Highlights:
-
•
Recent studies have explored the association of post-mRNA COVID-19 Vaccine-associated Myocarditis.
-
•
This is the most comprehensive study that assessed the association of myocarditis with all types of vaccines, including the non-mRNA vaccines (ChAdOx1-S, Ad26.COV2.S).
-
•
We conducted an exhaustive review of the reported myocarditis cases following COVID-19 vaccines using data from the EudraVigilance database, allowing readers to compare with other databases in terms of incidence rate and outcomes.
Keywords: COVID-19 vaccine, mRNA vaccine, Myocarditis, Myopericarditis
Abstract
Background
Myocarditis secondary to Coronavirus Disease 2019 (COVID-19) vaccination has been reported in the literature.
Objective
This study aimed to characterize the reported cases of myocarditis after COVID-19 vaccination based on age, gender, doses, and vaccine type from published literature and the EudraVigilance database.
Methods
We performed an analysis in the EudraVigilance database (until December 18, 2021) and a systematic review of published literature for reported cases of suspected myocarditis and pericarditis (until 30th June 2022) after the COVID-19 vaccination.
Results
EudraVigilance database analysis revealed 16,514 reported cases of myocarditis or pericarditis due to the vaccination with COVID-19 vaccines. The cases of myo- or pericarditis were reported predominantly in the age group of 18–64 (n = 12,214), and in males with a male-to-female (M: F) ratio of 1.7:1. The mortality among myocarditis patients was low, with 128 deaths (2 cases per 10.000.000 administered doses) being reported. For the systematic review, 72 studies with 1026 cases of myocarditis due to the vaccination with COVID-19 vaccines were included. The analysis of published cases has revealed that the male gender was primarily affected with myocarditis post-COVID-vaccination. The median (IQR) age of the myocarditis cases was 24.6 [19.5–34.6] years, according to the systematic review of the literature. Myocarditis cases were most frequently published after the vaccination with m-RNA vaccines and after the second vaccination dose. The overall mortality of published cases was low (n = 5).
Conclusion
Myocarditis is a rare serious adverse event associated with a COVID-19 vaccination. With early recognition and management, the prognosis of COVID-19 vaccine-induced myocarditis is favorable.
1. Introduction
The coronavirus-19 disease (COVID-19) is associated with significant morbidity and mortality, accounting for 6.45 million deaths and 593 million cases worldwide until August 2022 [1]. The advent of widespread vaccination has decreased the rate of infection spread, the severity of COVID-19, and mortality [2], [3]. However, COVID-19 vaccines have been associated with rare side effects, ranging from mild post-vaccination febrile illness to severe cases of thrombosis or rhabdomyolysis and cases of vaccine-related pericarditis and myocarditis [4], [5]. It is essential to characterize the rare side effects of the COVID-19 vaccine for public health messaging and their appropriate management.
In this paper, we aimed to analyze the reported cases of myo- or pericarditis from the EudraVigilance database and compare the data in relation to the reported and published cases of COVID-19 vaccine-related myopericarditis. In particular, we summarized patients’ demographics, the type of vaccines used, the spectrum of clinical presentations, diagnostic findings, and the prognosis of COVID-19 vaccine-related myocarditis. In addition, we characterized risk factors such as age and gender that may help to detect patients at risk of developing myocarditis after COVID-19 vaccinations. This review will assist healthcare providers by providing the most contemporaneous data related to COVID-19 vaccination with four different vaccine types and its association with myocarditis and help actively advocate for vaccination to a) promote herd immunity, b) decrease COVID-19 incidence and transmission, and c) lower COVID-19 related morbidity and mortality.
2. Methods
2.1. Search strategy
2.1.1. EudraVigilance database analysis
A search was performed in the EudraVigilance database for reported cases of suspected adverse events of myocarditis or pericarditis after the vaccination for COVID-19 until 18 December 2021. The EudraVigilance database relates to suspected side effects reported to EudraVigilance, the EU pharmacovigilance database designed for collecting reports of suspected side effects. The spontaneous cases reported to EudraVigilance come from patients and healthcare professionals and are submitted electronically by national medicines regulatory authorities and pharmaceutical companies.
2.1.2. A systematic review of the published literature
A comprehensive, systematic review of the published literature was conducted and reported following the Cochrane and PRISMA (Preferred reporting items for systematic review and Meta-analysis 2020 guidelines (please refer to Supplementary Fig. 1) [6]. A systematic literature search of electronic databases (Medline via PubMed, Scopus, Cochrane Library, and Google Scholar) for peer-reviewed articles conducted in humans and published in English from inception until June 2022 was performed. Boolean logic was used to perform a database search, and Boolean search operators “AND” and “OR” were used to link search terms. The following search terms were used: “SARS-CoV-2″ OR ”COVID-19″ AND “Vaccine” AND “Myocarditis'' OR ”Pericarditis“ OR ”Myopericarditis.“ We also screened all primary articles' bibliographies for additional cases. The study is registered in Prospero with a registration number CRD42021278080.
2.1.3. Study selection based on a systematic review
We included studies with a history of COVID-19 vaccination followed by reported myocarditis without any restrictions of timeframe. The studies were carefully screened and exported to the endnote reference library software (Clarivate), and the duplicates were removed. An additional manual check was carried out to cross-check for any remaining copies. All studies published were included in the review. A total of 1044 reports were extracted in the initial screening round. Two reviewers (VJ and DM) reviewed the papers based on title, keywords, and abstract. They (VJ and AJ) closely reviewed the articles that passed the initial screening to regulate their appropriateness for inclusion in the systematic analysis. A third reviewer (SN) checked and reviewed again and further assisted in the final study selection process.
2.1.4. Inclusion and exclusion criteria
The studies were reviewed to determine their suitability for inclusion in the systematic review. The following criteria were used for inclusion: studies published in English, either case reports or case series, observational studies, and studies including subjects of any age group who developed myocarditis after receiving the COVID-19 vaccine. We excluded all review articles, letters to the editor, short commentaries, and animal studies.
2.1.5. Data extraction, risk of bias assessment, and statistical analysis
The following data were extracted from the studies: timing from vaccination to symptoms onset, demographic data (study design, country, gender, and age), dosage, type of vaccine, length of hospital stay, laboratory data, imaging data, and comorbidities. Five authors assembled all available information in a shared spreadsheet (NB, RK, DM, SN, VJ). If any required data was missing, written in an incorrect format, or not reported in the paper, the corresponding authors of the respective papers were contacted via email for clarification. Supplementary material1 related to the main article was also investigated in such cases. The other authors conducted a quality assessment by evaluating the criteria for the diagnosis of myocarditis in each study and ensured the occurrence of COVID-19 vaccination preceded the diagnosis of myocarditis. Two investigators (JC and VJ) independently assessed the potential risk of bias using the Newcastle-Ottawa (NOS) scale for observational studies [7], and for case series and case reports, the checklist proposed by Murad et al. was used (Supplementary Table 4,5) [8]. Finally, descriptive statistics were used to summarize the data in this paper. The median and interquartile ranges were adopted to describe continuous variables, whereas frequencies and percentages were used for dichotomous data. All statistical analyses were conducted using the software R version 4.1.2 (available at https://cran.r-project.org/).
3. Results
3.1. EudraVigilance database analysis
According to the EudraVigilance database, 16,514 cases of vaccine-induced myocarditis or pericarditis were reported by 18th December 2021 (Table 1; Fig. 1). A total of 708 million COVID-19 vaccine doses were administered in the European Union/European Economic Area (EU/EEA) by 18th December 2021, according to the European Centre for Disease Prevention and Control.
Table 1.
Crude numbers of vaccine doses distributed and administered in the European Union/European Economic Area (EU/EEA) according to the European Centre for Disease Prevention and Control and reported cases of suspected myocarditis/pericarditis after the vaccination reported to the EudraVigilance database by December 18th, 2021.
| ASTRAZENECA CHADOX1 nCOV19 | PFIZER-BIONTECH TOZINAMERAN |
MODERN CX-024414 |
JANSSEN AD26. COV2. S | |
|---|---|---|---|---|
| Vaccine doses administered | 68.924.870 | 531.638.764 | 89.256.450 | 18.652.082 |
| Suspected cases of myocarditis | 537 | 6364 | 2392 | 141 |
| Suspected cases of pericarditis | 494 | 5234 | 1220 | 132 |
| Reported incidence of suspected myo- or pericarditis per administered doses | 0.0015 % | 0.0022 % | 0.0040 % | 0,0015 % |
| Reported suspected cases of myocarditis- or pericarditis per 1.000.000 administered doses | 15 | 22 | 40 | 15 |
| Male_(n) | 518 | 6949 | 2592 | 187 |
| Female_(n) | 490 | 4408 | 995 | 83 |
| Gender Not Specified_(n) | 23 | 241 | 25 | 3 |
| Myocarditis event reported by a healthcare professional | 450 | 4040 | 1865 | 92 |
| Myocarditis event reported by a non-healthcare professional | 125 | 2695 | 606 | 53 |
|
Myocarditis- or pericarditis. Age < 12 years |
1 | 15 | 0 | 0 |
|
Myocarditis- or pericarditis. age 12–17 years |
2 | 1336 | 101 | 0 |
|
Myocarditis- or pericarditis. age 18–64 years |
718 | 8370 | 2887 | 239 |
|
Myocarditis- or pericarditis. age 65–85 years |
210 | 844 | 269 | 23 |
|
Myocarditis- or pericarditis. age > 85 years |
7 | 92 | 10 | 1 |
| Myocarditis outcome. fatal | 13 | 79 | 27 | 9 |
| Myocarditis outcome. not recovered | 244 | 2057 | 719 | 45 |
| Myocarditis outcome. recovered | 88 | 1253 | 573 | 27 |
| Myocarditis outcome. recovering | 114 | 1720 | 634 | 23 |
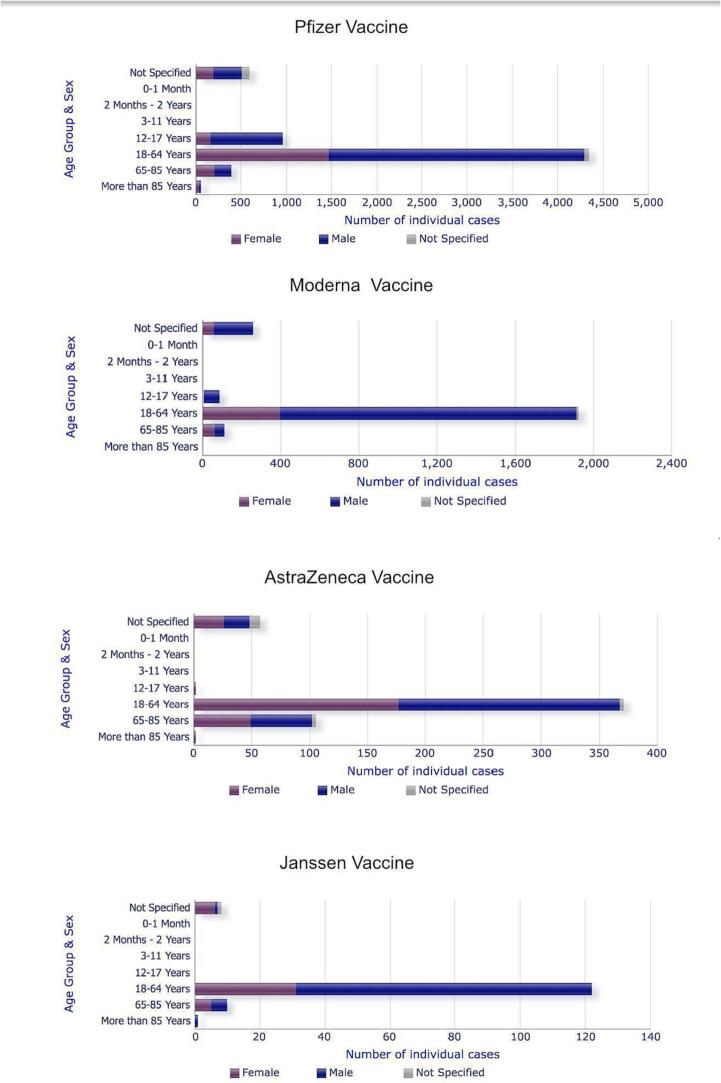
Fig. 1.
Age and gender distribution of suspected myocarditis with different COVID-19 vaccines according to EudraVigilance data analysis reported until December 18th, 2021. Across all the vaccine types, patients aged 18–64 years showed the highest prevalence of suspected myocarditis.
3.1.1. Pfizer-BioNTech (Tozinameran) vaccine
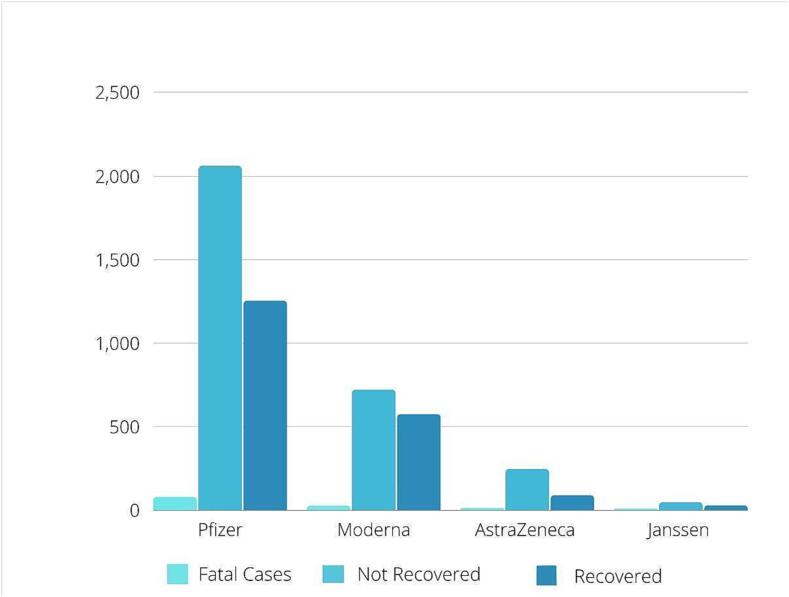
Among 531,638,764 Pfizer-BioNTech (Tozinameran) vaccine doses administered in the EU/EEA, 11,598 (0.0022 %; 22 cases per 1 million administered doses) cases of myocarditis or pericarditis due to the vaccination were reported to the EudraVigilance. The number of myocarditis cases was higher for males than females: 4,174 vs. 2,044 (2:1 ratio) (Fig. 1), and cases of pericarditis did not differ between males and females: 2,775 vs. 2,364 (1.1:1 ratio). The reported cases were predominantly in the age group of 18–64 years (n = 8370), followed by n = 1336 cases for patients in the age category 12–17 years. Fatal outcome among myocarditis cases was reported in 79 patients; 2057 cases were not recovered, and 1253 cases fully recovered at the time point of reporting (Table 1; Fig. 2).
Fig. 2.
Outcome analysis of Eudravigilance database on the reported cases of suspected myocarditis after the vaccination with COVID 19 vaccines. At the time point of reporting, the highest number of patients still did not recover from the disease across all vaccine types, followed by the number of patients recovered. The fatality rate was low for all vaccine types.
3.1.2. Moderna (CX-024414) vaccine
Among 89,256,450 Moderna (CX-024414) vaccine doses administered in the EU/EEA, 3612 (0.0040 %; 40 cases per 1 million administered doses) myocarditis or pericarditis cases due to the vaccination were reported to the EudraVigilance. The number of myocarditis cases was higher for males than females: 1,864 vs. 514 (3.6:1 ratio) (Fig. 1), and cases of pericarditis were also higher for males than females: 728 vs 481 (1.5:1 ratio). The reported cases were predominantly in the age group 18–64 years (n = 2887), followed by 269 cases in the age category 65–85 years. Fatal outcome among myocarditis cases was reported in 27 cases, 719 cases not recovered, and 573 cases fully recovered at the time point of reporting (Table 1; Fig. 2).
3.1.3. AstraZeneca (Chadox1 nCOV19) vaccine
Among 68,924,870 AstraZeneca (Chadox1 nCOV19) vaccine doses administered in the EU/EEA, 1031 (0.0015 %; 15 cases per 1 million administered doses) cases of suspected myocarditis or pericarditis due to the vaccination were reported to the EudraVigilance. There was no sex difference for the reported cases, with 266 reported myocarditis cases being males and 256 females (Fig. 1), and 252 reported pericarditis cases were males and 234 females. The predominant age group among these individuals was 18–64 years (718 suspected cases), followed by 210 cases in the age group of 65–85 years. Fatal outcome among myocarditis cases was reported for 13 cases, 244 cases not recovered, and 88 fully recovered at the time point of reporting (Table 1; Fig. 2).
3.1.4. Janssen (AD26. COV2. S) vaccine
Among 18,652,082 Janssen (AD26. COV2. S) doses administered in the EU/EEA, 273 (0.0015 %; 15 cases per 1 million administered doses) cases of suspected myocarditis or pericarditis due to the vaccination were reported to the EudraVigilance. There was a sex difference, with 98 reported myocarditis cases being males and 42 females (Fig. 1) and 89 reported pericarditis cases being males and 41 females. The 239 cases were in the age group of 18–64 years, followed by 23 cases in the age category of 65–85 years. Fatal outcome among myocarditis cases was reported for 9 cases, 45 cases not recovered, and 27 cases fully recovered at the time point of reporting (Table 1; Fig. 2).
3.2. Systematic review of the literature
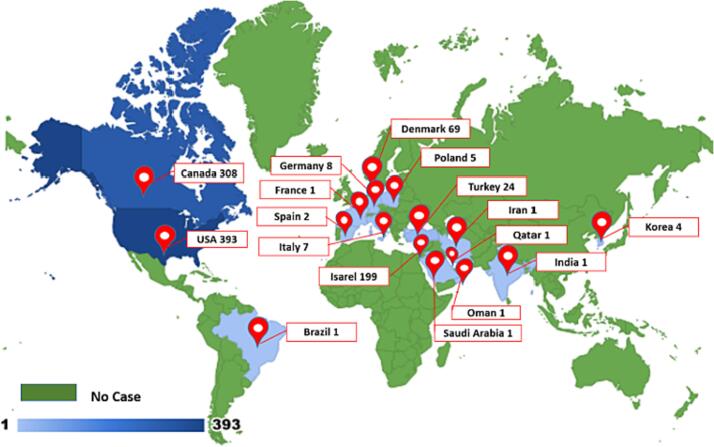
Of the 72 included studies, 37 case reports, 27 case series, 6 cohorts, and 2 cross-sectional studies were identified [4], [9], [10], [11], [12], [13], [14], [15], [16], [17], [18], [19], [20], [21], [22], [23], [24], [25], [26], [27], [28], [29], [30], [31], [32], [33], [34], [35], [36], [37], [38], [39], [40], [41], [42], [43], [44], [45], [46], [47], [48], [49], [50], [51], [52], [53], [54], [55], [56], [57], [58], [59], [60], [61], [62], [63], [64], [65], [66], [67], [68], [69], [70], [71], [72], [73], [74], [75], [76], [77], [78], [79]. Overall, more than 36 million vaccine doses were administered in the reported studies. In these reports, 1026 patients were identified to have developed myocarditis after being vaccinated against COVID-19: 85 % were male, and 15 % were female (Table 2). 393 (38 %) patients were from the United States, 199 (19 %) from Israel, 308 (30 %) from Canada, 69 (7 %) from Denmark, 24 (2.3 %) from Turkey, 8 (0.8 %) from Germany, 7 (0.7 %) from Italy, 5 (0.5 %) from Poland, 4 (0.4 %) from Korea, 2 (0.20 %) from Spain and 1 (0.10 %) patient from each Qatar, France, Saudi Arabia, Iran, Oman, India and Brazil (Table 2; Fig. 3). The median [IQR] age of patients was 24.6 [19.5–34.6] years. From the population reported with myocarditis after being vaccinated against COVID-19, the majority of cases were vaccinated with a Pfizer vaccine (n = 727), followed by the Moderna (n = 237), AstraZeneca (n = 3), and Jansen and Jansen (n = 3) (Table 2). Out of 1026 patients, data regarding the vaccination dose time point (first or second dose) was available for 781 patients. Based on that, 19 % of patients (n = 146) developed myocarditis after receiving the first dose of the vaccine, while 81 % (n = 635) received the second dose of the COVID-19 vaccine in the included study population (Table 2).
Table 2.
Characteristics of published cases with vaccine-associated myocarditis according to the systematic review (number n, percentages, median and interquartile range IQR. COPD = Chronic Obstructive Pulmonary Disease; OSA = Obstructive Sleep Apnea; IVIG = Intravenous immunoglobulin.
| Variable | All patients (*N = 1026) |
|---|---|
| Age, years | 24.6 [19.5–34.6] |
| Sex: | N = 820 |
| Female, n (%) | 123(15 %) |
| Male, n (%) | 697(85 %) |
| Type of vaccine: | N = 972 |
| Moderna, n (%) | 237(24 %) |
| Pfizer, n (%) | 727(75 %) |
| J&J, n (%) | 3(0.3 %) |
| AstraZeneca, n (%)Sinovac n [%]Covaxin n [%] | 3(0.3 %) 1 (0.1 %) 1 [0.1 %] |
| Total vaccine dose | N = 781 |
| Patient with 1st Dose, n (%)Patient with 2nd Dose, n (%) | 146 (19 %) 635 (81 %) |
| Vaccination to Symptom Onset (Days) | 3 (2–5) |
| Country: | N = 1026 |
| USA, n (%) | 393(38 %) |
| Italy, n (%) | 7(0.7 %) |
| Spain, n (%) | 2(0.20 %) |
| Israel, n (%) | 199(19 %) |
| Qatar, n (%) | 1(0.10 %) |
| Germany, n (%) | 8(0.8 %) |
| Korea, n (%) | 4(0.4 %) |
| Iran, n (%) | 1(0.10 %) |
| France Saudi Arabia Brazil Canada Denmark Turkey Oman Poland India |
1(0.10 %) 1(0.10 %) 1(0.10 %) 308(30 %) 69(7 %) 24 [2.3 %] 1 [0.1 %] 5 [0.5 %] 1 [0.1 %] |
| Comorbidities: | |
| Hypertension, n/N (%) | 17(21 %) |
| Diabetes Mellitus, n (%) | 6(8 %) |
| Hyperlipidemia, n (%) | 3(4 %) |
| Obesity, n (%) | 2(29 %) |
| Myocardial diseases, n (%) | 9(7 %) |
| Others | COPD-1, OSA-2, Hypothyroidism-2 |
| Symptoms: | |
| Chest Pain, n (%) | 465/496(94 %) |
| Tachycardia, n (%) | 34/36(94 %) |
| Fever, n (%) | 114/256(44 %) |
| Fatigue, n (%) | 44/134(33 %) |
| Headache, n (%) | 49/163(30 %) |
| Dyspnea, n (%) | 43/161(27 %) |
| Myalgia, n (%) | 67/173(38 %) |
| Laboratory values: | |
| Troponin I(ng/ml) | 8.77 [2.14–14.03] |
| CRP(mg/dl) | 4.36 [2.12–7.90] |
| ESR (mm/hr)BNP [pg/ml] | 21.5 [14–27.5] 271 [56–1865] |
| ECG Findings: | |
| ST-elevation, n (%) | 178/289(62 %) |
| ST depression, n (%) | 9/44(20 %) |
| T wave inversion, n (%) | 47(28 %) |
| Sinus rhythm, n (%) | 148/210(70 %) |
| Echocardiogram findings: | |
| Ejection Fraction (%) | 56 [53], [54], [55], [56], [57], [58], [59], [60], [61] |
| Normal Wall Motion, n (%) | 87/102(85 %) |
| Hypokinesia, n (%) | 59/100(59 %) |
| MRI findings | N = 619 |
| Myocardial Edema, n (%) | 222/368(60 %) |
| Late Gadolinium Enhancements, n (%) | 419/619(68 %) |
| Myocardial Fibrosis, n (%)Epicardial Fibrosis, n (%) | 5/11 (45 %)4 (100 %) |
| Biopsy, n | 8 |
| Management: | |
| Steroids/Corticosteroid, n (%) | 121/328(36 %) |
| Colchicine, n (%) | 96/328=(29 %) |
| NSAID, n (%) | 245/295(83 %) |
| IVIGASPIRIN | 57/305(19 %)37/140 (26 %) |
| Outcome | N = 273 |
| Mortality, n (%) | 5(2 %) |
| Survived, n (%) | 268 [98 %] |
| Length of hospitalization(days) | 3 (2–5) |
Fig. 3.
Geographical Distribution of Cases of Myocarditis after Vaccination (Country, No of cases) based on the systematic literature search.
3.3. The timing of symptom onset
The median timing of symptom onset of myocarditis was 3 (2–5) days. The median timing of symptom onset of myocarditis after the first and second dose of vaccination were 5.5 (3–11.5) and 3 (2–4) days, respectively.
3.4. Patient's comorbidities
Obesity (29 %) and hypertension (17 %) were the most common comorbidities. Data regarding comorbidities were rarely reported and are listed in (Supplementary Table 4,5).
3.5. Symptoms
Symptoms were variably reported in different studies, thus making the total number of patients with a particular symptom different for each symptom. The proportion of each symptom was: 94 % patients presented with chest pain (465/496), 94 % of patients presented with tachycardia (34/36), 44 % patients presented with fever (114/256), 38 % patients presented with myalgia (67/173), 33 % patients presented with fatigue (44/134), 30 % patients presented with a headache (49/163) and 27 % patients presented with dyspnea (42/160; Table 2).
3.6. Biomarkers
Data on biomarkers was available from 40 studies. The levels of the biochemical markers were expressed as median and interquartile range and were as follows: troponin I 8.77 [2.14–14.03] ng/mL, C-reactive protein [CRP] 4.36 [2.12–7.90] mg/dL; erythrocyte sedimentation rate (ESR) 21.5 [14–27.5] mm/hour; brain natriuretic peptide (BNP) 271 [56–1865] pg/ml (Table 2, Supplementary Table 2).
3.7. Electrocardiographic data
Electrocardiographic (ECG) data were variably reported in different studies: 62 % of patients presented with ST-elevation (178/289), 20 % of patients presented with ST depression (9/44), 28 % of patients had T wave inversion (47/169), and 70 % were reported to have a sinus rhythm on ECG (148/210) (Table 2, Supplementary Table 2).
3.8. Findings in cardiac imaging
Echocardiography findings were reported for 400 patients. The median left ventricular ejection fraction was 56 % [53], [54], [55], [56], [57], [58], [59], [60], [61] at the time point of diagnosis. In 87 of 102 patients, normal wall motion was reported, and in 59 of 100 patients, wall hypokinesis was described (59 %) (Table 2).
Cardiac Magnetic Resonance Imaging (CMRI) data, the gold-standard test for diagnosing myocarditis, was available for 619 patients (Supplementary Table 3). Myocardial edema was described in 60 % of cases (222/368). Late gadolinium enhancement was found in 68 % (419/619) of patients. Myocardial fibrosis was found in 5 of 11 patients (45 %), and epicardial fibrosis was reported in 4 of 4 patients (100 %). Biopsy was performed in only 8 patients (Table 2).
3.9. Treatment
The following drugs were used for the treatment of myocarditis: nonsteroidal anti-inflammatory drugs [NSAID] (overall: 83 %; 245/295), colchicine (29 %; 96/328), steroids (36 %) (121/328), intravenous immune globulin [IVIG] (19 %;57/305) (Table 2, Supplementary Table 3).
3.10. Outcome
The outcome was reported for 273 patients: 268 patients survived (98 %), and 5 patients died (2 %) [Pfizer = 3, Moderna = 1, Janssen = 1]. The median (IQR) length of hospitalization was short: 3 (2–5) days (Table 2, Supplementary Table 3).
4. Discussion
To our knowledge, this is the first research article combining the analysis of COVID-19 vaccination-associated myocarditis after the use of four different vaccine types (two mRNA- and two vector-based vaccine types) from the EudraVigilance database and the systematic review of the literature addressing the occurrence, symptomatology, clinical findings, and outcomes of myocarditis in COVID-vaccinated individuals.
The World Health Organization (WHO) and The Global Advisory Committee on Vaccine Safety (GACVS) reviewed initial reports of myocarditis after the administration of COVID-19 vaccination and issued an advisory statement on 26th May 2021 [80]. After this statement, more data in multiple countries have become available as the number of individuals receiving the COVID-19 vaccine and reporting myocarditis after vaccination increased. Suspected cases of myocarditis and pericarditis were reported to EudraVigilance in Europe. As of June 2021, 40.6 cases of myocarditis per million vaccine second doses administered among males and 4.2 cases per million-second vaccine doses administered among females in individuals 12–29 years of age were reported based on data from the US Vaccine Adverse Events Reporting System (VAERS) [81]. The reported numbers declined in males and females over 30 years to 2.4 and 1.0 per million-second vaccine doses, respectively [80]. As of 3 December 2021, the VAERS data search showed 1977 confirmed reports of myocarditis in all age groups post-COVID-19 vaccination. Most of these cases are reported in male adolescents and young adults after the second dose of the COVID-19 vaccine [80]. A study conducted from December 2020 to August 2021 on VAERS data reported 1991 reports of myocarditis among 192,405,448 persons receiving a total of 354,100,845 mRNA-based COVID-19 vaccines. They found a median age of 21 years (16–31 years) and a median time to symptom onset as 2 days (1–3 days) [1]. The reported rate of myocarditis in the study was highest after the second vaccination dose in adolescents and young men aged 18 to 24 years. The myocarditis was mild in most cases, and the most common treatment was non-steroidal anti-inflammatory drugs in 87 % of patients. A similar finding was reported in a study by Hajjo et al. in which myocarditis was mostly seen in the 18–29 years age group and after a second dose of vaccination [82]. Our findings are in agreement with the observed reports [83], with the affected median (IQR) age of individuals in our study of 24.6 years, predominantly males (85 %), and in 81 % of patients receiving a 2nd dose of COVID-19 vaccine.
Historically, post-vaccination myocarditis has been strongly associated with the smallpox vaccine [84], and isolated cases have been reported after influenza, tetanus, human papillomavirus, and hepatitis B vaccinations [85]. The exact pathophysiology of post-vaccination myocarditis remains unknown. Endomyocardial biopsy and autopsy reports have shown lymphocytic and eosinophilic infiltration adjoining myositis, suggesting an immune-mediated injury or hypersensitivity reaction [86]. As symptoms of post-COVID-19 vaccination myocarditis were mild with rapid clinical improvements, endomyocardial biopsies could not be performed to generate adequate clinical evidence [12], [87]. Although myocarditis is rare and tends to resolve spontaneously in most cases, it may lead to life-threatening arrhythmias, cardiomyopathy, and heart failure [88].
4.1. Sex
The EudraVigilance database analysis shows that cases of myo- or pericarditis were reported predominantly in males. The analysis of published cases in our systematic review also revealed that the male sex was primarily affected by myocarditis. This finding is synonymous with the study conducted on VAERS data that reported that males comprised 82 % of the myocarditis cases [89]. Although the causes and pathophysiology for the male predominance in post-COVID-19 vaccination myocarditis are currently unknown, increasing evidence suggests that sex and sex hormones profoundly impact immune responses. Following vaccination, females generally experience more adverse reactions than males and develop higher antibody responses. Indeed, protective antibody responses are twice as high in women as compared to men after vaccination against influenza, rubella, measles, mumps, hepatitis A and B, yellow fever, herpes simplex 2, dengue viruses, rabies, or smallpox [90]. These sex differences seem to depend on circulating estradiol levels and diminish with increasing age [91]. Generally, men are more likely to develop myocarditis and post-myocarditis heart failure than women [92] and an excess risk of myocarditis during SARS-CoV-2 infection has recently been described in young males [93]. These sex differences in immune responses in cardiac tissue seem to depend on age as a pro-inflammatory shift in aged female hearts, but not in male hearts, has recently been demonstrated [94]. Finally, a gender influence on the reporting of vaccine-related adverse events might also account for the lower incidence of COVID-19 vaccine-related myocarditis in women. Accordingly, Pilote and coworkers have recently shown that institutionalized bias against women in healthcare settings was positively associated with the male-to-female ratio of reported COVID-19 cases in countries with higher gender inequality [95]. In addition, a previous study suggested that diagnostic evaluation, including ECG, lab markers, echo, and MRI, were performed more commonly in males presenting with chest pain after vaccination, even though the prevalence of chest pain in females was higher than in males in that study [83].
4.2. Age
Our analysis shows that the median age for post-COVID vaccination myocarditis was 24.6 years. Our observations are similar to the results published in the current literature, showing that people younger than 30 years had a higher incidence of post-vaccine myocarditis [96], [97]. The number of cases in the EugraVigilance database was highest in the age group of 18–64, as individuals in these age categories received the highest number of doses and were the largest age group by size. CDC update from VAERS data reports similar findings where expected cases of myocarditis far exceed the observed number of cases post-COVID-19 vaccines. Maximum cases were reported in age groups of 18–24 years for both males and females, followed by the adolescent age group in males and 30–39 years in males. Extensive studies need to be conducted on an ongoing basis by sub-grouping age groups and stratifying them by the number of doses received to explain the differences in the incident rates by age [98].
4.3. Timing of myocarditis
Our systematic analysis of the literature confirms the results reported by the Center for Disease Control (CDC). The median interval of symptom onset was 3 [2], [3], [4], [5] days post-vaccination, with 92 % of patients experiencing symptom onset within seven days of vaccination, 96 % of patients meeting the criteria for myocarditis were hospitalized, experienced an acute clinical course with mild symptoms with a discharge rate of 95 % and 0 % mortality [81].
4.4. Outcome
Our study shows that the mortality of COVID-19 vaccine-related myocarditis is higher than the mortality associated with myocarditis caused by the COVID-19 infection [99]. Nevertheless, acute care physicians should consider myocarditis as a differential diagnosis in patients presenting with cardiac symptoms within 40 days of receiving COVID-19 vaccinations. Affected patients need to be followed up longitudinally on a global level to determine the cause and sequelae of post-vaccination myocarditis [80].
4.5. Differences in the incidence of myocarditis between the vaccines
Our report summarizes the reported incidence of myocarditis after COVID-19 vaccination with all four vaccine types used in Europe. It is not clear why there are differences in the incidence of myocarditis between the vaccines. Our study results show that the incidence of post-COVID-19 vaccine myocarditis is higher with m-RNA vaccines, particularly higher with Moderna Vaccine (40 cases per 1 million doses administered) vs Pfizer Vaccine (22 cases per 1 million doses administered). In contrast, our systematic literature shows that myocarditis cases were most frequently published after the vaccination with the Pfizer-BioNTech vaccine (n = 710). The EudraVigilance data correlate with the results from a cohort study that reported a pooled incidence rate ratio comparing Moderna and Pfizer as 1·43, with an excess risk of 29 cases per million doses in Moderna recipients compared with Pfizer recipients [97]. Additionally, Karlstad et al. conducted a cohort study on 23.1 million Nordic residents and reported that the second vaccine dose was associated with a higher risk of myocarditis. They reported a higher adjusted incident rate ratio (IRR) with the Moderna vs Pfizer vaccine: adjusted IRRs of 6.57 for the Moderna vaccine and 1.75 for the Pfizer vaccine [100]. Reports have also suggested that Pfizer-BioNTEch was used in 164 countries while Moderna was used in 11 countries [101]. Current literature suggests that the overall incidence rates of myocarditis after mRNA vaccines exceeded non-mRNA vaccines [100], [101].
One possible explanation for a lower incidence of myocarditis after the vector vaccine might be due to the replication of the incompetent adenovirus vectored COVID-19 vaccines showing a broad tissue tropism [102]. While little is known about the tissue tropism of mRNA-based vaccines, their instability at higher temperatures would suggest uptake predominantly at the site of administration, i.e., in the muscle tissue. Transcription of mRNA is followed by the expression of the spike protein on the cell surface. These high local concentrations of the antigen will coincide with the inevitable local muscle trauma at the vaccination site, which will result in the leakage of cytosolic muscle proteins [103]. It is possible that this coincidence will trigger autoreactive antibodies in susceptible individuals that cross-react with myocardial tissue. If this is the case, subcutaneous injection should be explored, which may increase the tolerability and cardiac safety of mRNA vaccines [104].
4.6. CMR for diagnosis of myo/pericarditis
Due to nonspecific clinical presentation and paucity of specific biomarkers, cardiac imaging stands out to play an important role in diagnosing myo/pericarditis. Many international guidelines have recommended CMR as a class I evidence [105], [106], [107]. CMR establishes the diagnosis of suspected myocarditis; hence, any patient after vaccination with clinical symptoms of myo/pericarditis should undergo CMR [108]. It is equally important to follow up vaccine-associated myocarditis with cardiac MRI despite showing good results of complete resolution of edema [109].
4.7. Strengths and Limitations
Apart from mRNA COVID-19 vaccines, this is the very first and most comprehensive review that has addressed the incidence of myocarditis after AstraZeneca vaccination. Secondly, we have included exhaustive European data from the EudraVigilance database and provide readers with a comparison with the USA VAERS database in terms of incidence rate and outcomes with different vaccine types. Our study has several limitations: a) due to the invasive nature of the procedure, a surge in COVID-19 cases, good response to conservative treatment with a self-limiting disease course, biopsy has not been performed in the majority of the available cases, the diagnosis of myocarditis or pericarditis following COVID-19 vaccination does not necessitate a diagnosis of vaccine-induced myo (or peri) carditis. The fatal outcome may not always have been due to myopericarditis.; b) missing or incompletely reported data for gender, age, and dose number may have caused discrepancies in the final outcomes- the use of a database/registry opens up the data to under-reporting (as not all would have been captured) and over-reporting (as suspected cases might have been proven to be another diagnosis altogether). and c) the exact number of mRNA vaccines administered for Pfizer and Moderna is not available, and we could not provide the myocarditis risk associated with specific mRNA vaccine types. Furthermore, data on the reported cases of myocarditis and the number of vaccine doses administered are coming from two different databases, which are not connected. Therefore, data on incidences reported for a specific age group according to the vaccine doses administered could not be calculated. Lastly, the majority of studies haven’t reported fulminant and non-fulminant myocarditis-related data. We were not able to report it in our analysis.
4.8. Future Directions
Future research needs to determine specific etiology and pathophysiology relating to the role of immune cells, molecular mimicry, and autoantibody formation in the development of COVID-19-acquired immunity post-COVID-19 infection and vaccination and myocardial injury. It is essential to characterize post-vaccination myocarditis structurally and functionally, both at a micro and macro level, using histopathology, immunochemistry, EKG, cardiac biomarkers, and imaging. The risk–benefit ratio of COVID-19 vaccinations for different genders, age groups, ethnicities, and genetic differences needs to be explored, and predisposing factors for the development of myocarditis with COVID-19 or its vaccination be identified. With the emergence of Omicron and Delta variants and 3rd COVID-19 booster of mRNA vaccines, we foresee additional booster vaccinations against COVID-19. Thus, characterization of clinical courses, short/long term outcomes, treatment, and management protocols need to be defined for post-COVID-19 vaccination myocarditis. In addition, the patients presenting with prolonged symptoms need to be followed up prospectively to identify the long-term effects of myocardial inflammation caused by the vaccine on the structure and function of the myocardium.
5. Conclusion
Our study suggests that myocarditis after COVID-19 vaccination is rare, mostly mild, and resolves with conservative treatment. As the risk of cardiac complications due to SARS-CoV-2 infection far exceeds the risks of rare vaccine-related transient myocarditis, which occurs predominantly in young male patients, our data supports COVID-19 vaccination in a general population. In the case of a vaccine-related event, reporting, monitoring of affected patients, and evaluation of reported data will provide evidence-based information to guide management and mitigate vaccine-associated myocyte inflammation.
Author contributions:
VJ and JMSM designed the study; DM, VJ, SN, KL, SM, NB, RK, GH, and JMSM extracted the data. VJ, SPA, KL, JEC, SN, and DT performed the screening and selection; AJ, VJ, JMSM, and DM contributed to the statistical analyses and interpretation of results; VJ, TK, SN, SPA, KL, JEC, CG, BJ, MAM, MP, ABS, VA and SM drafted the manuscript. All authors read and approved the final manuscript.
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
Statement of ethics: Not required.
Acknowledgment of Funding: None
Data availability statement: All data relevant to the study are included in the article or uploaded as supplementary information.
Statement of ethics: Not required.
Acknowledgment of Funding: None
Prospero Registration No: CRD42021278080
CRD42021278080
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.ijcha.2023.101280.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Annie F.H., Alkhaimy H., Nanjundappa A., Elashery A. Association between myocarditis and mortality in COVID-19 patients in a large registry. Mayo Clin Proc Innov Qual Outcomes. 2022;6:114–119. doi: 10.1016/j.mayocpiqo.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Heath P.T., Galiza E.P., Baxter D.N., Boffito M., Browne D., Burns F., et al. Safety and efficacy of NVX-CoV2373 Covid-19 vaccine. N. Engl. J. Med. 2021;385:1172–1183. doi: 10.1056/NEJMoa2107659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thomas S.J., Moreira E.D., Kitchin N., Absalon J., Gurtman A., Lockhart S., et al. Safety and efficacy of the BNT162b2 mRNA Covid-19 vaccine through 6 months. N. Engl. J. Med. 2021;385:1761–1773. doi: 10.1056/NEJMoa2110345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmitt P., Demoulin R., Poyet R., Capilla E., Rohel G., Pons F., et al. Acute Myocarditis after COVID-19 vaccination: a case report. Rev Médecine Interne. 2021;42:797–800. doi: 10.1016/j.revmed.2021.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hafeez M.U., Ikram M., Shafiq Z., Sarfraz A., Sarfraz Z., Jaiswal V., et al. COVID-19 vaccine-associated thrombosis with thrombocytopenia syndrome (TTS): a systematic review and post hoc analysis. Clin. Appl. Thromb. 2021;27 doi: 10.1177/10760296211048815. 107602962110488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., The P.R.I.S.M.A., et al. statement: an updated guideline for reporting systematic reviews. BMJ. 2020;2021 doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.G. Wells, B. Shea, D. O’Connell, j.e. Peterson, V. Welch, M. Losos, et al., The Newcastle–Ottawa Scale (NOS) for Assessing the Quality of Non-Randomized Studies in Meta-Analysis, 2000.
- 8.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological quality and synthesis of case series and case reports. BMJ Evid-Based Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montgomery J., Ryan M., Engler R., Hoffman D., McClenathan B., Collins L., et al. Myocarditis following immunization With mRNA COVID-19 vaccines in members of the US military. JAMA Cardiol. 2021;6:1202. doi: 10.1001/jamacardio.2021.2833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kim H.W., Jenista E.R., Wendell D.C., Azevedo C.F., Campbell M.J., Darty S.N., et al. Patients with acute myocarditis following mRNA COVID-19 vaccination. JAMA Cardiol. 2021;6:1196. doi: 10.1001/jamacardio.2021.2828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Marshall M., Ferguson I.D., Lewis P., Jaggi P., Gagliardo C., Collins J.S., et al. Symptomatic acute myocarditis in 7 adolescents after pfizer-BioNTech COVID-19 vaccination. Pediatrics. 2021;148 doi: 10.1542/peds.2021-052478. e2021052478. [DOI] [PubMed] [Google Scholar]
- 12.Mansour J., Short R.G., Bhalla S., Woodard P.K., Verma A., Robinson X., et al. Acute myocarditis after a second dose of the mRNA COVID-19 vaccine: a report of two cases. Clin. Imaging. 2021;78:247–249. doi: 10.1016/j.clinimag.2021.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dickey J.B., Albert E., Badr M., Laraja K.M., Sena L.M., Gerson D.S., et al. A series of patients with myocarditis following SARS-CoV-2 vaccination with mRNA-1279 and BNT162b2. J. Am. Coll. Cardiol. Img. 2021;14:1862–1863. doi: 10.1016/j.jcmg.2021.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shaw K.E., Cavalcante J.L., Han B.K., Gössl M. Possible association between COVID-19 vaccine and myocarditis. J. Am. Coll. Cardiol. Img. 2021;14:1856–1861. doi: 10.1016/j.jcmg.2021.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abu Mouch S., Roguin A., Hellou E., Ishai A., Shoshan U., Mahamid L., et al. Myocarditis following COVID-19 mRNA vaccination. Vaccine. 2021;39:3790–3793. doi: 10.1016/j.vaccine.2021.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nevet A. Acute myocarditis associated with anti-COVID-19 vaccination. Clin Exp Vaccine Res. 2021;10:196. doi: 10.7774/cevr.2021.10.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vidula M.K., Ambrose M., Glassberg H., Chokshi N., Chen T., Ferrari V.A., et al. Myocarditis and other cardiovascular complications of the mRNA-based COVID-19 Vaccines. Cureus. 2021 doi: 10.7759/cureus.15576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hudson B., Mantooth R., DeLaney M. Myocarditis and pericarditis after vaccination for COVID-19. J Am Coll Emerg Physicians Open. 2021;2:e12498. doi: 10.1002/emp2.12498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dionne A., Sperotto F., Chamberlain S., Baker A.L., Powell A.J., Prakash A., et al. Association of myocarditis with BNT162b2 messenger RNA COVID-19 vaccine in a case series of children. JAMA Cardiol. 2021;6:1446. doi: 10.1001/jamacardio.2021.3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schauer J., Buddhe S., Colyer J., Sagiv E., Law Y., Mallenahalli Chikkabyrappa S., et al. Myopericarditis after the pfizer messenger ribonucleic acid coronavirus disease vaccine in adolescents. J. Pediatr. 2021;238:317–320. doi: 10.1016/j.jpeds.2021.06.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tano E., San Martin S., Girgis S., Martinez-Fernandez Y., Sanchez V.C. Perimyocarditis in adolescents after pfizer-BioNTech COVID-19 vaccine. J Pediatr Infect Dis Soc. 2021;10:962–966. doi: 10.1093/jpids/piab060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chamling B., Vehof V., Drakos S., Weil M., Stalling P., Vahlhaus C., et al. Occurrence of acute infarct-like myocarditis following COVID-19 vaccination: just an accidental co-incidence or rather vaccination-associated autoimmune myocarditis? Clin. Res. Cardiol. 2021;110:1850–1854. doi: 10.1007/s00392-021-01916-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.King W.W., Petersen M.R., Matar R.M., Budweg J.B., Cuervo Pardo L., Petersen J.W. Myocarditis following mRNA vaccination against SARS-CoV-2, a case series. Am Heart J plus Cardiol Res Pract. 2021;8 doi: 10.1016/j.ahjo.2021.100042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rosner C.M., Genovese L., Tehrani B.N., Atkins M., Bakhshi H., Chaudhri S., et al. Myocarditis temporally associated with COVID-19 vaccination. Circulation. 2021;144:502–505. doi: 10.1161/CIRCULATIONAHA.121.055891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Abbate A., Gavin J., Madanchi N., Kim C., Shah P.R., Klein K., et al. Fulminant myocarditis and systemic hyperinflammation temporally associated with BNT162b2 mRNA COVID-19 vaccination in two patients. Int. J. Cardiol. 2021;340:119–121. doi: 10.1016/j.ijcard.2021.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Albert E., Aurigemma G., Saucedo J., Gerson D.S. Myocarditis following COVID-19 vaccination. Radiol Case Rep. 2021;16:2142–2145. doi: 10.1016/j.radcr.2021.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Deb A., Abdelmalek J., Iwuji K., Nugent K. Acute myocardial injury following COVID-19 vaccination: a case report and review of current evidence from vaccine adverse events reporting system database. J. Prim. Care Community Health. 2021;12 doi: 10.1177/21501327211029230. 215013272110292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Minocha P.K., Better D., Singh R.K., Hoque T. Recurrence of acute myocarditis temporally associated with receipt of the mRNA coronavirus disease 2019 (COVID-19) vaccine in a male adolescent. J. Pediatr. 2021;238:321–323. doi: 10.1016/j.jpeds.2021.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Watkins K., Griffin G., Septaric K., Simon E.L. Myocarditis after BNT162b2 vaccination in a healthy male. Am. J. Emerg. Med. 2021;50:815.e1–815.e2. doi: 10.1016/j.ajem.2021.06.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Habib M.B., Hamamyh T., Elyas A., Altermanini M., Elhassan M. Acute myocarditis following administration of BNT162b2 vaccine. Idcases. 2021;25:e01197. doi: 10.1016/j.idcr.2021.e01197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Muthukumar A., Narasimhan M., Li Q.-Z., Mahimainathan L., Hitto I., Fuda F., et al. In-Depth evaluation of a case of presumed myocarditis after the second dose of COVID-19 mRNA vaccine. Circulation. 2021;144:487–498. doi: 10.1161/CIRCULATIONAHA.121.056038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Singh B., Kaur P., Cedeno L., Brahimi T., Patel P., Virk H., et al. COVID-19 mRNA vaccine and myocarditis. Eur. J. Case Rep. Int. Med. 2021;16 doi: 10.12890/2021_002681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Isaak A., Feisst A., Luetkens J.A. Myocarditis following COVID-19 vaccination. Radiology. 2021;301:E378–E379. doi: 10.1148/radiol.2021211766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.McLean K., Johnson T.J. Myopericarditis in a previously healthy adolescent male following COVID-19 vaccination: a case report. Acad. Emerg. Med. 2021;28:918–921. doi: 10.1111/acem.14322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ehrlich P., Klingel K., Ohlmann-Knafo S., Hüttinger S., Sood N., Pickuth D., et al. Biopsy-proven lymphocytic myocarditis following first mRNA COVID-19 vaccination in a 40-year-old male: case report. Clin. Res. Cardiol. 2021;110:1855–1859. doi: 10.1007/s00392-021-01936-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Patrignani A., Schicchi N., Calcagnoli F., Falchetti E., Ciampani N., Argalia G., et al. Acute myocarditis following Comirnaty vaccination in a healthy man with previous SARS-CoV-2 infection. Radiol Case Rep. 2021;16:3321–3325. doi: 10.1016/j.radcr.2021.07.082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hasnie A.A., Hasnie U.A., Patel N., Aziz M.U., Xie M., Lloyd S.G., et al. Perimyocarditis following first dose of the mRNA-1273 SARS-CoV-2 (Moderna) vaccine in a healthy young male: a case report. BMC Cardiovasc. Disord. 2021;21:375. doi: 10.1186/s12872-021-02183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sulemankhil I., Abdelrahman M., Negi S.I. Temporal association between the COVID-19 Ad26.COV2.S vaccine and acute myocarditis: a case report and literature review. Cardiovasc. Revasc. Med. 2022;38:117–123. doi: 10.1016/j.carrev.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim I.-C., Kim H., Lee H.J., Kim J.Y., Kim J.-Y. Cardiac imaging of acute myocarditis following COVID-19 mRNA vaccination. J. Korean Med. Sci. 2021;36:e229. doi: 10.3346/jkms.2021.36.e229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Nassar M., Nso N., Gonzalez C., Lakhdar S., Alshamam M., Elshafey M., et al. COVID-19 vaccine-induced myocarditis: case report with literature review. Diabetes Metab. Syndr. 2021;15 doi: 10.1016/j.dsx.2021.102205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Witberg G., Barda N., Hoss S., Richter I., Wiessman M., Aviv Y., et al. Myocarditis after Covid-19 vaccination in a large health care organization. N. Engl. J. Med. 2021;385:2132–2139. doi: 10.1056/NEJMoa2110737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bautista García J., Peña Ortega P., Bonilla Fernández J.A., Cárdenes León A., Ramírez Burgos L., Caballero D.E. Acute myocarditis after administration of the BNT162b2 vaccine against COVID-19. Rev Esp Cardiol Engl Ed. 2021;74:812–814. doi: 10.1016/j.rec.2021.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Alania-Torres E., Morillas-Climent H., García-Escrivá A., Vinueza-Buitrón P., Poquet-Catalá I., Zorio E., et al. Case report: probable myocarditis after covid-19 mRNA vaccine in a patient with arrhythmogenic left ventricular cardiomyopathy. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.759119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Patel Y.R., Louis D.W., Atalay M., Agarwal S., Shah N.R. Cardiovascular magnetic resonance findings in young adult patients with acute myocarditis following mRNA COVID-19 vaccination: a case series. J. Cardiovasc. Magn. Reson. 2021;23:101. doi: 10.1186/s12968-021-00795-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diaz G.A., Parsons G.T., Gering S.K., Meier A.R., Hutchinson I.V., Robicsek A. Myocarditis and pericarditis after vaccination for COVID-19. J. Am. Med. Assoc. 2021;326:1210. doi: 10.1001/jama.2021.13443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.N. Gautam, P. Saluja, M. Fudim, K. Jambhekar, T. Pandey, S. Al’Aref, A Late Presentation of COVID-19 Vaccine-Induced Myocarditis. Cureus 2021. https://doi.org/10.7759/cureus.17890. [DOI] [PMC free article] [PubMed]
- 47.Verma A.K., Lavine K.J., Lin C.-Y. Myocarditis after Covid-19 mRNA vaccination. N. Engl. J. Med. 2021;385:1332–1334. doi: 10.1056/NEJMc2109975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ambati S., Colon M., Mihic M., Sanchez J., Bakar A. Acute Myopericarditis after COVID-19 Vaccine in Teenagers. Case Rep. Cardiol. 2021;2021:1–5. doi: 10.1155/2021/8268755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Onderko L., Starobin B., Riviere A.E., Hohl P.K., Phillips C.T., Morgan R.B., et al. Myocarditis in the setting of recent COVID-19 vaccination. Case Rep. Cardiol. 2021;2021:1–5. doi: 10.1155/2021/6806500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miqdad M.A., Nasser H., Alshehri A., Mourad A.R. Acute Myocarditis Following the Administration of the Second BNT162b2 COVID-19 Vaccine Dose. Cureus. 2021 doi: 10.7759/cureus.18880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.N.A zdaki, M. Farzad, Q.T. Long, interval and syncope after a single dose of COVID-19 vaccination: (a case report). Pan Afr Med J 40. 2021. https://doi.org/10.11604/pamj.2021.40.67.31546. [DOI] [PMC free article] [PubMed]
- 52.Nguyen T.D., Mall G., Westphal J.G., Weingärtner O., Möbius-Winkler S., Schulze P.C. Acute myocarditis after COVID-19 vaccination with mRNA-1273 in a patient with former SARS-CoV-2 infection. ESC Heart Fail. 2021;8:4710–4714. doi: 10.1002/ehf2.13613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi S., Lee S., Seo J.-W., Kim M., Jeon Y.H., Park J.H., et al. Myocarditis-induced Sudden Death after BNT162b2 mRNA COVID-19 Vaccination in Korea: Case Report Focusing on Histopathological Findings. J. Korean Med. Sci. 2021;36:e286. doi: 10.3346/jkms.2021.36.e286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Williams C.B., Choi J.-I., Hosseini F., Roberts J., Ramanathan K., Ong K. Acute Myocarditis Following mRNA-1273 SARS-CoV-2 Vaccination. CJC Open. 2021:1410–1412. doi: 10.1016/j.cjco.2021.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tailor P.D., Feighery A.M., El-Sabawi B., Prasad A. Case report: acute myocarditis following the second dose of mRNA-1273 SARS-CoV-2 vaccine. Eur Heart J - Case Rep. 2021 doi: 10.1093/ehjcr/ytab319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lim Y., Kim M.C., Kim K.H., Jeong I.-S., Cho Y.S., Choi Y.D., et al. Case report: acute fulminant myocarditis and cardiogenic shock after messenger RNA coronavirus disease 2019 vaccination requiring extracorporeal cardiopulmonary resuscitation. Front Cardiovasc Med. 2021;8 doi: 10.3389/fcvm.2021.758996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Das B.B., Kohli U., Ramachandran P., Nguyen H.H., Greil G., Hussain T., et al. Myopericarditis after messenger RNA coronavirus disease 2019 vaccination in adolescents 12 to 18 Years of Age. J. Pediatr. 2021;238:26–32.e1. doi: 10.1016/j.jpeds.2021.07.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Sara Facetti, Mario Giraldi, Andrea Lorenzo Vecchi, Silvia Rogiani, Daniele Nassiacos. Miocardite acuta in giovane adulto due giorni dopo vaccino Pfizer. G Ital Cardiol 2021. https://doi.org/10.1714/3689.36746. [DOI] [PubMed]
- 59.Riedel P.G., Sakai V.F., Toniasso S.D.C.C., Brum M.C.B., Fernandes F.S., Pereira R.M., et al. Heart failure secondary to myocarditis after SARS-CoV-2 reinfection: a case report. Int. J. Infect. Dis. 2021;113:175–177. doi: 10.1016/j.ijid.2021.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kim D., Choi J.H., Jang J.Y., So O., Cho E., Choi H., et al. A Case Report for Myopericarditis after BNT162b2 COVID-19 mRNA Vaccination in a Korean Young Male. J. Korean Med. Sci. 2021;36:e277. doi: 10.3346/jkms.2021.36.e277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mevorach D., Anis E., Cedar N., Bromberg M., Haas E.J., Nadir E., et al. Myocarditis after BNT162b2 mRNA Vaccine against Covid-19 in Israel. N. Engl. J. Med. 2021;385:2140–2149. doi: 10.1056/NEJMoa2109730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Jain S.S., Steele J.M., Fonseca B., Huang S., Shah S., Maskatia S.A., et al. COVID-19 vaccination-associated myocarditis in adolescents. Pediatrics. 2021;148 doi: 10.1542/peds.2021-053427. e2021053427. [DOI] [PubMed] [Google Scholar]
- 63.Simone A., Herald J., Chen A., Gulati N., Shen A.-Y.-J., Lewin B., et al. Acute Myocarditis following COVID-19 mRNA vaccination in adults aged 18 years or Older. JAMA. Intern. Med. 2021 doi: 10.1001/jamainternmed.2021.5511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Larson K.F., Ammirati E., Adler E.D., Cooper L.T., Hong K.N., Saponara G., et al. Myocarditis after BNT162b2 and mRNA-1273 vaccination. Circulation. 2021;144:506–508. doi: 10.1161/CIRCULATIONAHA.121.055913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Truong D.T., Dionne A., Muniz J.C., McHugh K.E., Portman M.A., Lambert L.M., et al. Clinically suspected myocarditis temporally related to COVID-19 vaccination in adolescents and young adults: suspected myocarditis after COVID-19 vaccination. Circulation. 2022;145:345–356. doi: 10.1161/CIRCULATIONAHA.121.056583. [DOI] [PubMed] [Google Scholar]
- 66.Buchan S.A., Seo C.Y., Johnson C., Alley S., Kwong J.C., Nasreen S., et al. Epidemiology of myocarditis and pericarditis following mRNA vaccination by vaccine product, schedule, and interdose interval among adolescents and adults in Ontario, Canada. JAMA Netw Open. 2022;5:e2218505. doi: 10.1001/jamanetworkopen.2022.18505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Husby A., Hansen J.V., Fosbøl E., Thiesson E.M., Madsen M., Thomsen R.W., et al. SARS-CoV-2 vaccination and myocarditis or myopericarditis: population based cohort study. BMJ. 2021:e068665. doi: 10.1136/bmj-2021-068665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bews H., Bryson A., Bortoluzzi T., Tam J.W., Jassal D.S. COVID-19 vaccination-induced myopericarditis: an imager’s perspective. CJC Open. 2022;4:497–500. doi: 10.1016/j.cjco.2022.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Patel P., Desai D., Ganta N., Tadepalli S., Kata P., Kanukuntla A., et al. Symptomatic myocarditis post COVID-19 vaccination. Cureus. 2022 doi: 10.7759/cureus.24052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Giray D., Epçaçan S. Acute myocarditis following COVID-19 mRNA vaccination: a paediatric case. Cardiol. Young. 2022;32:1178–1180. doi: 10.1017/S1047951121004698. [DOI] [PubMed] [Google Scholar]
- 71.Tiwari A., Karna G., Chakrabarti S.S., Panda P.K., Kaur U. Hyper-eosinophilic syndrome with myocarditis after inactivated SARSCoV-2 vaccination - a case study. Curr. Drug Saf. 2023;18:103–106. doi: 10.2174/1574886317666220509165317. [DOI] [PubMed] [Google Scholar]
- 72.Dursun A.D., Saricam E., Sariyildiz G.T., Iscanli M.D., Cantekin Ö.F. The evaluation of oxidative stress in the young adults with COVID-19 mRNA vaccines induced acute pericarditis- myopericarditis. Int J Gen Med. 2022;15:161–167. doi: 10.2147/IJGM.S347977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Al-Rasbi S., Al-Maqbali J.S., Al-Farsi R., Al Shukaili M.A., Al-Riyami M.H., Al Falahi Z., et al. Myocarditis, pulmonary hemorrhage, and extensive myositis with rhabdomyolysis 12 days after first dose of pfizer-BioNTech BNT162b2 mRNA COVID-19 vaccine: a case report. Am J Case Rep. 2022;23 doi: 10.12659/AJCR.934399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Etuk A.S., Jackson I.N., Panayiotou H. A rare case of myocarditis after the first dose of moderna vaccine in a patient with two previous COVID-19 infections. Cureus. 2022 doi: 10.7759/cureus.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sharbatdaran A., Chahal Y., Molaei M., Bhavsar D. A rare case of COVID-19 vaccine-induced myopericarditis in a young adult. Radiol Case Rep. 2022;17:1916–1920. doi: 10.1016/j.radcr.2022.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ansari U., Britsch S., Rogowski S., Duerschmied D., Papavassiliu T. Case Report: Transient Increase of CMR T1 Mapping Indices in a Patient With COVID-19 mRNA vaccine induced acute myocarditis. Front Cardiovasc Med. 2022;9 doi: 10.3389/fcvm.2022.880717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mohammed L.M., Dhillon V., Bong J.P., Patri J. Myocarditis secondary to COVID-19 mRNA vaccine: a case report. Cureus. 2022 doi: 10.7759/cureus.22345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Puchalski M., Kamińska H., Bartoszek M., Brzewski M., Werner B. COVID-19-Vaccination-Induced Myocarditis in Teenagers: case Series with Further Follow-Up. Int. J. Environ. Res. Public Health. 2022;19:3456. doi: 10.3390/ijerph19063456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.D’Angelo T., Cattafi A., Carerj M.L., Booz C., Ascenti G., Cicero G., et al. Myocarditis After SARS-CoV-2 Vaccination: A Vaccine-Induced Reaction? Can. J. Cardiol. 2021;37:1665–1667. doi: 10.1016/j.cjca.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.World Health Organization. COVID-19 subcommittee of the WHO Global Advisory Committee on Vaccine Safety (GACVS): updated guidance regarding myocarditis and pericarditis reported with COVID-19 mRNA vaccines n.d. https://www.who.int/news/item/09-07-2021-gacvs-guidance-myocarditis-pericarditis-covid-19-mrna-vaccines (accessed October 20, 2023).
- 81.Gargano J.W., Wallace M., Hadler S.C., Langley G., Su J.R., Oster M.E., et al. Use of mRNA COVID-19 Vaccine After Reports of Myocarditis Among Vaccine Recipients: Update from the Advisory Committee on Immunization Practices — United States, June 2021. MMWR Morb. Mortal. Wkly Rep. 2021;70:977–982. doi: 10.15585/mmwr.mm7027e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Hajjo R., Sabbah D.A., Bardaweel S.K., Tropsha A. Shedding the Light on Post-Vaccine Myocarditis and Pericarditis in COVID-19 and Non-COVID-19 Vaccine Recipients. Vaccines. 2021;9:1186. doi: 10.3390/vaccines9101186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Bozkurt B., Kamat I., Hotez P.J. Myocarditis with COVID-19 mRNA vaccines. Circulation. 2021;144:471–484. doi: 10.1161/CIRCULATIONAHA.121.056135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Su J.R., McNeil M.M., Welsh K.J., Marquez P.L., Ng C., Yan M., et al. Myopericarditis after vaccination, Vaccine Adverse Event Reporting System (VAERS), 1990–2018. Vaccine. 2021;39:839–845. doi: 10.1016/j.vaccine.2020.12.046. [DOI] [PubMed] [Google Scholar]
- 85.Mei R., Raschi E., Forcesi E., Diemberger I., De Ponti F., Poluzzi E. Myocarditis and pericarditis after immunization: gaining insights through the vaccine adverse event reporting system. Int. J. Cardiol. 2018;273:183–186. doi: 10.1016/j.ijcard.2018.09.054. [DOI] [PubMed] [Google Scholar]
- 86.Yamamoto H., Hashimoto T., Ohta-Ogo K., Ishibashi-Ueda H., Imanaka-Yoshida K., Hiroe M., et al. A case of biopsy-proven eosinophilic myocarditis related to tetanus toxoid immunization. Cardiovasc Pathol. 2018;37:54–57. doi: 10.1016/j.carpath.2018.10.003. [DOI] [PubMed] [Google Scholar]
- 87.Murphy J.G., Wright R.S., Bruce G.K., Baddour L.M., Farrell M.A., Edwards W.D., et al. Eosinophilic-lymphocytic myocarditis after smallpox vaccination. Lancet. 2003;362:1378–1380. doi: 10.1016/S0140-6736(03)14635-1. [DOI] [PubMed] [Google Scholar]
- 88.Nagano N., Yano T., Fujita Y., Koyama M., Hasegawa R., Nakata J., et al. Hemodynamic collapse after influenza vaccination: a vaccine-induced fulminant myocarditis? Can. J. Cardiol. 2020;36:1554.e5–1554.e7. doi: 10.1016/j.cjca.2020.05.005. [DOI] [PubMed] [Google Scholar]
- 89.Oster M.E., Shay D.K., Su J.R., Gee J., Creech C.B., Broder K.R., et al. Myocarditis Cases reported after mRNA-based COVID-19 vaccination in the US from December 2020 to August 2021. J. Am. Med. Assoc. 2022;327:331. doi: 10.1001/jama.2021.24110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Klein S.L., Jedlicka A., Pekosz A. The Xs and Y of immune responses to viral vaccines. Lancet Infect. Dis. 2010;10:338–349. doi: 10.1016/S1473-3099(10)70049-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Potluri T., Fink A.L., Sylvia K.E., Dhakal S., Vermillion M.S., Vom Steeg L., et al. Age-associated changes in the impact of sex steroids on influenza vaccine responses in males and females. npj Vaccines. 2019;4:29. doi: 10.1038/s41541-019-0124-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Fairweather D., Cooper L.T., Blauwet L.A. Sex and gender differences in myocarditis and dilated cardiomyopathy. Curr. Probl. Cardiol. 2013;38:7–46. doi: 10.1016/j.cpcardiol.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Dagan N., Barda N., Balicer R.D. Adverse effects after BNT162b2 VACCINE AND SARS-COV-2 INFECTION, ACCORDING TO AGE AND Sex. N. Engl. J. Med. 2021;385:2299. doi: 10.1056/NEJMc2115045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Barcena De Arellano M.L., Pozdniakova S., Kühl A.A., Baczko I., Ladilov Y., Regitz-Zagrosek V. Sex differences in the aging human heart: decreased sirtuins, pro-inflammatory shift and reduced anti-oxidative defense. Aging. 2019;11:1918–1933. doi: 10.18632/aging.101881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Tadiri C.P., Gisinger T., Kautzky-Willer A., Kublickiene K., Herrero M.T., Raparelli V., et al. The influence of sex and gender domains on COVID-19 cases and mortality. Can. Med. Assoc. J. 2020;192:E1041–E1045. doi: 10.1503/cmaj.200971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Ling R.R., Ramanathan K., Tan F.L., Tai B.C., Somani J., Fisher D., et al. Myopericarditis following COVID-19 vaccination and non-COVID-19 vaccination: a systematic review and meta-analysis. Lancet Respir. Med. 2022;10:679–688. doi: 10.1016/S2213-2600(22)00059-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wong H.-L., Hu M., Zhou C.K., Lloyd P.C., Amend K.L., Beachler D.C., et al. Risk of myocarditis and pericarditis after the COVID-19 mRNA vaccination in the USA: a cohort study in claims databases. Lancet. 2022;399:2191–2199. doi: 10.1016/S0140-6736(22)00791-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Su JR. Myopericarditis following COVID-19 vaccination : Updates from the Vaccine Adverse Event Reporting System (VAERS) 2021.
- 99.Jaiswal V., Sarfraz Z., Sarfraz A., Mukherjee D., Batra N., Hitawala G., et al. COVID-19 Infection and myocarditis: a state-of-the-art systematic review. J. Prim. Care Community Health. 2021;12 doi: 10.1177/21501327211056800. 215013272110568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Karlstad Ø., Hovi P., Husby A., Härkänen T., Selmer R.M., Pihlström N., et al. SARS-CoV-2 Vaccination and Myocarditis in a Nordic Cohort Study of 23 Million Residents. JAMA Cardiol. 2022;7:600. doi: 10.1001/jamacardio.2022.0583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Holder J. Tracking Coronavirus Vaccinations Around the World. N Y. Times. 2021 [Google Scholar]
- 102.Mendonça S.A., Lorincz R., Boucher P., Curiel D.T. Adenoviral vector vaccine platforms in the SARS-CoV-2 pandemic. npj Vaccines. 2021;6:97. doi: 10.1038/s41541-021-00356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Azzam Z., Krivoy N., Alroy G., Zinder O. Serum Creatine Kinase Levels after a Single Intramuscular Injection—Dependence on Injection Volume. Ann Clin Biochem Int J Lab Med. 1994;31:193–194. doi: 10.1177/000456329403100216. [DOI] [PubMed] [Google Scholar]
- 104.Karer M., Stiasny K., Zeitlinger M., Jilma B. Subcutaneous injection of mRNA vaccines against severe acute respiratory syndrome coronavirus 2: an option for severe bleeding disorders or anticoagulated patients? Blood Coagul. Fibrinolysis. 2021;32:423–424. doi: 10.1097/MBC.0000000000001048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.McMurray JJV, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, et al. ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: The Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology. Developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur Heart J 2012;33:1787–847. https://doi.org/10.1093/eurheartj/ehs104. [DOI] [PubMed]
- 106.Ezekowitz J.A., O’Meara E., McDonald M.A., Abrams H., Chan M., Ducharme A., et al. 2017 Comprehensive Update of the Canadian Cardiovascular Society Guidelines for the Management of Heart Failure. Can. J. Cardiol. 2017;33:1342–1433. doi: 10.1016/j.cjca.2017.08.022. [DOI] [PubMed] [Google Scholar]
- 107.Gulati M., Levy P.D., Mukherjee D., Amsterdam E., Bhatt D.L., Birtcher K.K., et al. AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR Guideline for the Evaluation and Diagnosis of Chest Pain: A Report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. Circulation. 2021;2021:144. doi: 10.1161/CIR.0000000000001029. [DOI] [PubMed] [Google Scholar]
- 108.Urzua Fresno C., Sanchez Tijmes F., Shaw K.E., Huang F., Thavendiranathan P., Khullar S., et al. Cardiac Imaging in Myocarditis: Current Evidence and Future Directions. Can. Assoc. Radiol. J. 2023;74:147–159. doi: 10.1177/08465371221119713. [DOI] [PubMed] [Google Scholar]
- 109.Fronza M., Thavendiranathan P., Karur G.R., Abdel-Qadir H., Udell J.A., Wald R.M., et al. Cardiac MRI and Clinical Follow-up in COVID-19 Vaccine–associated Myocarditis. Radiology. 2022;304:E48–E49. doi: 10.1148/radiol.220802. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.