Esophageal adenocarcinoma is on the rise in the West and yet its marginal survival rate has not changed in 30 years. Preemptive approaches, including radiofrequency ablation (RFA), and photodynamic therapy (PDT) for Barrett’s esophagus and dysplasia are achieving dramatic initial results. Although the long-term efficacy of these nonspecific ablative therapies is awaiting longitudinal studies, reports of recurrences are increasing. More targeted therapies, particularly directed at the stem cells of Barrett’s esophagus, demand knowing the origin of this intestinal metaplasia (IM). The prevailing concept holds that Barrett’s esophagus arises from the “transcommitment” of esophageal stem cells to produce an intestine-like epithelium. Given the remarkable frequency with which Barrett’s esophagus appears in Western populations, empirical data for such a transcommitment phenomenon have been surprisingly limited. An alternative explanation derives from the discovery of a discrete population of residual embryonic cells (RECs) existing at the gastroesophageal junction in normal individuals that expands and colonizes regions of the esophagus denuded by chronic reflux. These RECs form IM within days of esophageal injury, suggesting a novel mechanism of tumorigenesis. A corollary of this work is that the Barrett’s stem cell is distinct from that of the squamous epithelium and, once identified, will form the basis of new strategies for addressing Barrett’s and its related neoplasia.
The incidence of esophageal adenocarcinoma is rapidly increasing in Western countries. This is despite the introduction of sophisticated endoscopic techniques and our ability to readily monitor the presumed precursor lesion known as Barrett’s esophagus.1 Barrett’s esophagus is a columnar metaplasia emanating from the gastroesophageal junction that involves short (<3 mm) or long (>3 mm) regions of the esophagus. The definition of Barrett’s esophagus in the United States requires the presence of IM, marked by goblet cells and gene expression patterns typical of small intestine epithelium. By many criteria Barrett’s esophagus mirrors the appearance and progression of gastric IM to gastric adenocarcinoma common in Asia. Both are IMs triggered by chronic inflammation due to gastroesophageal reflux in Barrett’s esophagus or Helicobacter pylori infection in gastric IM. Both progress via the “Correa sequence” of IM, low- and high-grade dysplasia, and finally invasive adenocarcinoma.2 Both adenocarcinomas are highly aggressive, and when unresectable, are inured to any regimen of chemotherapy and kill most patients within a year of onset. Given this poor therapeutic response, it seems that early detection and perhaps eradication of these precursor lesions will be the only means of preventing deaths. If so, the Correa sequence of IM, low- and high-grade dysplasia, which evolves over 10–25 years, offers an intriguing window of therapeutic opportunity. To fully exploit this for preemptive therapeutics, it will be important to address two vulnerabilities revealed in the Correa sequence. The first is that most patients presenting with esophageal adenocarcinoma report no history of acid reflux and thus would not have triggered endoscopic evaluation.1 This fact underscores the need for simple and reliable screening tests for the at-risk yet asymptomatic segments of the population. A second problem with the Correa sequence is the difficulties in accurate endoscopic recognition of low-grade dysplasia. Although Barrett’s itself confers a 30- to 50-fold increased risk for esophageal adenocarcinoma (0.5%–1.0% conversion/year), it is generally believed that low-grade dysplasia guarantees the progression to esophageal adenocarcinoma although reports of the degree of risk vary. Some of this variability could reflect differences in the threshold for the diagnosis of low-grade dysplasia between pathologists and exclusion of mimics. When benign mimics of low-grade dysplasia are excluded, the risk of progression to either high-grade dysplasia or adenocarcinoma is as high as 85% at 9 years.3 Thus, arguably the most dangerous and actionable lesion—low-grade dysplasia—often eludes detection. We desperately need sensitive means of imaging-based detection of low-grade dysplasia to unleash preemptive therapeutic approaches.
If preemptive therapies are the future, what form will they take? The past 5 years have seen the aggressive introduction of RFA and PDT for killing Barrett’s esophagus and particularly those tissues having high-grade dysplasia.4 Both approaches are predicated on nonspecific annihilation of dysplastic Barrett’s glands before they progress. Reports of Barrett’s reemergence after RFA5 and PDT underscores the difficulty faced in any nonspecific ablative process that presumably must destroy each and every progenitor of IM for long-term success. Going forward on records of early success, both RFA and PDT will be optimized for achieving long-term suppression of dysplasia and Barrett’s esophagus itself. Whether recurrences are a consistent problem is unknown at present, but if so, the basis is likely to be the survival of a “stem cell” compartment within these precursor lesions, the nature of which has not been determined to date. Recently, however, “organoids” of Barrett’s tissue—self-replicating units of epithelial tissue—have been shown to undergo regenerative differentiation for multiple passages in 3-dimensional cultures, suggesting the existence of a progenitor or stem cell pool.6 The identity and molecular features of these stem cells thus are central to any future strategies for selective targeting of this population to effectively eradicate Barrett’s esophagus and low-grade dysplasia. The origin of the stem cells of Barrett’s esophagus has been the subject of considerable controversy over the past three decades, whose resolution will not only clarify the value of this targeting strategy, but offer an even earlier means of precluding the onset of Barrett’s metaplasia.
Cellular Origin of Barrett’s Metaplasia
It is estimated that between 1% and 2% of the US population has Barrett’s esophagus, translating to an enormous figure of 3–4 million individuals nationwide with a heightened risk for lethal esophageal adenocarcinoma. Given the frequent acquisition of this metaplasia, it would seem a robust mechanism for its generation exists and at this point would have been well scrutinized and defined. In contrast with these expectations, the origins of Barrett’s has gone through multiple renditions without achieving consensus in the field.7 The most obvious potential source for Barrett’s was the migration of gastric cardia epithelium in a process of repairing gastro-esophageal reflux-mediated damage to the adjacent esophageal epithelium. This otherwise convenient model of Barrett’s esophagus has been diluted by the absence of goblet cells, the sina qua non of Barrett’s in the United States, in gastric epithelium. With its demise, more complicated models were proposed. Probably the most radical was the colonization of the acid-damaged esophagus by circulating, multipotent bone marrow stem cells. The evidence in favor of this model was based on the incorporation of cells into IM from genetically tagged bone marrow transplants. However, although bone marrow stem cells have been linked to mesoderm-derived tissues, their potential to form epithelial populations, typically of ectoderm or endoderm origins, has not been established. Moreover, the pattern of incorporation of bone marrow cells in Barrett’s glands was not consistent with any role as progenitors to this metaplasia. By far, overall, the dominant concept for the origin of Barrett’s esophagus centers on the notion that acid reflux induces the esophageal squamous stem cells to switch their fate to generating columnar epithelia with intestinal characteristics.7 The initial empirical basis for the transcommitment hypothesis was not from Barrett’s esophagus at all, but rather from a transgenic mouse model that yielded gastric IM. In brief, Sagano et al generated a transgenic mouse in which caudal homeobox 2 (Cdx2), a gene implicated in intestinal cell differentiation, was expressed from a promoter active in gastric parietal cells.8 These mice showed evidence of gastric IM. As Cdx2 expression was reported in precursors of both gastric and esophageal adenocarcinoma, these findings were extrapolated to explain the origins of Barrett’s esophagus from esophageal squamous stem cells. Accordingly, multiple efforts expressed Cdx2 in murine esophagus and in human esophageal epithelia in vitro, although little evidence has emerged to support the concept that Cdx2 could “transcommit” esophageal cells to IM. The concept of stem cell transcommitment suggests a stem cell that normally gives rise to one set of differentiated cell types is altered to yield progeny with alternative cell fates. Attempts at transcommitting human esophageal epithelial cells to Barrett’s esophagus have employed bile salts and low pH of gastric fluids, although again the effects were limited. Given the near epidemic rates of Barrett’s esophagus in Western populations, it might have been imagined that ≥1 of these treatments could coax esophageal stem cells to Barrett’s esophagus. By way of counterexample, it was recently demonstrated that human airway stem cells readily transcommit to squamous metaplasia.9 Squamous metaplasia in the trachea and bronchi is tightly linked with a history of smoking and is thought to be a precursor to squamous carcinoma in the lung. However, it was unclear whether squamous metaplasia arose from rare, squamous stem cells among airway epithelial stem cells or from airway stem cells altered by carcinogen exposure. Using clonal lineages of tracheobronchial stem cells, Kumar et al9 showed that simple changes in growth conditions could direct 100% of these stem cells to assume 2 different fates: airway epithelium, including ciliated and goblet cells, or stratified squamous differentiation. Interestingly, stem cells from squamous tissue such as skin, esophagus, and cervical epithelium have been analyzed for >2 decades and yet no reports of such facile cell fate alterations have arisen. Thus, it seems that esophageal stem cells, as well as those of other squamous tissues, do not have an intrinsic capacity for transcommitting to columnar metaplasia that would explain the abundance of Barrett’s cases.
Origins of Barrett’s Revisited
The weaknesses in the 3 models for the cell of origin of Barrett’s esophagus and their limited predictive value signaled the need for additional models. We had previously cloned the p63 gene encoding a p53-like transcription factor and demonstrated that its expression was specific to stem cells of stratified epithelia including epidermis, esophagus, as well as mammary and prostate glands. In the functional characterization of the p63 gene, we generated p63 knockout (p63ko) mice and human epidermal stem cells, in which p63 was targeted by RNA interference. The results and conclusions from these studies were consistent and dramatic: p63 plays an essential role in the “stemness” of regenerative cells of stratified epithelia—specifically characteristics of immaturity and self-renewal.10,11 What this meant for the mouse was a loss, in utero, of all stratified epithelia owing to a nonregenerative differentiation of these stem cells. Thus, at birth, the squamous epithelium of the skin existed in only small patches of differentiated cells devoid of stem cells on an expanse of exposed dermis. Although the dynamics of the p63ko epidermis was analyzed in detail, we made only brief note of the metaplasia in the esophagus and proximal stomach, which are normally lined with a squamous epithelium. Daniely et al12 subsequently examined our p63 knockout mouse for upper airway development, but noted the metaplasia in the esophagus, which they described as being reminiscent of Barrett’s esophagus. They concluded, however, that this metaplasia arises from esophageal stem cells as a “reprogramming” owing to the deletion of p63. In contrast, our data showed that the p63ko epidermis, another squamous epithelium, undergoes a nonregenerative disintegration rather than a shift to a columnar metaplasia. Therefore, we found the conclusions from Daniely et al difficult to reconcile.
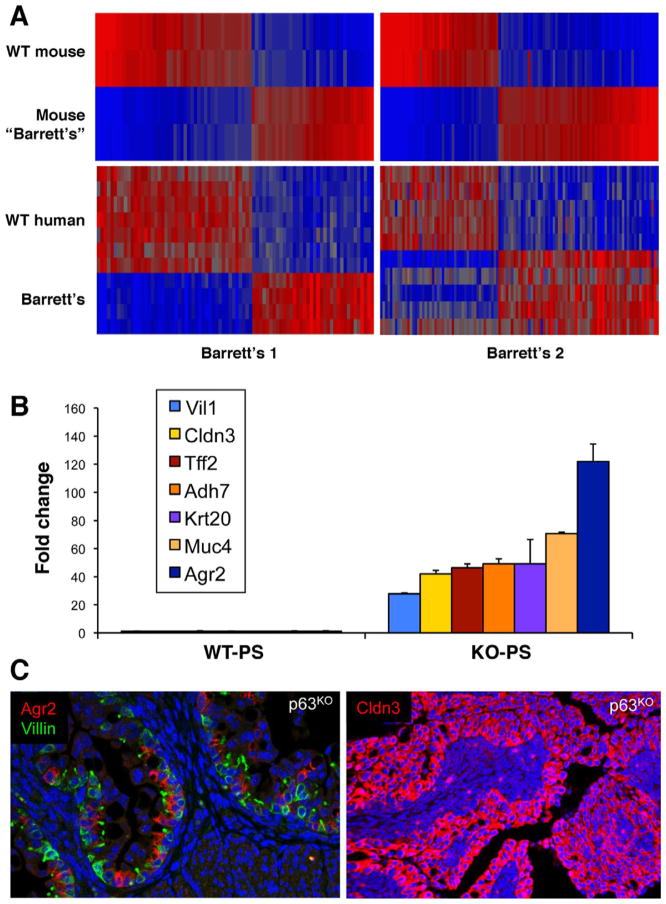
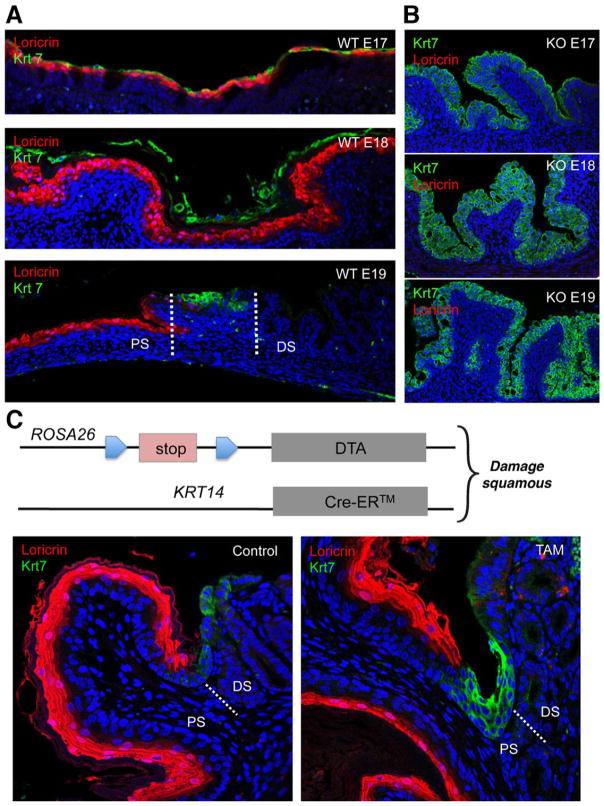
In preparation for a meeting organized by Xin Lu at Oxford University to deconstruct the Barrett’s problem, we set about to dissect the evolution of the Barrett’s-like metaplasia in the esophagus and proximal stomach in the p63 null mouse. This was a wonderful opportunity; we had not worked previously on Barrett’s and therefore had no dog in the fight and yet a potentially relevant model. Our first task was validating our model—how good is the p63ko metaplasia in mimicking Barrett’s? We started with whole genome expression analyses on late p63ko embryos (p63ko mice die at birth owing to loss of skin and all other stratified epithelia) compared with its wild-type counterpart. Not only did the gene expression profiles of the murine metaplasia broadly match that of human Barrett’s, but we were startled to find that the p63ko metaplasia had all of the typical markers that distinguished Barrett’s in humans but with much higher fold-changes (eg, 120-fold increase in Agr2) and lower P values13 (Figure 1). Immunofluorescence localization of these markers showed robust staining of the metaplasia in our mice as well as in sections of human Barrett’s (Figure 1C). Thus, our embryonic mouse model yielded better and more minable data for Barrett’s than could be obtained from human biopsies where Barrett’s glands were often contaminated by squamous islands and stromal tissues. In addition, our bioinformatics also included datasets from all other tissues in the gastrointestinal (GI) tract, which revealed that Barrett’s esophagus was distinct from small intestine and, therefore, not a simple transcommitment to intestinal fate. With confidence that the late p63ko embryos were yielding a Barrett’s-like metaplasia, the next question focused on its origin. By tracking back through embryogenesis in this mouse, it was apparent that even E14 p63ko embryos have a highly proliferative metaplasia, whereas the wild-type counterparts showed the expected, early squamous epithelium in the esophagus and proximal stomach. One day earlier, however, at E13, the culprit cells were identified as a simple columnar epithelium lining the proximal stomach and expressing metaplasia markers such as Car4 and Krt7. This line of cells along the basement membrane had to represent the “ground state” of the metaplasia in both the p63ko and the wild-type embryos, which of course begged the question as to why the wild-type embryos did not also develop metaplasia. The answer came from analysis of the p63 stem cells in the esophagus of the wild-type E13 embryos. These cells first appear as a small population of cells sequestered in the esophagus away from the Car4 cells lining the proximal stomach. One day later at E14, this p63+ population of squamous stem cells had greatly expanded in numbers and begun a posterior migration to and underneath the line of Car4 cells. In the process of undermining these Car4 cells, the p63+ cells displaced the Car4 cells from basement membrane. These displaced Car4 cells showed considerably less proliferation than those more proximally distributed, which were still attached to the basement membrane, consistent with a wealth of studies on epithelial cell biology. This displacement phenomenon does not happen in the p63ko embryos because p63 is required for the regenerative expansion of these squamous stem cells populations in the first place. Aside from the undermining process in wild-type embryos, which revealed why they do not develop metaplasia, it was the tracking of displaced Car4 cells through development that revealed the origins of Barrett’s in the adult. In particular, we followed the Car4 cells in the wild-type embryos through E15-E18, in which we detected Car4+(Krt7+) cells riding above the stratifying squamous cells until they were sloughed off at E17 (Figure 2A). We noted, however, that a group of these Car4/Krt7+ cells, designated here as RECs, remained precisely at the squamocolumnar junction of E18 embryos. In contrast, p63-null embryos showed extensive development of a Krt7+ metaplasia without evidence for a squamous population of cells (Figure 2B). Even in adult mice, RECs were a consistent feature of this junction. If RECs were retained at the junction, it was possible that they represent a source of cells for the initiation of Barrett’s esophagus. We tested this hypothesis by monitoring the activity of these Krt7+ cells in a mouse model in which the esophageal epithelium was damaged by the conditional expression of diphtheria toxin A subunit (Figure 2C). Significantly, damage to the adjacent squamous tissue of the esophagus triggered a rapid expansion and anterior march of these Krt7 cells from the SCJ to the esophagus. Although collateral damage of other squamous tissues in the Krt14-driven Cre recombinase in this experiment precluded analysis beyond 10 days, this model provided support for the notion that RECs are the source of Barrett’s esophagus.13
Figure 1.
Bioinformatics linking p63ko metaplasia with human Barrett’s. (A) Heatmaps of whole genome expression microarray data comparing differentially expressed genes in wild-type and p63ko proximal stomach with normal patient esophagus and those with Barrett’s esophagus. (B) Histogram of fold-changes of key Barrett’s markers in proximal stomach of wild-type versus p63ko E18 embryos. (C) Immunofluorescence micrograph showing expression of Agr2, Cldn3, and villin, key markers of human Barrett’s esophagus, in metaplasia of p63ko mouse. Figure 1A and B is provided with permission from Dr Yusuke Yamamoto. Figure 1C is reprinted with permission from Wang et al.16
Figure 2.
Dynamics of RECs in wild-type and p63ko mice. (A) Co-labeling of squamous (anti-loricrin, red) and RECs (anti-Krt7, green) in wild-type embryos showing suprasquamous disposition of RECs at E17, sloughing of RECs at E18, and retention of RECs at the squamocolumnar junction at E19. (B) Corresponding labeling of loricrin and Krt7 in p63ko embryos showing absence of squamous cells and the development of a Barrett’s-like metaplasia in late embryogenesis. (C) Schematic of mouse strain in which diphtheria toxin A subunit is conditionally expressed in Krt14-expressing squamous stem cells. Left, section through the squamocolumnar junction staining with anti-loricrin showing squamous tissue and anti-Krt7 showing junctional RECs. Right, section through junction of mouse after 7-day expression of DTA in squamous tissue showing redistribution of Krt7 cells toward squamous epithelium. Figure 2A and B reprinted with permission from Wang et al.16 Figure C is provided with permission from Dr Xia Wang.
A key question we faced was how predictive the mouse models we developed were for human disease. The bioinformatics revealed that the “mouse Barrett’s esophagus” was essentially the same as the human counterpart, with any differences explained by the fact that it was only four embryonic days in the making. This concept was bolstered by examining the esophagus of 22-week-old human fetuses, which showed a similar Krt7+ population of cells being displaced by the stratification of underlying squamous stem cells. In addition, sections through the gastroesophageal junction of patients without Barrett’s esophagus showed a similar population of Krt7+ cells residing precisely at this locale and these cells also stained with other markers of Barrett’s esophagus such as Muc4.13 The confirmation of these predictions from the mouse model in the human setting supports both the similarity between and the origins of the p63ko metaplasia and Barrett’s esophagus.
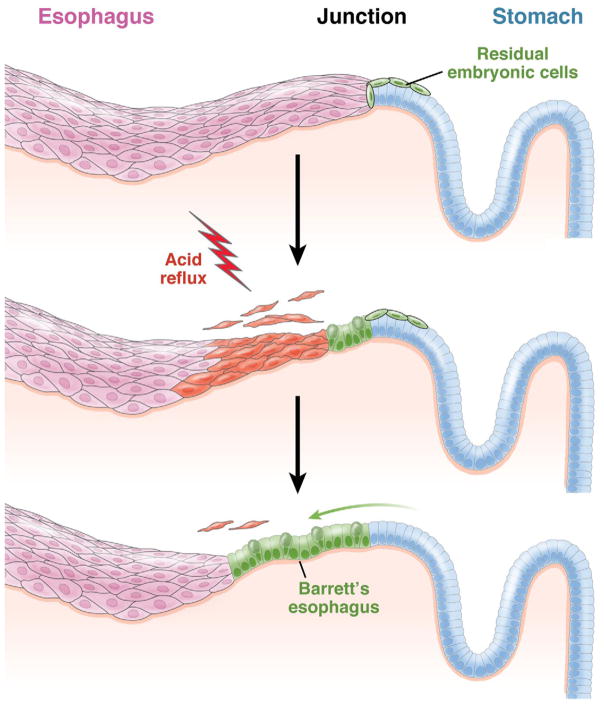
We have defined a discrete population of cells existing precisely at the gastroesophageal junction in both adult mice and humans that have been developmentally linked to the population of cells that initiate the Barrett’s-like metaplasia in the p63ko mouse. These RECs, which are likely supported by stem cells assembled along a circumferential line of basement membrane, share many markers of Barrett’s esophagus. Moreover, we have demonstrated that this population of cells in mice expands and migrates into the esophageal epithelium upon damage to the esophagus (Figure 3). The presence and dynamics of this residual embryonic population provide significant challenges to the dominant hypothesis for Barrett’s esophagus involving the transcommitment of esophageal squamous stem cells.
Figure 3.
Opportunistic expansion of RECs in response to gastroesophageal reflux disease. RECs are shown at the junction of the esophagus and stomach in normal adults and expand along the basement membrane vacated by the reflux-induced death of squamous esophageal cells to form Barrett’s esophagus.
Quante et al reported another model of Barrett’s-like metaplasia in mice based on the forced expression of interleukin-1β in the esophagus.14 Like the Wang et al paper, data from Quante et al showed that the metaplastic cells arose from the squamocolumnar junction rather than by transcommitment of esophageal stem cells. However, Quante et al used lineage tracing off the promoter of Lgr5, a gene linked in some studies to gastric stem cells, to argue that the metaplastic cells were derived from gastric cardia, the portion of stomach epithelium closest to the junction. Like all lineage tracing, the conclusions are only as good as the specificity of the promoter used, and whether the Lgr5 promoter has the necessary discrimination is unclear at present. Regardless of whether RECs or gastric cardia is the origin of the metaplasia seen in Barrett’s, Quante et al present a further challenge to the dominant transcomitment model.
Critics of the REC hypothesis (as well, apparently, of the recent gastric cardia hypothesis) have focused on a set of interesting surgical models in rats that can produce esophagitis, Barrett’s esophagus, and esophageal adenocarcinoma.15 Although esophagogastroduodenal anastomosis, the most popular variant, yields high rates of a Barrett’s-like metaplasia and adenocarcinoma, both are also present in esophagoduodenal (EDA) and esophagojejunal (EJA) anastomoses, in which the gastroesophageal junction and its population of RECs should be absent. Although on its surface the presence of the metaplasia and adenocarcinoma in the EDA and EJA models would dismiss the significance of a junctional population of embryonic cells, additional populations of RECs are likely distributed throughout the GI tract without regard to precise junctions. Evidence for this is that gastric IM, the precursor of gastric adenocarcinoma, is a close cousin of the IM in Barrett’s and can appear in an intermittent, diffuse pattern along the gastric epithelium of patients with H pylori gastritis. If this IM is also derived from rogue, minority populations of cells as proposed for Barrett’s esophagus, such cells, or similar populations distributed in the upper GI tract, might also be the source of metaplasia and adenocarcinoma in the EDA and EJA models. Of course, evidence is lacking for such diffusely distributed embryonic cells in the upper GI tract, and their existence and the general validity of the REC hypothesis will be linked.
Finally, a major supposition raised by the p63ko mouse is that Barrett’s metaplasia arises in the absence of a selectable mutation generally thought essential for the evolution of cancer precursors. The basis of this concept was that the Barrett’s-like metaplasia in this mouse appeared very rapidly between embryonic days 13 and 14, and by E18 had assumed the gene expression profile of human Barrett’s esophagus.13 Although epigenetic changes conceivably could occur during this brief window, the progression of RECs at the gastroesophageal junction to recognizable metaplasia seems much more linked to an opportunistic process of expanding across basement membrane otherwise occupied by esophageal squamous stem cells. Support for this concept, which could explain the high incidence of Barrett’s esophagus and gastric IM, will have to await the comparison of deep sequencing and copy number variation data of early Barrett’s esophagus and normal somatic cells from these patients.
There is a growing realization of the futility of treating highly aggressive cancers such as esophageal adenocarcinoma and the need for preemptive approaches to dysplasia and Barrett’s metaplasia itself. RFA and PDT are important directions here with very encouraging initial results that are likely to improve with optimized protocols. The jury is still out on the long-term effects of these ablative treatments and therefore additional strategies, coupled with broad screens to identify early lesions in patients without overt symptoms, are essential. In this regard, the resolution of the controversy surrounding the cellular origins of Barrett’s esophagus is more than an academic pursuit and likely to shape future approaches to blocking the Correa sequence. Therapeutically targeting stem cells of Barrett’s esophagus would be an ideal strategy, for the recently described REC hypothesis demands a Barrett’s stem cell unique from those of the surrounding esophagus and gastric cardia. Identifying these 3 populations of stem cells will set the stage for specific therapeutics.
Acknowledgments
The authors thank the members of the Xian-McKeon, Crum, and Ho laboratories for helpful discussions and support. This work was supported by the National Cancer Institute and General Medical Sciences of the NIH (RO1-GM083348 and R21CA124688), the European Research Council, the Genome Institute of Singapore, and the Biomedical Research Council and the National Medical Research Council, Singapore.
Abbreviations used in this paper
- EDA
esophagoduodenal
- EJA
esophagojejunal
- IM
intestinal metaplasia
- PDT
photodynamic therapy
- RECs
residual embryonic cells
- RFA
radiofrequency ablation
Footnotes
Conflicts of interest
The author discloses no conflicts.
References
- 1.Reid BJ, Li X, Galipeau PC, et al. Barrett’s oesophagus and oesophageal adenocarcinoma: time for a new synthesis. Nat Rev Cancer. 2010;10:87–101. doi: 10.1038/nrc2773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Correa P. Human gastric carcinogenesis: a multistep and multi-factorial process. First American Cancer Society Award Lecture on Cancer Epidemiology and Prevention. Cancer Res. 1992;52:6735–6740. [PubMed] [Google Scholar]
- 3.Curvers WL, ten Kate FJ, Krishnadath KK, et al. Low-grade dysplasia in Barrett’s esophagus: overdiagnosed and underestimated. Am J Gastroenterol. 2010;105:1523–1530. doi: 10.1038/ajg.2010.171. [DOI] [PubMed] [Google Scholar]
- 4.Garman KS, Shaheen NJ. Ablative therapies for Barrett’s esophagus. Curr Gastroenterol Rep. 2011;13:226–239. doi: 10.1007/s11894-011-0182-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vaccaro BJ, Gonzalez S, Poneros JM, et al. Detection of intestinal metaplasia after successful eradication of Barrett’s esophagus with radiofrequency ablation. Dig Dis Sci. 2011;56:1996–2000. doi: 10.1007/s10620-011-1680-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sato T, Stange DE, Ferrante M, et al. Long-term expansion of epithelial organoids from human colon, adenoma, adenocarcinoma, and Barrett’s epithelium. Gastroenterology. 2011;141:1762–1772. doi: 10.1053/j.gastro.2011.07.050. [DOI] [PubMed] [Google Scholar]
- 7.Souza RF, Krishnan K, Spechler SJ. Acid, bile, and CDX: the ABCs of making Barrett’s metaplasia. Am J Physiol Gastrointest Liver Physiol. 2008;295:G211–218. doi: 10.1152/ajpgi.90250.2008. [DOI] [PubMed] [Google Scholar]
- 8.Mutoh H, Hakamata Y, Sato K, et al. Conversion of gastric mucosa to intestinal metaplasia in Cdx2-expressing transgenic mice. Biochem Biophys Res Commun. 2003;294:470–479. doi: 10.1016/S0006-291X(02)00480-1. [DOI] [PubMed] [Google Scholar]
- 9.Kumar PA, Hu Y, Yamamoto Y, et al. Distal airway stem cells render alveoli in vitro and during lung regeneration following H1N1 influenza infection. Cell. 2011;147:525–538. doi: 10.1016/j.cell.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang A, Schweitzer R, Sun D, et al. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–718. doi: 10.1038/19539. [DOI] [PubMed] [Google Scholar]
- 11.Senoo M, Pinto F, Crum CP, et al. p63 is essential for the proliferative potential of stem cells in stratified epithelia. Cell. 2007;129:523–536. doi: 10.1016/j.cell.2007.02.045. [DOI] [PubMed] [Google Scholar]
- 12.Daniely Y, Liao G, Dixon D, et al. Critical role of p63 in the development of a normal esophageal and tracheobronchial epithelium. Am J Physiol Cell Physiol. 2004;287:C171–181. doi: 10.1152/ajpcell.00226.2003. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quante M, Bhagat G, Abrams JA, et al. Bile acid and inflammation activate gastric cardia stem cells in a mouse model of Barrett-like metaplasia. Cancer Cell. 2012;21:36–51. doi: 10.1016/j.ccr.2011.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pavlov K, Maley CC. New models of neoplastic progression in Barrett’s oesophagus. Biochem Soc Trans. 2010;38:331–336. doi: 10.1042/BST0380331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang X, Ouyang H, Yamamoto Y, et al. Residual embryonic cells as precursors of a Barrett’s-like metaplasia. Cell. 2011;145:1023–1035. doi: 10.1016/j.cell.2011.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]