Abstract
Background
Repetitive experiences of certain stresses evoke feedforward cardiovascular responses via central command (CC)--central signals from the higher brain. However, it is unclear whether the anticipatory cardiovascular responses before pain stimulation occur after repetitive pain experiences and how nitrous oxide/oxygen inhalation (N2O), a sedative widely used in dentistry, affects the responses. We tested the hypothesis that the repetitive cold pressor test (CPT) alters the anticipatory cardiovascular responses, which are attenuated by N2O.
Materials and methods
Beat-to-beat systolic (SBP) and diastolic blood pressure (DBP), heart rate (HR), and finger arterial stiffness (β-stiffness) were measured during the 5-min rest, 30-s countdown (CD) before CPT, 2-min CPT, and 3-min recovery (CPT[1st]) in 15 young adults [age, 28 ± 4 years]. The same protocols were repeated randomly with the second CPT (CPT + CC) or placebo test (PLCB + CC).
Results
SBP and DBP increased from baseline in CPT[1st] and CPT + CC under room air (RA) and 40 % N2O, while SBP was lower under N2O than under RA in CPT[1st]. HR in CPT[1st] was lower under N2O than under RA. The change (Δ) in HR was smaller during CPT[1st] than during CPT + CC under N2O, and a similar trend was observed under RA. ΔSBP by CD was lower under N2O than under RA in CPT[1st] but not in CPT + CC. HR increased with CD in CPT + CC but not in CPT[1st] under both RA and N2O. β-stiffness increased by CD regardless of the pain experience, while it was lower under N2O.
Conclusion
Repetitive pain experiences induce a feedforward HR increase. 40 % N2O decreases vascular stiffness, which may attenuate the anticipatory pressor response only when the feedforward HR increase does not exist.
Keywords: Arterial stiffness, Central command, Feedforward, Heart rate, Nitrous oxide, Pain
Highlights
-
•
Repetitive pain stimuli elicit a feedforward HR increase, a risk factor of cardiovascular events.
-
•
Nitrous oxide/oxygen inhalation decreased pain sensation and peripheral vascular stiffness.
-
•
Nitrous oxide/oxygen inhalation did not decrease HR after repetitive pain experiences.
-
•
It attenuated the pressor response via vasodilation only when HR increase does not exist.
-
•
Even when nitrous oxide sedation is applied in dentistry, to mitigate pain stress must be needed.
1. Introduction
Stress-induced pressor responses are the physiological processes that protect body tissue from damage, namely, the fight-or-flight response. It is also known that the responses will be elicited simply by exposure to the environment after repeated experiences of such stress [1]. For example, heart rate (HR) and blood pressure (BP) increase without peripheral sensory stimulation before exercise in athletes when placed under certain competitive conditions, reducing the oxygen debt incurred due to the latency of the typical responses after the onset of exercise, allowing for efficient oxygen delivery during exercise and immediate repayment of oxygen debt after exercise [2,3]. Such feedforward cardiovascular regulations are mediated by central command (CC) that consists of neural impulses from the motor cortex that irradiate to autonomic neurons in the brain stem, leading to parasympathetic withdrawal and sympathetic activation. CC are effective in life-threatening environments but are undesirable when people are required to stay still against unpleasant stimuli. If this feedforward control system enhances cardiovascular responses, it is more likely that the risk of cardiovascular events and behavioral disorders will increase in repeated stressful environments, such as dental treatments involving pain. However, it remains unknown whether repetitive pain experiences elicit feedforward cardiovascular responses and the underlying mechanism(s).
Nitrous oxide/oxygen (N2O) inhalation is a sedation technique widely used in the dental field for the disabled and medically compromised patients to reduce anxiety and pain sensation [4]. Recently, we applied a cold pressor test (CPT) to elicit a pressor response [5] in young adults and reported that the pressor response to CPT was attenuated with suppressed sympathetic activity when they inhaled 40 % N2O [6]. However, there have been no reports on whether N2O attenuates the feedforward pressor responses.
In this study, we hypothesized that repeated exposure to CPT would evoke centrally mediated feedforward cardiovascular responses and increase the magnitude of the pressor responses during stimulation. We also tested the hypothesis that 40 % N2O would modify the generation of feedforward cardiovascular responses induced by repeated CPT.
2. Materials and Methods
2.1. Participants
Fifteen healthy young adults participated in this study. They were non-smokers and had no overt history of cardiovascular, neuromuscular, or other chronic diseases. Patient were excluded if they were currently under medications and/or had a body mass index >30 kg m−2. The study performed according to the ethical principles of the Declaration of Helsinki. All participants were informed of the study protocol and provided written informed consent. Participants’ physical characteristics are shown in Table 1.
Table 1.
Physical characteristics.
| Variables | n = 15 |
|---|---|
| Sex (M:F) | 9 : 6 |
| Age, year | 28 ± 4 |
| Height, cm | 165.8 ± 9.4 |
| Weight, kg | 62.4 ± 13.9 |
| BMI, kg/m2 | 22.5 ± 3.6 |
Values are mean ± S.D.
2.2. Protocol and measurements
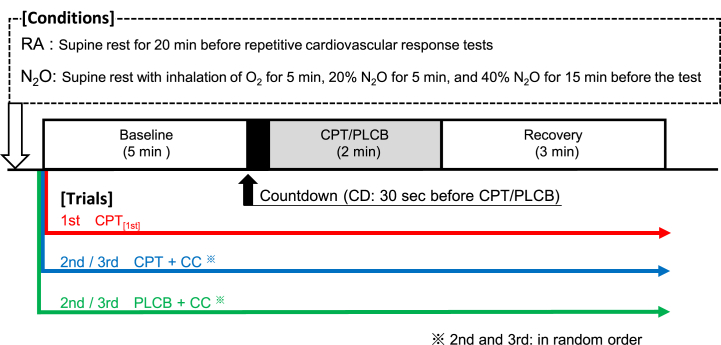
The experiment was performed ≥48 h after the last caffeinated or alcoholic beverage, ≥24 h after strenuous physical activity, and ≥6 h after a light meal. Fluid intake was ad libitum until 6 h before the experiment. The participant entered an environmentally controlled room with an ambient temperature of ∼28 °C and was placed in a supine position. Lead-II electrocardiogram, sphygmomanometry (model PVM-2703; Nihon Koden, Tokyo, Japan), pulse oximeter with photoplethysmography (BP-608 E V®; Colin, Tokyo, Japan), and finger-plethysmography (PORTAPRESTM®; FMS, Enschede, Netherlands) were appropriately attached. They were kept at rest until systolic (SBP) and diastolic BP (DBP) became stable within a range of 5 mmHg for at least three consecutive measurements. Thereafter, they underwent repetitive cardiovascular response tests under two conditions: inhalation of 40 % N2O and room air (RA) on separate days (Fig. 1). The repetitive cardiovascular response test consisted of three sequential trials: the 1st experience of pain stimulation by CPT in which the participants immersed their hand in cold water (∼4 °C) for 2 min (CPT[1st]), a 2nd CPT (CPT + central command (CC)), and a placebo stimulation with warm water (∼35 °C) (PLCB + CC). In each trial, baseline data were recorded for 5 min with the participants resting quietly, a countdown (CD) 30-s before stimulation to determine when CPT/PLCB would be applied, and CPT/PLCB followed by a 3-min recovery. After recovery, the participants rated their subjective pain levels using a Visual Analog Scale (VAS). CPT + CC and PLCB + CC were then repeated randomly in the same manner at 20-min intervals. Beat-to-beat BP waveforms, electrocardiograms, and percutaneous oxygen saturation were continuously recorded throughout the test. Arm cuff BP was measured at the 1st and 4th mins during baseline and 1st and 2nd mins during CPT/PLCB.
Fig. 1.
Timelines for the repetitive cardiovascular response tests in which the participants immersed their hand in cold water (∼4 °C) as pain stimulation for 2 min (CPT). The experiment consists of three trials: the first CPT (CPT[1st]), the second CPT (CPT + CC), and the placebo test with warm water (∼35 °C) (PLCB + CC) under two conditions: room air (RA) and 40 % nitrous oxide/oxygen (N2O) inhalation. Participants performed the 5-min baseline, 30-s countdown (CD) before the stimulation, 2-min CPT/PLCB, and 3-min recovery in each trial.
2.3. Data analysis
Data were stored at a sampling rate of 625 Hz using a data acquisition system (PowereLab®; ADInstruments, Sydney, Australia). The peak and nadir values of the arterial BP waveform were used as beat-to-beat SBP and DBP, respectively. HR per beat was calculated from the product of the reciprocal of the R–R interval and 60. These variables were averaged over 5 min at baseline and every 30 s for CD, CPT/PLCB, and recovery.
2.3.1. Peripheral arterial stiffness
Because the sympathetic nervous system regulates vascular tone [7], an index of peripheral arterial stiffness has been employed for the quantitative evaluation of sympathetic nerve activity. β-Stiffness was calculated using the formula described by Muneyasu et al. [8] from photoplethysmogram and beat-to-beat BP simultaneously measured on the finger and can be used as an index of sympathetic nerve activity because its frequency component is synchronized with HR and correlates with the low-frequency component of SBP as is sympathetic nerve activity. β-Stiffness has also been reported to show significant correlations between brain activity and subjective pain intensity [9,10].
2.3.2. Cardiac baroreflex sensitivity
Cardiac baroreflex sensitivity (BRS) was analyzed using the sequence method and power spectral analysis of the short-term HR and SBP variability by applying an autoregressive methodology [11].
2.4. Statistical analysis
Values are expressed as means ± S.D. All measures passed the normality and equality tests. VAS and cumulative hemodynamic changes during CPT/PLCB were compared using two-way repeated measures ANOVA with conditions [RA and N2O], trials [CPT[1st], CPT + CC, PLCB + CC], and interaction (conditions × trials) as factors. Absolute hemodynamics and changes from baseline (Δ) during CPT/PLCB were examined using two-way repeated measures ANOVA with time, trials, and interaction (time × trials) as factors within each condition and those with time, conditions, and interaction (time × conditions) as factors within each trial. BRS were compared using two-way repeated measures ANOVA with time, conditions, and interaction in each trial. If interactions and/or main factors were found to be significant, the Turkey's method was used as a post-hoc test for multiple comparisons. A p-value of <0.05 was considered statistically significant. Power and sample size calculations were based on a study by Isono et al. [6], showing the differences in increases in SBP during CPT between RA and N2O. For a repeated measure study design, at least 14 subjects need to be studied to reject the null hypothesis that the conditions of RA and N2O are equal with a power of 0.80 and a Type I error probability of 0.05.
3. Results
3.1. Subjective pain intensity
Table 2 lists subjective pain levels during CPT/PLCB in CPT[1st], CPT + CC, and PLCB + CC under RA and N2O. It was lower under N2O than under RA both in CPT[1st] (p = 0.013) and CPT + CC (p < 0.001), but not in PLCB. No difference was observed between CPT[1st] and CPT + CC either under N2O or RA.
Table 2.
VAS for pain intensity.
| RA | N2O | p-value (RA vs N2O) | |
|---|---|---|---|
| CPT[1st] | 74.7 ± 16.2 | 60.9 ± 18.4 | 0.013 |
| CPT + CC | 76.1 ± 13.9 | 50.7 ± 29.0 | <0.001 |
| PLCB + CC | 0.0 ± 0.0 | 0.0 ± 0.0 | 1.000 |
Values are mean ± S.D. VAS indicates visual analog scale. Each variable was compared between conditions under room air (RA) and 40%nitrous oxide (N2O) inhalation using Tukey test (n = 15).
3.2. Hemodynamics
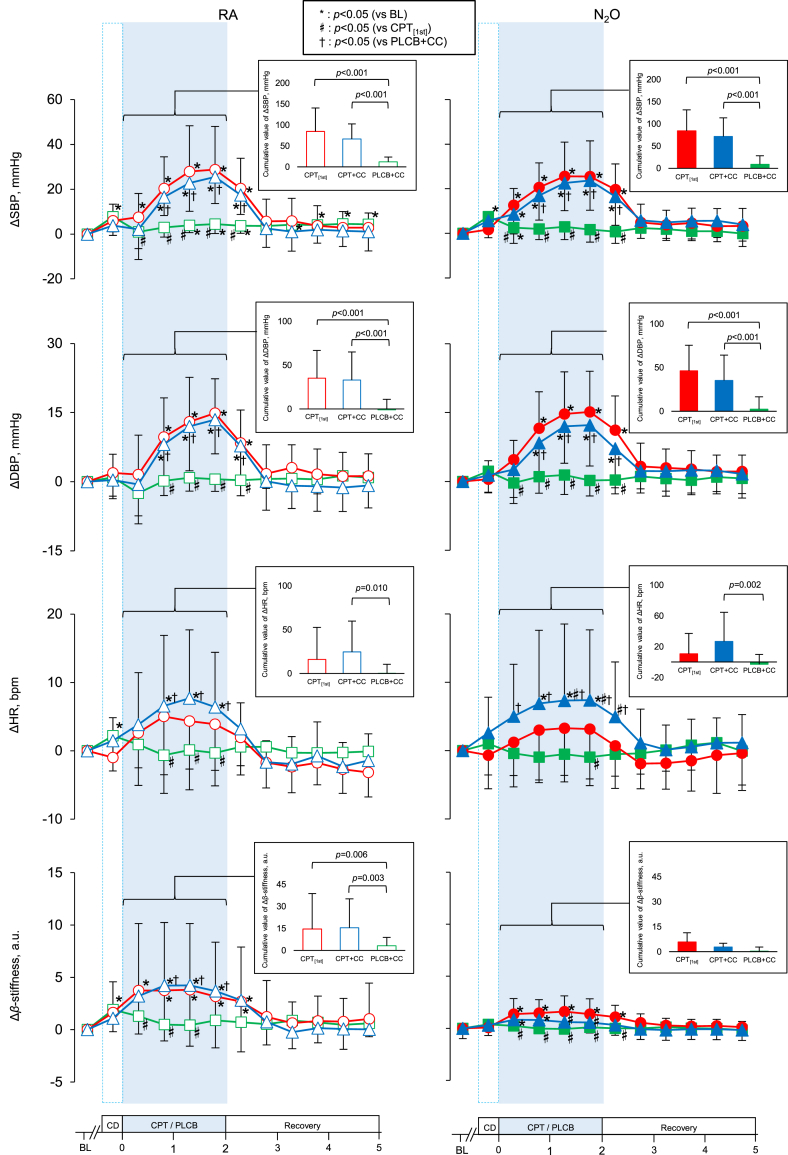
The cardiovascular variables at baseline and CPT/PLCB in each trial under RA and N2O are shown in Table 3. SBP and DBP significantly increased from baseline in CPT[1st] and CPT + CC under both conditions (ps < 0.001), while they remained unchanged in PLCB + CC. SBP during CPT/PLCB was lower under N2O than under RA in CPT[1st] (p = 0.020) but not in CPT + CC. SpO2 was significantly higher under N2O compared to RA during CPT/PLCB, but was similar during baseline. β-Stiffness significantly increased from baseline in CPT[1st] and CPT + CC under both conditions (ps < 0.05), while it was lower during CPT + CC under N2O than under RA. They remained unchanged in PLCB + CC under both conditions.
Table 3.
Cardiovascular variables during baseline and CPT/PLCB.
| RA |
N2O |
||||
|---|---|---|---|---|---|
| Baseline | CPT/PLCB | Baseline | CPT/PLCB | ||
| SBP, mmHg | CPT[1st] | 117.3 ± 10.8 | 135.8 ± 16.2* | 114.3 ± 8.1 | 129.5 ± 12.2*$ |
| CPT + CC | 119.4 ± 12.3 | 135.6 ± 17.8* | 117.1 ± 10.2 | 137.8 ± 14.9* | |
| PLCB + CC | 119.0 ± 13.4 | 121.9 ± 11.9 | 119.4 ± 9.6 | 124.1 ± 13.1 | |
| DBP, mmHg | CPT[1st] | 73.2 ± 9.3 | 88.7 ± 10.6* | 71.4 ± 4.9 | 86.3 ± 12.1* |
| CPT + CC | 78.0 ± 8.0 | 91.3 ± 12.5* | 74.3 ± 5.3 | 90.3 ± 11.3* | |
| PLCB + CC | 74.9 ± 6.2 | 75.0 ± 7.6 | 75.1 ± 4.9 | 76.8 ± 8.4 | |
| HR, bpm | CPT[1st] | 65.5 ± 9.1 | 71.6 ± 12.1 | 64.7 ± 10.8 | 62.5 ± 18.2$ |
| CPT + CC | 64.4 ± 7.6 | 71.0 ± 12.1 | 58.8 ± 18.9 | 70.4 ± 8.8 | |
| PLCB + CC | 63.7 ± 8.4 | 63.4 ± 8.6 | 62.7 ± 10.9 | 61.1 ± 10.1 | |
| SpO2, % |
CPT[1st] | 98.8 ± 1.1 | 99.1 ± 0.9 | 99.5 ± 0.6 | 99.7 ± 0.6$ |
| CPT + CC | 98.5 ± 1.1 | 99.1 ± 0.9 | 99.8 ± 0.4 | 99.9 ± 0.3$ | |
| PLCB + CC | 98.5 ± 1.0 | 98.7 ± 0.9 | 99.8 ± 0.4 | 99.8 ± 0.4$ | |
| β-stiffness, a.u. | CPT[1st] | 1.9 ± 1.6 | 5.7 ± 8.0* | 1.3 ± 0.5 | 2.8 ± 1.4* |
| CPT + CC | 3.0 ± 4.9 | 7.2 ± 9.1* | 1.2 ± 0.3 | 2.0 ± 0.8*$ | |
| PLCB + CC | 2.4 ± 3.2 | 2.9 ± 4.2 | 1.5 ± 0.7 | 1.4 ± 1.1 | |
Values are mean ± S.D. SBP indicates systolic blood pressure; DBP, diastolic blood pressure; HR, heart rate; SpO2, percutaneous oxygen saturation; CPT, cold pressor test; PLCB, placebo test; CC, central command. Each variable was compared between conditions under RA and N2O and between baseline and CPT/PLCB using two-way repeated ANOVA (n = 15). *, vs baseline at p < 0.05; $, vs RA at p < 0.05 by post-hoc test (Tukey test).
3.3. Cardiovascular responses
Fig. 2 demonstrates the responses of SBP, DBP, HR, and β-stiffness to stimulation. ΔSBP significantly increased from 0 to 30s in CPT[1st] and CPT + CC under both RA and N2O, while a significant increase in ΔDBP was observed from 30 to 60s. SBP at 60–90s and 90–120s in CPT[1st] and CPT + CC tended to be attenuated under N2O compared to RA (p = 0.122 and 0.082). ΔHR significantly increased in CPT + CC under both conditions but not in CPT[1st] under either condition. ΔHR at 60–90s and 90–120s was significantly lower in CPT[1st] than in CPT + CC under N2O (p = 0.044 and 0.033). Δβ-stiffness significantly increased from 0 to 30s in CPT[1st] and CPT + CC under RA and N2O, while the magnitude of the increase was smaller under N2O than under RA both in CPT[1st] and CPT + CC (p = 0.045 and 0.009).
Fig. 2.
Time course changes (Δ) from baseline in systolic blood pressure (SBP), diastolic blood pressure (DBP), heart rate (HR), and finger arterial stiffness (β-stiffness) and cumulative values during CPT/PLCB in CPT[1st], CPT + CC, and PLCB + CC under RA and N2O. Values are means ± S.D. *, vs BL at p < 0.05; #, vs CPT [1st] at p < 0.05; †, vs PLCB + CC at p < 0.05.
3.4. Feedforward cardiovascular changes
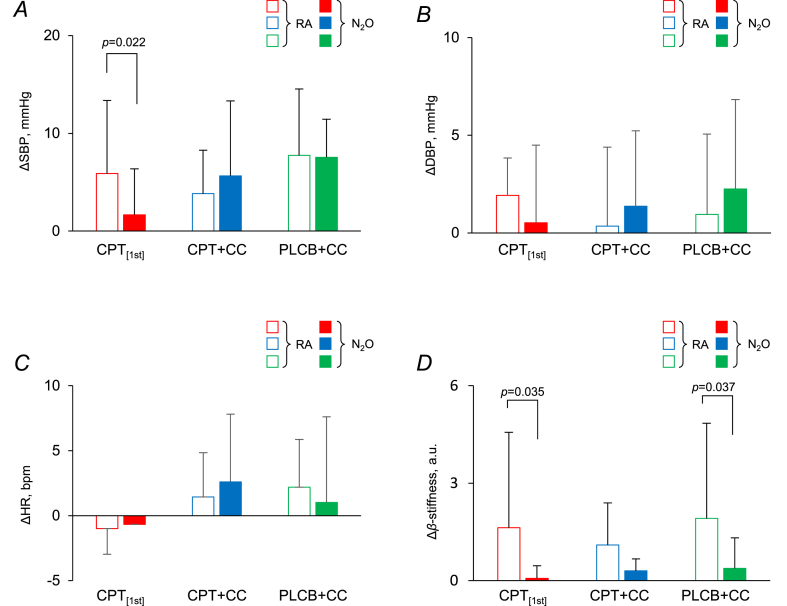
Fig. 3 shows the changes in cardiovascular variables induced by CD immediately before the onset of stimulation. SBP significantly increased in all trials under both conditions by CD except for CPT[1st] under N2O. ΔSBP was smaller under N2O than under RA in CPT[1st] (p = 0.022), while there were no differences between conditions in either CPT + CC or PLCB + CC. ΔDBP by CD did not show any differences between trials or conditions. ΔHR was increased by CD in CPT + CC and PLCB + CC, but not in CPT[1st], without any differences between RA and N2O conditions. Δβ-stiffness demonstrated similar relationships between conditions, being lower under N2O than under RA, among all trials.
Fig. 3.
Changes (Δ) in SBP (A), DBP (B), HR (C), and β-stiffness (D) by 30-s CD before the stimulation in CPT[1st], CPT + CC, and PLCB + CC under RA and N2O, respectively. Values are means ± S.D. A significant difference from RA is indicated at the level of p < 0.05 (Tukey test).
3.5. Relationship between subjective pain levels and cardiovascular effectors
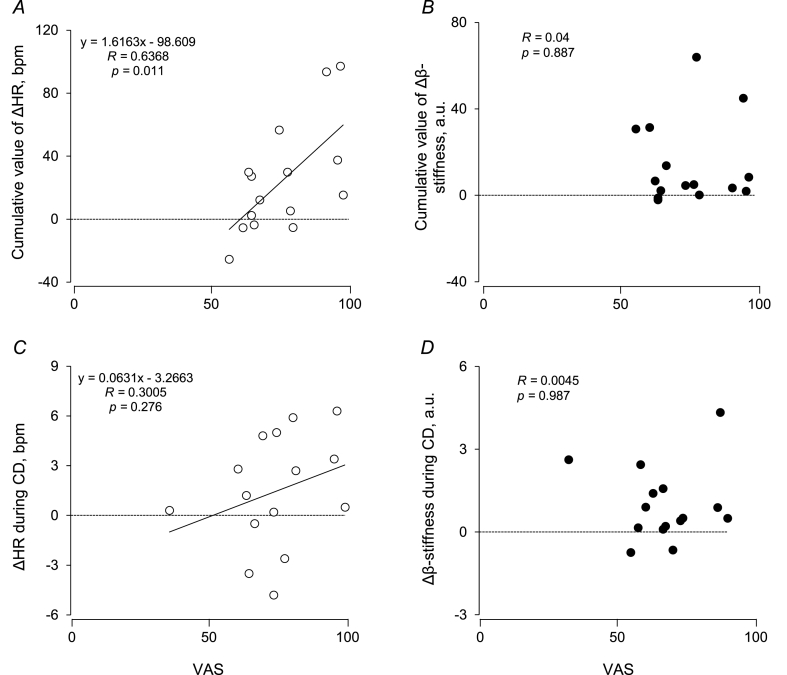
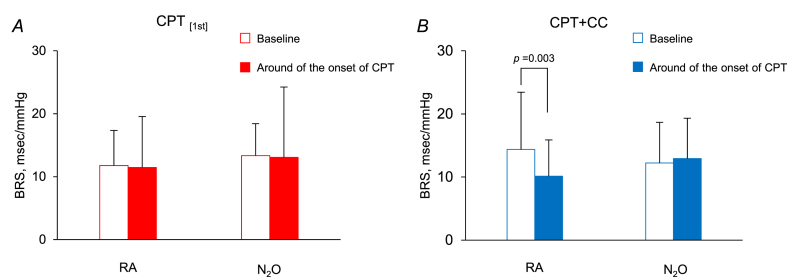
The cumulative value of ΔHR, but not Δβ-stiffness, was correlated with VAS in CPT + CC (r = 0.64 and r = 0.04) (Fig. 4). ΔHR, but not Δβ-stiffness, by CD in CPT + CC was correlated with VAS for previous CPT (r = 0.30 and < 0.01). Under RA, BRS decreased from the baseline in the 30-s around the onset of CPT/PLCB in CPT + CC (p = 0.003), but not in CPT[1st], while it remained unchanged in all trials under N2O (Fig. 5).
Fig. 4.
Relationship between subjective pain intensity and cardiovascular changes during pain stimulation and that between subjective pain intensity and feedforward cardiovascular changes after conditioning by experiencing pain stimulation. Linear regression analysis of the inter-individual relationships between visual analog scale (VAS) and cumulative values of ΔHR (A) and Δβ-stiffness (B) and those between VAS and ΔHR (C) and Δβ-stiffness (D) during CD under RA. Bivariate correlations were examined using the Pearson correlation coefficient.
Fig. 5.
Cardiac baroreflex sensitivity (BRS) determined from the sequence method of the short-term HR and SBP variabilities for 15 participants at baseline and around the onset of CPT/PLCB under RA and N2O in CPT[1st] (A) and CPT + CC (B), respectively. Values are means ± S.D. A significant difference from baseline at the level of p < 0.05 (Tukey test) was observed around the onset of CPT under RA in CPT + CC.
4. Discussion
The main findings of this study were as follows: 1) an increase in SBP immediately before the first experience of CPT was observed under RA, which disappeared under N2O, and this feedforward increase emerged after experiencing CPT under RA was not abolished by N2O; 2) the pressor response during CPT remained unchanged after experiencing CPT, and the magnitude of response was attenuated by N2O regardless of the experience; 3) the increase in HR immediately before and during CPT appeared after experiencing CPT but not before; and 4) β-stiffness increased immediately before and during CPT regardless of the experience, while it was lower under N2O in both trials. These results suggest that repeated pain stimuli affect the pressor response during and immediately before CPT via the changes in HR, which is altered by N2O inhalation via β-stiffness.
4.1. Conditioned responses and feedforward control to pain stimuli
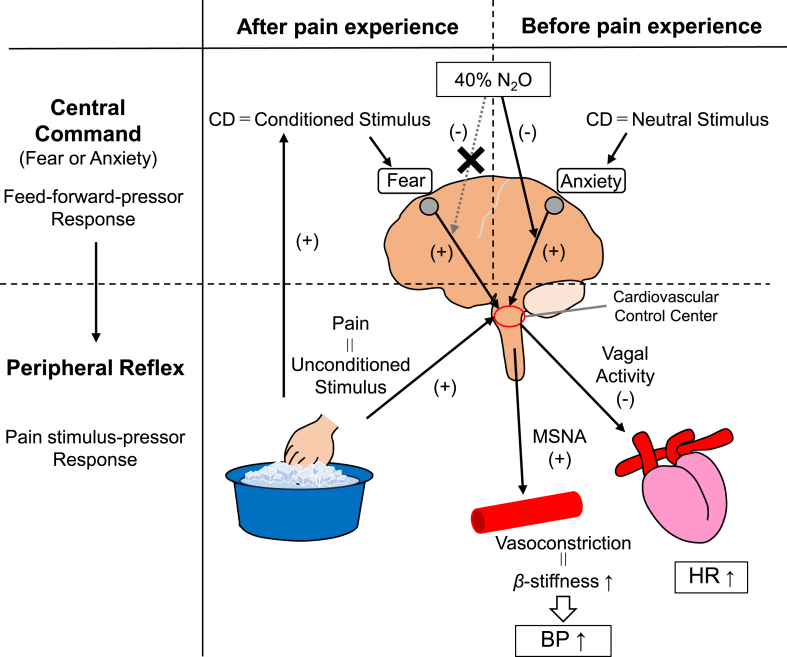
An increase in SBP immediately before the onset of stimulation appeared to be similar before and after experiencing CPT; however, this anticipatory SBP increase disappeared with N2O only when CPT was not experienced, indicating that there were two distinct processes of anticipatory cardiovascular responses depending on the experience of painful stimuli. Fanselow et al. [12] reported that when a neutral stimulus, an initially fearless environment, was experienced with subsequent unconditioned stimuli associated with fear/threat, the neutral stimulus changes to a conditioned stimulus, which induces a new central circuit that produces various physiological responses. Since CPT in this study was an unconditioned stimulus, CPT with CD in CPT[1st] was considered to cause conditioning CD with fear of pain, which depicted another pattern of anticipatory response in the subsequent trials. Although anxiety and fear are often confused because of the similar cardiovascular responses, their neural circuits are shown to be different [13]. Given that the response to painful stimuli is one of the series of fight-or-flight responses, the elevated SBP immediately before the first CPT in RA may be a different mechanism of “anxiety” than “fear.” Indeed, the increase in SBP by CD for CPT[1st] was suppressed by the anxiolytic effect of N2O. Conversely, there was no difference in ΔSBP during CD between RA and N2O after experiencing CPT, suggesting that feedforward pressor responses may occur through another neural circuit induced by the fear of a specific stimulation, which cannot be attenuated by N2O. Fig. 6 illustrates the co-relationship between central command before and after pain experience, peripheral reflex induced by pain stimulation, and the effectors involved in the pressor mechanism during CPT.
Fig. 6.
The schema illustrates the co-relationship between central command before and after pain experience during CD, peripheral reflex induced by pain stimulation, and the effectors involved in the pressor mechanism during CPT under RA and 40 % N2O.
4.2. Cardiovascular effectors involved in the feedforward mechanisms
In CPT[1st], an “anxious” condition, the pain intensity associated with CPT/PLCB was unknown to the participant, and finger β-stiffness increased with anticipatory SBP increase. Finger vascular tone is known to be innervated by the sympathetic nerve [14], which is activated by anxiety or emotional arousal to constrict the finger vessels [15]. Since CD is an arousal stimulus informing participants of anxiety, activated sympathetic nerves may increase peripheral vascular resistance, an effector of BP, and finger β-stiffness. The increase in finger β-stiffness occurred similarly before and after the experience of pain stimulus and seemed to be involved in anticipatory SBP increases. As N2O inhalation increases finger blood flow [16,17], decreased finger β-stiffness under N2O may be attributed to the effect of 40 % N2O inhalation. Furthermore, since this reduction was observed in both inexperienced and experienced trials, the vasodilation effect should influence peripherally, but not the higher cortex.
After experiencing CPT and learning the stimulus intensity, HR increased immediately before stimulation, indicating that the feedforward response may involve an HR increase. This association was also supported by the correlation between VAS and ΔHR by CD. Furthermore, as it has been reported that increases in HR are reflected in SBP more than in DBP [18,19], the post-experience HR increase appeared in SBP rather than DBP. The present findings of reduced BRS after experiencing pain may also be a congruent neurophysiological mechanism that facilitates the increase in HR by CD. Fisher et al. [20] reported that post-exercise muscle ischemia that stimulated peripheral chemoreceptors by metabolites and removed CC from the motor cortex decreased HR but kept higher sympathetic activity. In this study, CD conditioned by CPT evoked a feedforward increase in HR, suggesting that the elevated HR occurring after pain experience can be mediated by CC. 40 % N2O did not attenuate the anticipatory HR increase in CPT + CC or PLCB + CC, consistent with previous reports [21], implying that the activity of central circuits evoking feedforward HR control by conditioned stimuli is not suppressed by N2O. Taken together, it appears that 40 % N2O may induce vasodilation, which antagonizes vasoconstriction caused by anxiety, to prevent feedforward SBP increase before conditioning, but 40 % N2O may not affect HR increase caused by fear, to maintain feedforward SBP increase after conditioning, even with reduced β-stiffness.
4.3. Effects of central command and N2O on pressor response during CPT
Although HR during the second CPT was higher than the first for both RA and N2O, there was no difference in the degree of elevated BP, suggesting that the contribution of CC to the pressor response was negligibly small. However, the increase in β-stiffness during CPT was smaller under N2O than under RA, and accordingly, the increase in SBP was attenuated. Changes in vascular resistance have been reported to contribute to the pressor response during exercise more than changes in HR [22], which was also true in this study. Therefore, the pressor response during CPT should be due to an increase in vascular resistance. Shimizu et al. [23] have indicated that β-stiffness can be an important risk factor for cardiovascular events. Based on Fig. 2, since the increase in β-stiffness during CPT[1st] was not different from that during PLCB + CC under N2O, N2O inhalation sedation may reduce the cardiovascular risks associated with pain stimulation. Moreover, the attenuated pressor response during stimulation by N2O may be due to the suppression of the cardiovascular centers activating sympathetic nerves and/or the suppression of sympathetic nerve itself, but not suppression of CC. Indeed, we have demonstrated that 40 % N2O reduced the increase in sympathetic nerve activity directly measured using microneurography during CPT in humans [6].
4.4. Clinical implications and limitations
N2O inhibits sympathetic nerve activation, vasoconstriction, and pressor response—risk factors for mortality events [24], suggesting that 40 % N2O inhalation may be beneficial to avoid adverse events during dental treatments in patients with reduced cardiovascular reserves who are more likely to exceed safe limits [25]. However, N2O failed to suppress anticipatory hemodynamics induced by the conditioned stimulus. Even when inhalation sedation is applied, care must be taken to mitigate stress, such as painful stimuli, to prevent unpleasant reactions during treatment, especially for special needs patients who are prone to receiving conditioned stimuli because of hyperesthesia. The evaluations of VAS and finger β-stiffness used to estimate a quantification of subjective pain levels and sympathetic nerve activity included methodological limitations. It is necessary to apply brain fMRI and microneurography to directly measure the higher brain activity and sympathetic nerve activity to verify the underlying mechanism(s) responsible for hemodynamic exaggerations by conditioned stimuli in future research. It would provide appropriate strategies to reduce undesirable reactions during dental treatments. Although the actual power of this study was 0.72, slightly less than expected, we believe that the sample size was reasonable because recruiting a large number of subjects who agreed to inhale N2O was the challenge, and it did not alter the conclusions.
5. Conclusions
The experience of pain stimuli induces increases in HR as a feedforward mechanism of the anticipatory pressor response, which may be derived from the central command. 40 % N2O inhalation decreased subjective pain sensation and peripheral vascular stiffness but not the HR-mediated pressor response evoked by the pain experience. These results suggest that 40 % N2O may attenuate the pressor response only when the feedforward HR increase does not exist.
Ethics statement
This study was approved by the Ethical Review Committee of Hiroshima University (E−2042-2).
Data availability statement
The data that support the findings of this study are available on request from the corresponding author.
CRediT authorship contribution statement
Hironori Miyazaki: Writing – original draft, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization. Yoshifumi Nishio: Data curation. Kohta Miyahara: Data curation. Chiaki Furutani: Writing – review & editing. Ziqiang Xu: Writing – original draft, Formal analysis. Noboru Saeki: Writing – review & editing. Toshio Tsuji: Writing – review & editing, Writing – original draft, Methodology, Formal analysis. Yoshiyuki Okada: Writing – review & editing, Writing – original draft, Validation, Supervision, Project administration, Investigation, Funding acquisition, Formal analysis, Data curation, Conceptualization.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
This study was supported by the JSPS KAKENHI grant (18K09927) and Grant-in-Aid from Ryokufukai , Japan. We are also grateful to the study volunteers for their participation.
Contributor Information
Hironori Miyazaki, Email: hiro1906@hiroshima-u.ac.jp.
Yoshifumi Nishio, Email: nishio24@hiroshima-u.ac.jp.
Kohta Miyahara, Email: miyako800@gmail.com.
Chiaki Furutani, Email: chiakiii@hiroshima-u.ac.jp.
Ziqiang Xu, Email: ziqiangxu@hiroshima-u.ac.jp.
Noboru Saeki, Email: nsaeki@hiroshima-u.ac.jp.
Toshio Tsuji, Email: toshiotsuji@hiroshima-u.ac.jp.
Yoshiyuki Okada, Email: okay@hiroshima-u.ac.jp.
References
- 1.Ishii K., Matsukawa K., Liang N., Endo K., Idesako M., Asahara R., et al. Central command generated prior to arbitrary motor execution induces muscle vasodilatation at the beginning of dynamic exercise. J. Appl. Physiol. 2016;120:1424–1433. doi: 10.1152/japplphysiol.00103.2016. 1985. [DOI] [PubMed] [Google Scholar]
- 2.Goodwin G.M., McCloskey D.I., Mitchell J.H. Cardiovascular and respiratory responses to changes in central command during isometric exercise at constant muscle tension. J. Physiol. 1972;226:173–190. doi: 10.1113/jphysiol.1972.sp009979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaufman M.P. Control of breathing during dynamic exercise by thin fiber muscle afferents. J. Appl. Physiol. 2010;109:947–948. doi: 10.1152/japplphysiol.00892.2010. 1985. [DOI] [PubMed] [Google Scholar]
- 4.Use of nitrous oxide for pediatric dental patients. Pediatr. Dent. 2018;40:281–286. [PubMed] [Google Scholar]
- 5.Okada Y., Jarvis S.S., Best S.A., Edwards J.G., Hendrix J.M., Adams-Huet B., et al. Sympathetic neural and hemodynamic responses during cold pressor test in elderly blacks and whites. Hypertension. 2016;67:951–958. doi: 10.1161/HYPERTENSIONAHA.115.06700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isono K., Okada Y., Mitsui T., Masuda Y., Ogasawara T. Nitrous oxide inhalation attenuates an increase in muscle sympathetic nerve activity during cold stress. Faseb. J. 2015;29 [Google Scholar]
- 7.Okada Y., Kamijo Y., Okazaki K., Masuki S., Goto M., Nose H. Pressor responses to isometric biting are evoked by somatosensory receptors in periodontal tissue in humans. J. Appl. Physiol. 2009;107:531–539. doi: 10.1152/japplphysiol.91199.2008. 1985. [DOI] [PubMed] [Google Scholar]
- 8.Muneyasu T., Hirano H., Furui A., Soh Z., Nakamura R., Saeki N., et al. Cardiorespiratory synchronisation and systolic blood pressure correlation of peripheral arterial stiffness during endoscopic thoracic sympathectomy. Sci. Rep. 2021;11:5966. doi: 10.1038/s41598-021-85299-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji T., Arikuni F., Sasaoka T., Suyama S., Akiyoshi T., Soh Z., et al. Peripheral arterial stiffness during electrocutaneous stimulation is positively correlated with pain-related brain activity and subjective pain intensity: an fMRI study. Sci. Rep. 2021;11:4425. doi: 10.1038/s41598-021-83833-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsubara H., Hirano H., Hirano H., Soh Z., Nakamura R., Saeki N., et al. Quantitative evaluation of pain during electrocutaneous stimulation using a log-linearized peripheral arterial viscoelastic model. Sci. Rep. 2018;8:3091. doi: 10.1038/s41598-018-21223-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Parati G., Di Rienzo M., Mancia G. How to measure baroreflex sensitivity: from the cardiovascular laboratory to daily life. J. Hypertens. 2000;18:7–19. [PubMed] [Google Scholar]
- 12.Fanselow M.S. Neural organization of the defensive behavior system responsible for fear. Psychon. Bull. Rev. 1994;1:429–438. doi: 10.3758/BF03210947. [DOI] [PubMed] [Google Scholar]
- 13.Walker D.L., Toufexis D.J., Davis M. Role of the bed nucleus of the stria terminalis versus the amygdala in fear, stress, and anxiety. Eur. J. Pharmacol. 2003;463:199–216. doi: 10.1016/s0014-2999(03)01282-2. [DOI] [PubMed] [Google Scholar]
- 14.Nagasaka T., Hirata K., Mano T., Iwase S., Rossetti Y. Heat-induced finger vasoconstriction controlled by skin sympathetic nerve activity. J. Appl. Physiol. 1990;68:71–75. doi: 10.1152/jappl.1990.68.1.71. 1985. [DOI] [PubMed] [Google Scholar]
- 15.Bini G., Hagbarth K.E., Hynninen P., Wallin B.G. Regional similarities and differences in thermoregulatory vaso- and sudomotor tone. J. Physiol. 1980;306:553–565. doi: 10.1113/jphysiol.1980.sp013414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Roth G.I., Matheny J.L., Falace D.A., O'Reilly J.E., Norton J.C. Effect of age on the digit blood flow response to sedative concentrations of nitrous oxide. Anesth. Prog. 1984;31:17–22. [PMC free article] [PubMed] [Google Scholar]
- 17.Nakahara T., Yasumoto S., Jinnouchi Y., Hano K. [Concentrations of sevoflurane with and without nitrous oxide to block vasomotor reflexes to incision (MACBVR)] Masui. 2002;51:7–13. [PubMed] [Google Scholar]
- 18.Rudas L., Crossman A.A., Morillo C.A., Halliwill J.R., Tahvanainen K.U., Kuusela T.A., et al. Human sympathetic and vagal baroreflex responses to sequential nitroprusside and phenylephrine. Am. J. Physiol. 1999;276:H1691–H1698. doi: 10.1152/ajpheart.1999.276.5.h1691. [DOI] [PubMed] [Google Scholar]
- 19.Miyai N., Arita M., Miyashita K., Morioka I., Shiraishi T., Nishio I. Blood pressure response to heart rate during exercise test and risk of future hypertension. Hypertension. 2002;39:761–766. doi: 10.1161/hy0302.105777. [DOI] [PubMed] [Google Scholar]
- 20.Fisher J.P., Adlan A.M., Shantsila A., Secher J.F., Sorensen H., Secher N.H. Muscle metaboreflex and autonomic regulation of heart rate in humans. J. Physiol. 2013;591:3777–3788. doi: 10.1113/jphysiol.2013.254722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kawamura R., Stanley T.H., English J.B., Hill G.E., Liu W.S., Webster L.R. Cardiovascular responses to nitrous oxide exposure for two hours in man. Anesth. Analg. 1980;59:93–99. [PubMed] [Google Scholar]
- 22.Ogoh S., Fadel P.J., Monteiro F., Wasmund W.L., Raven P.B. Haemodynamic changes during neck pressure and suction in seated and supine positions. J. Physiol. 2002;540:707–716. doi: 10.1113/jphysiol.2001.013259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Shimizu K., Takahashi M., Shirai K. A huge earthquake hardened arterial stiffness monitored with cardio-ankle vascular index. J. Atherosclerosis Thromb. 2013;20:503–511. doi: 10.5551/jat.16097. [DOI] [PubMed] [Google Scholar]
- 24.Huggett R.J., Burns J., Mackintosh A.F., Mary D.A. Sympathetic neural activation in nondiabetic metabolic syndrome and its further augmentation by hypertension. Hypertension. 2004;44:847–852. doi: 10.1161/01.HYP.0000147893.08533.d8. [DOI] [PubMed] [Google Scholar]
- 25.Williamson J.D., Supiano M.A., Applegate W.B., Berlowitz D.R., Campbell R.C., Chertow G.M., et al. Intensive vs standard blood pressure control and cardiovascular disease outcomes in adults aged >/=75 Years: a randomized clinical trial. JAMA. 2016;315:2673–2682. doi: 10.1001/jama.2016.7050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author.