Abstract
Background
Prior evidence demonstrates that pulse pressure (PP), a surrogate marker of arterial stiffness, is an independent risk factor for mortality and major adverse cardiovascular (CV) events.
Objectives
The study aimed to identify the association of PP with death, myocardial infarction, and stroke among participants enrolled in large CV outcome clinical trials and determine if this association was impacted by pre-existing CV disease, or specific CV risk factors.
Methods
A total of 65,382 individuals, ages 19 to 98 years, that were enrolled in one of five CV outcome trials were analyzed. Baseline demographics, history, blood pressures, and medications were collected. Univariate and multivariable analyses were conducted to explore temporal patterns, risks, and adjusted survival rates.
Results
Mean baseline PP was 52 ± 12 mmHg. For every 10 mmHg increase in PP, there was an increased risk of death, stroke, or myocardial infarction (hazard ratio (HR) 1.11, 95 % CI 1.08 to 1.14, p < 0.001). Similarly, a PP ≥ 60 mmHg demonstrated an HR of 1.27 (95 % CI 1.19 to 1.36, p < 0.001) compared with PP < 60 mmHg. A similar association existed for all subgroups analyzed except for participants with a history of stroke where increasing PP did not increase risk (HR 1.02, 95 % CI 0.95 to 1.10, p = 0.53). PP was a better predictor of adverse outcomes when compared to both systolic and diastolic blood pressures using the AIC and C-index.
Conclusions
Among participants enrolled in CV outcome trials, baseline PP is associated with increased risk of death, myocardial infarction, and stroke for those with pre-existing CV disease and risk factors with the exception of a prior history of stroke.
Keywords: Pulse pressure, Hypertension, Cardiovascular outcomes, Clinical trial
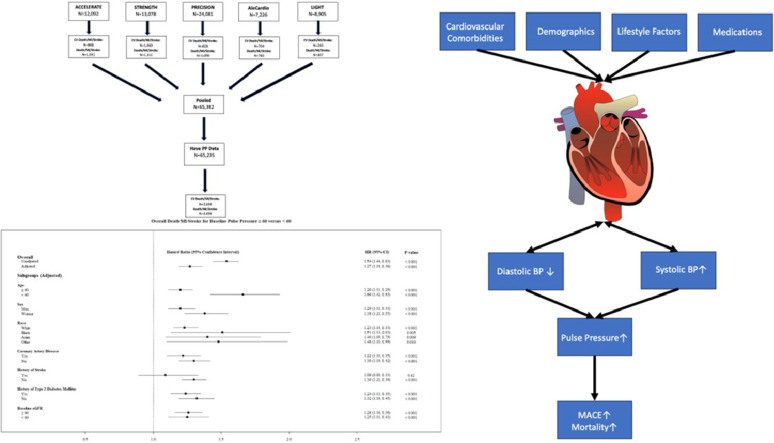
Central illustration legend: pulse pressure and risk of death, myocardial infarction, and stroke
(Top) Cohort flow chart demonstrating pooled cardiovascular outcome trial participants analyzed with adjudicated outcomes by trial and outcome. (Bottom) Forest plot of overall cohort and selected subgroups for hazard associated with having a baseline Pulse Pressure ≥ 60 mmHg. The overall population, as well as all subgroups, demonstrated increased hazard in the presence of an elevated Pulse Pressure with the exception of those with a prior history of stroke. (Right) Risk factors associated with pathophysiological modifications to the heart and their relation to traditional blood pressure measures that facilitate the rise in pulse pressure which is an independent predictive risk factor for death, myocardial infarction, and stroke. Abbreviations: PP, pulse pressure; BP, blood pressure; MACE, major adverse cardiovascular events; MI, myocardial infarction
.
1. Introduction
Contemporary American and European blood pressure (BP) guidelines share many similarities, however, there are differences [1]. Notably, American guidelines lack a discussion of isolated systolic hypertension and do not acknowledge that a wide pulse pressure (PP) is a marker of increased cardiovascular (CV) risk, whereas European guidelines denote that a PP ≥ 60 mm Hg portends increased CV risk particularly among middle-aged and older individuals [2], [3], [4]. A sustained high systolic BP combined with a low diastolic BP results in a wide PP and is an approximation of arterial stiffness and cardiac contractility [5]. Arterial stiffness typically increases due to age and chronic disease processes such as diabetes and kidney disease and causes an increase in pulse wave velocity [6,7]. Wide PP also appears to have genetic underpinnings [8]. Ultimately elevated systolic BP leads to an increase in cardiac afterload and arterial stretch, while a decreased diastolic BP contributes to a reduction in perfusion, and can compromise cardiac diastole resulting in increased risk for ischemic events in patients with pre-existing vascular disease [9]. Increased PP is associated with mortality and major adverse CV events in populations considered at high risk for CV disease, oftentimes outperforming systolic and diastolic BP in its predictive power [[10], [11], [12],13]. However, contrasting evidence has emerged in certain subpopulations suggesting that a wide PP does not necessarily portend worse outcomes [14,15].
American BP guidelines focus on the reduction of systolic BP due to the positive association between increasing systolic BP, risk of major adverse cardiovascular events, and overall mortality in many clinical trials and registries [16], [17], [18], [19]. However, with the aggressive reduction of systolic BP there is a characteristic decrease in diastolic BP, leading to paradoxical adverse outcomes in many retrospective analyses, a so-called J-curve, particularly among individuals with a wide pulse pressure [20,21].Clinicians may intuitively grasp that a wide PP portends a worse prognosis, but how much risk is impacted and in whom is this risk magnified is not clear.
The study's primary aim was to determine the association of PP to incident mortality, myocardial infarction (MI), and stroke, among contemporary CV outcome trial participants, and to determine if the association was impacted by common CV comorbidities. A secondary aim was to evaluate which BP measure, (systolic BP, diastolic BP, or PP) was most predictive of death, MI, and stroke.
2. Methods
2.1. Study population
The study population consisted of 65,382 patients pooled from five CV large outcome trials [22,23,24,25,26] each ranging between 7226 and 24,081 participants (Supplementary Appendix). Of these, 65,235 patients had both baseline systolic and diastolic BP which are needed to calculate baseline PP. Patients were stratified into groups based on baseline PP ≥ 60 mm Hg (n = 15,650; 24 %) or <60 mm Hg (n = 49,585; 76 %).
2.2. Clinical characteristics and outcomes
Baseline characteristics, including demographics, comorbidities, medication use, and laboratory values, were collected and harmonized across trials. Outcomes assessed included a composite of overall death, MI, or stroke and a separate composite of CV death, MI, or stroke. Median participant follow-up time was 970 days (approximately 2.7 years).
2.3. Statistical analysis
Baseline characteristics were compared between patients with a PP above and below 60 mm Hg using Student's t-test for continuous variables and chi-square testing for categorical variables. Mean ± standard deviation (SD) or frequency and percent are reported, respectively.
Cox proportional hazards regression was used to assess the association between baseline PP and outcomes. Hazard ratios (HR) with 95 % confidence intervals (CI) were reported. Baseline PP was considered as a continuous predictor variable (per 10 mmHg increase) and as a categorical predictor variable (≥60 mm Hg versus <60 mm Hg). For overall death, MI, and stroke, the list of covariates adjusted for included: age, sex, body mass index, race, smoking, coronary artery disease, peripheral arterial disease, type 2 diabetes mellitus, history of heart failure, stroke, transient ischemic attack, MI, percutaneous coronary intervention, coronary artery bypass grafting, baseline low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), eGFR, calcium channel blocker usage, statin usage, thiazide usage, mineralocorticoid receptor antagonist usage, and trial. For CV death, MI, and stroke, the list of covariates adjusted for included: age, sex, body mass index, race, smoking, coronary artery disease, peripheral arterial disease, type 2 diabetes mellitus, hypertension, history of heart failure, stroke, MI, percutaneous coronary intervention, coronary artery bypass grafting, baseline LDL-C, HDL-C, eGFR, calcium channel blocker usage, statin usage, mineralocorticoid receptor antagonist usage, and trial.
Additionally, Kaplan–Meier (KM) survival curves were generated for baseline PP groups with ≥ 60 mm Hg versus <60 mm Hg. Outcomes were compared between groups using the log-rank test. Finally, baseline PP, systolic BP, and diastolic BP models were compared on Akaike Information Criterion (AIC) and C-index to determine which model had the better fit and most prediction power.
Statistical analysis was performed using SAS version 9.4 (SAS Institute Inc., Cary, NC). KM curves were generated in SigmaPlot version 11.0 (Systat Software Inc., San Jose, CA). The spline curves and forest plot were created in R version 4.0.0 (The R Foundation for Statistical Computing, Vienna, Austria). The flow charts were produced in Word for Microsoft 365 version 2303 (Microsoft Corporation, Redmond, WA). All tests are two-tailed with a 0.05 significance level.
3. Results
3.1. Baseline data
Baseline characteristics are reported in Table 1 and are stratified by a baseline PP ≥ ≥ 60 mm Hg or <60 mm Hg. Among the 65,235 patients, the average age was 62.8 ± 9.2 years, 45.4 % were female, 77.9 % were White, 8.4 % were Black, 7.8 % were Asian, and 13.2 % of the cohort identified as Hispanic/Latino. The cohort had an average systolic BP of 128.2 ± 14.1 mm Hg, average diastolic BP of 76.1 ± 8.9 mm Hg, and an average PP of 52.1 ± 12.1 mm Hg (Table 1.).
Table 1.
Baseline characteristics of pooled cardiovascular outcome trial participants.
| Total | Baseline pulse pressure < 60 mmHg | Baseline pulse pressure ≥ 60 mmHg | P-value | |
|---|---|---|---|---|
| Frequency | 65,235 | 49,585 | 15,650 | |
| Age (years) | 62.8 ± 9.2 | 61.5 ± 9.1 | 67.0 ± 8.3 | <0.001 |
| Systolic BP (mmHg) | 128.2 ± 14.1 | 123.8 ± 11.4 | 142.2 ± 12.6 | <0.001 |
| Diastolic BP (mmHg) | 76.1 ± 8.9 | 76.8 ± 8.5 | 73.7 ± 9.8 | <0.001 |
| Pulse pressure (mmHg) | 52.1 ± 12.1 | 47.0 ± 7.7 | 68.5 ± 8.6 | <0.001 |
| Female | 29,587 (45.4) | 22,785 (46.0) | 6802 (43.5) | <0.001 |
| Body mass index | 32.3 ± 6.7 | 32.4 ± 6.7 | 32.1 ± 6.5 | <0.001 |
| Race | <0.001 | |||
| White | 50,725 (77.9) | 38,210 (77.1) | 12,515 (80.1) | |
| Black | 5496 (8.4) | 4582 (9.3) | 914 (5.9) | |
| Asian | 5076 (7.8) | 3848 (7.8) | 1228 (7.9) | |
| Other | 3854 (5.9) | 2888 (5.8) | 966 (6.2) | |
| Ethnicity | <0.001 | |||
| Hispanic/latino | 8060 (13.2) | 6321 (13.6) | 1739 (12.1) | |
| Non-hispanic/latino | 52,860 (86.8) | 40,245 (86.4) | 12,615 (87.9) | |
| Current smoker | 10,571 (16.2) | 8717 (17.6) | 1854 (11.9) | <0.001 |
| Coronary artery disease | 20,319 (31.2) | 14,500 (29.3) | 5819 (37.3) | <0.001 |
| Peripheral artery disease | 4042 (6.2) | 2432 (4.9) | 1610 (10.3) | <0.001 |
| Type 2 diabetes | 33,224 (50.9) | 24,257 (48.9) | 8967 (57.3) | <0.001 |
| History of hypertension | 54,537 (83.8) | 40,394 (81.7) | 14,143 (90.6) | <0.001 |
| History of CHF | 6318 (9.7) | 4584 (9.2) | 1734 (11.1) | <0.001 |
| History of stroke | 3529 (5.4) | 2353 (4.7) | 1176 (7.5) | <0.001 |
| History of TIA | 2087 (3.2) | 1442 (2.9) | 645 (4.1) | <0.001 |
| History of MI | 16,113 (24.7) | 11,843 (23.9) | 4270 (27.3) | <0.001 |
| Acute coronary syndrome | 22,829 (35.1) | 16,789 (34.0) | 6040 (38.7) | <0.001 |
| History of PCI | 16,738 (25.7) | 11,954 (24.1) | 4784 (30.6) | <0.001 |
| History of CABG | 7101 (10.9) | 4613 (9.3) | 2488 (15.9) | <0.001 |
| eGFR (ml/min/1.73 m2) | 83.5 ± 25.4 | 85.1 ± 25.3 | 78.5 ± 25.0 | <0.001 |
| ≥60 | 55,012 (84.5) | 42,848 (86.6) | 12,164 (78.0) | |
| 30–59 | 9801 (15.1) | 6498 (13.1) | 3303 (21.2) | |
| <30 | 267 (0.4) | 132 (0.3) | 135 (0.9) | |
| Aspirin | 39,318 (60.3) | 29,184 (58.9) | 10,134 (64.8) | <0.001 |
| Beta blockers | 32,912 (50.5) | 23,864 (48.1) | 9048 (57.8) | <0.001 |
| Calcium channel blockers | 15,086 (23.1) | 10,089 (20.3) | 4997 (31.9) | <0.001 |
| Statins | 50,977 (78.1) | 37,819 (76.3) | 13,158 (84.1) | <0.001 |
| ACE inhibitors and ARBs | 45,145 (69.2) | 33,542 (67.6) | 11,603 (74.1) | <0.001 |
| Thiazide diuretics | 13,927 (21.3) | 10,279 (20.7) | 3648 (23.3) | <0.001 |
| Mineralocorticoids | 2784 (4.3) | 2170 (4.4) | 614 (3.9) | 0.015 |
Values are mean ± SD or n (%). Abbreviations: ACE, angiotensin converting enzyme; ARB, angiotensin II receptor blocker; BP, blood pressure; CABG, coronary artery bypass graft; CHF, congestive heart failure; TIA, transient ischemic attack; eGFR, estimated glomerular filtration rate; MI, myocardial infarction; PCI, percutaneous coronary intervention.
Patients with a PP ≥ 60 mmHg tended to be older, with an average age of 61.5 ± 9.1 years compared to PP < 60 mmHg with an average age of 67.0 ± 8.3 years (p < 0.001). Additionally, there was a higher incidence of most CV comorbidities, including a history of coronary artery disease (37.3 % vs. 29.3 %), type 2 diabetes mellitus (57.3 % vs. 48.9 %), heart failure (11.1 % vs. 9.2 %), stroke (7.5 % vs. 4.7 %), transient ischemic attack (4.1 % vs. 2.9 %), MI (27.3 % vs. 23.9 %), and acute coronary syndrome (38.7 % vs. 34.0 %) among patients with a PP ≥ 60 mm Hg compared to PP < 60 mm Hg (all p < 0.001). Also, 22.0 % of patients with a PP ≥ 60 mm Hg had an eGFR < 60 ml/min/1.73 m2 compared to 13.4 % with PP < 60 mm Hg and were more likely to use all types of antihypertensive and cardiovascular medications except mineralocorticoid receptor antagonists.
3.2. Outcomes
Death, MI, or stroke occurred in 4494 pooled trial participants (6.9 %). CV death, MI, or stroke occurred in a total of 3694 participants (5.7 %). Patients with a PP ≥ 60 mmHg versus PP < 60 mmHg had a significantly higher overall death, MI, or stroke rate (9.9 % vs. 5.9 %, p < 0.001) and CV death, MI, or stroke rate (8.2 % vs. 4.9 %, p < 0.001).
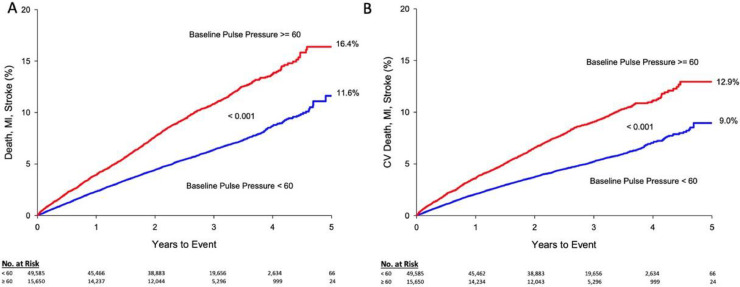
Kaplan-Meier estimates of survival are shown in Fig. 1. After 1-year, overall death, MI, or stroke was 3.9 % for baseline PP ≥ 60 mm Hg and 2.3 % for baseline PP < 60 mmHg (p < 0.001). After 5 years, the occurrence was 16.4 % among patients with a baseline PP ≥ 60 mmHg, compared with 11.6 % for baseline PP < 60 mmHg (p < 0.001).The composite of CV death, MI, or stroke incidence was increased in patients with a baseline PP ≥ 60 mmHg, 3.6 %, compared to 2.1 % for baseline PP < 60 mm Hg (p < 0.001) at 1-year and was 12.9 % compared with 9.0 % after 5 years (p < 0.001). Median patient follow-up time was 970 days (∼2.7 years) and follow up-range was between 1 and 2710 days (∼7.4 years).
Fig. 1.
Mortality and adverse cardiovascular events survival curves by baseline pulse pressure (mmHg).
Long-term survival of the pooled cardiovascular outcome trial participants, including the numbers at risk, stratified by baseline pulse pressure (≥60 vs. <60 mm Hg) for A. Overall Death, MI, and Stroke and B. CV Death, MI, and Stroke. Both survival curves demonstrate that an elevated baseline pulse pressure is indicative and predictive of worse outcomes. Cumulative KM estimates are given at 5 years for each group. The number at risk each year is displayed under each figure. Abbreviations: CV, cardiovascular; MI, myocardial infarction.
Cox regression analysis was performed for the overall cohort and across multiple subgroups, grouped by age, sex, race, history of prior CV disease, or presence of CV risk factors (diabetes and chronic kidney disease). PP was treated as a continuous predictor variable (Table 2) as well as a categorical predictor variable (Central Illustration). For every 10 mm Hg increase in PP, there was an increased risk of overall death, MI, or stroke (unadjusted HR 1.20, 95 % CI 1.17 to 1.23, p < 0.001). For every 10 mm Hg increase in PP, there was an identical increased risk of CV death, MI, or stroke (unadjusted HR 1.20, 95 % CI 1.17 to 1.23, p < 0.001).
Table 2.
Association of baseline pulse pressure per 10 mmHg increase with adverse outcomes.
| Overall Death/MI/Stroke* |
CV Death/MI/Stroke⁎⁎ |
|||
|---|---|---|---|---|
| Hazard ratio (95 % CI) | P-value | Hazard ratio (95 % CI) | P-value | |
| Overall (Unadjusted) | ||||
| Unadjusted | 1.20 (1.17, 1.23) | <0.001 | 1.20 (1.17, 1.23) | <0.001 |
| Adjusted | 1.11 (1.08, 1.14) | <0.001 | 1.12 (1.09, 1.15) | <0.001 |
| Subgroups (Adjusted) | ||||
| Age | ||||
| ≥60 | 1.09 (1.07, 1.12) | <0.001 | 1.11 (1.07, 1.14) | <0.001 |
| <60 | 1.18 (1.11, 1.24) | <0.001 | 1.17 (1.11, 1.24) | <0.001 |
| Sex | ||||
| Men | 1.08 (1.05, 1.11) | <0.001 | 1.09 (1.05, 1.12) | <0.001 |
| Women | 1.15 (1.11, 1.20) | <0.001 | 1.17 (1.12, 1.22) | <0.001 |
| Race | ||||
| White | 1.10 (1.07, 1.13) | <0.001 | 1.11 (1.08, 1.14) | <0.001 |
| Black | 1.14 (1.03, 1.26) | 0.011 | 1.17 (1.05, 1.30) | 0.004 |
| Asian | 1.16 (1.07, 1.26) | <0.001 | 1.14 (1.05, 1.25) | 0.003 |
| Other race | 1.16 (1.04, 1.29) | 0.006 | 1.15 (1.02, 1.29) | 0.023 |
| Ethnicity | ||||
| Hispanic/latino | 1.14 (1.05, 1.24) | 0.003 | 1.16 (1.06, 1.28) | 0.002 |
| Non-hispanic/latino | 1.12 (1.09, 1.15) | <0.001 | 1.13 (1.10, 1.17) | <0.001 |
| Medical history | ||||
| CAD | 1.10 (1.06, 1.14) | <0.001 | 1.11 (1.07, 1.16) | <0.001 |
| No CAD | 1.11 (1.08, 1.15) | <0.001 | 1.12 (1.08, 1.16) | <0.001 |
| Stroke | 1.02 (0.95, 1.10) | 0.53 | 1.00 (0.92, 1.08) | 0.99 |
| No stroke | 1.12 (1.09, 1.15) | <0.001 | 1.13 (1.10, 1.17) | <0.001 |
| Type 2 diabetes mellitus | 1.10 (1.07, 1.14) | <0.001 | 1.12 (1.08, 1.16) | <0.001 |
| No type 2 diabetes mellitus | 1.12 (1.08, 1.16) | <0.001 | 1.12 (1.07, 1.17) | <0.001 |
| eGFR ≥ 60 | 1.10 (1.07, 1.14) | <0.001 | 1.11 (1.07, 1.15) | <0.001 |
| eGFR < 60 | 1.10 (1.06, 1.15) | <0.001 | 1.12 (1.07, 1.17) | <0.001 |
Adjusted for age, sex, body mass index, race, smoking, coronary artery disease, peripheral arterial disease, type 2 diabetes mellitus, history of heart failure, stroke, transient ischemic attack, myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, baseline low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), eGFR, calcium channel blocker usage, statin usage, thiazide usage, mineralocorticoid receptor antagonist usage, trial.
Adjusted for age, sex, body mass index, race, smoking, coronary artery disease, peripheral arterial disease, type 2 diabetes mellitus, hypertension, history of heart failure, stroke, myocardial infarction, percutaneous coronary intervention, coronary artery bypass grafting, baseline low density lipoprotein-cholesterol (LDL-C), high density lipoprotein-cholesterol (HDL-C), eGFR, calcium channel blocker usage, statin usage, mineralocorticoid receptor antagonist usage, trial.
Abbreviations: CAD, coronary artery disease; CV, cardiovascular; eGFR, estimated glomerular filtration rate; MI, myocardial infarction.
Analyzed subgroups are seen in Table 2 and the Supplementary Appendix. A wide PP was associated with higher risk in patients < 60 years of age compared with age ≥ 60. Age ≥ 60 was associated with a HR of 1.09 (1.07 to 1.12, p < 0.001) for overall death, MI, or stroke and a HR of 1.11 (1.07 to 1.14, p < 0.001) for CV death, MI, or stroke while age < 60 was associated with a HR of 1.18 (1.11 to 1.24, p < 0.001) for overall death, MI, stroke and a HR of 1.17 (1.11–1.24, p < 0.001) for CV death, MI, or stroke. Although increasing PP was a risk factor in both men and women, every 10 mm Hg increase in PP was associated with higher risk in women (HR 1.15, 95 % CI 1.11 to 1.20, p < 0.001) compared with men (HR 1.08, 95 % CI 1.05–1.11, p < 0.001). While most subgroups demonstrated an increased risk as PP increased, PP was not associated with adverse outcomes among individuals with a history of stroke (HR 1.00, 95 % CI 0.92 to 1.08, p < 0.001).
Univariate and multivariable models generated in this analysis for PP, systolic BP, and diastolic BP were evaluated using the AIC and C-index (Table 3). From this, in both the univariate and multivariable adjusted models, PP had a lower AIC and a higher C-Index compared to other models based on SBP and DBP - lower AIC and higher C-Index indicate better fitting and a more predictive model.
Table 3.
AIC and C-index of predictive univariate and multivariable models of pulse pressure upon mortality.
| Univariate model | Multivariable model | |
|---|---|---|
| AIC (lower is better) | ||
| Baseline PP | 68,085 | 63,692 |
| Baseline SBP | 68,187 | 63,709 |
| Baseline DBP | 68,273 | 63,759 |
| C-index (higher is better) | ||
| Baseline PP | 0.5714 | 0.7075 |
| Baseline SBP | 0.5570 | 0.7071 |
| Baseline DBP | 0.5092 | 0.7030 |
Abbreviation: AIC, akaike information criterion; DBP, diastolic blood pressure; PP, pulse pressure; SBP, systolic blood pressure.
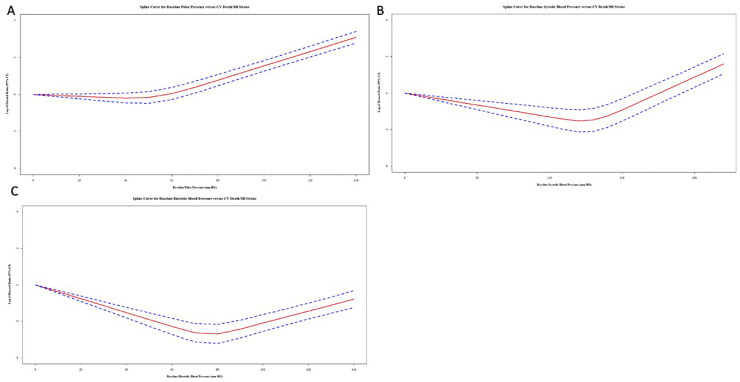
The unadjusted spline curves obtained from univariate models of PP, systolic BP, and diastolic BP are presented in Fig. 2 and illustrate the relationship between baseline PP, systolic BP, and diastolic BP with CV death, MI, and stroke. J-curves are apparent for all models with lowest hazard associated with a PP of 40 mmHg, systolic BP of 120 mmHg, and a diastolic BP of 80 mmHg and hazards increasing below and above these thresholds.
Fig. 2.
Spline curves of log-transformed cox proportional hazard ratios for selected blood pressure measures (mmHg) versus cardiovascular death, myocardial infarction and stroke.
Spline curves of pooled cardiovascular outcome trial participants, including the 95 % confidence interval, for A. Baseline Pulse Pressure B. Baseline Systolic BP C. Baseline Diastolic BP. All curves demonstrate characteristic J-curves. Abbreviations: BP, blood pressure.
A forest plot demonstrates the HR with 95 % CI comparing baseline PP ≥ 60 mm Hg versus PP < 60 mm Hg on overall death, MI, stroke rate. These HR are provided for the overall cohort as well as by the subgroups of age, sex, race, coronary artery disease, history of stroke, history of type 2 diabetes mellitus, and baseline eGFR group (Central Illustration).
4. Discussion
This study is among the first to use pooled CV outcome trial populations to assess the predictive nature of baseline PP to determine the risk of adverse outcomes among various patient subgroups [27,28]. Among CV outcome trial participants, a wide PP at trial enrollment was predictive of death, MI, and stroke with a high degree of significance. Comparing univariate and multivariable models, the baseline PP outperforms systolic BP and diastolic BP in model fit and predictive power. Most patient subgroups demonstrated similar degrees of increased risk due to a wide PP, which corroborates population cohort studies and analyses of participants in hypertension randomized clinical trials [29]. The overall findings are similar to an analysis of the REACH (Reduction of Atherothrombosis for Continued Health) registry which found, that in addition to PP offering enhanced predictive ability of mortality and major adverse CV events in a high-risk population, it offered prognostic information beyond other blood pressure measures [30].
Certain subgroups demonstrating the greatest associated risk with widening PP were women and individuals under the age of 60. The significance of wide PP demonstrating 18 % increased risk of death, stroke, or MI, for each 10 mm Hg increase in PP among younger individuals is important given conflicting prior data. A 2001 analysis of Framingham Heart Study participants demonstrated that among individuals less than 50 years of age, PP was not a predictor of adverse outcomes, whereas a 2020 analysis of adults aged 18–40 did demonstrate an independent association with mortality with higher PP [28,31]. The difference in risk associated with wide PP in women (15 % per 10 mm Hg increase) compared with men (8 %) is also notable given that the largest study of PP and CV disease related mortality only studied men [32]. It is important to acknowledge the differences in physiology between men and women and the more vital role PP may play in predicting risk among women [33]. The protective effects of estrogen in pre- and peri-menopausal women are well studied in the reduction of vascular deterioration, blood pressure, and PP. The decrease in estrogen in post-menopausal women may cause vascular inflammation and damage moreso than the decrease in testosterone in men [34,35,36]. To our knowledge this is the largest cohort of women to be analyzed regarding the association between PP and adverse outcomes.
Among patients with a prior stroke (n = 3540), PP did not predict death, myocardial infarction, or stroke. Opposing evidence also exists in this population. A 2017 study assessing the association of PP with adverse outcomes after ischemic stroke studied a similar number of patients (n = 4195) and demonstrated an increased risk of a composite endpoint of recurrent vascular events and all-cause mortality after a median follow-up of 23.5 months [37]. Notably the composite endpoint was broader than was assessed in our study and included stroke, TIA, acute coronary syndrome, and peripheral vascular disease. Also, the 2017 study was a predominantly Chinese population. A smaller study that performed 24-h ambulatory blood pressure monitoring (n = 219) in patients following an acute stroke reported no association between PP and mortality after a median follow-up of 2.5 years [38].
Other markers of vascular stiffness may provide value in addition to PP. An analysis of the Rotterdam study found that adding aortic pulse wave velocity provided additional predictive value above cardiovascular risk factors, measures of atherosclerosis, and PP [39]. However, in the absence of central BP monitoring or invasive techniques to obtain arterial stiffness directly, the calculation of PP from brachial BP monitoring serves as a capable surrogate of arterial stiffness with it being highly correlated with more invasive and involved methods [40].
Because arterial stiffness and PP portend such an increased risk of adverse CV events, strategies to reduce arterial stiffness have been tested in the hopes that reducing arterial stiffness may be a potential new therapeutic target [41]. Recent trials demonstrate the superiority of sacubitril/valsartan, compared to angiotensin receptor blockers alone, with respect to lowering central aortic blood pressure and PP [42]. However, among individuals with heart failure with reduced ejection fraction sacubitril-valsartan, compared with enalapril, did not significantly reduce central aortic stiffness [43].
4.1. Study limitations
This study was limited by the retrospective design and the aggregation of trials requiring a harmonization of cohorts and study definitions. Additionally, the BP obtained at baseline was a one-time research BP measurement that may not represent BP trajectory throughout a trial, and it may be biased in patients with white-coat hypertension or a white coat effect. BP measurements were standardized in each trial protocol, however between trials, BP measurement protocols varied, and as such could add a potential source of bias in pooling participant BP values across trials. The present study analyzed participants in cardiovascular outcomes trials, which enrolled individuals at elevated cardiovascular risk. Extrapolation to individuals without elevated cardiovascular risk may not be appropriate.
5. Conclusion
This pooled analysis of CV outcome trial participants summarizes the impact of baseline PP on the risk of death, MI, and stroke in the overall population studied and in various high-risk subgroups. A wide PP is associated with increased mortality and risk of stroke and MI when considered as both a continuous (per 10 mm Hg increase) and as a categorical (PP ≥ 60 mm Hg and PP < 60 mm Hg) variable, even when adjusted for comorbidities. Even as a single baseline measurement, PP must be considered among the best independent markers of short and long-term CV risk.
Declaration of Competing Interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:
Luke Laffin reports a relationship with Medtronic that includes: consulting or advisory. Luke Laffin reports a relationship with Eli Lilly and Company that includes: consulting or advisory. Luke Laffin reports a relationship with Mineralys Therapeutics, Inc. that includes: board membership. Luke Laffin reports a relationship with AstraZeneca Pharmaceuticals LP that includes: funding grants. Luke Laffin reports a relationship with CRISPR Therapeutics that includes: consulting or advisory. Luke Laffin reports a relationship with LucidAct Health that includes: equity or stocks. Luke Laffin reports a relationship with Gordy Health that includes: equity or stocks.
Footnotes
Funding: No funding to disclose.
Disclosures: Luke J. Laffin has been a consultant and/or served on steering committees for Medtronic, Lilly, Mineralys Therapeutics, AstraZeneca, and Crispr Therapeutics; has received research funding from AstraZeneca; and has ownership interest in LucidAct Health and Gordy Health. The other authors have no disclosures.
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.ajpc.2023.100623.
Appendix. Supplementary materials
References
- 1.Bakris G., Ali W., Parati G. ACC/AHA versus ESC/ESH on hypertension guidelines: JACC guideline comparison. J Am Coll Cardiol. 2019;73(23):3018–3026. doi: 10.1016/j.jacc.2019.03.507. [DOI] [PubMed] [Google Scholar]
- 2.Whelton P.K., et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA guideline for the prevention, detection, evaluation, and management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association task force on clinical practice guidelines. Circulation. 2018;138(17):e484–e594. doi: 10.1161/CIR.0000000000000596. [DOI] [PubMed] [Google Scholar]
- 3.Williams B., et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology and the European Society of Hypertension. J Hypertens. 2018;36(10):1953–2041. doi: 10.1097/HJH.0000000000001940. [DOI] [PubMed] [Google Scholar]
- 4.Bakris G., Sorrentino M. Redefining hypertension—Assessing the new blood-pressure guidelines. N Engl J Med. 2018;378(6):497–499. doi: 10.1056/NEJMp1716193. [DOI] [PubMed] [Google Scholar]
- 5.Dart A.M., Kingwell B.A. Pulse pressure—A review of mechanisms and clinical relevance. J Am Coll Cardiol. 2001;37(4):975–984. doi: 10.1016/s0735-1097(01)01108-1. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell G.F. Arterial stiffness and wave reflection: biomarkers of cardiovascular risk. Artery Res. 2009;3(2):56–64. doi: 10.1016/j.artres.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zieman S.J., Melenovsky V., Kass D.A. Mechanisms, pathophysiology, and therapy of arterial stiffness. Arterioscler Thromb Vasc Biol. 2005;25(5):932–943. doi: 10.1161/01.ATV.0000160548.78317.29. [DOI] [PubMed] [Google Scholar]
- 8.Wain L.V., et al. Genome-wide association study identifies six new loci influencing pulse pressure and mean arterial pressure. Nat Genet. 2011;43(10):1005–1011. doi: 10.1038/ng.922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Stead L.G., et al. Impact of acute blood pressure variability on ischemic stroke outcome. Neurology. 2006;66(12):1878–1881. doi: 10.1212/01.wnl.0000219628.78513.b5. [DOI] [PubMed] [Google Scholar]
- 10.Böhm M., et al. Achieved diastolic blood pressure and pulse pressure at target systolic blood pressure (120–140 mmHg) and cardiovascular outcomes in high-risk patients: results from ONTARGET and TRANSCEND trials. Eur Heart J. 2018;39(33):3105–3114. doi: 10.1093/eurheartj/ehy287. [DOI] [PubMed] [Google Scholar]
- 11.Benetos A., et al. Mortality and cardiovascular events are best predicted by low central/peripheral pulse pressure amplification but not by high blood pressure levels in elderly nursing home subjects. J Am Coll Cardiol. 2012;60(16):1503–1511. doi: 10.1016/j.jacc.2012.04.055. [DOI] [PubMed] [Google Scholar]
- 12.Gillebert T.C. Pulse pressure and blood pressure components: is the sum more than the parts? Eur J Prev Cardiol. 2018;25(5):457–459. doi: 10.1177/2047487318755805. [DOI] [PubMed] [Google Scholar]
- 13.Warren J., et al. Impact of pre-procedural blood pressure on long-term outcomes following percutaneous coronary intervention. J Am Coll Cardiol. 2019;73(22):2846–2855. doi: 10.1016/j.jacc.2019.03.493. [DOI] [PubMed] [Google Scholar]
- 14.Saladini F., et al. Office pulse pressure is a predictor of favorable outcome in young- to middle-aged subjects with stage 1 hypertension. Hypertension. 2017;70(3):537–542. doi: 10.1161/HYPERTENSIONAHA.117.09516. [DOI] [PubMed] [Google Scholar]
- 15.Pastor-Barriuso R., et al. Systolic blood pressure, diastolic blood pressure, and pulse pressure: an evaluation of their joint effect on mortality. Ann Intern Med. 2003;139(9):731–739. doi: 10.7326/0003-4819-139-9-200311040-00007. [DOI] [PubMed] [Google Scholar]
- 16.Alderman M.H. A new model of risk: implications of increasing pulse pressure and systolic blood pressure on cardiovascular disease. J Hypertens Suppl. 1999;17(5):S25–S28. [PubMed] [Google Scholar]
- 17.Haider A.W., Larson M.G., Franklin S.S., Levy D. Systolic blood pressure, diastolic blood pressure, and pulse pressure as predictors of risk for congestive heart failure in the Framingham Heart Study. Ann Intern Med. 2003;138(1):10–16. doi: 10.7326/0003-4819-138-1-200301070-00006. [DOI] [PubMed] [Google Scholar]
- 18.Kannel W.B. Elevated systolic blood pressure as a cardiovascular risk factor. Am J Cardiol. 2000;85(2):251–255. doi: 10.1016/s0002-9149(99)00635-9. [DOI] [PubMed] [Google Scholar]
- 19.Weber M.A., et al. Systolic blood pressure and cardiovascular outcomes during treatment of hypertension. Am J Med. 2013;126(6):501–508. doi: 10.1016/j.amjmed.2013.01.007. [DOI] [PubMed] [Google Scholar]
- 20.McEvoy J.W., et al. Diastolic blood pressure, subclinical myocardial damage, and cardiac events. J Am Coll Cardiol. 2016;68(16):1713–1722. doi: 10.1016/j.jacc.2016.07.754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Flint A.C., et al. Effect of systolic and diastolic blood pressure on cardiovascular outcomes. New Engl J Med. 2019;381(3):243–251. doi: 10.1056/NEJMoa1803180. [DOI] [PubMed] [Google Scholar]
- 22.Lincoff A.M., et al. Evacetrapib and cardiovascular outcomes in high-risk vascular disease. N Engl J Med. 2017;376(20):1933–1942. doi: 10.1056/NEJMoa1609581. [DOI] [PubMed] [Google Scholar]
- 23.Nicholls S.J., et al. Effect of high-dose omega-3 fatty acids vs. corn oil on major adverse cardiovascular events in patients at high cardiovascular risk: the STRENGTH randomized clinical trial. JAMA. 2020;324(22):2268–2280. doi: 10.1001/jama.2020.22258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nissen S.E., et al. Cardiovascular safety of celecoxib, naproxen, or ibuprofen for arthritis. N Engl J Med. 2016;375(26):2519–2529. doi: 10.1056/NEJMoa1611593. [DOI] [PubMed] [Google Scholar]
- 25.Lincoff A.M., et al. Effect of aleglitazar on cardiovascular outcomes after acute coronary syndrome in patients with type 2 diabetes mellitus: the AleCardio randomized clinical trial. JAMA. 2014;311(15):1515–1525. doi: 10.1001/jama.2014.3321. [DOI] [PubMed] [Google Scholar]
- 26.Nissen S.E., et al. Effect of naltrexone-bupropion on major adverse cardiovascular events in overweight and obese patients with cardiovascular risk factors: a randomized clinical trial. JAMA. 2016;315(10):990–1004. doi: 10.1001/jama.2016.1558. [DOI] [PubMed] [Google Scholar]
- 27.Nargesi A.A., et al. Nonlinear relation between pulse pressure and coronary heart disease in patients with type 2 diabetes or hypertension. J Hypertens. 2016;34(5):974–980. doi: 10.1097/HJH.0000000000000866. [DOI] [PubMed] [Google Scholar]
- 28.Franklin S.S., et al. Does the relation of blood pressure to coronary heart disease risk change with aging? Circulation. 2001;103(9):1245–1249. doi: 10.1161/01.cir.103.9.1245. [DOI] [PubMed] [Google Scholar]
- 29.Pareek M., et al. Pulse pressure, cardiovascular events, and intensive blood-pressure lowering in the systolic blood pressure intervention trial (SPRINT) Am J Med. 2019;132(6):733–739. doi: 10.1016/j.amjmed.2019.01.001. [DOI] [PubMed] [Google Scholar]
- 30.Selvaraj S., et al. Pulse pressure and risk for cardiovascular events in patients with atherothrombosis. J Am Coll Cardiol. 2016;67(4):392–403. doi: 10.1016/j.jacc.2015.10.084. [DOI] [PubMed] [Google Scholar]
- 31.Li J., et al. Association of pulse pressure with all-cause mortality in young adults. Postgrad Med J. 2020;96(1138):461–466. doi: 10.1136/postgradmedj-2019-137070. [DOI] [PubMed] [Google Scholar]
- 32.Domanski M., et al. Pulse pressure and cardiovascular disease-related mortality: follow-up study of the multiple risk factor intervention trial (MRFIT) JAMA. 2002;287(20):2677–2683. doi: 10.1001/jama.287.20.2677. [DOI] [PubMed] [Google Scholar]
- 33.Wenger N.K., et al. Call to action for cardiovascular disease in women: epidemiology, awareness, access, and delivery of equitable health care: a presidential advisory from the American heart association. Circulation. 2022;145(23):e1059–e1071. doi: 10.1161/CIR.0000000000001071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Xing D., et al. Estrogen and mechanisms of vascular protection. Arterioscler Thromb Vasc Biol. 2009;29(3):289–295. doi: 10.1161/ATVBAHA.108.182279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smulyan H., et al. Comparative effects of aging in men and women on the properties of the arterial tree. J Am Coll Cardiol. 2001;37(5):1374–1380. doi: 10.1016/s0735-1097(01)01166-4. [DOI] [PubMed] [Google Scholar]
- 36.London G.M., et al. Influence of sex on arterial hemodynamics and blood pressure. Role of body height. Hypertension. 1995;26(3):514–519. doi: 10.1161/01.hyp.26.3.514. [DOI] [PubMed] [Google Scholar]
- 37.Su N., et al. Pulse pressure within 3 months after ischemic stroke is associated with long-term stroke outcomes. Am J Hypertens. 2017;30(12):1189–1195. doi: 10.1093/ajh/hpx121. [DOI] [PubMed] [Google Scholar]
- 38.Robinson T.G., et al. Twenty-four hour systolic blood pressure predicts long-term mortality following acute stroke. J Hypertens. 2001;19(12):2127–2134. doi: 10.1097/00004872-200112000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Mattace-Raso F.U.S., et al. Arterial stiffness and risk of coronary heart disease and stroke. Circulation. 2006;113(5):657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- 40.McEniery C.M., et al. Endothelial function is associated with pulse pressure, pulse wave velocity, and augmentation index in healthy humans. Hypertension. 2006;48(4):602–608. doi: 10.1161/01.HYP.0000239206.64270.5f. [DOI] [PubMed] [Google Scholar]
- 41.Safar M.E., Blacher J., Jankowski P. Arterial stiffness, pulse pressure, and cardiovascular disease—Is it possible to break the vicious circle? Atherosclerosis. 2011;218(2):263–271. doi: 10.1016/j.atherosclerosis.2011.04.039. [DOI] [PubMed] [Google Scholar]
- 42.Williams B., et al. Effects of sacubitril/valsartan versus olmesartan on central hemodynamics in the elderly with systolic hypertension. Hypertension. 2017;69(3):411–420. doi: 10.1161/HYPERTENSIONAHA.116.08556. [DOI] [PubMed] [Google Scholar]
- 43.Desai A.S., et al. Effect of sacubitril-valsartan vs. enalapril on aortic stiffness in patients with heart failure and reduced ejection fraction: a randomized clinical trial. JAMA. 2019;322(11):1077–1084. doi: 10.1001/jama.2019.12843. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.