Abstract
While data driven estimates of the global burden of disease for some vaccine preventable diseases (VPDs) are limited, aggregate case numbers of VPDs are reported annually by country in the Joint Reporting Form (JRF). We examined pertussis surveillance data in the JRF, and vaccine coverage estimates, in comparison to measles, which is a priority disease for elimination and eradication efforts and is supported by the WHO Global Measles and Rubella Laboratory Network. In 2012, highest pertussis case numbers and incidence were reported from high income countries with high vaccine coverage, discordant with countries that had low vaccine coverage. Use of laboratory diagnostics for pertussis cases varied among countries. In contrast, highest reported numbers of measles cases and incidences tended to occur in low income countries. These observations imply poor quality global surveillance data for some VPDs, limiting capacity for monitoring global epidemiology or making vaccination policy decisions. Efforts are needed to improve the availability of quality surveillance data for all VPDs.
Keywords: Surveillance, Pertussis, Measles, Vaccine preventable disease, Data
1. Introduction
Global or multi-national surveillance standards exist for numerous vaccine preventable diseases (VPDs) including polio, measles, yellow fever, and rotavirus, as well as some vaccine preventative causes of invasive bacterial disease, meningitis, and encephalitis [1–6]. While the geographic scope and sensitivity of these surveillance systems may differ, use of consistent case definitions, standardized core variables, laboratory networks, and performance indicators allow for evaluation of surveillance data quality. Based on these, it is possible to draw conclusions about the epidemiology of disease, which in turn can be used for program monitoring and making vaccine policy decisions.
By contrast, the availability of reliable surveillance data for some VPDs – notably, pertussis, diphtheria, and tetanus – is limited and lacks the surveillance and laboratory standardization of other priority diseases. From a global perspective, annual number of reported VPD cases are provided by member countries to the World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF) in the Joint Reporting Form (JRF). These numbers are meant to represent all cases, including clinically, epidemiologically and laboratory confirmed cases [7]. The JRF also requests reporting of the number of cases for which laboratory testing was performed and the number of cases which were laboratory confirmed.
Pertussis is highly transmissible and incidence rates continue to be high; transmission is presumed to occur in all regions of the world. Measles has received significant attention among international partners because of elimination and mortality reduction efforts [2]. In addition, measles surveillance is supported by the WHO Global Measles and Rubella Laboratory Network, which serves >180 countries and follows standardized laboratory procedures for confirming suspected cases [8]. To better understand the current surveillance data quality situation for pertussis, as a typical VPD, in comparison to measles, which has a well-established global surveillance system, we compared surveillance data for these two diseases from the same sources.
2. Methods
In the JRF, 3 fields are collected pertaining to each VPD: total cases (including clinically, epidemiologically, and laboratory confirmed), number of cases tested by laboratory investigation, and number of cases confirmed by laboratory investigation [7]. For both pertussis and measles, we examined completeness of reporting data for all countries and graphically evaluated incidence per country, with WHO/UNICEF estimates of coverage with three doses of diphtheria-tetanus-pertussis (DPT) vaccine (DTP3 coverage) (for pertussis) and the first dose of measles vaccine (MCV1 coverage) (for measles) [9] among <1 year olds for member states in 2012 (updated, as of July 13, 2013) [7], and World Bank Gross National Income per capita, Atlas method (GNI) [10]. Annual incidence was calculated as the number cases reported on the JRF divided by the WHO population estimate for the associated country [11]. Because of strong outlier data, we additionally examined in-detail countries with high case counts, incidence, and lowest vaccine coverage, for pertussis and measles. To account for potential annual fluctuations due to periodic outbreaks, we similarly examined incidence over a 5 year time period (2008–2012). Finally, to assess the consistency in the reporting of laboratory-based surveillance for pertussis, we evaluated completeness of pertussis data for number of cases tested by laboratory investigation, and number of cases confirmed by laboratory investigation, as well as the consistency of these numbers with total cases reported.
3. Results
Among 194 member states, 34 did not provide data on pertussis cases and an additional 52 reported zero pertussis cases during 2012. In contrast, only 11 countries did not provide measles surveillance data in 2012. Although 62 countries reported zero cases of measles, many of these are nations documented to have met their measles elimination targets.
The 2012 country-level incidence of pertussis was compared with DTP3 coverage and GNI (Fig. 1). Interestingly, many countries with high relative incidence of reported pertussis were countries with high DTP3 coverage. Similarly, many of the countries with high incidence had high GNI. When similar plots were examined for measles (Fig. 2), there was not a clear trend between reported measles incidence and vaccine coverage. However, in contrast to pertussis, high measles incidence occurred almost exclusively in countries with low GNI; this difference between pertussis and measles is particularly apparent among outlying (high) incidence countries on these graphs.
Fig. 1.
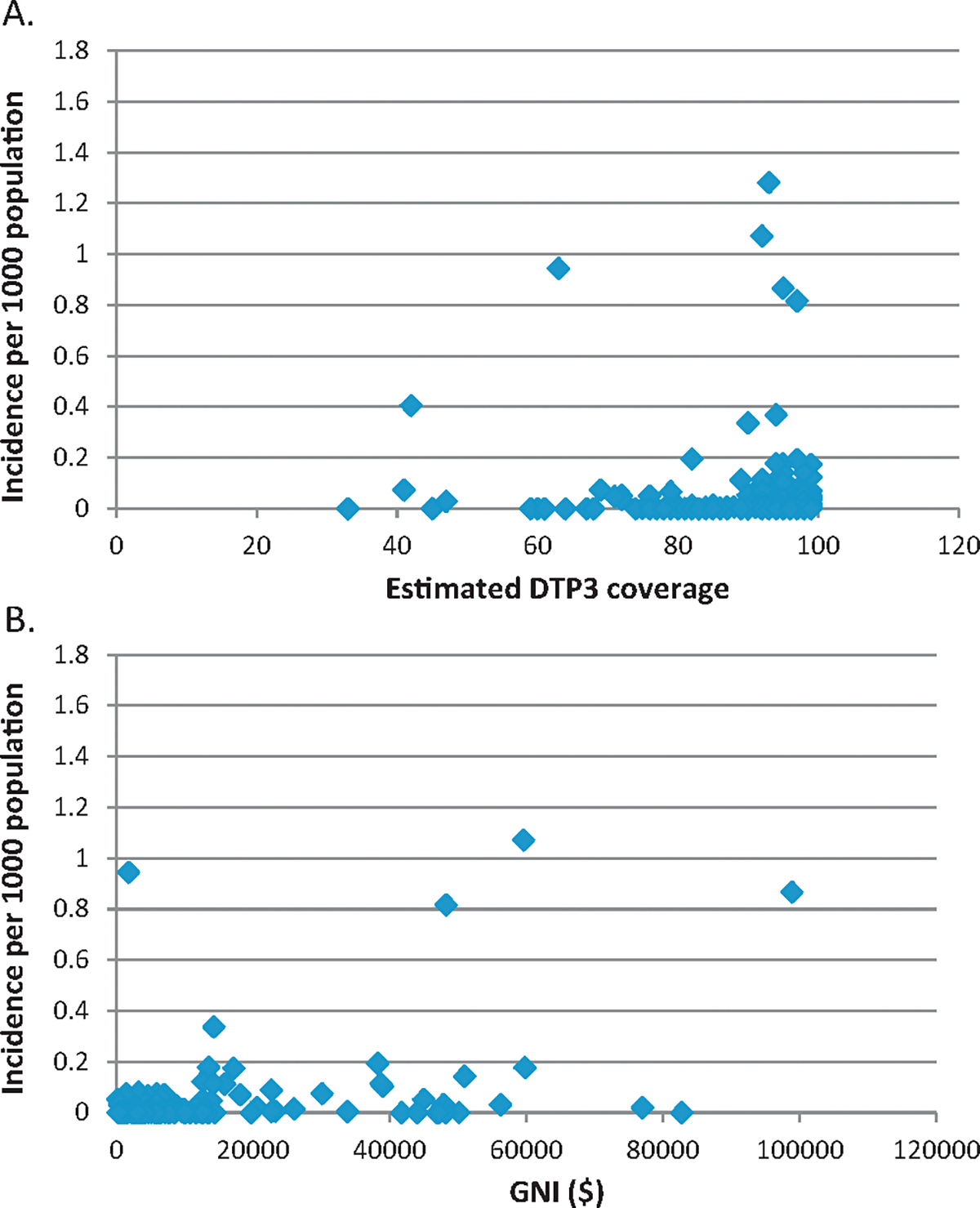
Country-level pertussis incidence per 1000 population, 2012, in comparison to estimated DTP3 coverage, 2012 (A) and GNI ($), 2012 (B).
Fig. 2.
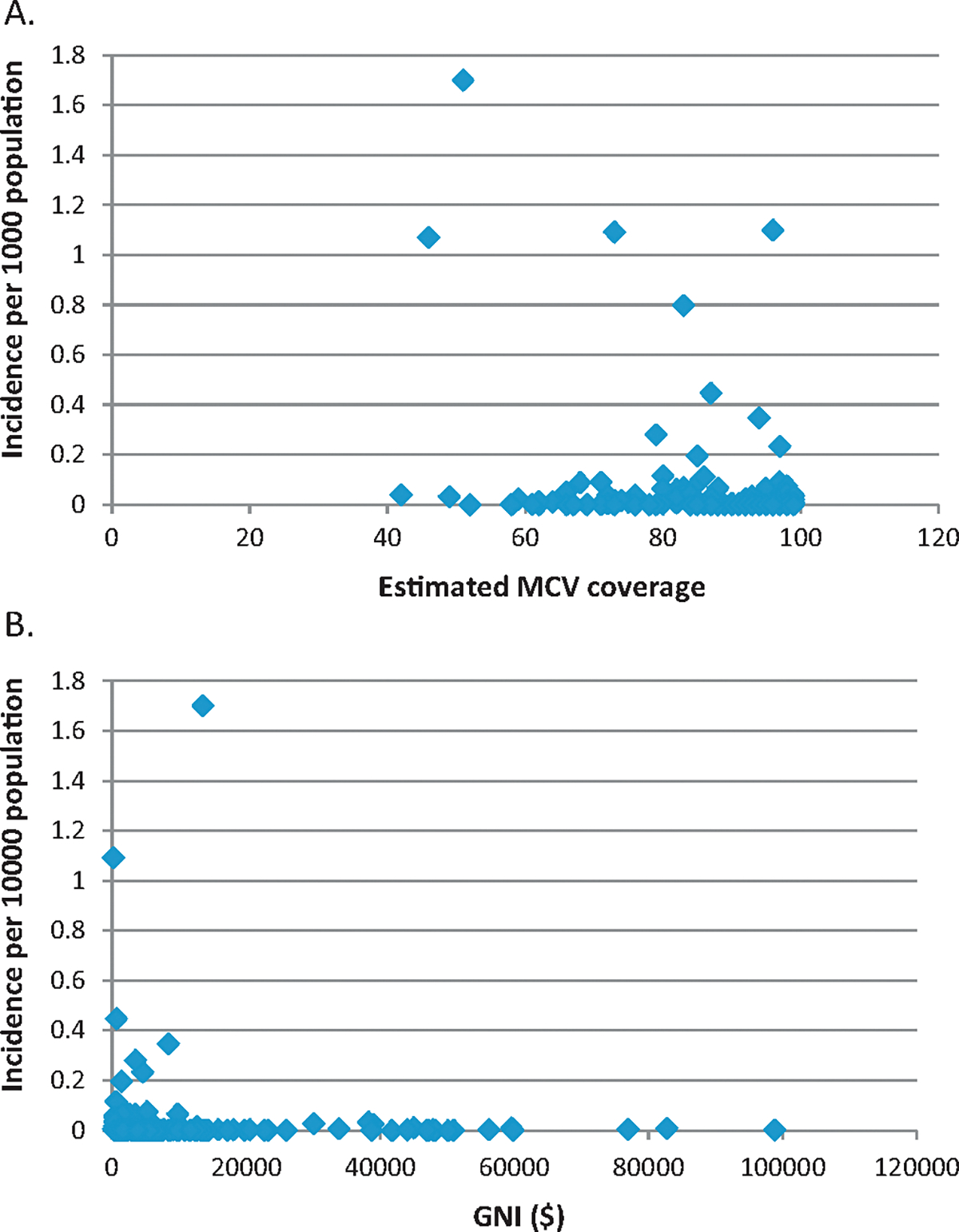
Country-level measles incidence per 1000 population, 2012, in comparison to estimated MCV coverage, 2012 (A) and GNI ($), 2012 (B).
Based on these observations, we further examined country-specific data for countries with highest case counts, incidence, and lowest vaccine coverage. For pertussis, highest reported case counts and incidence rates in 2012 tended to occur in high income countries (Tables 1a and 1b), as demonstrated by gross national income per capita (GNI) among the 10 counties with highest pertussis counts (median GNI = $22,450) and highest pertussis incidence (median GNI = $30,620). However, countries with the lowest estimated DTP3 coverage were more likely to be low income countries (Table 1c). For instance, median GNI among the 10 countries with lowest estimated DTP3 coverage was $740. Highest reported pertussis incidence from 2008 to 2012 commonly occurred in high income countries with high DTP3 coverage (median GNI of 10 countries with highest pertussis incidence = $28,310; median estimated DTP3 coverage = 94%, in the mid-point year of 2010) (Table 1d).
Table 1a.
Countries with the highest pertussis case counts, as reported on 2012 JRF.
| Country | Pertussis case count, 2012 JRF | Annual pertussis incidence, per 1000 populationa | Estimated DTP3 coverageb, 2012 | GNI, ($), 2012c |
|---|---|---|---|---|
|
| ||||
| India | 44,154 | 0.036 | 72 (85) | 1530 |
| Australia | 23,855 | 1.07 | 92 (92) | 59,570 |
| Netherlands | 13,552 | 0.82 | 97 (97) | 48,250 |
| United Kingdom of Great Britain and Northern Ireland | 11,980 | 0.19 | 97 (97) | 38,250 |
| Nigeria | 11,628 | 0.07 | 41 (57) | 1430 |
| Russian Federation | 7220 | 0.05 | 97 (97) | 12,700 |
| Papua New Guinea | 6472 | 0.94 | 63 (63) | 1790 |
| Chile | 5762 | 0.37 | 90 (90) | 14,280 |
| New Zealand | 5598 | 1.28 | 93 (93) | 30,620* |
| Canada | 4845 | 0.14 | 95 (95) | 50,970 |
Table 1b.
Countries with the highest annual incidence of pertussis, 2012.
| Country | Pertussis case count, 2012 JRF | Annual pertussis incidence, per 1000 populationa | Estimated DTP3 coverageb, 2012 | GNI, ($), 2012c |
|---|---|---|---|---|
|
| ||||
| New Zealand | 5598 | 1.28 | 93 (93) | 30,620* |
| Australia | 23,855 | 1.07 | 92 (92) | 59,570 |
| Papua New Guinea | 6472 | 0.94 | 63 (63) | 1790 |
| Norway | 4231 | 0.87 | 95 (95) | 98,860 |
| Netherlands | 13,552 | 0.82 | 97 (97) | 48,250 |
| Somalia | 3784 | 0.41 | 42 (61) | NA |
| Israel | 2730 | 0.37 | 94 (NA) | 28,930* |
| Chile | 5762 | 0.34 | 90 (90) | 14,280 |
| Yemen | 4699 | 0.20 | 82 (82) | 1110* |
| United Kingdom of Great Britain and Northern Ireland | 11,980 | 0.19 | 97 (97) | 38,250 |
Table 1c.
Countries with the lowest estimated DTP3 coverage, 2012.
| Country | Pertussis case count, 2012 JRF | Annual pertussis incidence, per 1000 populationa | Estimated DTP3 coverageb, 2012 | GNI, ($), 2012c |
|---|---|---|---|---|
|
| ||||
| Equatorial Guinea | 0 | 0 | 33 (41) | 13,560 |
| Nigeria | 11,628 | 0.07 | 41 (57) | 1430 |
| Somalia | 3784 | 0.41 | 42 (61) | NA |
| Syrian Arab Republic | 4 | <0.01 | 45 (64) | 2610† |
| Chad | Not reported | 45 (72) | 740 | |
| Central African Republic | 124 | 0.03 | 47 (59) | 490 |
| South Sudan | Not reported | 59 (68) | 650 | |
| Guinea | Not reported | 59 (99) | 460 | |
| Haiti | 0 | 0 | 60 (81) | 760 |
| Ethiopia | Not reported | 61 (83) | 410 | |
Table 1d.
Countries with the highest pertussis incidence, 2008–2012.
| Country | Total pertussis case count, 2008–2012 JRF | Annualized pertussis incidence, 2008–2012, per 1000 populationa | Estimated DTP3 coverageb, 2010 | GNI, ($), 2010c |
|---|---|---|---|---|
|
| ||||
| Australia | 140,160 | 1.26 | 92 (92) | 46,320 |
| Norway | 20,930 | 0.86 | 93 (93) | 86,850 |
| Papua New Guinea | 18,978 | 0.55 | 56 (70) | 1300 |
| Netherlands | 38,053 | 0.46 | 97 (97) | 48,580 |
| Estonia | 3036 | 0.45 | 94 (94) | 14,150 |
| New Zealand | 8708 | 0.40 | 93 (93) | 28,310 |
| Switzerland | 14,300 | 0.37 | 96 (96) | 73,350 |
| Israel | 9670 | 0.26 | 94 (NA) | 27,270 |
| Somalia | 8300 | 0.18 | 45 (64) | NA |
| Slovenia | 1695 | 0.17 | 96 (96) | 23,910 |
The bold value represents the column which was used to select the countries that are listed in the table.
Annual incidence is calculated as the number of pertussis cases reported on the 2012 JRF [7] divided by the WHO population estimate for the associated country [11] (In Table 1d, the 5 year total incidence was divided by 5, to represent average annual incidence).
UNICEF/WHO estimate of DTP3 coverage [9]. Numbers in parentheses represent country official figures on theJRF.
GNI = World Bank Gross National Income percapita, Atlas method [10] (*estimate from 2011, †estimate from 2010).
As with the DTP3 coverage rates, lowest MCV1 coverage rates tended to occur in lower income countries (median GNI among the 10 countries with lowest coverage = $760); however, in contrast with pertussis, the highest measles case counts and incidence rates were in less-developed countries (median GNI among 10 highest case count countries = $1450; median GNI among 10 highest incidence countries = $3500) (Tables 2a, 2b, and 2c), suggesting that functional measles surveillance systems are in place among low income countries. Similarly, over the 5 year time period of 2008–2012, highest reported measles incidence tended to occur among low income countries (median GNI of 10 countries with highest measles incidence = $1170) (Table 2d).
Table 2a.
Countries with the highest measles case counts, as reported on 2012 JRF.
| Country | Measles case count, 2012 JRF | Annual measles incidence, per 1000 populationa | Estimated 1st dose measles coverageb | GNI ($), 2012c |
|---|---|---|---|---|
|
| ||||
| Democratic Republic of the Congo | 72,029 | 1.09 | 73 (84) | 220 |
| India | 18,668 | 0.02 | 74 (85) | 1530 |
| Indonesia | 15,489 | 0.06 | 80 (84) | 3420 |
| Ukraine | 12,746 | 0.28 | 79 (79) | 3500 |
| Somalia | 9983 | 1.07 | 46 (49) | NA |
| Sudan (the) | 8523 | 0.20 | 85 (85) | 1450 |
| Pakistan | 8046 | 0.05 | 83 (89) | 1260 |
| Romania | 7450 | 0.35 | 94 (94) | 8420 |
| Burkina Faso | 7362 | 0.45 | 87 (87) | 670 |
| Nigeria | 6447 | 0.04 | 42 (78) | 1430 |
Table 2b.
Countries with the highest annual incidence of measles, 2012.
| Country | Measles case count, 2012 JRF | Annual measles incidence, per 1000 populationa | Estimated 1st dose measles coverageb | GNI ($), 2012c |
|---|---|---|---|---|
|
| ||||
| Equatorial Guinea | 1190 | 1.70 | 51 (34) | 13,560 |
| Nauru | 11 | 1.10 | 96 (96) | |
| Democratic Republic of the Congo | 72,029 | 1.09 | 73 (84) | 220 |
| Somalia | 9983 | 1.07 | 46 (49) | NA |
| Djibouti | 709 | 0.80 | 83 (83) | NA |
| Burkina Faso | 7362 | 0.45 | 87 (87) | 670 |
| Romania | 7450 | 0.35 | 94 (94) | 8420 |
| Ukraine | 12,746 | 0.28 | 79 (79) | 3500 |
| Angola | 4458 | 0.23 | 97 (97) | 4580 |
| Sudan (the) | 8523 | 0.20 | 85 (85) | 1450 |
Table 2c.
Countries with the lowest estimated 1st dose measles coverage, 2012.
| Country | Measles case count, 2012 JRF | Annual measles incidence, per 1000 populationa | Estimated 1st dose measles coverageb | GNI ($), 2012c |
|---|---|---|---|---|
|
| ||||
| Nigeria | 6447 | 0.04 | 42 (78) | 1430 |
| Somalia | 9983 | 1.07 | 46 (49) | NA |
| Central African Republic | 141 | 0.03 | 49 (65) | 490 |
| Equatorial Guinea | 1190 | 1.70 | 51 (34) | 13,560 |
| Vanuatu | 0 | 0 | 52 (94) | 3080 |
| Guinea | 6 | <0.01 | 58 (99) | 460 |
| Haiti | 0 | 0 | 58 (66) | 760 |
| Mali | 341 | 0.02 | 59 (85) | 660 |
| Syrian Arab Republic (the) | 13 | <0.01 | 61 (78) | 2610† |
| South Sudan | 1952 | 62 (70) | 650 | |
Table 2d.
Countries with the highest measles incidence, 2008–2012.
| Country | Total measles case count, 2008–2012 JRF | Annualized measles incidence, 2008–2012, per 1000 populationa | Estimated 1st dose measles coverageb, 2010 | GNI, ($), 2010c |
|---|---|---|---|---|
|
| ||||
| Malawi | 118,790 | 1.59 | 93 (93) | 340 |
| Burkina Faso | 65,246 | 0.79 | 92 (63) | 600 |
| Democratic Republic of the Congo | 223,756 | 0.68 | 74 (87) | 190 |
| Bulgaria | 24,412 | 0.65 | 97 (97) | 6320 |
| Namibia | 7379 | 0.65 | 75 (75) | 4430 |
| Somalia | 28,490 | 0.61 | 46 (68) | NA |
| Equatorial Guinea | 1704 | 0.49 | 51 (51) | 9840 |
| Zambia | 30,050 | 0.46 | 96 (97) | 1080 |
| Lesotho | 2839 | 0.26 | 85 (70) | 1170 |
| Iraq | 36,344 | 0.23 | 75 (89) | 4380 |
The bold value represents the column which was used to select the countries that are listed in the table.
Annual incidence is calculated as the number measles cases reported on the JRF [7] divided by the WHO population estimate for the associated country [11] (In Table 2d, the 5 year total incidence was divided by 5, to represent average annual incidence).
UNICEF/WHO estimate of 1st dose measles-containingvaccine coverage [9]. Numbers in parentheses represent country official figures on the JRF.
GNI = World Bank Gross National Income percapita, Atlas method [10] (†estimate from 2010).
In the 2012 JRF, 45 countries reported having performed laboratory testing for at least one suspected pertussis case. In the section for aggregate number of laboratory-confirmed cases, 162 of 194 countries provided information (including zero cases), among which 58 listed at least one laboratory-confirmed case (4 countries reported having tested at least one suspect case, but did not report any laboratory confirmed pertussis cases, and 17 countries reported having laboratory-confirmed cases, but listed zero cases or had absent data on the number of cases tested). Sixteen of the 58 countries with laboratory-confirmed pertussis cases had the same number of total and laboratory-confirmed pertussis cases, suggesting that only laboratory-confirmed cases were reported on the JRF. Five countries reported a larger number of laboratory-confirmed cases than total pertussis cases. Among the ten countries with the highest total reported pertussis cases in 2012 (Table 1a), one country did not provide any information in the section for reporting laboratory-confirmed cases, five countries listed zero laboratory-confirmed cases, and four countries reported having laboratory-confirmed pertussis cases. Taken together, these observations suggest that pertussis surveillance data reported on the JRF represents a mix of clinical and laboratory confirmed cases, with reporting standards varying among countries.
4. Discussion
The country-level discordance between pertussis incidence and DTP3 coverage, and limited sensitivity (86 of 194 countries provided no data or reported zero pertussis cases), suggests poor quality pertussis surveillance data reported using the JRF. This is supported by the observation that high pertussis incidence is common in high income countries, which may be related to the availability of increased resources for disease detection and reporting. The comparison of country-level disease data for the entire population with estimated DTP3 coverage must be undertaken with caution. Estimates of vaccination coverage among children <1 year of age are indicative of population immunity of a limited age cohort, and may not be representative of the entire population. Additionally, as the number of cases deceases, as is the case for measles in many countries, there may be an increased effort to detect all cases. However, measles surveillance data from a comparable source are suggestive of a functional global surveillance system, including in low-income countries, and are more consistent with global monitoring efforts and surveillance standards [2,8].
The WHO case definition for pertussis has two classifications: a clinical case, based only on clinical symptoms, and a laboratory-confirmed case, although the clinical symptoms as well as the laboratory tests used for confirmation may vary among countries [12]. In many countries, the total number of pertussis cases reported in the 2012 JRF was larger than the number of laboratory-confirmed cases; however, a small proportion of countries reported the same number of total and laboratory-confirmed pertussis cases, which points toward exclusive use of the laboratory-confirmed case definition and possible underestimation of the burden of disease. This underscores the variability in the use of case definitions and lack of data standardization for pertussis data in the JRF. Finally, the observation that many of the countries with high reported pertussis incidence did not provide any information on laboratory confirmation, although many of these higher income countries presumably have diagnostic laboratory capacity, prevents us from drawing conclusions about the reporting criteria used for many pertussis cases reported through the JRF.
We opted to focus our analysis on pertussis surveillance data, in comparison to measles. This decision was based on the estimated burden of global disease [13], transmissibility of Bordetella pertussis among humans, as well as the fact that pertussis epidemiology is one of determinants of the primary immunization schedule. Pertussis data are generally reported as aggregate numbers of cases, as is the case of diphtheria and tetanus, in comparison to measles, in which case-based reporting commonly occurs. Hence, it is likely that the reporting of the three diseases may have similar issues.
Why should we care about surveillance data quality for VPDs such as pertussis? First, surveillance data are crucial for monitoring disease incidence and for assessing the performance of the immunization program. Disease outbreaks serve as an opportunity to identify susceptible pockets or communities with low population immunity that may be missed through routine monitoring of vaccination coverage alone. These data are also critical for informing immunization policies and strategies, including the number and timing of immunization doses, as well as better understanding the severity of illnesses and whether additional public health interventions are warranted.
Currently, data on some VPDs reported through the JRF are insufficient for assessing epidemiologic trends or for informing immunization policies or strategies. Because of uncertainties about the quality of reported data, as well as the absence of more detailed information on disease epidemiology (such as the age and geographic distribution of cases), aggregate reports of pertussis cases in the JRF are not routinely used for global monitoring. Previous estimates of global pertussis burden have relied on mathematical models, in contrast to direct use of surveillance data [13]; however, the limitation of these models and the absence of high quality, representative, epidemiologic data from low and middle income countries is well recognized.
Recently there has been a resurgence of pertussis in several industrialized countries with well-performing surveillance and reporting systems, most of which use acellular pertussis vaccine. This has led to questions about waning immunity of these vaccines and the need for additional booster doses [14–18]. The observations in this report may partially be explained by the use of acellular pertussis in these countries. Importantly, the poor quality global surveillance data for pertussis makes it difficult to derive firm conclusions on the use of acellular pertussis vaccine or the need for additional booster doses.
Previous strategies have discussed the need for improving surveillance for all VPDs [19]. Challenges exist for improving surveillance data quality for some VPDs. Active surveillance and available laboratory diagnostic capacity are lacking in many countries [20]. Furthermore, variation in disease presentation and differences in case definitions may impact the sensitivity, specificity, and comparability of surveillance data among countries [12,21]. We encourage further discussion and exploration among agencies and member countries to improve the quality, and effective use of VPD surveillance data, in order to inform policy decisions and ensure proper immunization program monitoring, as well as to consider implementing outbreak investigation, with laboratory confirmation, to better describe the local disease epidemiology and allow more informed decisions on vaccine policies and strategies.
Acknowledgments
We thank Kathleen Wannemuehler, Jacqueline Gindler and Peter Bloland for helpful comments.
References
- [1].Tracking progress towards global polio eradication 2011–2012. Releve epidemiologique hebdomadaire/Section d’hygiene du Secretariat de la Societe des Nations 2013;88(April (15)):153–60. [PubMed] [Google Scholar]
- [2].Progress in global control and regional elimination of measles, 2000–2011. Releve epidemiologique hebdomadaire/Section d’hygiene du Secretariat de la Societe des Nations 2013;88(January (3)):29–36. [PubMed] [Google Scholar]
- [3].Nsubuga P, Perry H, Embrey K, Duale S. In: Kasolo F, Roungou JB, Perry H, editors. Technical guidelines for integrated disease surveillance and response in the African region. 2nd ed. Geneva: WHO; 2010. [Google Scholar]
- [4].WHO. Global invasive bacterial vaccine preventable diseases (IB-VPD) information and surveillance bulletin; 2013. http://www.who.int/nuvi/surveillance/IB_VPD_bulletin_Jan_Jun_2012_Final.pdf
- [5].WHO. Global rotavirus information and surveillance bulletin; 2013. http://www.who.int/nuvi/surveillance/RV_bulletin_Jan_June_2012_Final.pdf
- [6].Centers for Disease C, Prevention. Expanding poliomyelitis and measles surveillance networks to establish surveillance for acute meningitis and encephalitis syndromes – Bangladesh, China, and India, 2006–2008. MMWR Morbidity and Mortality 2012;61(December (49)):1008–11 [weekly report]. [PubMed] [Google Scholar]
- [7].WHO. WHO/UNICEF joint reporting process; 2013, http://www.who.int/immunization/monitoringsurveillance/routine/reporting/reporting/en/.
- [8].Featherstone DA, Rota PA, Icenogle J, Mulders MN, Jee Y, Ahmed H, et al. Expansion of the global measles and rubella laboratory network 2005–09. Journal of Infectious Diseases 2011;204(Suppl. 1):S491–8. [DOI] [PubMed] [Google Scholar]
- [9].Burton A, Monasch R, Lautenbach B, Gacic-Dobo M, Neill M, Karimov R, et al. WHO and UNICEF estimates of national infant immunization coverage: methods and processes. Bulletin of the World Health Organization 2009;87(July (7)):535–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Group TWB. World DataBank. World development indicators; 2013, http://data.worldbank.org/indicator/NY.GNP.PCAP.CD. [Google Scholar]
- [11].WHO. Demographic and socioeconomic statistics: population by country (recent years); 2013, http://apps.who.int/gho/data/view.main.POP2040ALL?lang=en. [Google Scholar]
- [12].Cherry JD, Tan T, Wirsing von Konig CH, Forsyth KD, Thisyakorn U, Greenberg D, et al. Clinical definitions of pertussis: summary of a global pertussis initiative roundtable meeting, February 2011. Clinical Infectious Diseases: An Official Publication of the Infectious Diseases Society of America 2012;54(June (12)):1756–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Crowcroft NS, Stein C, Duclos P, Birmingham M. How best to estimate the global burden of pertussis? Lancet Infectious Diseases 2003;3(July (7)):413–8. [DOI] [PubMed] [Google Scholar]
- [14].Tartof SY, Lewis M, Kenyon C, White K, Osborn A, Liko J, et al. Waning immunity to pertussis following 5 doses of DTaP. Pediatrics 2013;131(April (4)):e1047–52. [DOI] [PubMed] [Google Scholar]
- [15].Misegades LK, Winter K, Harriman K, Talarico J, Messonnier NE, Clark TA, et al. Association of childhood pertussis with receipt of 5 doses of pertussis vaccine by time since last vaccine dose, California, 2010. JAMA: The Journal of the American Medical Association 2012;308(November (20)): 2126–32. [DOI] [PubMed] [Google Scholar]
- [16].Klein NP, Bartlett J, Rowhani-Rahbar A, Fireman B, Baxter R. Waning protection after fifth dose of acellular pertussis vaccine in children. New England Journal of Medicine 2012;367(September (11)):1012–9. [DOI] [PubMed] [Google Scholar]
- [17].Cherry JD. Epidemic pertussis in 2012 – the resurgence of a vaccine-preventable disease. New England Journal of Medicine 2012;367(August (9)):785–7. [DOI] [PubMed] [Google Scholar]
- [18].Sheridan SL, Ware RS, Grimwood K, Lambert SB. Number and order of whole cell pertussis vaccines in infancy and disease protection. JAMA: The Journal of the American Medical Association 2012;308(August (5)):454–6. [DOI] [PubMed] [Google Scholar]
- [19].Dabbagh A, Eggers R, Cochi S, Dietz V, Strebel P, Cherian T. A new global framework for immunization monitoring and surveillance. Bulletin of the World Health Organization 2007;85(December (12)):904–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Guiso N, Liese J, Plotkin S. The Global Pertussis Initiative: meeting report from the fourth regional roundtable meeting, France, April 14–15, 2010. Human Vaccines 2011;7(April (4)):481–8. [DOI] [PubMed] [Google Scholar]
- [21].Ghanaie RM, Karimi A, Sadeghi H, Esteghamti A, Falah F, Armin S, et al. Sensitivity and specificity of the World Health Organization pertussis clinical case definition. International Journal of Infectious Diseases: IJID: Official Publication of the International Society for Infectious Diseases 2010;14(December (12)):e1072–5. [DOI] [PubMed] [Google Scholar]


