Summary
Several genetic variants linked to COVID-19 have been identified by host genomics researchers. Further advances in this research will likely play a role in the clinical management and public health control of future infectious disease outbreaks. The implementation of genetic testing to identify host genomic risk factors associated with infectious diseases raises several ethical, legal, and social implications (ELSIs). As an important stakeholder group, health professionals can provide key insights into these ELSI issues. In 2021, a cross-sectional online survey was fielded to US health professionals. The survey explored how they view the value and ethical acceptability of using COVID-19 host genomic information in three main decision-making settings: (1) clinical, (2) public health, and (3) workforce. The survey also assessed participants’ personal and professional experience with genomics and infectious diseases and collected key demographic data. A total of 603 participants completed the survey. A majority (84%) of participants agreed that it is ethically acceptable to use host genomics to make decisions about clinical care and 73% agreed that genetic screening has an important role to play in the public health control of COVID-19. However, more than 90% disagreed that it is ethically acceptable to use host genomics to deny resources or admission to individuals when hospital resources are scarce. Understanding stakeholder perspectives and anticipating ELSI issues will help inform policies for hospitals and public health departments to evaluate and perhaps adopt host genomic technologies in an ethically and socially responsible manner during future infectious disease outbreaks.
Keywords: COVID-19, ethical, legal and social issues (ELSI), ethics, host genomics, infectious disease, public health, stakeholders, health professionals, survey, clinical
The survey findings show that participants agree that it is ethically acceptable to use host genomic information in clinical care but disagree that it is acceptable to use for triaging patients and/or scarce resources. Health professionals’ perspectives are important to inform the implementation of host genomics in infectious-disease management.
Introduction
It has long been recognized that human beings are not uniformly affected by infectious agents or pathogens.1 While many factors (e.g., age, co-morbidities, socioeconomic status) can shape an individual’s risk of exposure to the pathogen and subsequent infection, there is growing evidence that host (or human) genomic factors play a role in several characteristics related to infectious disease response and treatment.2 Host genomics influence a range of infectious disease characteristics, such as susceptibility or resistance to infection, transmissibility of infection (e.g., super-spreaders), persistence of viral/bacterial infection, developing severe disease, developing debilitating long-term sequelae or outcomes, and mortality from the infectious disease.3,4,5 Host genomics could also play a role in determining whether an individual has serious side effects from or non-response to medications, treatments, and even vaccines.6,7,8,9
The COVID-19 pandemic, coupled with advancements in genomic technologies, has tremendously accelerated the pace of host genomics research.4,10,11,12 Within a mere 2 weeks of the first reported cases in January 2020, the sequence of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was published.13 By June, the findings from the initial large-scale genome-wide association studies (GWASs) were published, revealing that genetic variants or mutations on chromosome 3 were linked to severe and/or hospitalized cases of COVID-19.14,15 Furthermore, researchers have identified several other genes, such as IFNAR2 (OMIM: 602376), TYK2 (OMIM: 176941), OAS1 (OMIM: 164350), OAS2 (OMIM: 603350), OAS3 (OMIM: 603351), DPP9 (OMIM: 608258), and CCR2 (OMIM: 601267), as being implicated in COVID-19 critical illness.15,16,17,18 A collaborative host genomics study involving meta-analyses of nearly 50,000 individuals across 19 countries found four loci associated with SARS-CoV-2 infection and nine loci associated with COVID-19 severity.19 These and other advances in research may play a role in enabling genomics-based precision medicine and precision public health approaches.20,21 It may not be long before host genomic information is considered for use in clinical and public health decision-making settings to manage and control infectious-disease outbreaks (unpublished data).
While genetic testing was previously limited to rare monogenic conditions, advances in genomic science and technology have broadened testing services for a range of conditions and are no longer limited to high-risk individuals.22 As such, predictive infectious-disease-related host genetic testing and/or genomic screening to understand an individual’s or population’s risk will likely become available and play an important role in future outbreak control.23 This use of host genomics in the infectious-disease context raises several ethical, legal, and social implications (ELSIs). Prior to the pandemic, studies primarily from the Johns Hopkins Center for Bridging Infectious Disease, Genomics, and Society (BRIDGES) identified ELSI issues raised by the potential application of both pathogen and host genomic information across a range of infectious diseases that have large-scale public health consequences.3 However, few empirical studies have been published about stakeholder perspectives on the use of host genomics during an outbreak. A pre-COVID pilot survey focused on the use of host genomics in managing Ebola was conducted exclusively among infectious-disease-trained clinical staff.24 Other studies have also focused primarily on understanding the perspectives of health professionals who are experts in infectious diseases and/or genomics.25 The COVID pandemic has shown that health professionals from various backgrounds are an important stakeholder group since they will be at the forefront of implementing host genomics.24
The study reported here uses COVID-19 as an example to understand the perspectives of a much broader group of health professionals. The pandemic has exposed most of the current health professional workforce to infectious-disease care and control, opening a window of opportunity to solicit the opinions of a broad group of multidisciplinary health professionals—including those who are not experts or previously trained in infectious-disease care and/or genomics—regarding the use of infectious-disease-related genetic testing. In addition to expanding the stakeholder group, this study considered the use of host genomics in three settings: (1) a clinical setting involving hospital-based management of infectious diseases care that includes acute or inpatient services but not ambulatory or outpatient services; (2) a public health setting, defined as involving local- or state-based public health departments; and (3) a workforce setting specifically referring to the health professional workforce and their employers. Understanding health professionals’ perspectives on the use of host genomics across these three settings can help to anticipate and study the ELSI questions that arise. Decision makers may use the findings from the study to develop ethically and socially responsible policies regarding the use of host genomics.
Subjects and methods
The Johns Hopkins Medical Institutions Institutional Review Board (IRB) reviewed and acknowledged this study as exempt (IRB00289756). Since survey respondents could only proceed to the survey questions after reading the instructions regarding the voluntary and confidential nature of the survey, completion of the survey implied informed consent.
Study population and survey recruitment
The study population was health professionals from the 10 Regional Emerging Special Pathogen Treatment Centers (RESPTCs) in the US and their partners. The RESPTCs are part of the National Emerging Special Pathogens Training and Education Center (NETEC) network, which was set up in 2014 by the Office of the Assistant Secretary of Preparedness and Response to provide care for individuals infected with high-consequence pathogens in response to the 2014–2016 Ebola outbreak.26 All of the 10 RESPTCs are partnered with their larger health systems as well as their local and state public health departments.26
The initial strategy was to recruit survey participants primarily from these 10 RESPTCs and their partnering institutions, including the Johns Hopkins RESPTC. These included 10 large US hospitals and their local and state public health departments. Three of the coauthors (S.J., G.G., B.G.) led a breakout session at the 2021 NETEC Virtual Summit, which is an annual conference involving the members of all the RESPTCs. The breakout session served as a platform to engage with the RESPTC members, describe the survey study, and identify champions at each of the RESPTCs for survey recruitment. Following the NETEC Summit, the online survey link was sent to all the attendees from the breakout session, and they were asked to share the link with their colleagues. The survey link was also emailed to the directors of the RESPTCs, who forwarded it to the employee listservs at their centers and partnering institutions. The directors were reminded by email and during one of their monthly meetings to distribute the survey link.
At the Johns Hopkins RESPTC, the survey link was sent directly to the physicians’ and nurses’ listservs, after receiving the appropriate institutional approvals. It was also sent to administrators at the Maryland Department of Health, a partnering institution of the Johns Hopkins RESPTC, and they forwarded the link to both their state and local public health listservs. There was very low uptake among public health professionals at the health departments. As a result, the recruitment strategy was expanded to try to increase public health professional representation by sharing the survey link with the Doctor of Public Health (DrPH) student listservs at Johns Hopkins Bloomberg School of Public Health and relevant Facebook groups of public health professionals.
At the end of the survey, all respondents had a chance to enter into a raffle for a $50 Amazon gift card; if interested, they were asked to provide their email address. Once the survey was closed, 50 participants were selected randomly as raffle winners and were emailed the gift card.
Survey development
We developed an online cross-sectional survey based on the pre-COVID pilot survey instrument.24 Existing items from the pilot survey were used and adapted in relevant sections; the survey instrument was also informed by ongoing COVID-19 host genomics research.10,14,15,16,17,19,24,27,28,29,30,31 The survey questionnaire included 107 items grouped into seven sections. The questionnaire began with background information about host genomic research and its role in the COVID-19 pandemic. Respondents were asked to read this material and assume that meaningful host genomic risk factors, such as clinically relevant genetic variants with large effect sizes, were identified for COVID-19 and that there was an accurate COVID-19 genetic test. They then completed a series of questions regarding their views about the usefulness and ethical acceptability of using COVID-19 host genomics to respond to the current pandemic in three main decision-making settings: (1) clinical, (2) public health, and (3) workforce. These settings were previously identified by Geller et al. as important to think about during the COVID-19 pandemic, but there was no empirical evidence to support the possible use of host genomics across these settings.4 This paper only focuses on the key findings from six of the relevant sections (see Table 1).
Table 1.
Relevant survey sections
| Sections Examples of question topics |
Number of questionsa | |
|---|---|---|
| 1 | Genetic testing in the clinical setting Use of COVID-19 host genomic information to make decisions related to:
|
14 |
| 2 | Genomic screening in the public health setting Use of COVID-19 host genomic information to make decisions about:
|
21 |
| 3 | Genetic testing of health professionals in the workforce setting Use of employee COVID-19 host genomic information by employers to make decisions about:
|
29b |
| 4 | Genomics knowledge, training, and experience
|
3 |
| 5 | COVID-19 and infectious-disease experience
|
6 |
| 6 | Demographics
|
10 |
Each item in a scale is counted as a separate question.
This section included four items that were general questions related to non-COVID diseases (i.e., Ebola and heart disease).
Participants were asked to rate their agreement with the ethical acceptability of using COVID-19 host genomics in general and specific decision-making scenarios within each of the three settings. Response options followed a four-point Likert scale: strongly disagree, disagree, agree, and strongly agree. In the clinical setting (see section 1 in Table 1), the survey questions focused on the potential use of genetic testing to make decisions related to hospital admission, clinical care, and the allocation of scarce resources, such as ventilators, certain medications, and experimental treatments. For example, participants rated the following statement: “For patients who are admitted, when decisions have to be made about the use of scarce hospital resources (e.g., ventilators, certain medications, experimental treatments), it is ethically acceptable to prioritize resources for patients with higher genetic risk of severe COVID-19.” Participants also rated the usefulness of each of the following types of genetic tests in influencing the COVID-19 clinical care: an individual’s increased risk of developing severe COVID-19, developing long COVID, serious side effects from specific treatments or medications or vaccines, not responding to specific treatments or medications or vaccines, and mortality from COVID-19. They rated the usefulness of the genetic test on a four-point scale: not at all useful, minimally useful, moderately useful, or very useful.
In the public health setting (see section 2 in Table 1), the survey questions focused on the potential use of host genomic screening protocols to make decisions about various COVID-19 related public health measures. These measures included quarantine or stay-at-home measures, school closure policies, travel restrictions, immigration restrictions, prioritization of vaccine distribution, and vaccine waivers. For example, participants rated the following statement: “It is ethically acceptable for human genomic information to be used in public health practice to decide quarantine or stay-at-home measures (e.g., individuals at lower genetic risk of severe disease do not have to stay at home).” The usefulness of different types of public health genomic screening programs was similarly assessed. The health professionals rated the usefulness of each of the following type of genomic screening program in shaping public health practice during COVID-19: an individual’s increased risk of contracting COVID-19 (i.e., susceptibility), transmissibility of infection, developing severe COVID-19, developing long COVID, serious side effects from vaccines, not responding to vaccines, and mortality from COVID-19; as well as an individual’s decreased risk of contracting COVID-19 (i.e., resistance).
Last, in the workforce setting (see section 3 in Table 1), the survey questions focused on what employers should or should not be allowed to do using employee’s COVID-19 host genomic information. For example, participants rated the following: “Your employer should be allowed to use information about your genetic risk of severe COVID-19 to determine whether or not you should be assigned as a first responder or frontline worker.” Respondents were also asked which of the different types of genetic tests described in the previous section they would choose to take and whether they would use the results from those tests to help make decisions about what work assignments they would accept. These questions had three answer choices: yes, no, and maybe. In this section, participants were also asked if the nature of the infectious disease affected their answers in order to understand whether their views would change if it was a different infectious disease than COVID-19.
The survey included both quantitative and qualitative components, as each of the above sections included free-response questions in which participants were asked to describe their ethical reasoning for or against using infectious-disease-related genetic testing in each of the settings. In addition to the three sections already described, participants were asked about their personal and professional experience with genomics and infectious diseases (see sections 4 and 5 in Table 1). Demographic data (see section 6 in Table 1) were also collected on all participants.
The Qualtrics (Qualtrics Provo, UT) survey platform was used to develop the instrument. The instrument was iteratively revised during several feedback rounds within the project team and tested with a small sample (n = 10) of colleagues, including physicians, nurses, epidemiologists, and graduate students in medicine and public health. The test respondents provided detailed feedback to improve the survey instrument. We revised our survey based on the feedback and validated the final instrument. The survey was fielded from September 2021 to September 2022.
Data analysis
The distributions and ranges of all variables were examined. The four-point Likert scale responses were dichotomized (strongly disagree/disagree or strongly agree/agree; not at all/minimally useful or moderately/very useful; not at all/minimally confident or moderately/highly confident; and a little/no amount of additional training or a moderate/high amount of additional training). Participants were asked to write in their general job titles (e.g., epidemiologist, nurse, physician). These responses were re-coded into seven broad categories: nurse practitioner, physician, physician assistant, public health, registered nurse, resident, and other.
The survey data were analyzed using standard descriptive and bivariate statistics. Frequency distributions were used to document the perspectives of the survey participants on the ethical acceptability of the potential use of COVID-19 host genomic information across the three decision-making settings. Chi-squared tests were used to assess whether health professionals’ perspectives on the ethical acceptability of using host genomic information were associated with exposure to and/or experience with genetic testing, past infectious diseases, and/or COVID-19 as well as other demographic characteristics, such as age, gender, race, and job title. In cases where the bivariate cross-tabulations had cell counts less than five, Fisher’s exact tests were performed. For all the analyses, the statistical significance was set at p < 0.05. The analyses were performed using Stata, version 17 (StataCorp, College Station, TX). Answers to open-ended survey questions were summarized by identifying themes within the data, which were derived inductively. The qualitative findings are reported in the results and representative quotes are provided in the supplemental information to illustrate salient themes.
Results
Sociodemographic characteristics of the study population
In total, 603 health professionals participated in the survey. As shown in Table 2, a majority of the participants self-identified as female (77%), White (78%), and non-Hispanic (91%). Participants varied by age with 53% between 25 and 44 years. A large majority of participants worked in the state of Maryland (74%) and/or worked in a clinical setting for a public or private hospital (90%). Over 50% of the participants were registered nurses, 21% were physicians, 11% were advanced practice providers (including nurse practitioners and physician assistants), and only 4% were public health professionals.
Table 2.
Survey participant characteristics
| Participant characteristics | n (%) |
|---|---|
| Gender | |
| Female | 467 (77.45) |
| Male | 114 (18.91) |
| Non-binary/third gender | 6 (1.00) |
| Missing or prefer not to answer | 16 (2.65) |
| Age (in years) | |
| 18–24 | 33 (5.47) |
| 25–34 | 156 (25.87) |
| 35–44 | 161 (26.70) |
| 45–54 | 110 (18.24) |
| 55–64 | 107 (17.74) |
| 65 or older | 27 (4.48) |
| Missing or prefer not to answer | 9 (1.49) |
| Race | |
| White only | 472 (78.28) |
| Asian | 55 (9.12) |
| Black or African American | 20 (3.32) |
| Othera | 20 (3.32) |
| Missing or prefer not to answer | 36 (5.97) |
| Ethnicity | |
| Not of Hispanic, Latino/a/x, or Spanish origin | 549 (91.04) |
| Hispanic, Latino/a/x, or Spanish origin | 35 (5.80) |
| Missing or prefer not to answer | 19 (3.15) |
| US state | |
| Maryland | 444 (73.63) |
| Otherb | 102 (16.92) |
| Missing or prefer not to answer | 57 (9.45) |
| Work setting | |
| Clinical setting | 542 (89.88) |
| Public health setting | 31 (5.14) |
| Both settings | 22 (3.65) |
| Other setting | 5 (0.83) |
| Missing or prefer not to answer | 3 (0.50) |
| Employer type | |
| Public or private hospital | 541 (89.72) |
| Public health departmentc | 35 (5.80) |
| Other | 24 (3.98) |
| Missing or prefer not to answer | 3 (0.50) |
| General job title | |
| Registered nurse | 325 (53.90) |
| Physician | 126 (20.90) |
| Advanced practice providerd | 69 (11.44) |
| Medical resident | 23 (3.81) |
| Public health | 23 (3.81) |
| Other | 23 (3.81) |
| Missing or prefer not to answer | 14 (2.32) |
Race: “other” includes 16 (2.65%) who selected multiple races, two (0.33%) Middle Eastern or North African, and two (0.33%) Native American.
US State: “other” includes 58 (9.62%) participants from FL; 18 (2.99%) participants from DC; five (0.83%) participants from NY; four (0.66%) each from NE and WA; two (0.33%) each from CA and TX; and one (0.17%) each from CO, DE, GA, IL, MA, MN, MO, OR, and VA.
Employer type: “public health department” includes 24 (3.98%) from local public health department, six (1%) from state, and five (0.83%) from federal.
General job title: “advanced practice provider” includes 48 (7.96%) nurse practitioners and 21 (3.48%) physician assistants.
Experience with genomics, COVID-19, and other infectious diseases
In order to better characterize survey participants, they were asked about their personal and professional experience with genomics and infectious diseases, including COVID-19 (see Table 3). Personal experience was described as the participant or anyone close to the participant ever having any experience with genetic testing and/or genetic conditions, such as having a heritable disease in the family and/or taking an ancestry genetic test from 23andMe or another company. Nearly half (47%) of respondents reported having personal experience with genomics. Although 68% reported feeling not at all or minimally confident in their genomics knowledge, only 50% reported wanting a high or moderate amount of additional training in host genomics.
Table 3.
Participants’ genomics and infectious disease experiences
| Participant experiences | n (%)a |
|---|---|
| Personal experience with genomics | |
| No | 312 (51.74) |
| Yes | 282 (46.77) |
| Confidence in your genomics knowledge | |
| Not at all or minimally confident | 409 (67.83) |
| Moderately or highly confident | 187 (31.01) |
| Additional training you would want about the human genetics of infectious disease | |
| A high or moderate amount of additional training | 305 (50.58) |
| A little or no amount of additional training | 294 (48.76) |
| Professional experience with infectious disease | |
| No experience | 267 (44.28) |
| <1 year | 38 (6.30) |
| 1–5 years | 109 (18.08) |
| 6–10 years | 57 (9.45) |
| >10 years | 128 (21.23) |
| Worked during past infectious-disease outbreaks | |
| No | 288 (47.76) |
| Yes | 309 (51.24) |
| Direct interaction with COVID-19-positive patients and/or samples as part of your work | |
| No | 141 (23.38) |
| Yes | 458 (75.95) |
| Testing positive for SARS-CoV-2 | |
| No | 513 (85.07) |
| Yes | 87 (14.43) |
| PPE shortage experienced by your institution | |
| No | 163 (27.03) |
| Yes | 436 (72.31) |
| PPE restriction imposed by your employer | |
| No | 98 (16.25) |
| Yes | 501 (83.08) |
Numbers may not add to totals because of missing information.
With regard to infectious-disease experience, 44% of respondents reported having no professional experience in the field of infectious-disease clinical management and/or public health control, while 21% reported having more than 10 years of experience. Similarly, 48% reported not having worked during any other infectious-disease outbreaks and/or interacted with infected individuals before COVID-19. Regardless of their professional or past experience with infectious diseases, 76% of participants reported having interacted directly with COVID-19-positive individual(s) and/or sample(s) as part of their work. Only 14% reported ever testing positive for SARS-CoV-2. All participants were also asked whether their institution or employer experienced a shortage of personal protective equipment (PPE) or imposed restrictions on the use of PPE. A majority of participants (72%) reported that their institution experienced PPE and/or mask shortages at some point during the pandemic and 83% reported that their employer imposed restriction(s) on their use of PPE, such as re-using their own PPE and/or extended use of PPE.
Attitudes toward the use of COVID-19 host genomic information
The results in this section are organized based on the three different decision-making settings and health professionals’ perspectives of using COVID-19 host genomics in each of those settings. Only 22% of the respondents reported that their answers would change depending on the nature of the infectious disease and 57% reported that their views would be the same regardless of the infectious disease.
Clinical settings
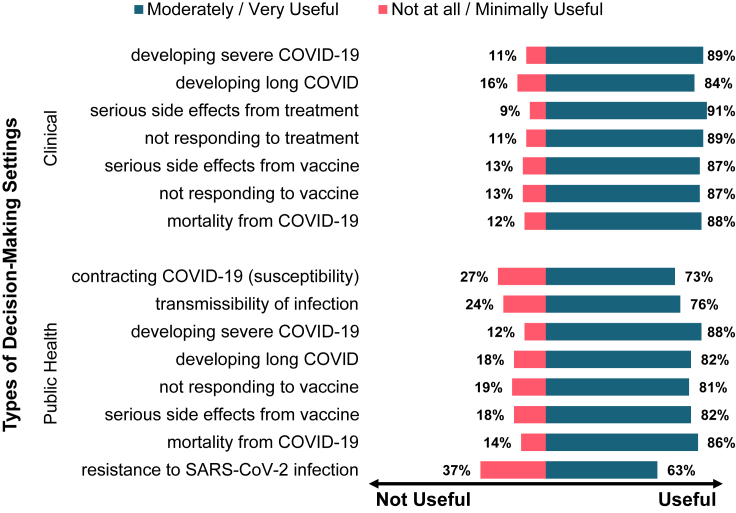
The overwhelming majority of survey participants rated each of the seven kinds of genetic tests as moderately/very useful for influencing the clinical care of COVID-19 patients (see Table S1 for detailed results). More than 90% of participants thought that a genetic test to identify an individual’s increased risk of developing serious side effects from specific treatments or medications is moderately or very useful, while only 9% thought that it is not at all or minimally useful (see Figure 1). Health professionals were asked their perspectives on the ethical acceptability of the use of COVID-19 host genomic information in general and specific decision-making scenarios within the clinical setting (see Table S2). In a general scenario when resources are not scarce, 84% agreed that it is ethically acceptable to use host genomic information along with other information to make decisions about the clinical care of a COVID-19 patient (see Figure 2A). However, only 63% agreed that it is acceptable to use host genomics for hospital admission decisions during COVID-19. They were also asked about more specific instances of using host genomic information to make resource allocation decisions when resources are scarce during an infectious outbreak (see Figure 2B). Over 90% of the participants disagreed that individuals with a higher genetic risk of severe COVID-19 should be denied admission or resources should be withheld from them when resources are scarce. However, 65% agreed it is acceptable to prioritize admission and 63% agreed it is acceptable to prioritize resources for individuals with a higher genetic risk of severe COVID-19.
Figure 1.
Health professionals’ views on the usefulness of COVID-19 host genomics to identify risk of infectious-disease characteristics
Figure 2.
Health professionals’ views on the ethical acceptability of using COVID-19 host genomic information
Many survey participants also provided text responses to explain their ethical reasoning for the choices they made. Examples of key themes identified in these responses are illustrated in Table S3. While most healthcare professionals found host genomics to be useful, they also believed that it is not ethical to use it to deny clinical care or admission under any circumstances. Others believed that host genomics could be used to guide treatment but not for making decisions regarding prioritizing, withholding, or denying resources. Several concerns related to the use of host genomics were also identified. Participants expressed concerns about potential discrimination, stigma, disparities, inequities, and racism, particularly if adequate safeguards are not in place before using host genomic information. They also cautioned that genomic risk is only one of several factors that must be considered in managing infectious diseases. Some participants also emphasized the importance of prioritizing the clinical signs and symptoms presented clearly by the individual over results from predictive genetic testing. Last, the need for stronger evidence demonstrating the increased accuracy of genetic tests in predicting outcomes was highlighted.
Public health settings
Participants were asked to rate the usefulness of eight different types of genetic screening programs for influencing public health practice during COVID-19 (see Table S1). Similar to the findings in the clinical settings, a majority of participants rated each of the screening programs as moderately/very useful (see Figure 1). A public health genetic screening program to identify individuals who are resistant to SARS-CoV-2 infection was rated as moderately/very useful by 63% of the participants. As shown Figure 2A, 73% of participants agreed that genetic screening has an important role to play in the public health control of COVID-19, while only 31% agreed that it would interfere with the overall goals of public health (see Table S2 for details). When health professionals were asked whether they think it is ethically acceptable for host genomic information to be used in public health practice to decide specific measures (see Figure 2B), 59% disagreed that it is acceptable to use it to decide quarantine or stay-at-home measures and/or school closure policies. For example, respondents disagreed that individuals at lower genetic risk of severe disease do not have to stay at home or that schools can remain open for students and teachers at lower genetic risk of severe disease. A higher number of participants also disagreed with using COVID-19 host genomic information to decide travel (66%) or immigration (68%) restrictions. However, only 25% disagreed that it is acceptable to use host genomics to prioritize the distribution of vaccines in a public health setting (e.g., individuals who are at higher genetic risk of severe disease are prioritized for vaccines). Similarly, 55% of participants agreed with its use to decide vaccine waivers (e.g., individuals who are at higher genetic risk of adverse reactions to vaccines are excused from being vaccinated).
Participants were provided the option of describing their ethical reasoning regarding the use of genomic screening programs in public health, with some expressing concerns while others identified potential benefits (see Table S3 for illustrative quotes). The primary theme was that genomic risk is a deeply personal matter and should only inform individuals’ decisions about their health and behavior. However, a few participants believed it could be used for restrictive measures by public health authorities if the genetic test was accurate and reliable. Various concerns were raised about using genomic screening to determine quarantine and other restrictive measures in public health. These concerns included discrimination, disparities, racism, stigma, and distrust of public health authorities among the public. Some participants argued that restricting individuals based on their genomics would be an infringement of basic human rights and freedoms. Additionally, some feared that individuals found to be at low genetic risk for COVID-19 severity might engage in risky behavior and that host genomics could undermine solidarity in public health efforts.
Workforce settings
As shown in Figure 2A, 84% of participants disagreed that their employers should be allowed to mandate genetic testing of their health professional workforce to inform work assignment decisions. However, 91% of participants agreed that their employers should be allowed to offer them confidential genetic testing for relevant COVID-19 outcomes as an option if they are directly involved in COVID-19 clinical care and/or public health control. Health professionals were also asked about specific scenarios in which employers use employees’ personal genetic risk information to determine work assignment and level of PPE available to them during an outbreak (see Figure 2B). Only about a quarter of the participants agreed that their employers should be allowed to use their personal genetic information to make workforce decisions. An overwhelming majority reported that they will or may choose to take any of the 10 types of genetic tests if they are offered to them in the future to understand their own genetic risk related to COVID-19 (see Table S4). However, less than 45% of the participants reported that they will not use the results from the genetic test(s) to help make decisions about what work assignments they would accept.
In the text responses, participants elaborated on their disagreement with employers having access to their personal genomic information and making employment decisions based on it (see Table S3 for illustrative quotes). They expressed concerns about the confidentiality of their data, potential discrimination by employers, and worsening the problem of health professional shortages. Several participants also emphasized that genomic risk should not be the basis for the allocation of PPE, as it is the employer’s responsibility to ensure that all employees have access to PPE. Finally, some participants reported that knowing their host genomics could lead to increased anxiety, while others stated that it would not affect their employment decisions, as they accepted the risks associated with their profession when they chose to become health professionals.
Associations between ethical acceptability and the health professionals’ experience with genomics and infectious disease
Additional bivariate analyses were performed to understand whether participants’ perspectives on the ethical acceptability of using host genomic information in either the clinical or public health setting were associated with any of the following eight factors: (1) confidence in their genomics knowledge, (2) personal experience with genomics, (3) professional experience with infectious disease, (4) whether they worked during past infectious-disease outbreaks prior to the COVID-19 pandemic, (5) whether they had direct interaction with COVID-19-positive individuals as part of their work, (6) whether they ever tested positive for SARS-CoV-2, (7) whether their institution experienced PPE shortage, and (8) whether their institution had imposed PPE restrictions. Among these eight factors, only four were found to have statistically significant associations with ethical acceptability and the associations varied by type of setting (see Table 4). These four factors were confidence in genomics knowledge, experience with past infectious outbreaks, direct interaction with COVID-19-positive individuals, and experience with PPE restrictions. In the clinical setting, except for participants’ confidence in their genomics knowledge, all three of the remaining factors were significantly associated with the ethical acceptability of using host genomics to make decisions about hospital admission and clinical care (see Table 4). For example, there was a statistically significant association between ethical acceptability of using host genomics in the clinical setting and the health professional’s experience with past infectious-disease outbreaks. Those who had worked during a past outbreak and/or interacted with infected individuals before COVID-19 were slightly less likely to agree that it is ethically acceptable to use host genomics to make clinical decisions. Around 88% of those who had not worked during a past outbreak agreed that it is acceptable to use it in clinical care, and a slightly lower proportion (81%) of individuals with past experience agreed such use was acceptable (chi-squared test, p < 0.05).
Table 4.
Associations between ethical acceptability and the health professionals’ experience with genomics and infectious disease
| Confidence in your genomics knowledge |
Worked during past infectious-disease outbreaks |
Direct interaction with COVID-19-positive individuals as part of your work |
PPE restriction imposed by your employer |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Not at all or minimally confident |
Moderately or highly confident |
p valueb | No |
Yes |
p valueb | No |
Yes |
p valueb | No |
Yes |
p valueb | |
| n (%)a | n (%)a | n (%)a | n (%)a | n (%)a | n (%)a | n (%)a | n (%)a | |||||
| Clinical setting | ||||||||||||
| It is ethically acceptable to use host genomic information along with other information to make decisions about patient admission during COVID-19 | – | – | 0.2 | – | – | <0.05 | – | – | <0.05 | – | – | 0.5 |
| Strongly disagree/disagree | 144 (35.29) | 76 (40.64) | – | 94 (32.75) | 126 (40.78) | – | 64 (45.39) | 158 (34.57) | – | 39 (40.21) | 182 (36.33) | – |
| Strongly agree/agree | 264 (64.71) | 111 (59.36) | – | 193 (67.25) | 183 (59.22) | – | 77 (54.61) | 299 (65.43) | – | 58 (59.79) | 319 (63.67) | – |
| It is ethically acceptable to use host genomic information along with other information to make decisions about the clinical care of COVID-19 patients | – | – | 0.1 | – | – | <0.05 | – | – | 0.1 | – | – | <0.05 |
| Strongly disagree/disagree | 57 (13.97) | 36 (19.35) | – | 35 (12.24) | 59 (19.09) | – | 28 (19.86) | 66 (14.47) | – | 22 (22.68) | 71 (14.2) | – |
| Strongly agree/agree | 351 (86.03) | 150 (80.65) | – | 251 (87.76) | 250 (80.91) | – | 113 (80.14) | 390 (85.53) | – | 75 (77.32) | 429 (85.8) | – |
| Public health setting | ||||||||||||
| Genetic screening has an important role to play in the public health control of COVID-19 | – | – | <0.0001 | – | – | 0.9 | – | – | 0.1 | – | – | 0.8 |
| Strongly disagree/disagree | 88 (21.57) | 70 (37.43) | – | 76 (26.39) | 82 (26.62) | – | 30 (21.28) | 130 (28.45) | – | 25 (25.51) | 134 (26.8) | – |
| Strongly agree/agree | 320 (78.43) | 117 (62.57) | – | 212 (73.61) | 226 (73.38) | – | 111 (78.72) | 327 (71.55) | – | 73 (74.49) | 366 (73.2) | – |
| Genetic screening would interfere with the overall goals of public health control of COVID-19 | – | – | <0.001 | – | – | 0.7 | – | – | 0.1 | – | – | 0.5 |
| Strongly disagree/disagree | 303 (74.45) | 109 (58.29) | – | 201 (70.3) | 212 (68.83) | – | 105 (74.47) | 309 (67.76) | – | 70 (72.16) | 344 | – |
| Strongly agree/agree | 104 (25.55) | 78 (41.71) | – | 86 (29.97) | 96 (31.17) | – | 36 (25.53) | 147 (32.24) | – | 27 (27.84) | 156 | – |
| Workforce setting | ||||||||||||
| Your employer should be allowed to mandate genetic testing | – | – | 0.2 | – | – | 0.7 | – | – | 0.4 | – | – | 0.2 |
| Strongly disagree/disagree | 334 (82.27) | 161 (86.56) | – | 240 (84.21) | 256 (83.12) | – | 115 (81.56) | 383 (84.36) | – | 75 (78.95) | 422 (84.40) | – |
| Strongly agree/agree | 72 (17.73) | 25 (13.44) | – | 45 (15.79) | 52 (16.88) | – | 26 (18.44) | 71 (15.64) | – | 20 (21.05) | 78 (15.60) | – |
| Your employer should be allowed to offer you confidential genetic testing | – | – | <0.001 | – | – | 0.9 | – | – | 0.9 | – | – | 0.8 |
| Strongly disagree/disagree | 24 (5.91) | 28 (15.14) | – | 25 (8.77) | 28 (9.12) | – | 13 (9.22) | 40 (8.83) | – | 9 (9.47) | 43 (8.62) | – |
| Strongly agree/agree | 382 (94.09) | 157 (84.86) | – | 260 (91.23) | 279 (90.88) | – | 128 (90.78) | 413 (91.17) | – | 86 (90.53) | 456 (91.38) | – |
All the percentages in this table are row percentages.
All p values estimated with a chi-squared test.
On the other hand, in the public health and workforce setting, the only factor that was significantly associated with ethical acceptability is participants’ confidence in their genomics knowledge. Among those who were not at all or minimally confident, 78% agreed that genetic screening has an important role to play in the public health control of COVID-19 and only 26% agreed that genetic screening would interfere with the overall goals of public health. Among those who were moderately or highly confident, only 63% of health professionals agreed that genetic screening is important for public health (chi-squared test, p < 0.0001) and 42% agreed that it interferes with the goals of public health (chi-squared test, p < 0.001). In the workforce setting, among those who were not at all or minimally confident, 94% agreed that employers should be allowed to offer employees confidential genetic testing services. This proportion decreased to 85% agreeing that employers should offer confidential genetic testing among those who were moderately or highly confident in their genomics knowledge (chi-squared test, p < 0.001). Last, the idea of whether employers should be allowed to mandate genetic testing was not significantly associated with any of the factors related to genomics and infectious-disease experience.
Discussion
As genetic testing expands into the infectious-disease setting, health professionals will be at the forefront of implementing host genomics, whether for clinical management or public health control of an infectious outbreak. Our findings provide valuable insights into how health professionals think about the issues surrounding the implementation of host genomic information in clinical, public health, and workforce settings.
Across the three decision-making settings, survey participants were more favorable toward the ethical acceptability of using COVID-19 host genomics to inform decisions in the clinical and public health settings compared to the workforce setting. An overwhelming majority believed that genetic testing and screening programs to identify those at increased risk of COVID-19 severity as well as non-response to or serious side effects from specific treatments or medications is useful for influencing clinical care and public health practice. While our findings are specific to COVID-19, a large majority of participants, both with and without prior experience with infectious-disease outbreaks, self-reported that their answers about ethical acceptability of using host genomics would remain the same regardless of the infectious disease. As such, these results could be relevant in making decisions about the management of future infectious-disease outbreaks if and when clinically meaningful host genetic variants are discovered. However, it is important to note that, while our study did not collect data on other infectious-disease examples, there are studies previously published that discuss how the ethical and policy implications of using host genomics could differ based on the type of infectious disease.3
In the clinical setting, there was greater support for using genomic information to prioritize scarce resources for individuals with higher genetic risk of severe COVID-19 than for withholding resources or denying them admission. The current sample of health professionals, surveyed 18–26 months into the COVID-19 pandemic, have a clear preference for using genomic information to prioritize rather than withhold resources. This was likely influenced by their pandemic experiences,32,33 as more information about an individual’s risk could enable better decision making in scarce resource situations. However, while an overwhelming majority disagreed with the ethical acceptability of using host genomics in the denial or withholding of resources, there was more fragmentation among the participants’ responses to the ethical acceptability of using it for prioritization of scarce resources. Many participants still expressed concerns about its use even for prioritization or triage of resources and the need for safeguards. Specifically, they were apprehensive that relying too heavily on genomic risk factors could lead to an oversight of other important clinical risk factors as well as potentially exacerbating healthcare disparities and inequities. For example, participants expressed concerns that only individuals with high socioeconomic status may be able to afford genetic testing and/or that all genetic variants across diverse populations may not be identified, leading to testing favoring only certain groups of people. Therefore, it is important to strike a balance between genomic and other conventional risk factors and to ensure that the benefits of host genomic testing are accessible to all individuals, regardless of their socioeconomic status or other factors that may affect their access to healthcare.
In the workforce setting, health professionals have expressed strong support for confidential genetic testing in the workplace while opposing the use of personal genetic information by employers to inform employment decisions.25 This may be mostly due to fears about genetic discrimination and the belief that genetic risk alone is insufficient to make decisions about work assignments. While health professionals may be interested in knowing their personal genetic risk and supporting precision medicine and/or precision public health approaches to control outbreaks, many were also wary of employers having access to employee genetic information. Some participants expressed distrust in their employers’ ability to keep personal genomic data confidential and were concerned that it might be used for discriminatory purposes. Several also worry that employers may use employee genomic risk as an excuse to deny them PPE, which they believe should be universal as PPE has been very effective in preventing infections. While the Genetic Information Nondiscrimination Act (GINA) prohibits employers from using genetic information in employment decisions, it is possible that authorities could waive GINA’s genetic privacy provisions during a public health emergency such as a pandemic.3 Further research is needed to understand the extent of GINA’s protections during an infectious-disease outbreak, when the use of employees’ genetic testing results may be justified in order to protect public health, and it is unclear if the declaration of a public health emergency could be used to override GINA’s prohibitions in these scenarios.5,34,35
Our study also explores health professionals’ perspectives on the use of COVID-19 host genomics in the public health setting. Although the majority of participants worked in a clinical setting, they were able to provide important insights about the use of host genomic screening programs in the public health setting. Given the widespread impact of the COVID-19 pandemic, hospital-based health professionals were functioning as both clinicians and public health providers. A majority of participants disagreed with the use of genomic information in most of the public health situations queried. They felt that it is not ethically acceptable to use genomic screening programs to decide various public health measures, such as immigration restriction, travel restriction, school closure, and quarantine. They cited concerns about furthering discrimination, increasing risky behavior among low-risk individuals, and undermining the solidarity needed during public health crises. Regardless, more than a third of the participants still agreed that COVID-19 host genomics can be used for deciding various policies and this is a significant proportion. Additional studies may be needed to further interrogate why these health professionals support the use of host genomics for public health decisions.
The only area in the public health setting that had a lot of support was measures related to vaccine prioritization and waivers. The widespread acceptance of using genomic screening programs to decide prioritization of vaccine distribution and vaccine waivers may be due to the timing of the survey since it was fielded in late 2021 when the COVID-19 vaccine rollouts were just beginning and discussions about vaccine prioritization were top of mind. That respondents—most of whom were working in the clinical setting—were only supportive of using genomic information for vaccine distribution might reflect their greater familiarity with that particular public health scenario. Additional research that focuses on public health professionals may provide further insights into how such information should be used to make decisions about quarantine, travel restrictions, and other public health measures.
Although we were surprised to find no significant differences in the perspectives of those who had personal experience with genomics compared to those who did not, it is possible that the survey did not account for the nature or quality of their experiences. Whether participants’ experiences were negative or positive might have had a greater influence on their views than simply whether or not they had any personal experience. Similarly, health professionals’ views were not directly correlated with their specific COVID-19 experiences, such as testing positive for SARS-CoV-2 or experiencing a PPE shortage at their workplace. However, in the public health setting, participants with greater confidence in their genomics knowledge expressed apprehension about using host genomics. Moreover, in the clinical setting, participants with professional experience with past infectious outbreaks were slightly less inclined to use host genomics. This suggests that those who are more familiar with genomics and/or infectious diseases tend to be more cautious about using host genomics to make decisions. These findings highlight the importance of considering other factors that drive health professionals’ decisions about the ethical acceptability of using host genomics. Future research should explore these factors in greater detail to better understand why health professionals agree or disagree with the ethical acceptability of using host genomics.
Although factors explored here regarding experience with genomics and infectious disease do not appear to be driving their thinking, a majority of health professionals think that it is ethically acceptable to use host genomics in clinical and public health settings. They believe that it could play an important role in the management of infectious outbreaks.
Limitations
This cross-sectional survey examines health professionals’ perspectives at only one point in time. Given that it was an opt-in survey where participants volunteered to participate, this creates selection bias as those who participate may be systematically different from those who do not, and the study was unable to identify the characteristics of those who opted not to participate.
Additionally, the survey used non-probability sampling methods, which severely undermines the generalizability of the results as the survey participants are not representative of all health professionals in the US. The survey response may also be subject to social desirability bias, especially questions related to ethical acceptability of various scenarios using host genomic information. The identification of new COVID-19 variants also coincided with when the survey was distributed to other RESPTCs and public health departments, and this also contributed to the low uptake of the survey, especially among public health professionals. We also encountered several logistical challenges with recruitment, which led to a low number of participants recruited outside of the DC-Maryland-Virginia region. Due to these reasons, these findings are a snapshot of the views of hospital-based health professionals primarily from this area. These data cannot adequately represent the views of public health professionals and cannot be generalized to other settings or professions. While we had hoped for a much more geographically and professionally diverse dataset, the analyses are limited by the sample and the results may considerably differ for health professionals from other areas and institutions that may have experienced very different COVID-19 measures, especially with regard to PPE shortages and direct interaction with COVID-19-positive individuals.
Conclusions
Despite these limitations, this study provides important empirical evidence about stakeholders’ views that should be taken into consideration before precision approaches are implemented in clinical, public health, and/or workforce settings. Future research should also ask these questions in a more nationally representative sample as well as strive to understand the perspectives of other important and diverse stakeholder groups, such as public health professionals, on these issues. Decision makers can use these findings to develop ethically informed implementation guidelines and health policies. These may include increasing the education and training of health professionals in the area of host genomics before they are asked to implement it in their clinical or public health practice.
Data and code availability
The raw individual survey data supporting the current study have not been deposited in a public repository because of data privacy and confidentiality laws but anonymized data are available from the corresponding author on request.
Acknowledgments
The authors would like to thank the members of the Johns Hopkins Biocontainment Unit, the BRIDGES Center, and the Johns Hopkins Berman Institute of Bioethics for their assistance in identifying the issues explored in this survey. We are very grateful to all the health professionals who participated in this survey, especially during a pandemic. We also thank everyone who helped distribute our survey, specifically the teams at the RESPTCs and the Maryland Department of Health. This work was supported by grant number 1RM1HG009038 from the National Human Genome Research Institute. The funders had no involvement in the design, implementation, analysis, interpretation, or reporting of this study.
Author contributions
All authors were involved in the development of the survey, methods, review, and editing of the manuscript. S.J. led the data analysis and the original manuscript draft writing.
Declaration of interests
The authors declare no competing interests.
Footnotes
Supplemental information can be found online at https://doi.org/10.1016/j.xhgg.2023.100255.
Web resources
BRIDGES Center, https://bioethics.jhu.edu/research-and-outreach/projects/center-for-bridging-infectious-disease-genomics-and-society-bridges/
The COVID-19 Host Genetics Initiative, https://www.covid19hg.org/
COVID-19 Genomics and Precision Health Information (GPH), https://phgkb.cdc.gov/PHGKB/coVInfoStartPage.action
Genetic Information Nondiscrimination Act of 2008, http://www.healthinfolaw.org/federal-law/GINA
Online Mendelian Inheritance in Man (OMIM): http://www.omim.org/
Supplemental information
References
- 1.Malik S. Genomics Research: The Underpinning of Infectious Disease Prevention and Control Strategies. Public Health Genomics. 2013;16:3. doi: 10.1159/000346772. [DOI] [PubMed] [Google Scholar]
- 2.Thierry A.R. Host/genetic factors associated with COVID-19 call for precision medicine. Precis. Clin. Med. 2020;3:228–234. doi: 10.1093/pcmedi/pbaa026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Geller G., Dvoskin R., Thio C.L., Duggal P., Lewis M.H., Bailey T.C., Sutherland A., Salmon D.A., Kahn J.P. Genomics and infectious disease: a call to identify the ethical, legal and social implications for public health and clinical practice. Genome Med. 2014;6:106. doi: 10.1186/s13073-014-0106-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Geller G., Duggal P., Thio C.L., Mathews D., Kahn J.P., Maragakis L.L., Garibaldi B.T. Genomics in the era of COVID-19: ethical implications for clinical practice and public health. Genome Med. 2020;12:95. doi: 10.1186/s13073-020-00792-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Boyce A.M., Garibaldi B.T. Genomics and High-Consequence Infectious Diseases: A Scoping Review of Emerging Science and Potential Ethical Issues. Health Secur. 2019;17:62–68. doi: 10.1089/hs.2018.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rauch A., Rohrbach J., Bochud P.Y. The recent breakthroughs in the understanding of host genomics in hepatitis C. Eur. J. Clin. Invest. 2010;40:950–959. doi: 10.1111/j.1365-2362.2010.02337.x. [DOI] [PubMed] [Google Scholar]
- 7.Clark P.J., Thompson A.J. Host genomics and HCV treatment response. J. Gastroenterol. Hepatol. 2012;27:212–222. doi: 10.1111/j.1440-1746.2011.06918.x. [DOI] [PubMed] [Google Scholar]
- 8.Poland G.A., Ovsyannikova I.G., Kennedy R.B. Pharmacogenomics and Vaccine Development. Clin. Pharmacol. Ther. 2021;110:546–548. doi: 10.1002/cpt.2288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valdés-Fernández B.N., Duconge J., Espino A.M., Ruaño G. Personalized health and the coronavirus vaccines—Do individual genetics matter? Bioessays. 2021;43 doi: 10.1002/bies.202100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.COVID-19 Host Genetics Initiative The COVID-19 Host Genetics Initiative, a global initiative to elucidate the role of host genetic factors in susceptibility and severity of the SARS-CoV-2 virus pandemic. Eur. J. Hum. Genet. 2020;28:715–718. doi: 10.1038/s41431-020-0636-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Juengst E.T., Van Rie A. Transparency, trust, and community welfare: towards a precision public health ethics framework for the genomics era. Genome Med. 2020;12:98. doi: 10.1186/s13073-020-00800-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bruce J., Johnson S.B. Exploring the ethics of genetic prioritisation for COVID-19 vaccines. Eur. J. Hum. Genet. 2022;30:875–879. doi: 10.1038/s41431-022-01058-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wu F., Zhao S., Yu B., Chen Y.-M., Wang W., Song Z.-G., Hu Y., Tao Z.-W., Tian J.-H., Pei Y.-Y., et al. A new coronavirus associated with human respiratory disease in China. Nature. 2020;579:265–269. doi: 10.1038/s41586-020-2008-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Severe Covid-19 GWAS Group. Ellinghaus D., Degenhardt F., Bujanda L., Buti M., Albillos A., Invernizzi P., Fernández J., Prati D., Baselli G., et al. Genomewide Association Study of Severe Covid-19 with Respiratory Failure. N. Engl. J. Med. 2020;383:1522–1534. doi: 10.1056/NEJMoa2020283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pairo-Castineira E., Clohisey S., Klaric L., Bretherick A.D., Rawlik K., Pasko D., Walker S., Parkinson N., Fourman M.H., Russell C.D., et al. Genetic mechanisms of critical illness in COVID-19. Nature. 2021;591:92–98. doi: 10.1038/s41586-020-03065-y. [DOI] [PubMed] [Google Scholar]
- 16.Guarino B. 2020. Scientists Pinpoint Genes Common Among Those with Severe Covid-19 Cases.https://www.washingtonpost.com/science/2020/12/14/covid-genes-illness-severity/ The Washington Post. [Google Scholar]
- 17.Zhang Q., Bastard P., Liu Z., Le Pen J., Moncada-Velez M., Chen J., Ogishi M., Sabli I.K.D., Hodeib S., Korol C., et al. Inborn errors of type I IFN immunity in patients with life-threatening COVID-19. Science. 2020;370 doi: 10.1126/science.abd4570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duncan C.J.A., Mohamad S.M.B., Young D.F., Skelton A.J., Leahy T.R., Munday D.C., Butler K.M., Morfopoulou S., Brown J.R., Hubank M., et al. Human IFNAR2 deficiency: Lessons for antiviral immunity. Sci. Transl. Med. 2015;7:307ra154. doi: 10.1126/scitranslmed.aac4227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niemi M.E.K., Karjalainen J., Liao R.G., Neale B.M., Daly M., Ganna A., Pathak G.A., Andrews S.J., Kanai M., Veerapen K., et al. Mapping the human genetic architecture of COVID-19. Nature. 2021;600:472–477. doi: 10.1038/s41586-021-03767-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khoury M.J., Iademarco M.F., Riley W.T. Precision Public Health for the Era of Precision Medicine. Am. J. Prev. Med. 2016;50:398–401. doi: 10.1016/j.amepre.2015.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Velmovitsky P.E., Bevilacqua T., Alencar P., Cowan D., Morita P.P. Convergence of Precision Medicine and Public Health Into Precision Public Health: Toward a Big Data Perspective. Front. Public Health. 2021;9:561873. doi: 10.3389/fpubh.2021.561873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Laberge A.-M., Richer J., Ravitsky V. Toward Broader Genetic Contextualism: Genetic Testing Enters the Age of Evidence-Based Medicine. Am. J. Bioeth. 2019;19:77–79. doi: 10.1080/15265161.2018.1544315. [DOI] [PubMed] [Google Scholar]
- 23.Milne R. Societal considerations in host genome testing for COVID-19. Genet. Med. 2020;22:1464–1466. doi: 10.1038/s41436-020-0861-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gerber J.E., Geller G., Boyce A., Maragakis L.L., Garibaldi B.T. Genomics in Patient Care and Workforce Decisions in High-Level Isolation Units: A Survey of Healthcare Workers. Health Secur. 2021;19:318–326. doi: 10.1089/hs.2020.0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Walker A., Boyce A., Duggal P., Thio C.L., Geller G. Genomics and Infectious Diseases: Expert Perspectives on Public Health Considerations regarding Actionability and Privacy. Ethics Hum. Res. 2020;42:30–40. doi: 10.1002/eahr.500051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kraft C.S., Kortepeter M.G., Gordon B., Sauer L.M., Shenoy E.S., Eiras D.P., Larson L., Garland J.A., Mehta A.K., Barrett K., et al. The Special Pathogens Research Network: Enabling Research Readiness. Health Secur. 2019;17:35–45. doi: 10.1089/hs.2018.0106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Casanova J.-L., Su H.C., COVID Human Genetic Effort. Abel L., Aiuti A., Almuhsen S., Arias A.A., Bastard P., Biggs C., Bogunovic D., et al. A Global Effort to Define the Human Genetics of Protective Immunity to SARS-CoV-2 Infection. Cell. 2020;181:1194–1199. doi: 10.1016/j.cell.2020.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cucinotta D., Vanelli M. WHO Declares COVID-19 a Pandemic. Acta Biomed. 2020;91:157–160. doi: 10.23750/abm.v91i1.9397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shereen M.A., Khan S., Kazmi A., Bashir N., Siddique R. COVID-19 infection: Emergence, transmission, and characteristics of human coronaviruses. J. Adv. Res. 2020;24:91–98. doi: 10.1016/j.jare.2020.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rasmussen S.A., Abul-Husn N.S., Casanova J.-L., Daly M.J., Rehm H.L., Murray M.F. The intersection of genetics and COVID-19 in 2021: preview of the 2021 Rodney Howell Symposium. Genet. Med. 2021;23:1001–1003. doi: 10.1038/s41436-021-01113-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wickenhagen A., Sugrue E., Lytras S., Kuchi S., Noerenberg M., Turnbull M.L., Loney C., Herder V., Allan J., Jarmson I., et al. A prenylated dsRNA sensor protects against severe COVID-19. Science. 2021;374 doi: 10.1126/science.abj3624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chemali S., Mari-Sáez A., El Bcheraoui C., Weishaar H. Health care workers’ experiences during the COVID-19 pandemic: a scoping review. Hum. Resour. Health. 2022;20:27. doi: 10.1186/s12960-022-00724-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Butler C.R., Webster L.B., Diekema D.S., Gray M.M., Sakata V.L., Tonelli M.R., Vranas K.C. Perspectives of Triage Team Members Participating in Statewide Triage Simulations for Scarce Resource Allocation During the COVID-19 Pandemic in Washington State. JAMA Netw. Open. 2022;5 doi: 10.1001/jamanetworkopen.2022.7639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lewis M.H. Should Genetic Testing for Variants Associated with Influenza Infection Be Mandatory for Health Care Employees? AMA J. Ethics. 2018;20:E819–E825. doi: 10.1001/amajethics.2018.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Field R.I., Orlando A.W., Rosoff A.J. Genetics and COVID-19: How to Protect the Susceptible. Trends Genet. 2021;37:106–108. doi: 10.1016/j.tig.2020.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The raw individual survey data supporting the current study have not been deposited in a public repository because of data privacy and confidentiality laws but anonymized data are available from the corresponding author on request.