.3 mM l-methionine.
Measured 24 h postinoculation on leaves maintained under wet conditions.
Measured 29 h postinoculation after inoculation for 24 h under wet conditions; leaves were allowed to dry for an additional 5 h.
ND, not detectable under the conditions of the experiment.
Values with different letters were significantly different (P < 0.05) within each column.
Values with different letters in parentheses were significantly different (P < 0.05) across each row.
—, not done.
DISCUSSION
The first unique step in methionine biosynthesis in all organisms is the acylation of homoserine (Fig. 1). This step is catalyzed by homoserine O-succinyltransferase, such as is encoded by metA in E. coli, or by homoserine O-acetyltransferase, such as is encoded by MET2 in S. cerevisiae (42). We have identified two genes in P. syringae, designated metX and metW, that are required for methionine biosynthesis and have examined their role in the biosynthetic pathway. The predicted amino acid sequence of MetX shows strong homology to several homoserine O-acetyltransferases, including those of L. meyeri, H. influenzae, and at least four fungal species but shows no significant homology to the MetA proteins of E. coli or S. typhimurium. This homology to O-acetyltransferases and lack of homology to O-succinyltransferases strongly suggests that O-acetylhomoserine is the first intermediate in the methionine biosynthetic pathway in P. syringae. In contrast, Foglino et al. (15) concluded that O-succinylhomoserine is the first intermediate in the methionine biosynthetic pathway in P. aeruginosa based on their finding that metA from E. coli complemented a P. aeruginosa methionine auxotroph and that homocysteine was detected in a cell extract after addition of O-succinylhomoserine but not after addition of O-acetylhomoserine. These results suggest that two organisms as closely related as P. syringae and P. aeruginosa, which are in the same rRNA homology group but distinct DNA homology groups (39), may synthesize methionine via distinctive pathways. Alternatively, sequence homology among these transferase enzymes may poorly indicate substrate utilization; however, the strong homology among several known homoserine O-acetyltransferases and their poor relatedness to several known homoserine O-succinyltransferases does not favor this possibility.
Auxotrophs with an inactivated metX or metW gene exhibited growth on cystathionine, indicating that these genes are involved in either of the first two steps of the pathway. As described above, homology studies indicated that metX encodes the first enzyme in the pathway, specifically the conversion of homoserine to an acylated homoserine derivative. Although the conversion of this acylated homoserine derivative to cystathionine would be an obvious function to predict for MetW, the biochemical and genetic evidence is equivocal. Lack of growth on an acylated homoserine derivative, as was observed with O-succinylhomoserine, may not indicate an inability of the compound to support growth, since acylated homoserine derivatives are often not transported into cells (15). Similarly, although MetW does not appear to be similar to any known protein, including all sequenced cystathionine synthase genes, this lack of homology may simply result from a lack of similarity between functionally analogous proteins. A striking homology was observed between metW and an unidentified upstream ORF with divergent transcription from the metY-metX operon of L. meyeri (6). Interestingly, this operon in L. meyeri is responsible for both the acylation of homoserine and the conversion of the acylated homoserine derivative to cystathionine.
Our sequence and mutational analysis of the metXW locus strongly suggest that the pathway for methionine biosynthesis in P. syringae most closely resembles the methionine biosynthetic pathway shown for the fungus Neurospora crassa (Fig. 1B). First, the acylated homoserine intermediate in P. syringae appears to be O-acetylhomoserine, based on the MetX homology comparisons, rather than O-succinylhomoserine as in E. coli (41) and P. aeruginosa (15). Second, the growth of the P. syringae methionine auxotrophs on cystathionine provides evidence that P. syringae synthesizes methionine through a transsulfuration pathway, although it does not dismiss the possibility that P. syringae can also utilize a direct sulfhydrylation pathway. While methionine biosynthesis in E. coli occurs only through transsulfuration, P. aeruginosa (15) as well as most fungi, including S. cerevisiae, N. crassa, and Aspergillus nidulans (41), are capable of synthesizing methionine through either transsulfuration or direct sulfhydrylation. In these organisms, one pathway tends to be strongly favored. Interestingly, there appear to be differences among P. aeruginosa strains, since methionine mutants of P. aeruginosa 1 grew on cystathionine (7) while metA or metZ mutants of P. aeruginosa PAO1 did not (15). Third, similar to L. meyeri metYX, transcription of P. syringae metXW was not repressed by methionine. The absence of such methionine repression has also been reported for the fungus A. immersus (18). Last, the genetic evidence indicates that the P. syringae metXW locus has homologs in some, but not all, gram-negative bacteria. Specifically, in hybridization studies, we found that the P. syringae metXW locus hybridized to genomic DNA from all P. syringae pathovars and all Pseudomonas species examined, but not to that of the enteric organisms E. coli and Pantoea agglomerans or to three species of the nonenteric genus Xanthomonas.
The results of this study and previous studies (3, 4) demonstrate that methionine biosynthesis plays a critical role in bacterial fitness on leaf surfaces. Previous studies examined the epiphytic behavior of an auxotrophic marker-exchange mutant designated MX7, which was derived from the fitness-reduced methionine auxotroph mutant 42 (33). MX7 exhibited abundant growth on moist leaves, indicating that leaves could provide sufficient methionine to support bacterial growth under at least some conditions. In contrast, MX7 exhibited poor survival following the drying of the leaf surfaces (3) and following spray application onto plants under warm, dry field conditions (4), demonstrating that methionine biosynthesis is required for optimum fitness on dry or drying leaf surfaces. In this study, we found that MX7 carries a transposon insertion in metX, and since metW is downstream of metX and is probably cotranscribed, MX7 is likely deficient in the expression of both metX and metW. The reduced fitness of MX7(pVΩ7Δ), a metX mutant that contains an active metW, and SPC102, a metW mutant that contains an active metX, demonstrates that metX and metW are each required for optimum epiphytic fitness. The reduced fitness of six other independently isolated methionine auxotrophs of P. syringae demonstrates that methionine prototrophy, rather than alternative functions of MetX and MetW, directly contributes to epiphytic fitness.
Methionine prototrophy, unlike other amino acid prototrophies, appears to be specifically required for bacterial fitness under stressful environmental conditions. Of approximately 54 uncharacterized auxotrophs screened for reduced epiphytic fitness (33), only three, one each for methionine, tryptophan, and isoleucine-valine, were sufficiently reduced in fitness to be identified as epiphytic fitness mutants. The reduced fitness of the tryptophan auxotroph (3), as well as other tryptophan auxotrophs that have been identified (data not shown), probably resulted from a tryptophan limitation on both wet and dry leaf surfaces, since tryptophan has been found to be among the least abundant amino acids in leaf exudates from almost all plant species examined, including Phaseolus vulgaris (37, 51). We have found that several other amino acid auxotrophs, including histidine and leucine auxotrophs, exhibited wild-type growth and survival on both wet and dry leaf surfaces (data not shown). These studies demonstrate that methionine prototrophy is unique in playing a role in the fitness of epiphytic bacteria.
In exploring the nature of this role, we must consider several possible mechanisms by which an inability to synthesize methionine could result in reduced fitness on leaves under stressful environmental conditions. First, it is possible that low methionine concentrations on leaves could restrict bacterial growth. Application of exogenous methionine to leaves resulted in very small increases in the population sizes of both MX7 and the wild-type strain B728a under moist conditions, demonstrating a possible methionine limitation for growth, but such application did not improve bacterial survival under environmentally stressful conditions. We have previously proposed the possibility that the parental strain responds to the drying of the leaf surface by initiating high levels of protein synthesis that mediate survival. In such a case, small intracellular pool sizes of methionine could restrict the ability of a methionine auxotroph to synthesize proteins either fast enough or in high enough quantities to mediate survival, and application of exogenous methionine may not influence these intracellular pool sizes rapidly enough to cause a detectable difference.
A second possibility regarding the role of methionine prototrophy in epiphytic fitness is that it contributes directly to the ability of the bacteria to tolerate chemical and/or physical stresses in their microenvironment. For example, Gläser et al. (17) discovered a relationship between methionine biosynthesis and osmotolerance in S. cerevisiae. Specifically, they identified a gene, designated HAL2, whose overexpression resulted in improved growth under salt stress; they subsequently found that it was identical to MET22, a gene involved in methionine biosynthesis. Further studies showed that methionine supplementation improved the tolerance of wild-type yeast to high concentrations of NaCl and LiCl. Interestingly, we have found a potential relationship between osmotolerance and epiphytic fitness. We showed in a previous study (33) that 14 of 82 epiphytic fitness mutants of P. syringae exhibited reduced osmotolerance. Thus, methionine prototrophy may contribute to optimum epiphytic fitness by contributing to bacterial tolerance to high salt concentrations, such as may be present in the nutrient-laden water film on a leaf surface.
In summary, we isolated and sequenced two P. syringae loci, designated metX and metW, and examined their roles in methionine biosynthesis and in bacterial fitness on leaf surfaces. Evidence based on sequence homology, as well as on biochemical and genetic regulation studies, suggest that the pathway for methionine biosynthesis in P. syringae most closely resembles the methionine biosynthetic pathway in the fungus N. crassa and is distinct from P. aeruginosa. That is, an acylhomoserine intermediate is formed and assimilation of sulfur is by transsulfuration. We also presented evidence that methionine prototrophy is required for optimum epiphytic fitness, and that this requirement is particularly pronounced under environmentally stressful conditions, suggesting a role for methionine prototrophy in bacterial stress tolerance.
ACKNOWLEDGMENTS
We thank Gabriella Cirvilleri for the generous gift of the pLK2 plasmid. We thank Andrew Jackson for thoughtful counsel and expert advice. We thank John Wagner and Karen Scholthoff for helpful discussions. We thank Mavis Hendson for her generosity in sharing her extensive culture collection. We also thank Remy Fèllay and Reid Frederick for advice on Tn3-Spice transcriptional assays.
REFERENCES
- 1.Anderson D M, Mills D. The use of transposon mutagenesis in the isolation of nutritional and virulent mutants in two pathovars of Pseudomonas syringae. Phytopathology. 1985;75:104–108. [Google Scholar]
- 2.Baroni M, Livian S, Martegani E, Alberghina L. Molecular cloning and regulation of the expression of the MET2 gene of Saccharomyces cerevisiae. Gene. 1986;46:71–78. doi: 10.1016/0378-1119(86)90168-x. [DOI] [PubMed] [Google Scholar]
- 3.Beattie G A, Lindow S E. Survival, growth, and localization of epiphytic fitness mutants of Pseudomonas syringae on leaves. Appl Environ Microbiol. 1994;60:3790–3798. doi: 10.1128/aem.60.10.3790-3798.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Beattie G A, Lindow S E. Comparison of the behavior of epiphytic fitness mutants of Pseudomonas syringae under controlled and field conditions. Appl Environ Microbiol. 1994;60:3799–3808. doi: 10.1128/aem.60.10.3799-3808.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender C L, Cooksey D A. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J Bacteriology. 1986;165:534–541. doi: 10.1128/jb.165.2.534-541.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bourhy P, Martel A, Margarita D, Saint Girons I, Belfaiza J. Homoserine O-acetyltransferase, involved in the Leptospira meyeri methionine biosynthetic pathway, is not feedback inhibited. J Bacteriol. 1997;179:4396–4398. doi: 10.1128/jb.179.13.4396-4398.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calhoun D H, Feary T W. Transductional analysis of Pseudomonas aeruginosa methionineless auxotrophs. J Bacteriol. 1969;97:210–216. doi: 10.1128/jb.97.1.210-216.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cami B, Clepet C, Patte J C. Evolutionary comparisons of three enzymes of the threonine biosynthetic pathway among several microbial species. Biochimie. 1993;75:487–495. doi: 10.1016/0300-9084(93)90115-9. [DOI] [PubMed] [Google Scholar]
- 9.Coplin D L, Sequeira L, Hanson R S. Pseudomonas solanacearum: virulence of biochemical mutants. Can J Microbiol. 1974;20:519–529. doi: 10.1139/m74-080. [DOI] [PubMed] [Google Scholar]
- 10.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics: a manual for genetic engineering. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 11.Ditta G W, Stanfield S, Corbin D, Helinski D R. Broad host range DNA cloning system for gram-negative bacteria: construction of a gene bank of Rhizobium meliloti. Proc Natl Acad Sci USA. 1980;27:7347–7351. doi: 10.1073/pnas.77.12.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ercolani G L. Bacterial canker of tomato. III. The effect of auxotrophic mutation on the virulence of Corynebacterium michiganense (E.F. Sm.) Jens. Phytopathol Mediterr. 1970;9:145–150. [Google Scholar]
- 13.Ewbank E, Maraite H. Conversion of methionine to phytotoxic 3-methylthiopropionic acid by Xanthomonas campestris pv. manihotis. J Gen Microbiol. 1990;136:1185–1189. [Google Scholar]
- 14.Fawcett T W, Bartlett S G. An effective method for eliminating “artifact banding” when sequencing double-stranded DNA templates. BioTechniques. 1990;9:46–48. [PubMed] [Google Scholar]
- 14a.Fèllay, R. Unpublished data.
- 15.Foglino M, Borne F, Bally M, Ball G, Patte J C. A direct sulfhydrylation pathway is used for methionine biosynthesis in Pseudomonas aeruginosa. Microbiology. 1985;141:431–439. doi: 10.1099/13500872-141-2-431. [DOI] [PubMed] [Google Scholar]
- 16.Garber E D. Further observations on biochemical mutants of Pseudomonas tabaci. Bot Gaz. 1959;120:157–161. [Google Scholar]
- 17.Gläser H U, Thomas D, Gaxiola R, Montrichard F, Surdin-Kerjan Y, Serrano R. Salt tolerance and methionine biosynthesis in Saccharomyces cerevisiae involve a putative phosphatase gene. EMBO J. 1993;12:3105–3110. doi: 10.1002/j.1460-2075.1993.tb05979.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Goyon C, Faugeron G, Rossignol J. Molecular cloning and characterization of the met2 gene from Ascobolus immersus. Gene. 1988;63:297–308. doi: 10.1016/0378-1119(88)90533-1. [DOI] [PubMed] [Google Scholar]
- 19.Günther E, Petruschka L, Herrmann H. Reverse transsulfuration pathway in Pseudomonas aeruginosa. Z Allg Mikrobiol. 1979;19:439–442. doi: 10.1002/jobm.3630190610. [DOI] [PubMed] [Google Scholar]
- 20.Gutiérrez S, Velasco J, Fernandez F J, Martin J F. The cefG gene of Cephalosporium acremonium is linked to the cefEF gene and encodes a deacetylcephalosporin C acetyltransferase closely related to homoserine O-acetyltransferase. J Bacteriol. 1992;174:3056–3064. doi: 10.1128/jb.174.9.3056-3064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gutterson N I, Layton T J, Ziegle J S, Warren G J. Molecular cloning of genetic determinants for inhibition of fungal growth by a fluorescent pseudomonad. J Bacteriol. 1986;169:696–703. doi: 10.1128/jb.165.3.696-703.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Haefele D M, Webb R. The use of ultrasound to facilitate the harvesting and quantification of epiphytic and phytopathogenic microorganisms. Phytopathology. 1982;72:947. [Google Scholar]
- 23.Hansen J, Kielland-Brandt M C. Saccharomyces carlsbergensis contains two functional MET2 alleles similar to homologues from S. cerevisiae and S. monacensis. Gene. 1994;140:33–40. doi: 10.1016/0378-1119(94)90727-7. [DOI] [PubMed] [Google Scholar]
- 24.Hendson M, Hildebrand D C, Schroth M N. Distribution among pseudomonads of sequences homologous to the rutin glycosidase and β-glucosidase genes of Pseudomonas viridiflava. Phytopathology. 1992;82:1230–1233. [Google Scholar]
- 25.Ish-Horowicz D, Burke J F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981;9:2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs S E, Habish H A, Dadd A H. Studies on induced mutants of Corynebacterium fascians and on their pathogenicity in comparison with that of “natural” strains. Ann Appl Biol. 1965;56:161–170. doi: 10.1111/j.1744-7348.1965.tb01225.x. [DOI] [PubMed] [Google Scholar]
- 27.Kerr D S, Flavin M. The regulation of methionine biosynthesis and the nature of cystathionine gamma-synthase in Neurospora. J Biol Chem. 1970;245:1842–1855. [PubMed] [Google Scholar]
- 28.King E O, Wood M K, Raney D E. Two simple media for the demonstration of pyocyanin and fluorescein. J Lab Clin Med. 1954;44:301–307. [PubMed] [Google Scholar]
- 29.Lindgren P B, Frederick R, Govindarajan A G, Panopoulos N J, Staskawicz B J, Lindow S E. An ice nucleation reporter gene system: identification of inducible pathogenicity genes in Pseudomonas syringae pv. phaseolicola. EMBO J. 1989;8:2990–3001. doi: 10.1002/j.1460-2075.1989.tb03508.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindgren P B, Peet R C, Panopoulos N J. Gene cluster of Pseudomonas syringae pv. phaseolicola controls pathogenicity on bean plants and hypersensitivity on nonhost plants. J Bacteriol. 1986;168:512–522. doi: 10.1128/jb.168.2.512-522.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lindow S E. Novel method for identifying bacterial mutants with reduced epiphytic fitness. Appl Environ Microbiol. 1993;59:1586–1592. doi: 10.1128/aem.59.5.1586-1592.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lindow S E. Bacterial ice nucleation measurements. In: Klement Z, Rudolf K, Sands D, editors. Methods in phytobacteriology. Budapest, Hungary: Akademia Kiado; 1990. pp. 428–434. [Google Scholar]
- 33.Lindow S E, Andersen G L, Beattie G A. Characteristics of insertional mutants of Pseudomonas syringae with reduced epiphytic fitness. Appl Environ Microbiol. 1993;59:1593–1601. doi: 10.1128/aem.59.5.1593-1601.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lippincott J A, Webb J H, Lippincott B B. Auxotrophic mutation and infectivity of Agrobacterium tumefaciens. J Bacteriol. 1965;90:1155–1156. doi: 10.1128/jb.90.4.1155-1156.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loper J E, Lindow S E. Lack of evidence for in situ fluorescent pigment production by Pseudomonas syringae pv. syringae on bean leaf surfaces. Phytopathology. 1987;77:1449–1454. [Google Scholar]
- 36.Michaeli S, Mevarech M, Ron E Z. Regulatory region of the metA gene of Escherichia coli K-12. J Bacteriol. 1984;160:1158–1162. doi: 10.1128/jb.160.3.1158-1162.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morgan J V, Tukey H B., Jr Characterization of leachate from plant foliage. Plant Physiol. 1964;39:590–593. doi: 10.1104/pp.39.4.590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Okazaki H, Shiio I. Methionine biosynthesis in Brevibacterium flavum: properties and essential role of O-acetylhomoserine sulfhydrylase. J Biochem. 1982;91:1163–1171. doi: 10.1093/oxfordjournals.jbchem.a133799. [DOI] [PubMed] [Google Scholar]
- 39.Palleroni N J. Family I. Pseudomonadaceae. In: Krieg N R, Holt J G, editors. Bergey’s manual of systematic bacteriology. Vol. 1. Baltimore, Md: Williams & Wilkins; 1988. pp. 141–213. [Google Scholar]
- 40.Ronald S, Farinha M A, Allan B J, Kropinski A M. Cloning and physical mapping of transcriptional regulatory (sigma) factors from Pseudomonas aeruginosa. In: Galli E, Silver S, Witholt B, editors. Pseudomonas: molecular biology and biotechnology. Washington, D.C: American Society for Microbiology; 1992. pp. 249–257. [Google Scholar]
- 41.Rowbury R J. Methionine biosynthesis and its regulation. In: Hermann K M, Somerville R L, editors. Amino acids: biosynthesis and genetic regulation. Reading, Mass: Addison-Wesley Publishing Co.; 1983. pp. 191–211. [Google Scholar]
- 42.Rowbury R J, Woods D D. O-succinylhomoserine as an intermediate in the synthesis of cystathionine by Escherichia coli. J Gen Microbiol. 1964;36:341–358. doi: 10.1099/00221287-36-3-341. [DOI] [PubMed] [Google Scholar]
- 43.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 44.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Savin M A, Flavin M. Cystathionine synthesis in yeast: an alternative pathway for homocysteine biosynthesis. J Bacteriol. 1972;112:299–303. doi: 10.1128/jb.112.1.299-303.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schulte R, Bonas U. A Xanthomonas pathogenicity locus is induced by sucrose and sulfur-containing amino acids. Plant Cell. 1992;4:79–86. doi: 10.1105/tpc.4.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Somylai G, Hevesi M, Bánfalvi Z, Klement Z, Kondorosi A. Isolation and characterization of non-pathogenic and reduced virulence mutants of Pseudomonas syringae pv. phaseolicola induced by Tn5 transposon insertions. Physiol Mol Plant Pathol. 1986;29:369–380. [Google Scholar]
- 48.Staskawicz B J, Dahlbeck D, Keen N, Napoli C. Molecular characterization of cloned avirulence genes from race 0 and race 1 of Pseudomonas syringae pv. glycinea. J Bacteriol. 1987;169:5789–5794. doi: 10.1128/jb.169.12.5789-5794.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Swanson B T, Wilkins H F, Kennedy B W. Factors affecting ethylene production by some plant pathogenic bacteria. Plant Soil. 1979;51:19–26. [Google Scholar]
- 50.Vali G. Quantitative evaluation of experimental results on the heterogeneous freezing nucleation of supercooled liquids. J Atmos Sci. 1971;28:402–406. [Google Scholar]
- 51.Weibull J, Ronquist F, Brishammar S. Free amino acid composition of leaf exudates and phloem sap: a comparative study in oats and barley. Plant Physiol. 1990;92:222–226. doi: 10.1104/pp.92.1.222. [DOI] [PMC free article] [PubMed] [Google Scholar]