Abstract
It has been hypothesized that reducing the bioenergetic costs of gut inflammation as an explanation for the effect of antibiotic growth promoters (AGPs) on animal efficiency, framing some observations but not explaining the increase in growth rate or the prevention of infectious diseases. The host's ability to adapt to alterations in environmental conditions and to maintain health involves managing all physiological interactions that regulate homeostasis. Thus, metabolic pathways are vital in regulating physiological health as the energetic demands of the host guides most biological functions. Mitochondria are not only the metabolic heart of the cell because of their role in energy metabolism and oxidative phosphorylation, but also a central hub of signal transduction pathways that receive messages about the health and nutritional states of cells and tissues. In response, mitochondria direct cellular and tissue physiological alterations throughout the host. The endosymbiotic theory suggests that mitochondria evolved from prokaryotes, emphasizing the idea that these organelles can be affected by some antibiotics. Indeed, therapeutic levels of several antibiotics can be toxic to mitochondria, but subtherapeutic levels may improve mitochondrial function and defense mechanisms by inducing an adaptive response of the cell, resulting in mitokine production which coordinates an array of adaptive responses of the host to the stressor(s). This adaptive stress response is also observed in several bacteria species, suggesting that this protective mechanism has been preserved during evolution. Concordantly, gut microbiome modulation by subinhibitory concentration of AGPs could be the result of direct stimulation rather than inhibition of determined microbial species. In eukaryotes, these adaptive responses of the mitochondria to internal and external environmental conditions, can promote growth rate of the organism as an evolutionary strategy to overcome potential negative conditions. We hypothesize that direct and indirect subtherapeutic AGP regulation of mitochondria functional output can regulate homeostatic control mechanisms in a manner similar to those involved with disease tolerance.
Key words: antibiotic growth promoters, growth, productive efficiency, hormesis, poultry
INTRODUCTION
For over 70 yr antibiotics were used for improving growth promotion in food-producing animals including poultry (Castanon, 2007). However, increased antibiotic resistance in food animals has led to the complete ban on antibiotic growth promoters (AGPs) in animal feed by the European Union in 2006 (E.U. regulation 1831/2003/EC; European Commission, 2003) and subsequent FDA request for the voluntary removal of medically important AGPs from animal feed in the United States (Thanner et al., 2016). Remarkably, for all the years of using antibiotics as growth promoters, no consensus, based on scientific evidence, was ever described as the mechanism(s) of action of AGPs.
The accumulated evidence thus far has separated the discussion into 2 fundamental targets about AGPs and their mechanism(s) of action: the microbiota and the host. However, it is not easy to determine the consequences of microbiota or host response modulation (and vice versa) by the action of AGP (or any other compound) since the intricate connection within the microbial community and this with the host and the environment (including stress, activity level and diet) results in massive, interconnected responses. For example, the intrinsic variability of the gut microbiota and the levels of antimicrobial resistance that certain groups of bacteria may develop or carry against the antimicrobial being used would induce a broad number of unequal effects on the even more complex physiological interactions between the different sections of the intestine with the rest of the interconnected organs, such as the liver and brain, regulating motor actions, metabolism, immune system and even behavior. Nevertheless, beyond this complexity, it is still striking that different antibiotics with diverse mechanisms and targets of action, induce the similar overall outcome in the host, encouraging the search for an alternative hypothesis for animal growth promotion and feed efficiency by in-feed antimicrobials at low doses.
In the present report, we will analyze the connection between AGPs and the intestinal ecosystem from the perspective of systems biology. As the list of alternatives to antibiotics increase, there has been little evaluation on just what made antibiotics such efficient growth promoters. Understanding the interactions between the key components of the intestinal ecosystem can provide a holistic assessment of the growth promoting process of antibiotics in poultry. Therefore, we propose an alternative hypothesis intended to provide a simple framework for the fundamental mechanism behind the benefit of antibiotics on animal efficiency, considering growth rate, feed conversion and animal health and how to integrate these mechanisms into the development of novel alternatives to antibiotics.
AGPS IN POULTRY
The growth promoter effect of antibiotics was discovered in the 1940s, when beneficial effects on production efficiency in poultry and swine were observed (Dibner and Richards, 2005). Some years later, their use as animal feed additives without prescription was approved in the United States and other countries. Since then, antibiotics were supplemented in animal feed at subtherapeutic doses to improve growth and feed conversion efficiency and to prevent infections (Castanon, 2007).
Traditionally, at least 4 main mechanisms have been considered to explain the effect of antimicrobials on animal growth, mostly linked to their antimicrobial effects: reduced nutrient use by microorganisms; reduction of bacterial metabolites; increased absorption and use of nutrient by a thinner-walled gut, and inhibition of subclinical infections (Gaskins et al., 2002; Dibner and Richards, 2005). Also, AGPs are considered to have a key role in reducing low-level inflammation and immunologic stress (Dal Pont et al., 2021), eventually by a direct anti-inflammatory effect (Niewold, 2007).
Assuming that only the antimicrobial activity of AGPs is responsible for improving animal performance, the development of antimicrobial resistance should have an evident effect in reducing the benefits associated with AGP. The frequent use of AGP in poultry production may well be selected for microbiota that are less responsive to their effects and thus their efficacy may have moderated after all this time. Accordingly, a reduction in the effectiveness of AGP in the last 30 yr produced by increasing levels of antimicrobial resistance was previously suggested (Laxminarayan et al., 2016). The initial work of Rosen (1995), analyzing available data of AGPs being used in poultry and pig production from mid ’40s (when AGPs were initially used) up to mid ’90s, concluded that they were effective 72% of the time. However, when comparing these data with a meta-analysis performed with information available from 1990 to 2020 (Maria Cardinal et al., 2019), the expected negative variation of AGP efficacy was not observed. In effect, the numeric results seem to be similar to those reported by Rosen (1995) with data from 1945 to 1990s.
AGP Doses in Poultry
It is important to highlight that AGPs are administered at subtherapeutic concentrations, probably far below the reported minimum inhibitory concentrations (MIC) for many bacterial species (Broom, 2017). MIC represents the lowest concentration of an antimicrobial that has any visible effect on target bacteria. However, bacterial growth, functionality, or metabolism could still be affected by those subinhibitory concentrations of antibiotics, causing possible competitive disadvantages for some bacteria species, such as highly adapted pathogenic bacteria. Many antibiotics can induce changes in bacteria metabolism by different mechanisms of action depending not only on bacteria species involved but also on the characteristics of others surrounding microorganisms (Broom, 2017). However, antimicrobial resistance should still affect these mechanisms of inhibition or metabolic alteration. Considering the complexity and diversity of the gut microbiota and the possible alterations produced by AGPs, proposing a simple and unique explanation for the different classes of antimicrobials showing growth-promoting effects is highly difficult to provide, even more considering the diverse mechanism of action of those antibiotics. Further, positive effects of several classes of antibiotic used as growth promoters on the progression and resolution of infectious diseases cannot be explained by their direct antibacterial activities alone (Tauber and Nau, 2008) suggesting that other effects of AGPs in addition to their antimicrobial activity must be involved in the growth-promoting effects.
AGPs and Hormesis
The toxic effects of certain substances are induced as the inhibition of some biological process above a threshold level. More than 130 yr ago, Schulz H., Uber Hefegifte. Pfligers Arch. Ges. Physiol., 42, 1888, 517-541. observed that several toxic substances had the opposite effect at lower concentrations in stimulating growth and respiration of yeast, suggesting that another component could exist beyond the toxic relationship. These and other early studies supported the formulation Arndt-Schulz Law or Hueppe's Rule, which stated that substances which inhibit biological processes at lethal levels may be expected to stimulate them at lower levels. This concept was observed in many other different biological systems becoming the Arndt-Schulz Law generally accepted for most toxic substances. However, over the years, the Arndt-Schulz Law began to be analyzed more and more critically since it could not be considered a general law and it was not providing explanatory capability itself with the result that it gradually fell into disuse, and the concept of toxic stimulatory effects of toxic substances was disregarded. Alternatively, in the field of pharmacology and toxicology, the “dose response threshold” has become widely accepted. Nowadays, the linear no-threshold risk model is commonly accepted and used by regulatory bodies as a basis for formulating public health policies though it simplistically assumes that the risk of adverse biological effects increases linearly as the total dose of stressor increases.
Southam and Ehrlich (1943) described the effect of a natural antibiotic in cedar wood that inhibited the growth of wood decaying fungi, but when given at low levels it had the opposite effect. They proposed the term "hormesis" to describe "a stimulatory effect of subinhibitory concentrations of any toxic substance on any organism." Hormesis, from Greek hórmēsis "rapid motion, eagerness,” is a term used to refer to a biphasic (or triphasic) dose response of an organism to an agent (which can be a chemical compound or an environmental factor) and it is characterized by a low-dose stimulation or beneficial effect and a high-dose inhibitory or toxic effect (Mattson, 2008). An assessment of the historical and current dose response literature exposes that hormesis manifestation is general and it can be observed commonly from plants to humans independently of level of biological organization (i.e., cell, organ, and organism), endpoint, inducing agent and mechanism. In the field of biology, hormesis can be defined as an adaptive compensatory response of cells and organisms to a moderate stress that follows the initial disturbance of homeostasis. The term is often used to describe a contradictory low-dose beneficial effects of stressors, but this paradox is clearly associated with prejudices about what is good and bad, and cognitively predisposed to associate the simple cause-effect relationships. Dose response models are one example of this conceptual oversimplification of biological reality. Examples include ischemic preconditioning, exercise, dietary energy restriction and exposures to low doses of certain biological, chemical, or physical agents.
In general, hormesis can be outlined as a central evolutionary strategy structured within the limits of biological “plasticity,” defined as “the ability of individual genotypes to produce different phenotypes when exposed to different environmental conditions” (Pigliucci et al., 2006). Plasticity allows the organism to rapidly respond and maintain reproductive fitness under variable conditions and the degree of phenotypic adaptive change and type of phenotypic alternative can vary depending on environmental conditions. Hence, hormesis can be seen as a common regulatory strategy for biological resource allocation and a component of biological plasticity, where high dose of stressors damages a biological system, while a low dose of the same substance generates a positive response in several physiologic functions, from cell growth to behavior. It must be also considered that organisms respond in a hormetic manner to signals that indicate stress, toxicity, or disruptions in homeostasis (Calabrese, 2008). Both antibiotics and their metabolites exhibited toxicity to nontarget organisms. Therefore, the concept of hormesis can be associated to explain the effects of in-feed antibiotics in animal productive efficiency. Considering the several factors contributing to productive efficiency, the effect would depend on at least 3 apparently individual but highly interrelated contributors: growth rate, feed conversion and animal health.
Therefore, in-feed antibiotics can be considered stressors that induce a condition of hormesis in individuals, affecting different physiological responses of the animal that alter the dynamics of resource allocation.
AGPs, Hormesis, and Growth
The growth rate is one of the characteristic parameters that is considered to evaluate the productive efficiency in chickens and other animals and that the AGP improves. Hormesis and growth rate have been described in early reports in prokaryotes as well as in eukaryotes. The Nobel prize recipient, Charles Richet, in 1905 to 1907 showed the stimulatory effects of low concentrations of formalin, a range of metals and brief exposure to radium in bacteria and lactic fermentation (Stebbing, 1982). Hotchkiss (1923) investigated the effects of various cations upon the growth of “Bacterium coli” (Escherichia coli), describing that 15 of the 23 chlorides compounds tested had the effect of stimulating bacterial growth. Other authors described similar effects in the growth of Staphylococcus after 16 h exposure to various concentrations of penicillin, and refer similarities also with sulfonamides, cyanide, pyrithiamine, and some narcotics (Miller et al., 1945; Pratt and Dufrenoy, 1948). Hobby and Dawson (1944) investigating the rate of bacterial growth as influencing the action of penicillin report the bacterial count after 24 h of refrigeration in a media containing red blood cells was much higher in the presence of 10 and 100 units of penicillin than in the presence of 0.1 unit or in the control tubes. Welch et al. (1946) found that at certain concentration levels of streptomycin injected intraperitoneally into mice infected with Salmonella, increases rather than decreases the fatality rate. Similarly, but working with penicillin, Randall et al. (1947) observed that relatively high doses of this antibiotic could improve the survival of mice infected with Salmonella but at relatively lower doses could exert a stimulating effect on the death rate of infected mice. Enhancement of growth by streptomycin has also been reported for several bacteria, including Mycobacterium tuberculosis (Spendlove et al., 1948), Meningococcus (Miller and Bohnhoff, 1947; Rake, 1948), E. coli (Rake, 1948), Staphylococcus aureus, E. coli, Pseudomonas aeruginosa, Proteus spp. (Paine and Finland, 1948) and several sporogenic and nonsporogenic bacteria (Curran and Evans, 1947).
In eukaryotes, Southam and Ehrlich (1943) reported an apparent stimulation in the growth of otherwise sensitive organisms at certain concentrations of an antibiotic substance extracted from the heartwood of western red cedar (Thuja plicata). The material they were using, probably phenolic in nature, produced this stimulatory action with various fungi and that the degree of hormesis became less as the culture aged. Hessayson (1953) observed that while high concentrations of an antibiotic produced by the fungus Trichothecium roseum was able to inhibit mycelial growth of Fusarium oxysporum in soil, much lower concentrations had the effect of stimulating growth. Antibiotic streptomycin has been found to stimulate fungal growth at subinhibitory concentrations (Roessler and Herbst, 1946; Campbell and Saslaw, 1949; Loefer et al., 1952). Antibiotics have also been found to stimulate the growth of protozoan populations. Neomycin at lower concentrations increased population size of the ciliated protozoan Tetrahymena gelii population after 72 h although the individual size was smaller than control (Blumberg and Loefer, 1952). Similar outcomes were observed with the antibiotic aureomycin and then chlortetracycline with Euglena gracilis, an abundant and well-studied water living protist (Robbins et al., 1951).
In plants, Nickell and Finlay (1954) showed that antibiotics (penicillin, terramycin, streptomycin, thiolutin, bacitracin) at low concentrations can stimulate plant growth in tissue culture, standard laboratory seed germination, and seed germination and subsequent growth in soil. The effect of some of the antibiotics tested showed stimulation at low levels and inhibition at higher levels, including oxytetracycline, streptomycin, and chloromycetin (Nickell and Finlay, 1954). The analysis of the in vitro results suggests that effects were direct on the plant cells since microorganisms were not present.
In animals, several studies have shown that antibiotics widely used in livestock, that is, tetracyclines, can stimulate cell growth at low concentration over a narrow range but also cause adverse effects such as interruption of mitochondrial proteostasis and physiology in several animals ranging from roundworms, fruit flies, and mice to human cell lines at higher levels (Wang et al., 2015). In 1956, a hormetic dose response was observed in growth of poultry fed with various antibiotics (Luckey et al., 1956). It was a nonexpected result as they were trying to reduce bacteria load to observe how animal growth was affected by an unknown vitamin produced by bacteria. Subsequently, these results were also described in pigs, cattle and humans. Indeed, the use of antibiotics as AGP is considered advantageous in commercial production to improve animal growth and feed conversion and reduced morbidity and mortality due to clinical and subclinical diseases. AGPs are considered the “gold standard” of performance-enhancing feed additives although at high concentrations they are toxic, fitting to the principles of hormesis. The average growth improvement has been estimated to be between 4 and 8%, and feed utilization was improved by 2 to 5% (Ewing and Cole, 1994; Dahiya et al., 2006), although it depends on the growth period and the environmental conditions. For example, in younger animals, AGPs can improve growth rates up to 16% and feed efficiency by up to 7% (Cromwell, 2002).
The explanation behind AGP and growth can be found in the proposed theoretical model of growth hormesis (Stebbing, 1998) that states that hormesis represents an overcompensation to a disruption in homeostasis. Many organisms adjust their developmental growth, metabolism, and behavior to promote survival and reproduction, sensing and responding to changes in internal and external environmental conditions (Koyama et al., 2020). The occurrence of what appears to be a consistent response of growth promotion in single-cell and complex organisms after exposure to a variety of toxic agents, including antibiotics, points to a basic shared mechanism operating at cellular or subcellular level to overcome potential threat produced after sensing signals of risk. This mechanism seems to be universal and fundamental of most living organisms and most likely appeared during early evolution to adjust organism's fitness to stressors.
AGPS AND ENVIRONMENTAL STRESSORS AT THE ORGANISM LEVEL
Environmental Stressors
The gut has the greatest surface area separating the environmentally exposed lumen and the internal subepithelial tissue and therefore continually exposed to infectious and noninfectious triggers. Similarly, these external elements have a dramatic effect on gut bacterial composition (Feye et al., 2020; Bindari and Gerber, 2022). Other factors as breed, age, sex of the bird, and management practices affects the intestinal ecosystem (Lumpkins et al., 2008; Oakley et al., 2014; Ballou et al., 2016; Lee et al., 2019; Feye et al., 2020; Duangnumsawang et al., 2022; Emami et al., 2022) although environmental factors as diet, temperature, litter quality, etc. appear to play a much more dominant role in the development of the intestinal ecosystem, modulating animal health while regulating the immune system and metabolism (Pan and Yu, 2014; Kogut, 2019; Nobs et al., 2020; Emami et al., 2021; Patra and Kar, 2021; Emami et al., 2022; Lee et al., 2022). Several environmental factors can play an important role for modifying the inflammatory state.
AGPs and Inflammation
Inflammation is a highly regulated physiological response mechanism against deleterious stimuli that are infectious, toxic, or immune in origin. Regulated inflammation is essential to eliminate harmful stimuli. Yet, if the trigger remains, an inappropriate chronic inflammatory response can occur that leads to disrupted tissue function, organ failure, and mortality. Inflammation is the most prevalent manifestation of host innate defense in reaction to alterations from an infectious or noninfectious insult in tissue homeostasis (Medzhitov, 2021). Inflammation and energy metabolism are strictly linked as this profound interconnection has been driven during evolutive process (Ottaviani et al., 2007). To maintain energy homeostasis and sustain developmental growth, organisms adapt their metabolism according to the available resources (Koyama et al., 2020; Lee et al., 2022). The intensified feed intakes and nutrient excesses in modern animal production are likely to predispose these animals to a particularly chronic, inflammatory state that triggers unnecessary energy costs (Kogut et al., 2018). Sterile and metabolic inflammation are, typically, chronic, low-grade inflammatory states resulting from innate immune system stimulation by noninfectious cellular components and metabolites, including several feed components (Teirlynck et al., 2009). Nutrients, such as fatty acids, or metabolites produced by the gut microbiota, also represent metabolic perturbations of intestinal epithelial cells as they can impair mitochondrial function (Guerbette et al., 2022). High-energy diets themselves can trigger gut inflammation in poultry. For example, high levels of glucose induce oxidative stress in cells of the gastrointestinal tract (Bhor and Sivakami, 2003; Powell et al., 2004). High concentrations of glucose induce basal inducible nitric oxide synthase (iNOS) promoter activity through changes in intracellular glutathione, an intracellular antioxidant molecule, and nuclear factor κB (NF-κB) activity, an oxidative-stress-responsive transcription factor (Kwon et al., 1995, 1998; Hattori et al., 2000; Powell et al., 2004).
Based on an evolutionary perspective, it was conceptualized that immune and stress responses functionally overlap and that classic “antigens” (viruses and bacteria, as well as feed components) can be considered stressors that later were likely major selective forces for the evolution of the immune system (Ostan et al., 2008). Consequently, the phenomenon of hormesis can be supposed to arise for protecting the living organisms from deleterious effects as it is done, for example, by the immune system, but preceding this and collaborating with it.
Medzhitov (2021) recently acknowledged that “because infection and injury-induced inflammation are the most prominent and most studied forms of the response, we may have skewed our understanding of inflammation according to these extreme conditions.” In commercial poultry production, this reductionist view of inflammation has dominated our thinking. Consequently, the use of AGPs in poultry feeds has restricted our understanding of the physiological roles of inflammation in maintaining and monitoring tissue homeostasis (Broom and Kogut, 2018; Kogut et al., 2018). More explicitly, the removal of AGPs from animal feeds has provided evidence that environmental stressors, especially noninfectious in nature, has resulted in the increased recognition of low-grade, chronic inflammation in the intestine (Dal Pont et al., 2021). This chronic stimulation of the immune system impairs the ability of the animal to reach 100% of its genetic potential given that nutrients are diverted away from growth to support the inflammatory response. Therefore, in hindsight, AGPs were never truly used to control enteric infections (Dal Pont et al., 2021) given that intestinal infections predominantly induce acute but not chronic inflammation. Further evidence that AGP “masked” inflammation has been shown by the increased incidence of Clostridium perfringens-mediated necrotic enteritis worldwide since the removal of AGPs from poultry diets (Van Immerseel et al., 2004). Clostridium perfringens is a normal commensal of the chicken intestine even in diets provided in-feed AGP (Fasina and Lillehoj, 2019).
Disease Resistance and Disease Tolerance
Ayres (2020) has provided an interesting concept that “health is an active process that enables an organism to adapt to fluctuations in its intrinsic and extrinsic environments to maintain health or recover to a healthy state after disease occurs.” This definition of health recognizes a difference between defense mechanisms such as those centered on immune functions that antagonize infections (disease resistance) and ability of the host to limit the pathological damage caused by both the stressor and the host immune response via physiological mechanisms that promote health (disease tolerance) (Ayres, 2020; Medzhitov, 2021).
Although disease resistance and disease tolerance can be either uncoupled (independent responses), positively correlated (involving same genes and mechanisms), or negatively correlated (traded off) processes (Balard et al., 2020), the optimal extent of both defense systems is determined by the costs of resistance and tolerance (Sheldon and Verhulst, 1996). From an ecological perspective, mounting a resistance defense is energetically costly to the host, but the maintenance of tolerance mechanisms can also be disadvantageous to other functions of the organism (Stowe et al., 2000; Råberg et al., 2009). The artificial genetic selection to obtain animals with desirable productive traits usually moves the optimal balance of these defenses naturally selected (Broggi et al., 2016) which result in qualitatively and quantitatively differences between components of the immune system as the different types of immune responses may have different energy demands. Disease resistance requires the development and maintenance of a fully competent immune system (as well as the potential response to external stimuli) with an important metabolic cost for the animal (Råberg et al., 2002) but the use of nutritional inputs for the organization of animal immune responses involves not only the maintenance of the body capability to respond to the infection, but also later during the infection, when the immune defenses are upregulated (Siva-Jothy and Thompson, 2002; Rahnamaeian et al., 2015). The energetic support of the immune system significantly affects productivity as it is conditioned by the partitioning of dietary nutrients from skeletal muscle mass toward metabolic responses (Johnson, 1997).
The health problems and enhanced disease sensitivity of modern broilers compared to older breeds are believed to be the result of dysfunction of the specific cellular and humoral immune system (Yunis et al., 2000; Koenen et al., 2002; Cheema et al., 2003) as indicated by an increased susceptibility to infectious diseases and reduced adaptive immune responsiveness (Cheema et al., 2003). Under healthy conditions (clean environment) it is a remarkable benefit but under challenging condition, the greater susceptibility of these animals to develop subclinical or clinical infections becomes a comparatively greater threat to productive efficiency as mounting an immune response (conditional on its magnitude) is more energetically costly than maintaining the immune system (Derting and Compton, 2003). Even worse, the final step during infection, the disease, results in important metabolic costs associated with loss of function in the affected tissues or organs produced by auto-immune damage and the repairing of this damage after the infection. At this point the number and magnitude of episodes of infectious diseases become an important threat to productive efficiency in artificially selected poultry chickens. Preventing the development of an immune response and then the damage of the disease seems to be crucial for the largest energy savings in modern poultry chickens, and to reduce mortality rate.
The widespread nature of hormetic responses includes disease resistance and tolerance of the host and both are implicated in the control of diseases. According to the concept of hormesis, low-dose preconditioning by proinflammatory or other harmful signals, can modify a subsequent response to the same or alternative insults. For example, bacteria sepsis results from a deregulated response of the host to infection (Cao et al., 2019) and, concordantly with the hormesis notion, low doses of harmful agents would stimulate cytoprotective pathways that can be protective against a secondary event. In other words, low stress stimulus can influence both resistance and tolerance responses to same or different hazards.
Disease Resistance. Infectious and noninfectious environmental stressors usually induce inflammation and strongly affect feed conversion efficiency in broilers. AGPs added to feed certainly improve feed conversion and reduce mortality (Rosen, 1995), suggesting that antibiotics at subinhibitory concentrations largely control or prevent infectious diseases. As stated above, it is interesting that AGPs can reduce the incidence of gut diseases without killing pathogens. Typically, the reduction of the incidence of infectious diseases includes strategies which basically aim the reduction of the number of pathogens such as disinfection, vaccination, and the use of therapeutical antimicrobial drugs (Medzhitov et al., 2012; Soares et al., 2017). Such approach of microbial killing, referred as “disease resistance,” is also used by the host which includes the response of the immune system and all downstream events. Therefore, disease resistance can be defined as the ability to inhibit or limit infection, while tolerance do not limit infection but reduce or offset its fitness consequences and lastly the sum of both defines a host's defensive capacity. Until relatively a few years ago, the survival to infectious diseases has been believed to be entirely dependent on the ability of the host to fight against the pathogen through resistance mechanisms (Schneider and Ayres, 2008).
Disease Tolerance. Disease tolerance, an evolutionary conserved defense strategy against infection that does not exert a direct negative effect on the pathogen load (McCarville and Ayres, 2018), has been relatively recently recognized as an important mechanism for animal defense. Disease tolerance has been shown to provide protection against different classes of pathogens (Råberg et al., 2007; Gozzelino et al., 2012; Rodrigue-Gervais et al., 2014), including bacteria (Larsen et al., 2010).
Disease tolerance as a defense strategy, sustains host homeostasis whether triggered by an infection or environmental stressor. Disease tolerance relies on stress and damage responses that confer tissue damage control (Soares et al., 2014), that is, support the functional output of host tissues to maintain vital homeostasis compatible with survival (Chovatiya and Medzhitov, 2014; Kotas and Medzhitov, 2015). Stress and damage responses sense and react to variations in environmental cues or to damage imposed to cellular macromolecules and organelles, respectively (Soares et al., 2014). There are specific mechanisms to deal with unnecessary immune response suggested by Ayres and Schneider (2012) that involve “ignorance” of immune effector as host response actively block immune detection (deliberate) or by lack of receptors for recognizing a benign/mutualistic microbe (passive); or “silence” by keeping immune response off until needed (moving response threshold); and the ratio of microbe/host damage for an immune effector controls tolerance. If a host cannot detect a microbe, the host will not raise an immune response that can cause pathology. Also, host microbiota modulation that balances beneficial/pathogenic microorganisms could be included as a disease tolerance mechanism. Host-microbial interactions involve commensals and mutualists, so a sufficient resistance response to pathogens while maintaining a homeostatic relationship with beneficial and harmless microbes is required. Some mutualistic bacteria can promote host tolerance by puzzling mechanisms as, that is, support of integrity of the physical separation of gut pathogens from systemic organs (Ewaschuk et al., 2008) or sensing environmental changes and intercommunicating with the host (Jones et al., 2015; Xu et al., 2022). The components of the tolerance defense system include host encoded mechanisms preventing the onset or supporting resolution of immunopathology in host tissues (Ayres and Schneider, 2012). Many of these tolerance mechanisms may well be affected by AGP to increase animal health, and concomitantly productivity, including modulation of excessive immune/inflammatory response and stress response, damage repair, and cellular regeneration mechanisms (Ayres and Schneider, 2012; Soares et al., 2017). The principal mechanisms involve host metabolic responses that sustain fundamental homeostatic controls within a capacity compatible with survival (Ayres, 2020; Troha and Ayres, 2020). Key to this defense strategy is the ability of the host to sense and adapt to disparities in nutrients, such as iron and glucose.
A similar concept to disease tolerance has been applied to the capacity of various tissues to "tolerate" or resist damage from inflammatory activity (Wu and Reddy, 2017), as those observed from noninfectious immune damage that would include sterile and metabolic inflammation. Tissue tolerance mechanisms could include features that depend on cellular stress responses and the manifestation includes mechanisms that facilitate the expansion of their homeostatic range (Wu and Reddy, 2017). These cellular stress resistance pathways are essential for survival and are conserved in insects, animals, and humans (Fontana et al., 2010). The fact that these compounds can induce stress resistance in different animal species led to the proposition that many organisms have evolved to sense unfavorable environmental conditions and protect themselves against a threatening unfavorable environment. All eukaryotic organisms detect and correct perturbations in homeostasis by way of different surveillance mechanisms. Sensing of homeostasis deviations might be also a general mechanism used by the host to detect premature signs of infections to enhance the ability to evaluate the threat and produce a suitable defense response (Colaço and Moita, 2016). Organelle dysfunction caused by pathogens, chemical and physical insults, or dietary components, rapidly triggers a compensatory response (Sawa et al., 2016) as low doses of any damaging agent perturbating cell homeostasis and inducing stress response does. Such stress responses are also key to trigger an effective immune response (Colaço and Moita, 2016) as well as cytoprotective responses that buffer stress and damage (Shore and Ruvkun, 2013). The induction of these stress resistance pathways following consumption of determined dietary or pharmacological compounds reflects the typical hormesis response, in which small doses of stress-inducing compounds produce health benefits due to overcompensation of homeostatic mechanisms in living organisms (Calabrese, 2001).
AGPs and Disease Tolerance
As mentioned before, it seems that the growth promoting activities of AGPs cannot be explained via their direct antimicrobial effects per se. Recently, it was reported that antibiotics, such as tetracycline, has an off-target side effect that provokes a perturbation of host cellular function that appears to represent a significant signal to initiate disease tolerance mechanisms against an influenza viral infection (Mottis et al., 2022). Specifically, the authors revealed the host's ability to detect a homeostatic disruption of the mitochondria functional activity (mitohormesis) to induce a mild mitochondrial stress of respiratory host cells that initiated the disease tolerance mechanisms. The events initiated by a disruption of homoeostasis played a role in limiting tissue damage, activated negative-feedback pathways that ceased the inflammatory response, and initiated tissue repair mechanisms resulting in a return to the steady state.
In summary, the components of the tolerance defense system include host encoded mechanisms preventing the onset or supporting resolution of immunopathology in host tissues. These pathways sustain fundamental homeostatic controls within a capacity compatible with survival and may well be affected by AGP.
AGPS AND ENVIRONMENTAL STRESSORS AT THE CELLULAR LEVEL
AGPs and Mitochondrial Stress Response
The endosymbiotic theory suggests that mitochondria, as the chloroplasts, have evolved from endosymbiotic prokaryotes. This endosymbiotic origin supports the idea that these organelles are also vulnerable to antibiotics. Indeed, there are several reports describing the toxic effects of several antibiotics to mitochondria homeostasis (Wang et al., 2015). Antibiotics of the families of the aminoglycosides, amphenicols, lincosamides, macrolides, oxazolidinones, streptogramins—all known as inhibitors of bacterial protein synthesis—also block mitochondrial polypeptide synthesis, often without a parallel effect on the cytoplasmic ribosome. Cephalosporins are closely related to the inhibition of mitochondrial substrate uptake and the enhancement of mitochondrial-derived ROS production (Suntur et al., 2005). Aminoglycoside antibiotics are thought to cause mitochondrial dysfunction; streptomycin can alter mitochondria function and bind to mitochondrial ribosome (Holliday, 2005; Itoh et al., 2022); and gentamicin behaves as an uncoupler of the electron transport chain (O'Reilly et al., 2019). Clindamycin and erythromycin compromise the activity of the mitochondrial respiratory chain (Prajapati et al., 2019). On isolated rat liver mitochondria, zinc-bacitracin, flavomycin, and chlortetracycline were found to interfere with mitochondrial energy metabolism (Nohl and Hegner, 1977). Using an in vitro translation system from bovine mitochondria, tetracycline was shown to have similar inhibitory effects on both Escherichia coli and bovine mitochondrial protein synthesis, but the mitochondrial system was more resistant to tiamulin, macrolides, virginiamycin, fusidic acid, and kirromycin than the E. coli system. Also, quinupristin/dalfopristin, streptogramin antibiotics like virginiamycin, binds to the large mitoribosomal subunit, inhibiting mitochondrial protein synthesis and functionally dysregulating oxidative phosphorylation complexes (Sighel et al., 2021). Penicillin produces mitochondria energy metabolic disorders, affecting basic respiration capacity, maximal respiration capacity, respiration potential, and inhibition of ATP generation (Hu et al., 2021). Many of these antibiotics were found to interfere with mitochondria homeostasis have a clear effect as growth promoters. Bunyan et al. (1977) analyzed 55 antimicrobial compounds as growth promoters, finding that some of them (cephalosporins, all penicillins, some aminoglycosides, clindamycin, lincomycin, vancomycin, spectinomycin, rifampycin, oxytetracycline, chlortetracycline, erythromycin, tylosin, flavomycin, virginiamycin, and bacitracin) have growth promotion effects. Others had little effect (polymyxin B, novobiocin, cycloserine, phophonomycin, and sodium fusidate) or were inactive (chloramphenicol, nalidixic acid, neomycin, trimethoprim, shuphadizine). Therefore, antibiotics can be considered an important mitochondrion hazard at toxic concentrations. However, at lower concentrations and, according to the concept of hormesis, low-dose preconditioning of cells by harmful signals can modify a subsequent response to the same or alternative insults. The mitochondrial hormetic effect of AGP explains their benefits in productive efficiency, therefore effects might be produced in mitochondria of intestinal mucosa as there is no absorption of most AGP from the normal gastrointestinal tract (Butaye et al., 2003).
Mitochondria, AGPs, and Growth in Poultry
Mitochondrial hormesis has been observed after an acute exposure to stress as it can stimulate adaptive mitochondrial responses that improve mitochondrial function and resistance to stress. At sub therapeutic doses, the effects of antibiotics in mitochondria of intestinal epithelium can spread systemically as mitochondrial stress response coordinates an array of adaptive responses in the organism (Shpilka and Haynes, 2018; Mottis et al., 2019). This adaptive response originates in the mitochondria, senses and responds to changes in internal and external environmental conditions to promote survival and reproduction of the animal. Durieux et al. (2011) showed that the positive effect of a mild mitochondrial stress is not restricted to the affected cell or tissue but also spreads to distal ones, thus conferring a global resistance and survival advantage to the whole organism. The signaling of a localized mitochondrial perturbation produced by AGPs in the intestinal mucosa can spread through the organism via mitochondrial stress-induced cytokines or soluble mediators (mitokines). These soluble molecules (protein, peptide or other) produced and secreted in response to a mitochondrial stress response are able to elicit either an adaptive or a compensatory response in distal cells not directly affected by the stressful event/stimulus. Several mitokines have been identified, including the neuronal peptide FLP2 in C. elegans (Berendzen et al., 2016; Shao et al., 2016), the well-known fibroblast growth factor 21 (FGF21) and growth differentiation factor 15 (GDF15), and adrenomedullin2 (ADM2) in mammals, although several other molecules and candidates have been suggested (Lv et al., 2016; Kang et al., 2017; Kim et al., 2017). Feed stressor in the human intestinal epithelium that induce oxidative stress response and the unfolded protein response, both central pathways of the mitohormetic response, produced increased expression of the mitokines GDF15 and ADM2 in jejunal crypts (Liszt et al., 2022). Also, feed components can activate the integrated stress response which leads to increased circulating GDF15 levels (Patel et al., 2019), an endocrine acting mitokine with metabolic actions. Although almost any cell or tissue can express GDF15 in response to various forms of stress (Hsiao et al., 2000; Appierto et al., 2009; Yang et al., 2010; Park et al., 2012; Chung et al., 2017), this evidence suggest that epithelial mitochondria respond to dietary stimulus influencing other cells and tissues beyond the gut.
Mitochondria, AGPs, Feed Efficiency, and Host Defenses
Besides growth rate, mitochondrion also emerges as an important player that defines many of the effects of AGP associated with feed efficiency, as mitochondria are a convergent signaling hub that regulates diverse developmental, environmental, and pathological stimuli. Hormesis induced by AGPs as a stressor would lead to improved battle against other stresses, which may at least partly account for some hormetic effects (Rattan, 2004; Le Bourg, 2011; Cañuelo et al., 2012; Schmeisser et al., 2013; Anderson et al., 2016). Both cell and tissue tolerance in the gut produced after hormetic mitochondria stimulation seems to be key to improve feed efficiency and to reduce mortality. Mitochondria have a central role in the energy metabolism, homeostasis, and energy balance in the gut, but it is also an important source of cellular reactive oxygen species (ROS) because electrons can leak from the respiratory chain and react with molecular oxygen to produce a superoxide anion (Demine et al., 2019). Because of active oxidative metabolism, in particular complex I and III of the electron transport chain (ETC), mitochondria are also a major source of ROS in cells (Chen and Zweier, 2014) and ROS are responsible for disrupting cellular homeostasis and inflammation. The imbalance between the production of ROS and the ability to neutralize them in living organisms results in oxidative stress and any excess of oxidative stress will result in gastrointestinal pathophysiology, including inflammation and apoptosis of epithelial cells, which would affect the intestinal functioning (Bhattacharyya et al., 2014), negatively affecting animal efficiency. As the gastrointestinal tract is inevitably exposed to foreign substances and microbial pathogens, the oxidative stress of the intestinal epithelium becomes highly relevant for animal efficiency. The gastrointestinal tract represents only 5% of the total body weight but consumes 20% of the whole-body oxygen (Blachier et al., 2009), indicating a high metabolic activity and a key role for mitochondria. Recent growing evidence has emphasized the extended roles of mitochondria in modulating oxidative stress as well as innate immune responses (Chen et al., 2018) standing interconnected at multiple levels (Mills et al., 2017). In this sense, mitochondrion is directly linked to the pathophysiology of metabolic inflammation (Diaz-Vegas et al., 2020). The coordinated response to mild mitochondrial stress, as that of AGPs in intestinal epithelial cells, seems to leave the cell less susceptible to subsequent perturbations (Yun and Finkel, 2014). Also, it was proposed that an independent pathway of tolerance can be initiated by inhibition of mitochondrial protein synthesis, as many antibiotics do, leading to perturbations of electron transport chain function (Colaço et al., 2021). If mitochondrion becomes less susceptible to many other stressors after AGPs stimulation, then the threshold level to trigger immune responses, including inflammation, would be higher and cell, tissue, and animal tolerance, increased. Therefore, mitochondrial hormesis and AGPs seem to be important to activate disease tolerance.
Mitochondria and Nuclear Factor Erythroid 2-Related Factor 2 (Nrf2)
Pioneer work in C. elegans reveals that genetic defect in the ETC (Dillin et al., 2002) or inhibition of mitochondrial protein synthesis (Houtkooper et al., 2013) results in extended lifespan, an evolutive strategy to improve organism performance as could be increased growth or reproduction. In mice, metabolic benefits arising from inhibition of ETC activity have been reported in the context of obesity and insulin resistance (Pospisilik et al., 2007; Chung et al., 2017; Masand et al., 2018). For instance, an acute oxidative stress via mitochondrial superoxide production stimulates the activation of endogenous antioxidant gene transcription regulated by the redox sensitive transcription factor Nrf2, resulting in an adaptive hormetic response. Nrf2 is a transcription factor and a key regulator of cellular redox state that regulates the cellular defense against toxic, oxidative or electrophilic stress through the expression of more than 250 genes involved in metabolism, drug detoxification and oxidative stress response, which is associated with the pathogenesis of inflammatory diseases (Furfaro et al., 2016). Calabrese and Kozumbo (2021) proposed a generalized mechanism for hormetic dose responses based on the redox-activated transcription factor Nrf2 and its upregulation of an integrative system of endogenous antioxidant and anti-inflammatory adaptive responses. In fact, many examples of low doses of toxic substances that activate Nrf2, enhance cellular resiliency, diminish damage, and elevate thresholds, have been reported. According to the authors, the intrinsic characteristics of Nrf2 via dose-dependent hierarchical processes are responsible for the enhanced biological resilience and produce the quantitative features of the hormetic dose response. Transient activation of Nrf2 in response to stress influences cellular pathways that maintain homeostasis if the damage caused by toxic stimuli is repairable. Nrf2 affects multiple aspects of mitochondrial metabolism and function, from nutrient absorption, anabolic metabolism, macromolecular biosynthesis to energy metabolism that supports cell growth and proliferation.
Activation of Nrf2 results in cells having greater resistance to chemical insults and inflammatory challenges. When ROS levels are low, Nrf2 exists in bound form in the cytoplasm with its inhibitor, Keap1, which suppresses Nrf2 activity by targeting it for constitutive polyubiquitination by a Cullin3-based E3 ligase complex resulting in proteasomal degradation (Kobayashi et al., 2004). The inhibitor Keap1 contains redox-sensitive cysteine residues which react during oxidative stresses conditions, removing the inactivation of Nrf2 by changes in the functional conformation of Keap1. Then, Nrf2 translocate to the nucleus, binds to Antioxidant Response Element sequences, and starts the transcription of a series of antioxidant enzymes and detoxifying proteins, known as the “Phase 2 detoxification response” (Suzuki et al., 2013). The expression level of Nrf2 is particularly high in the detoxification organs or tissues which directly counter the environment, such as the intestine (Kobayashi et al., 2004). The Nrf2/Keap1 axis also plays a fundamental role in the development of gastrointestinal tract and the maintenance of its proper functionality, at least as it was observed in mammals (Piotrowska et al., 2021). In the gut, besides protecting against oxidative stress, it is involved in the regulation of inflammatory pathways and tight junction proteins (Wen et al., 2019). The Nrf2-Keap1 pathway plays a key role in resisting intestinal mucosal injury as it was observed that in epithelial cells the nuclear translocation of Nrf2 significantly suppressed ROS generation and enhanced cell survival (Rodríguez-Ramiro et al., 2012). The protective effect of Nrf2 in maintaining the barrier has been proved in various experimental models, including Salmonella Typhimurium infection in mice (Theiss et al., 2009), and burn and brain induced intestinal barrier damage (Chen et al., 2016; Liu et al., 2017) or severe sepsis (Yu et al., 2017). Chickens fed with AGPs had shown to increase the expression of Nrf2 (Wang et al., 2022). Nontoxic levels of ROS induced by cefotaxime activated the Nrf2 pathway without cytotoxicity (Yamada et al., 2020). As a generalization, antibiotics impair mitochondria leading to elevated ROS levels (Suárez-Rivero et al., 2021).
As conceptually developed above, mitochondria stress and hormesis induced by low doses of antimicrobials would lead to elevated cytoprotection and disease tolerance, resulting in increased growth rate, feed conversion efficiency and death rate.
In summary, many of the antibiotics that have a clear effect as growth promoters showed to interfere with mitochondria homeostasis and, according to the concept of hormesis, low-dose preconditioning of cells by harmful signals can modify a subsequent response to the same or alternative insults.
The mitochondrial hormetic effect of AGP explains their benefits in productive efficiency. Signaling of a localized mitochondrial perturbation produced by AGPs in the intestinal mucosa can spread through the organism via mitochondrial stress-induced cytokines or soluble mediators (mitokines).
If mitochondrion become less susceptible to many other stressors after AGPs stimulation, then the threshold level to trigger immune responses, including inflammation, would be higher and cell, tissue, and animal tolerance, increased. Therefore, mitochondrial hormesis and AGPs seems to be important to activate disease tolerance, resulting in increased growth rate, feed conversion efficiency and death rate. The redox sensitive transcription factor Nrf2 would be one of the dependent pathways.
AGPS AND ENVIRONMENTAL STRESSORS AND THE MICROBIOTA
Microbiota play a critical role in productive efficiency of poultry chickens, providing nutrients and energy, and maintaining host health, modulating immune responses, and modulating the competitive exclusion of pathogens, particularly in the small intestine. According to the review of Iavicoli et al. (2021), the exposure of bacteria to different types of antibiotics, either administered individually or in mixtures, is capable of exerting hormetic effects, independently of the bacteria characteristics or the type of antibiotic used. The likely rationale idea supporting this outcome, and concordantly to the hormesis effects observed with many other organisms, is that the initial disruption of the homeostasis by an external stressor determines an adaptive response, which is opposite to the expected outcome (Calabrese and Baldwin, 2002). These antibiotics in bacteria should exert an inhibition of metabolic processes, but it can produce a stimulation at lower doses, principally as a defensive tactic to increase their resilience. It must be considered that although different bacterial strains can implement a hormetic response as a defensive strategy, the type of response that emerges can be substantially different, corresponding to the biological characteristics of each microorganism and the stressor that comes into play, such as the type of antibiotic and its inhibition mechanisms. Also, considering that usually the dose associated with the stimulation is commonly less than 50-fold with respect to the toxic threshold and antimicrobial compound will show differential levels of toxicity for each bacterial species (i.e., MIC), the result will be a disparity in the eventual stimulation of the microbial community components. Moreover, the composition and dynamics of gut microbiota is strongly influenced by how the stimulated microorganisms interact with the host and other components of the microbiota, and the consequent impact on environmental conditions. Therefore, it is reasonable to hypothesize that part of the observed microbiome modulation in productive animals by AGPs or alternatives with antimicrobial properties could be the result of direct stimulation rather than inhibition of determined microbial species.
Although all these studies show differences in microbiota modulation, they are only a scant description of the complexity and variability that exists within the very diverse feeding and management conditions in animal production. Beyond assumptions about ceca microbiota impact, there is no direct evidence to assume that these microbiome changes in the ceca are responsible or, the opposite, a consequence, of AGP related efficiency effects. Furthermore, there are no assessments to what extent these changes in the cecal microbiota could be a significant part of improving animal performance under different diet quality and compositions. In fact, the most significant microbial changes produced by virginiamycin, one of the most used antibiotic growth promoters in the poultry industry for disease prevention and growth promotion, induce the most significant microbial responses in the small intestine (Dumonceaux et al., 2006; Pourabedin et al., 2015). Pourabedin et al. (2015) found that AGP virginiamycin improved feed conversion ratio of broiler chickens and related to greater abundance of specific bacteria genera in the ileum with no changes in relative abundances in the ceca, or overall microbial diversity at either site. It is plausible that in many observations, cecal microbiota changes associated with animal efficiency were the consequence (or at least partially) of other nutritional/physiological changes more closely associated with the nutrient digestion and absorption efficiency in the upper gut. For example, if the nutritional quality of feed and the digestive efficiency are high, then it is not illogical to consider that the role of ceca microbiota under such conditions is not decisive to improve animal efficiency. Consequently, the role of ceca microbiota in animal efficiency would be highly variable and depend, at least, on the quality of the feed as well as the upper gut physiological condition.
The small intestine is the longest part of the chicken gastrointestinal system that is specialized for nutrient absorption, and it occurs mainly in the ileum which exhibits high numbers of Lactobacillus sp. (Witzig et al., 2015). Intestinal bacteria can stimulate ROS production in epithelial cells similarly to pathogen-induced respiratory burst on phagocytic cells (Jones et al., 2013; Alam et al., 2014). This enzymatically produced ROS in the epithelial cells by oxidase family members is stimulated by pathogenic or symbiotic bacteria, especially members of the lactobacilli taxon. These changes promote cell proliferation and migration (Wentworth et al., 2010, 2011), healing (Swanson et al., 2011), and modifies epithelial NF-κB signaling (Kumar et al., 2007). The microbiota, despite having a valuable relationship with the host, is ultimately an extrinsic influencer, and the ROS stimulated by microbes are a potent stressor that functions as a signaling agent.
The host intrinsic mechanisms that mediate nonimmune symbiont-induced effects are largely unknown. Oxidative stress response is also a central component in bacteria to microbial adaptability and pathogenicity (Riboulet et al., 2007). Apart from endogenously generated ROS during metabolic activity of bacteria, exogenous elements like environment, host, and antimicrobials can trigger reactions that elevate ROS levels. This is an important association that is critical for the formation, metabolism, development, and functioning of adaptive resistance. It has been proposed that members of the genus lactobacilli might have developed symbiotic relationships in which microbially induced ROS generation functions as a bacterial signal transducer in host gene regulatory events that potentiate multiple effects in different biological systems (Jones et al., 2015). The ability of lactobacilli to induce redox signals in epithelial cells appears to be a highly conserved hormetic adaptation to prepare cells for exogenous stimuli (Jones et al., 2015) although the impact of lactobacilli on host physiology appears to be strain specific (Storelli et al., 2011). Symbiont-induced ROS may also activate epithelial Nrf2 pathway signaling, and thereby mechanistically mediate the beneficial influences of these constituents of intestinal microbiota. The Nrf2 pathway is a highly conserved system for transducing exogenous stimuli into eukaryotic transcriptional responses and it has been suggested that Nrf2 pathway could be important as a signaling conduit between the Lactobacilli and the eukaryotic host, as fly, mice or chickens. Thus, hormesis, as a response to xenobiotic and environmental stimuli, evidently extends to the acquisition and adaptation to exogenous microbiota, illustrating a mechanism of coevolution and symbiosis between host cell/tissue/organs and microbes.
The Howitz and Sinclair xenohormesis hypothesis suggests that organisms respond to stress signaling molecules produced by third organisms, thus activating defense pathways to tolerate to expected adverse environmental conditions (Lamming et al., 2004; Howitz and Sinclair, 2008). According to this hypothesis, bacteria in the gut susceptible to AGPs could get a competitive advantage from antibiotic sublethal stimulation, and eventually release noxious molecules to the host, which could increase their stress signal and increase their defense and reduce inflammation threshold.
It has long been recognized that the xenobiotic detoxification functions of the liver evolved to deal with this exposure to diet-derived molecules arriving from the intestine. Recent studies have revealed that these pathways not only are sensitive to changes in the diet but also may be altered by peptides and small molecules derived from the gut-resident microbiota (Macpherson et al., 2016). Multiple signaling pathways, including those involved in bile acid biosynthesis (Dawson and Karpen, 2015; Jiang et al., 2015; Foley et al., 2019), the urea cycle (Stewart and Smith, 2005), and choline metabolism (Wang et al., 2011; Romano et al., 2015; Schugar et al., 2018), have been shown to be sensitive to changes to the microbial communities of the gut. It was observed that some gut-resident members of genus lactobacilli can stimulate hepatic Nrf2 and protect against oxidative liver injury (Saeedi et al., 2020). It can have important consequences for animal health and productivity, as overconsumption of carbohydrates induces oxidative stress and lipid accumulation in the liver, and subsequently induces the inflammatory response (Tan et al., 2018).
Therefore, to summarize, microbiome modulation in productive animals by AGPs could be the result of direct stimulation rather than inhibition of determined microbial species. Bacteria in the gut susceptible to AGPs could get a competitive advantage from antibiotic sublethal stimulation, and eventually release noxious molecules to the host, which could increase their stress signal and increase their defense and reduce inflammation threshold.
HYPOTHESIS AND PERSPECTIVES
It has been proposed that AGPs may work by having anti-inflammatory effects on intestinal inflammatory cells (Niewold, 2007). The close contact of the gastrointestinal mucosa with the microbiota results in a persistent physiological inflammation (Kogut et al., 2018), but also excessive nutrient intake or chemical, physical, metabolic stimuli can activate low-grade, persistent inflammation. Chronic low-grade intestinal inflammation has a negative impact on animal productivity by impairing nutrient absorption and allocation of nutrients for growth (Broom and Kogut, 2018; Dal Pont et al., 2021). Therefore, the hypothesis was that reducing the energetic costs of gut inflammation may be an explanation for the effect of AGPs on animal efficiency. Although this hypothesis is useful for framing some observations, it does not explain the increase in growth rate or the prevention of some diseases.
According to Ayres (2020), the ability to control and maintain health involves managing all the physiological interactions that regulate homeostasis of an organism. These “physiological health” regulatory mechanisms, which include such variables as growth and development, energy management, thermoregulation, and oxidative control, support the host's ability to function and adapt to alterations in environmental conditions. Metabolic pathways are the most vital in regulating physiological health since satisfying the energetic demands of the host guides most biological functions. Mitochondria are widely considered the metabolic heart of the cell because of their pivotal roles in energy metabolism and oxidative phosphorylation, the pathways for biosynthesis of macromolecules, and in regulating basic cellular processes, including cell growth, innate immune responses, cell differentiation, and cell death (Casanova et al., 2023). Mitochondria have developed surveillance mechanisms that recognize physiological alterations in tissues/cells homeostasis and initiate signaling pathways that can regulate immunometabolic function and homeostasis (Yun and Finkel, 2014). Thus, mitochondria can be considered a central hub of signal transduction pathways that receive messages about the health and nutritional states of the host cells and tissues and, in response, direct cellular and tissue physiological alterations throughout the host (Shen et al., 2022).
Therapeutic levels of antibiotics can damage mitochondria (Miller and Singer, 2022), but subtherapeutic levels may improve mitochondrial function and defense mechanisms by inducing an adaptive response of the cell, which can be spread to other distant cells by means of mitokines, coordinating an array of adaptive responses in the organism that coordinate the adjustment of the whole organism to stress (Quirós et al., 2016). These adaptive responses, originating in the mitochondria as a response to changes in internal and external environmental conditions, can promote growth rate of the animal as an evolutionary strategy to overcome potential negative environmental conditions for the species.
The microbiota, despite having a valuable relationship with the host at several levels, is ultimately also an extrinsic influencer in close contact with intestinal epithelial cells. Metabolites or derived by-products secreted by microbes can be potent stressor that functions as a signaling agent to simulate an adaptive response at mitochondrial level. In such sense, subtherapeutic levels of antimicrobials, in contrast to their higher inhibitory concentrations, can also produce a stimulation of certain microbial strains as result of a hormetic defensive tactic to increase their resilience. Therefore, it is reasonable to propose that part of the observed microbiome modulation by subinhibitory concentration of antimicrobial molecules could be the result of direct stimulation rather than inhibition of certain microbial species.
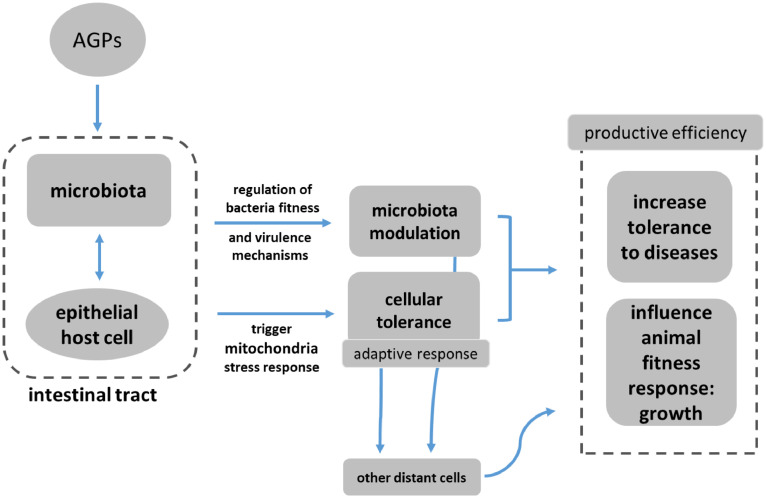
Thus, we hypothesize that direct and indirect subtherapeutic AGP regulation of mitochondria functional output can regulate homeostatic control mechanisms in a manner similar to those involved with disease tolerance (Figure 1). In any case, these tolerance mechanisms promote growth rate increase and a dynamic equilibrium in the intestine reducing the energetic cost of gut inflammation and prevent the onset or later support for the resolution of immunopathology, improving feed efficiency, and diminishing death rate.
Figure 1.
Schematic representation of the hypothesis proposed to explain how AGPs could improve productive efficiency in chickens. Subtherapeutic levels of antibiotics modulate microbiota and mitochondrial function, increasing cellular defense mechanisms by inducing an adaptive response. This mechanism can promote growth rate of the organism as an evolutionary strategy to overcome potential negative conditions.
DISCLOSURES
The authors declare that they have no competing financial interests or personal relationships that could inappropriately influence the present work.
REFERENCES
- Alam A., Leoni G., Wentworth C.C., Kwal J.M., Wu H., Ardita C.S., Swanson P.A., Lambeth J.D., Jones R.M., Nusrat A., Neish A.S. Redox signaling regulates commensal-mediated mucosal homeostasis and restitution and requires formyl peptide receptor 1. Mucosal Immunol. 2014;7:645–655. doi: 10.1038/mi.2013.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson E.N., Corkins M.E., Li J.C., Singh K., Parsons S., Tucey T.M., Sorkaç A., Huang H., Dimitriadi M., Sinclair D.A., Hart A.C. C. elegans lifespan extension by osmotic stress requires FUdR, base excision repair, FOXO, and sirtuins. Mech. Ageing Dev. 2016;154:30–42. doi: 10.1016/j.mad.2016.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appierto V., Tiberio P., Villani M.G., Cavadini E., Formelli F. PLAB induction in fenretinide-induced apoptosis of ovarian cancer cells occurs via a ROS-dependent mechanism involving ER stress and JNK activation. Carcinogenesis. 2009;30:824–831. doi: 10.1093/carcin/bgp067. [DOI] [PubMed] [Google Scholar]
- Ayres J.S. The biology of physiological health. Cell. 2020;181:250–269. doi: 10.1016/j.cell.2020.03.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayres J.S., Schneider D.S. Tolerance of infections. Annu. Rev. Immunol. 2012;30:271–294. doi: 10.1146/annurev-immunol-020711-075030. [DOI] [PubMed] [Google Scholar]
- Balard A., Jarquín-Díaz V.H., Jost J., Mittné V., Böhning F., Ďureje L., Piálek J., Heitlinger E. Coupling between tolerance and resistance for two related Eimeria parasite species. Ecol. Evol. 2020;10:13938–13948. doi: 10.1002/ece3.6986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ballou A.L., Ali R.A., Mendoza M.A., Ellis J.C., Hassan H.M., Croom W.J., Koci M.D. Development of the chick microbiome: how early exposure influences future microbial diversity. Front. Vet. Sci. 2016;3:2. doi: 10.3389/fvets.2016.00002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendzen K.M., Durieux J., Shao L.W., Tian Y., Kim H.E., Wolff S., Liu Y., Dillin A. Neuroendocrine coordination of mitochondrial stress signaling and proteostasis. Cell. 2016;166:1553–1563.e1510. doi: 10.1016/j.cell.2016.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya A., Chattopadhyay R., Mitra S., Crowe S.E. Oxidative stress: an essential factor in the pathogenesis of gastrointestinal mucosal diseases. Physiol. Rev. 2014;94:329–354. doi: 10.1152/physrev.00040.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhor V.M., Sivakami S. Regional variations in intestinal brush border membrane fluidity and function during diabetes and the role of oxidative stress and non-enzymatic glycation. Mol. Cell. Biochem. 2003;252:125–132. doi: 10.1023/a:1025599126840. [DOI] [PubMed] [Google Scholar]
- Bindari Y.R., Gerber P.F. Centennial review: factors affecting the chicken gastrointestinal microbial composition and their association with gut health and productive performance. Poult. Sci. 2022;101:1–19. doi: 10.1016/j.psj.2021.101612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blachier F., Boutry C., Bos C., Tomé D. Metabolism and functions of L-glutamate in the epithelial cells of the small and large intestines. Am. J. Clin. Nutr. 2009;90:814S–821S. doi: 10.3945/ajcn.2009.27462S. [DOI] [PubMed] [Google Scholar]
- Blumberg A., Loefer J.B. Effect of neomycin on two species of free-living protozoa. Physiol. Zool. 1952;25:276–282. [Google Scholar]
- Broggi J., Soriguer R.C., Figuerola J. Transgenerational effects enhance specific immune response in a wild passerine. Peer J. 2016;31:e1766. doi: 10.7717/peerj.1766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broom L.J. The sub-inhibitory theory for antibiotic growth promoters. Poult. Sci. 2017;96:3104–3108. doi: 10.3382/ps/pex114. [DOI] [PubMed] [Google Scholar]
- Broom L.J., Kogut M.H. The role of the gut microbiome in shaping the immune system of chickens. Vet. Immunol. Immunopathol. 2018;204:44–51. doi: 10.1016/j.vetimm.2018.10.002. [DOI] [PubMed] [Google Scholar]
- Bunyan J., Jeffries L., Sayers J.R., Gulliver A.L., Coleman K. Antimicrobial substances and chick growth promotion: the growth-promoting activities of antimicrobial substances, including fifty-two used either in therapy or as dietary additives. Br. Poult. Sci. 1977;18:283–294. [Google Scholar]
- Butaye P., Devriese L.A., Haesebrouck F. Antimicrobial growth promoters used in animal feed: effects of less well known antibiotics on gram-positive bacteria. Clin. Microbiol. Rev. 2003;16:175–188. doi: 10.1128/CMR.16.2.175-188.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calabrese E.J. Overcompensation stimulation: a mechanism for hormetic effects. Crit. Rev. Toxicol. 2001;31:425–470. doi: 10.1080/20014091111749. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J. Converging concepts: adaptive response, preconditioning, and the Yerkes-Dodson Law are manifestations of hormesis. Ageing Res. Rev. 2008;7:8–20. doi: 10.1016/j.arr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J., Baldwin L.A. Defining hormesis. Hum. Exp. Toxicol. 2002;21:91–97. doi: 10.1191/0960327102ht217oa. [DOI] [PubMed] [Google Scholar]
- Calabrese E.J., Kozumbo W.J. The hormetic dose-response mechanism: Nrf2 activation. Pharmacol. Res. 2021;167 doi: 10.1016/j.phrs.2021.105526. [DOI] [PubMed] [Google Scholar]
- Campbell C.C., Saslaw S. Enhancement of growth of certain fungi by streptomycin. Proc. Soc. Exp. Biol. Med. 1949;70:562. doi: 10.3181/00379727-70-16993. [DOI] [PubMed] [Google Scholar]
- Cañuelo A., Gilbert-López B., Pacheco-Liñán P., Martínez-Lara E., Siles E., Miranda-Vizuete A. Tyrosol, a main phenol present in extra virgin olive oil, increases lifespan and stress resistance in Caenorhabditis elegans. Mech. Ageing Dev. 2012;133:563–574. doi: 10.1016/j.mad.2012.07.004. [DOI] [PubMed] [Google Scholar]
- Cao C., Yu M., Chai Y. Pathological alteration and therapeutic implications of sepsis-induced immune cell apoptosis. Cell Death Dis. 2019;10:782. doi: 10.1038/s41419-019-2015-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova A., Wevers A., Navarro-Ledesma S., Pruimboom L. Mitochondria: it is all about energy. Front. Physiol. 2023;14 doi: 10.3389/fphys.2023.1114231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castanon J.I. History of the use of antibiotic as growth promoters in European poultry feeds. Poult. Sci. 2007;86:2466–2471. doi: 10.3382/ps.2007-00249. [DOI] [PubMed] [Google Scholar]
- Cheema M.A., Qureshi M.A., Havenstein G.B. A comparison of the immune response of a 2001 commercial broiler with a 1957 randombred broiler strain when fed representative 1957 and 2001 broiler diets. Poult. Sci. 2003;82:1519–1529. doi: 10.1093/ps/82.10.1519. [DOI] [PubMed] [Google Scholar]
- Chen Z., Zhang Y., Ma L., Ni Y., Zhao H. Nrf2 plays a pivotal role in protection against burn trauma-induced intestinal injury and death. Oncotarget. 2016;7:19272–19283. doi: 10.18632/oncotarget.8189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y., Zhou Z., Min W. Mitochondria, oxidative stress and innate immunity. Front. Physiol. 2018;9:1487. doi: 10.3389/fphys.2018.01487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y.R., Zweier J.L. Cardiac mitochondria and reactive oxygen species generation. Circ. Res. 2014;114:524–537. doi: 10.1161/CIRCRESAHA.114.300559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chovatiya R., Medzhitov R. Stress, inflammation, and defence of homeostasis. Mol. Cell. 2014;54:281–288. doi: 10.1016/j.molcel.2014.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung H.K., Ryu D., Kim K.S., Chang J.Y., Kim Y.K., Yi H.S., Kang S.G., Choi M.J., Lee S.E., Jung S.B. Growth differentiation factor 15 is a myomitokine governing systemic energy homeostasis. J. Cell Biol. 2017;216:149–165. doi: 10.1083/jcb.201607110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaço H.G., Barros A., Neves-Costa A., Seixas E., Pedroso D., Velho T., Willmann K.L., Faisca P., Grabmann G., Yi H.S., Shong M., Benes V., Weis S., Köcher T., Moita L.F. Tetracycline antibiotics induce host-dependent disease tolerance to infection. Immunity. 2021;54:53–67.e7. doi: 10.1016/j.immuni.2020.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colaço H.G., Moita L.F. Initiation of innate immune responses by surveillance of homeostasis perturbations. FEBS J. 2016;283:2448–2457. doi: 10.1111/febs.13730. [DOI] [PubMed] [Google Scholar]
- Cromwell G.L. Why and how antibiotics are used in swine production. Anim. Biotechnol. 2002;13:7–27. doi: 10.1081/ABIO-120005767. [DOI] [PubMed] [Google Scholar]
- Curran H.R., Evans F.R. Stimulation of sporogenic and nonsporogenic bacteria by traces of penicillin or streptomycin. Proc. Soc. Exp. Biol. Med. 1947;64:231–233. doi: 10.3181/00379727-64-15753. [DOI] [PubMed] [Google Scholar]
- Dahiya J.P., Wilkie D.C., van Kessel A.G., Drew M.D. Potential strategies for controlling necrotic enteritis in broiler chickens in post-antibiotic era. J. Anim. Feed Sci. Technol. 2006;29:60–88. [Google Scholar]
- Dal Pont G.C., Belote B.L., Lee A., Bortoluzzi C., Eyng C., Sevastiyanova M., Khadem A., Santin E., Farnell Y.Z., Gougoulias C., Kogut M.H. Novel models for chronic intestinal inflammation in chickens: intestinal inflammation pattern and biomarkers. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.676628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawson P.A., Karpen S.J. Intestinal transport and metabolism of bile acids. J. Lipid Res. 2015;56:1085–1099. doi: 10.1194/jlr.R054114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demine S., Renard P., Arnould T. Mitochondrial uncoupling: a key controller of biological processes in physiology and diseases. Cells. 2019;8:795. doi: 10.3390/cells8080795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derting T.L., Compton S. Immune response, not immune maintenance, is energetically costly in wild white-footed mice (Peromyscus leucopus) Physiol. Biochem. Zool. 2003;76:744–752. doi: 10.1086/375662. [DOI] [PubMed] [Google Scholar]
- Diaz-Vegas A., Sanchez-Aguilera P., Krycer J.R., Morales P.E., Monsalves-Alvarez M., Cifuentes M., Rothermel B.A., Lavandero S. Is mitochondrial dysfunction a common root of noncommunicable chronic diseases? Endocr. Rev. 2020;41:bnaa005. doi: 10.1210/endrev/bnaa005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dibner J.J., Richards J.D. Antibiotic growth promoters in agriculture: history and mode of action. Poult. Sci. 2005;84:634–643. doi: 10.1093/ps/84.4.634. [DOI] [PubMed] [Google Scholar]
- Dillin A., Hsu A.L., Arantes-Oliveira N., Lehrer-Graiwer J., Hsin H., Fraser A.G., Kamath R.S., Ahringer J., Kenyon C. Rates of behavior and aging specified by mitochondrial function during development. Science. 2002;298:2398–2401. doi: 10.1126/science.1077780. [DOI] [PubMed] [Google Scholar]
- Duangnumsawang Y., Zentek J., Vahjen W., Tarradas J., Boroojeni F.G. Alterations in bacterial metabolites, cytokines, and mucosal integrity in the caecum of broilers caused by feed additives and host-related factors. Front. Physiol. 2022;13 doi: 10.3389/fphys.2022.935870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumonceaux T.J., Hill J.E., Hemmingsen S.M., Van Kessel A.G. Characterization of intestinal microbiota and response to dietary virginiamycin supplementation in the broiler chicken. Appl. Environment. Microbiol. 2006;72:2815–2823. doi: 10.1128/AEM.72.4.2815-2823.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durieux J., Wolff S., Dillin A. The cell-non-autonomous nature of electron transport chain-mediated longevity. Cell. 2011;144:79–91. doi: 10.1016/j.cell.2010.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Greene E.S., Kogut M.H., Dridi S. Heat stress and feed restriction distinctly affect performance, carcass and meat yield, intestinal integrity, and inflammatory (chemo) cytokines in broiler chickens. Front. Physiol. 2021;12 doi: 10.3389/fphys.2021.707757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emami N.K., Schreier L.L., Greene E., Tabler T., Orlowski S.K., Anthony N.B., Proszkowiec-Weglarz M., Dridi S. Ileal microbial composition in genetically distinct chicken lines reared under normal or high ambient temperatures. Anim. Microbiome. 2022;4:28. doi: 10.1186/s42523-022-00183-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Commission Regulation (EC) No 1831/2003 of the European Parliament and of the Council of 22 September 2003 on additives for use in animal nutrition. Off. J. Eur. Union. 2003;L268:29–43. [Google Scholar]
- Ewaschuk J.B., Diaz H., Meddings L., Diederichs B., Dmytrash A., Backer J., Looijer-van Langen M., Madsen K.L. Secreted bioactive factors from Bifidobacterium infantis enhance epithelial cell barrier function. Am. J. Physiol. Gastrointest. Liver Physiol. 2008;295:G1025–G1034. doi: 10.1152/ajpgi.90227.2008. [DOI] [PubMed] [Google Scholar]
- Ewing W.N., Cole D.J.A. The Living Gut: An Introduction to Micro-Organisms in Nutrition. Context Publications; Dungannon, Northern Ireland: 1994. [Google Scholar]
- Fasina Y.O., Lillehoj H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019;98:188–198. doi: 10.3382/ps/pey390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feye K.M., Baxter M.F.A., Tellez-Isaias G., Kogut M.H., Ricke S.C. Influential factors on the composition of the conventionally raised broiler gastrointestinal microbiomes. Poult. Sci. 2020;99:653–659. doi: 10.1016/j.psj.2019.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley M.H., O'Flaherty S., Barrangou R., Theriot C.M. Bile salt hydrolases: gatekeepers of bile acid metabolism and host-microbiome crosstalk in the gastrointestinal tract. PLoS Pathog. 2019;15 doi: 10.1371/journal.ppat.1007581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontana L., Partridge L., Longo V.D. Extending healthy life span – from yeast to humans. Science. 2010;328:321–326. doi: 10.1126/science.1172539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furfaro A.L., Traverso N., Domenicotti C., Piras S., Moretta L., Marinari U.M., Pronzato M.A., Nitti M. The Nrf2/HO-1 axis in cancer cell growth and chemoresistance. Oxid. Med. Cell. Longev. 2016 doi: 10.1155/2016/1958174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaskins H.R., Collier C.T., Anderson D.B. Antibiotics as growth promotants: mode of action. Anim. Biotechnol. 2002;13:29–42. doi: 10.1081/ABIO-120005768. [DOI] [PubMed] [Google Scholar]
- Gozzelino R., Andrade B.B., Larsen R., Luz N.F., Vanoaica L., Seixas E., Coutinho A., Cardoso S., Rebelo S., Poli M., Barral-Netto M., Darshan D., Kühn L.C., Soares M.P. Metabolic adaptation to tissue iron overload confers tolerance to malaria. Cell Host Microbe. 2012;12:693–704. doi: 10.1016/j.chom.2012.10.011. [DOI] [PubMed] [Google Scholar]
- Guerbette T., Boudry G., Lan A. Mitochondrial function in intestinal epithelium homeostasis and modulation in diet-induced obesity. Mol. Metab. 2022;63 doi: 10.1016/j.molmet.2022.101546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattori Y., Hattori S., Sato N., Kasai K. High-glucose-induced nuclear factor kB activation in vascular smooth muscle cells. Cardiol. Res. 2000;46:188–197. doi: 10.1016/s0008-6363(99)00425-3. [DOI] [PubMed] [Google Scholar]
- Hessayson D.G. Fungitoxins in the soil: II. Trichothecin, its production and inactivation in unsterilized soils. Soil Sci. 1953;75:395–404. [Google Scholar]
- Hobby G.L., Dawson M.H. Relationship of penicillin to sulfonamide action. Proc. Soc. Exp. Biol. Med. 1944;56:184–186. [Google Scholar]
- Holliday R. Streptomycin, errors in mitochondria and ageing. Biogerontology. 2005;6:431–432. doi: 10.1007/s10522-005-4911-2. [DOI] [PubMed] [Google Scholar]
- Hotchkiss M. Studies on salt action. VI. The stimulating and inhibitive effect of certain cations upon bacterial growth. J. Bacteriol. 1923;8:141. doi: 10.1128/jb.8.2.141-162.1923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houtkooper R.H., Mouchiroud L., Ryu D., Moullan N., Katsyuba E., Knott G., Williams R.W., Auwerx J. Mitonuclear protein imbalance as a conserved longevity mechanism. Nature. 2013;497:451–457. doi: 10.1038/nature12188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz K.T., Sinclair D.A. Xenohormesis: sensing the chemical cues of other species. Cell. 2008;133:387–391. doi: 10.1016/j.cell.2008.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao E.C., Koniaris L.G., Zimmers-Koniaris T., Sebald S.M., Huynh T.V., Lee S.J. Characterization of growth-differentiation factor 15, a transforming growth factor beta superfamily member induced following liver injury. Mol. Cell. Biol. 2000;20:3742–3751. doi: 10.1128/mcb.20.10.3742-3751.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu F., Wu Y., Liu C., Zhu Y., Ye S., Xi Y., Cui W., Bu S. Penicillin disrupts mitochondrial function and induces autophagy in colorectal cancer cell lines. Oncol. Lett. 2021;22:691. doi: 10.3892/ol.2021.12952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iavicoli I., Fontana L., Agathokleous E., Santocono C., Russo F., Vetrani I., Fedele M., Calabrese E.J. Hormetic dose responses induced by antibiotics in bacteria: a phantom menace to be thoroughly evaluated to address the environmental risk and tackle the antibiotic resistance phenomenon. Sci. Total Environ. 2021;798 doi: 10.1016/j.scitotenv.2021.149255. [DOI] [PubMed] [Google Scholar]
- Itoh Y., Khawaja A., Singh V., Naschberger A., Rorbach J., Amunts A. Structural basis of streptomycin off-target binding to human mitoribosome. bioRxiv. 2022 02.02.478878. [Google Scholar]
- Jiang C., Xie C., Li F., Zhang L., Nichols R.G., Krausz K.W., Cai J., Qi Y., Fang Z.Z., Takahashi S., Tanaka N., Desai D., Amin S.G., Albert I., Patterson A.D., Gonzalez F.J. Intestinal farnesoid X receptor signaling promotes nonalcoholic fatty liver disease. J. Clin. Invest. 2015;125:386–402. doi: 10.1172/JCI76738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R.W. Inhibition of growth by pro-inflammatory cytokines: an integrated view. J. Anim. Sci. 1997;75:1244–1255. doi: 10.2527/1997.7551244x. [DOI] [PubMed] [Google Scholar]
- Jones R.M., Desai C., Darby T.M., Luo L., Wolfarth A.A., Scharer C.D., Ardita C.S., Reedy A.R., Keebaugh E.S., Neish A.S. Lactobacilli modulate epithelial cytoprotection through the Nrf2 pathway. Cell Rep. 2015;12:1217–1225. doi: 10.1016/j.celrep.2015.07.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones R.M., Luo L., Ardita C.S., Richardson A.N., Kwon Y.M., Mercante J.W., Alam A., Gates C.L., Wu H., Swanson P.A., Lambeth J.D., Denning P.W., Neish A.S. Symbiotic lactobacilli stimulate gut epithelial proliferation via Nox-mediaterd generation of reactive oxygen species. EMBO J. 2013;32:3017–3028. doi: 10.1038/emboj.2013.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang S.G., Yi H.S., Choi M.J., Ryu M.J., Jung S., Chung H.K., Chang J.Y., Kim Y.K., Lee S.E., Kim H.W., Choi H., Kim D.S., Lee J.H., Kim K.S., Kim H.J., Lee C.H., Oike Y., Shong M. ANGPTL6 expression is coupled with mitochondrial OXPHOS function to regulate adipose FGF21. J. Endocrinol. 2017;233:105–118. doi: 10.1530/JOE-16-0549. [DOI] [PubMed] [Google Scholar]
- Kim S.J., Xiao J., Wan J., Cohen P., Yen K. Mitochondrially derived peptides as novel regulators of metabolism. J. Physiol. 2017;595:6613–6621. doi: 10.1113/JP274472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi A., Kang M.I., Okawa H., Ohtsuji M., Zenke Y., Chiba T., Igarashi K., Yamamoto M. Oxidative stress sensor Keap1 functions as an adaptor for Cul3-based E3 ligase to regulate proteasomal degradation of Nrf2. Mol. Cell. Biol. 2004;24:7130–7139. doi: 10.1128/MCB.24.16.7130-7139.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen M.E., Boonstra-Blom A.G., Jeurissen S.H. Immunological differences between layer- and broiler-type chickens. Vet. Immunol. Immunopathol. 2002;89:47–56. doi: 10.1016/s0165-2427(02)00169-1. [DOI] [PubMed] [Google Scholar]
- Kogut M.H. The effect of microbiome modulation on the intestinal health of poultry. Anim. Feed Sci. Technol. 2019;250:32–40. [Google Scholar]
- Kogut M.H., Genovese K.J., Swaggerty C.L., He H., Broom L. Inflammatory phenotypes in the intestine of poultry: not all inflammation is created equal. Poult. Sci. 2018;97:2339–2346. doi: 10.3382/ps/pey087. [DOI] [PubMed] [Google Scholar]
- Kotas M.E., Medzhitov R. Homeostasis, inflammation, and disease susceptibility. Cell. 2015;160:816–827. doi: 10.1016/j.cell.2015.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koyama T., Texada M.J., Halberg K.A., Rewitz K. Metabolism and growth adaptation to environmental conditions in Drosophila. Cell Mol. Life Sci. 2020;77:4523–4551. doi: 10.1007/s00018-020-03547-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A.H.Wu, Collier-Hyams L.S., Hansen J.M., Li T., Yamoah K., Pan Z-Q., Jones D.P., Neish A.S. Commensal bacteria modulate cullin-dependent signaling via generation of reactive oxygen species. EMBO J. 2007;26:4457–4466. doi: 10.1038/sj.emboj.7601867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon G., Corbett J.A., Hauser S., Hill J.R., Turk J., McDaniel M.L. Evidence for involvement of the proteasome complex (26S) and NFkappaB in IL-1 beta-induced nitric oxide and prostanglandin production by rat islets and RINm5F cells. Diabetes. 1998;47:583–591. doi: 10.2337/diabetes.47.4.583. [DOI] [PubMed] [Google Scholar]
- Kwon G., Corbett J.A., Rodi C.P., Sullivan P., McDaniel M.L. Interleukin-1 beta-induced nitric oxide synthase expression by rat pancreatic beta-cells: evidence fir the involvement of nuclear factor kappa B in the signalling mechamism. Endocrinology. 1995;136:4790–4795. doi: 10.1210/endo.136.11.7588208. [DOI] [PubMed] [Google Scholar]
- Lamming D.W., Wood J.G., Sinclair D.A. Small molecules that regulate lifespan: evidence for xenohormesis. Mol. Microbiol. 2004;53:1003–1009. doi: 10.1111/j.1365-2958.2004.04209.x. [DOI] [PubMed] [Google Scholar]
- Larsen R., Gozzelino R., Jeney V., Tokaji L., Bozza F.A., Japiassú A.M., Bonaparte D., Cavalcante M.M., Chora A., Ferreira A., Marguti I., Cardoso S., Sepúlveda N., Smith A., Soares M.P. A central role for free heme in the pathogenesis of severe sepsis. Sci. Transl. Med. 2010;2:51ra71. doi: 10.1126/scitranslmed.3001118. [DOI] [PubMed] [Google Scholar]
- Laxminarayan R., Matsoso P., Pant S., Brower C., Røttingen J.A., Klugman K., Davies S. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387:168–175. doi: 10.1016/S0140-6736(15)00474-2. [DOI] [PubMed] [Google Scholar]
- Le Bourg E. A cold stress applied at various ages can increase resistance to heat and fungal infection in aged Drosophila melanogaster flies. Biogerontology. 2011;12:185–193. doi: 10.1007/s10522-010-9309-0. [DOI] [PubMed] [Google Scholar]
- Lee M.D., Ipharraguerre I.R., Arsenault R.J., Lyte M., Lyte J.M., Humphrey B., Angel R., Korver D.R. Informal nutrition symposium: leveraging the microbiome (and the metabolome) for poultry production. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., La T.M., Lee H.J., Choi I.S., Song C.S., Park S.Y., Lee J.B., Lee S.W. Characterization of microbial communities in the chicken oviduct and the origin of chicken embryo gut microbiota. Sci. Rep. 2019;9:6838. doi: 10.1038/s41598-019-43280-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszt K.I., Wang Q., Farhadipour M., Segers A., Thijs T., Nys L., Deleus E., Van der Schueren B., Gerner C., Neuditschko B., Ceulemans L.J., Lannoo M., Tack J., Depoortere I. Human intestinal bitter taste receptors regulate innate immune responses and metabolic regulators in obesity. J. Clin. Invest. 2022;132 doi: 10.1172/JCI144828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Bao Z., Xu X., Chao H., Lin C., Li Z., Liu Y., Wang X., You Y., Liu N., Ji J. Extracellular signal-regulated kinase/nuclear factor-erythroid2-like2/heme oxygenase-1 pathway-mediated mitophagy alleviates traumatic brain injury-induced intestinal mucosa damage and epithelial barrier dysfunction. J. Neurotrauma. 2017;34:2119–2131. doi: 10.1089/neu.2016.4764. [DOI] [PubMed] [Google Scholar]
- Loefer J.B., Bieberdorf F.W., Weichlein R.G. Inhibition and Enhancement of the Growth of Fungi with Streptomycin. Bull. Torrey Bot. Club. 1952;79:242–250. [Google Scholar]
- Luckey T.D., Gordon H.A., Wagner M., Reyniers J.A. Growth of germ-free birds fed antibiotics. Antibiot. Chemother. 1956;VI:36–40. [PubMed] [Google Scholar]
- Lumpkins B.S., Batal A.B., Lee M. The effect of gender on the bacterial community in the gastrointestinal tract of broilers. Poult. Sci. 2008;87:964–967. doi: 10.3382/ps.2007-00287. [DOI] [PubMed] [Google Scholar]
- Lv Y., Zhang S.Y., Liang X., Zhang H., Xu Z., Liu B., Xu M.J., Jiang C., Shang J., Wang X. Adrenomedullin 2 enhances beiging in white adipose tissue directly in an adipocyte-autonomous manner and indirectly through activation of M2 macrophages. J. Biol. Chem. 2016;291:23390–23402. doi: 10.1074/jbc.M116.735563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macpherson A.J., Heikenwalder M., Ganal-Vonarburg S.C. The liver at the nexus of host-microbial interactions. Cell Host Microbe. 2016;20:561–571. doi: 10.1016/j.chom.2016.10.016. [DOI] [PubMed] [Google Scholar]
- Maria Cardinal K., Kipper M., Andretta I., Machado Leal Ribeiro A. Withdrawal of antibiotic growth promoters from broiler diets: performance indexes and economic impact. Poult. Sci. 2019;98:6659–6667. doi: 10.3382/ps/pez536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masand R., Paulo E., Wu D., Wang Y., Swaney D.L., Jimenez-Morales D., Krogan N.J., Wang B. Proteome imbalance of mitochondrial electron transport chain in brown adipocytes leads to metabolic benefits. Cell Metabol. 2018;27:616–629.e4. doi: 10.1016/j.cmet.2018.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson M.P. Hormesis defined. Ageing Res. Rev. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarville J.L., Ayres J.S. Disease tolerance: concept and mechanisms. Curr. Opin. Immunol. 2018;50:88–93. doi: 10.1016/j.coi.2017.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. The spectrum of inflammatory responses. Science. 2021;374:1070–1075. doi: 10.1126/science.abi5200. [DOI] [PubMed] [Google Scholar]
- Medzhitov R., Schneider D.S., Soares M.P. Disease tolerance as a defense strategy. Science. 2012;335:936–941. doi: 10.1126/science.1214935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C.P., Bohnhoff M. Development of streptomyciii-resistainvta rianits of Meningococcus. Science. 1947;105:620–621. doi: 10.1126/science.105.2737.620. [DOI] [PubMed] [Google Scholar]
- Miller W.S., Green C.A., Kitchen H. Biphasic action of penicillin and other sulphonamide similarity. Nature. 1945;155:210–211. [Google Scholar]
- Miller M., Singer M. Do antibiotics cause mitochondrial and immune cell dysfunction? A literature review. J. Antimicrob. Chemother. 2022;77:1218–1227. doi: 10.1093/jac/dkac025. [DOI] [PubMed] [Google Scholar]
- Mills E.L., Kelly B., O'Neill L.A.J. Mitochondria are the powerhouses of immunity. Nature Immunol. 2017;18:488–498. doi: 10.1038/ni.3704. [DOI] [PubMed] [Google Scholar]
- Mottis A., Herzig S., Auwerx J. Mitocellular communication: shaping health and disease. Science. 2019;366:827–832. doi: 10.1126/science.aax3768. [DOI] [PubMed] [Google Scholar]
- Mottis A., Li T.Y., El Alam G., Rapin A., Katsyuba E., Liaskos D., D'Amico D., Harris N.L., Grier M.C., Mouchiroud L., Nelson M.L., Auwerx J. Tetracycline-induced mitohormesis mediates disease tolerance against influenza. J. Clin. Investig. 2022;132 doi: 10.1172/JCI151540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nickell L.G., Finlay A.C. Growth modifiers, antibiotics and their effects on plant growth. J. Agric. Food Chem. 1954;2:178–182. [Google Scholar]
- Niewold T.A. The nonantibiotic anti-inflammatory effect of antimicrobial growth promoters, the real mode of action? A hypothesis. Poult. Sci. 2007;86:605–609. doi: 10.1093/ps/86.4.605. [DOI] [PubMed] [Google Scholar]
- Nobs S.P., Zmora N., Elinav E. Nutrition regulates innate immunity in health and disease. Annu. Rev. Nutr. 2020;40:189–219. doi: 10.1146/annurev-nutr-120919-094440. [DOI] [PubMed] [Google Scholar]
- Nohl H., Hegner D. The effects of some nutritive antibiotics on the respiration of rat liver mitochondria. Biochem. Pharmacol. 1977;26:433–437. doi: 10.1016/0006-2952(77)90203-9. [DOI] [PubMed] [Google Scholar]
- Oakley B.B., Lillehoj H.S., Kogut M.H., Kim W.K., Maurer J.J., Pedroso A., Lee M.D., Collett S.R., Johnson T.J., Cox N.A. The chicken gastrointestinal microbiome. FEMS Microbiol. Lett. 2014;360:100–112. doi: 10.1111/1574-6968.12608. [DOI] [PubMed] [Google Scholar]
- O'Reilly M., Young L., Kirkwood N.K., Richardson G.P., Kros C.J., Moore A.L. Gentamicin affects the bioenergetics of isolated mitochondria and collapses the mitochondrial membrane potential in cochlear sensory hair cells. Front. Cell. Neurosci. 2019;13:416. doi: 10.3389/fncel.2019.00416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostan R., Bucci L., Capri M., Salvioli S., Scurti M., Pini E., Monti D., Franceschi C. Immunosenescence and immunogenetics of human longevity. Neuroimmunomodulation. 2008;15:224–240. doi: 10.1159/000156466. [DOI] [PubMed] [Google Scholar]
- Ottaviani E., Malagoli D., Franceschi C. Common evolutionary origin of the immune and neuroendocrine systems: from morphological and functional evidence to in silico approaches. Trends Immunol. 2007;28:497–502. doi: 10.1016/j.it.2007.08.007. [DOI] [PubMed] [Google Scholar]
- Paine T.F., Finland M. Streptomycin-sensitive, -dependent, and -resistant bacteria. Science. 1948;107:143–144. doi: 10.1126/science.107.2771.143. [DOI] [PubMed] [Google Scholar]
- Pan D., Yu Z. Intestinal microbiome of poultry and its interaction with host and diet. Gut Microb. 2014;5:108–119. doi: 10.4161/gmic.26945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park S.H., Choi H.J., Yang H., Do K.H., Kim J., Kim H.H., Lee H., Oh C.G., Lee D.W., Moon Y. Two in-and-out modulation strategies for endoplasmic reticulum stress-linked gene expression of pro-apoptotic macrophage-inhibitory cytokine 1. J. Biol. Chem. 2012;287:19841–19855. doi: 10.1074/jbc.M111.330639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel S., Alvarez-Guaita A., Melvin A., Rimmington D., Dattilo A., Miedzybrodzka E.L., Cimino I., Maurin A.C., Roberts G.P., Meek C.L., Virtue S., Sparks L.M., Parsons S.A., Redman L.M., Bray G.A., Liou A.P., Woods R.M., Parry S.A., Jeppesen P.B., Kolnes A.J., Harding H.P., Ron D., Vidal-Puig A., Reimann F., Gribble F.M., Hulston C.J., Farooqi I.S., Fafournoux P., Smith S.R., Jensen J., Breen D., Wu Z., Zhang B.B., Coll A.P., Savage D.B., O'Rahilly S. GDF15 provides an endocrine signal of nutritional stress in mice and humans. Cell Metab. 2019;29:707–718.e8. doi: 10.1016/j.cmet.2018.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patra A.K., Kar I. Heat stress on microbiota composition, barrier integrity, and nutrient transport in gut, production performance, and its amelioration in farm animals. J. Anim. Sci. Technol. 2021;63:211–247. doi: 10.5187/jast.2021.e48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pigliucci M., Murren C.J., Schlichting C.D. Phenotypic plasticity and evolution by genetic assimilation. J. Exp. Biol. 2006;209(Pt. 12):2362–2367. doi: 10.1242/jeb.02070. [DOI] [PubMed] [Google Scholar]
- Piotrowska M., Swierczynski M., Fichna J., Piechota-Polanczyk A. The Nrf2 in the pathophysiology of the intestine: molecular mechanisms and therapeutic implications for inflammatory bowel diseases. Pharmacol. Res. 2021;163 doi: 10.1016/j.phrs.2020.105243. [DOI] [PubMed] [Google Scholar]
- Pospisilik J.A., Knauf C., Joza N., Benit P., Orthofer M., Cani P.D., Ebersberger I., Nakashima T., Sarao R., Neely G., Esterbauer H., Kozlov A., Kahn C.R., Kroemer G., Rustin P., Burcelin R., Penninger J.M. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131:476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- Pourabedin M., Guan L., Zhao X. Xylo-oligosaccharides and virginiamycin differentially modulate gut microbial composition in chickens. Microbiome. 2015;3:15. doi: 10.1186/s40168-015-0079-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powell L.A., Warpeha K.M., Xu W., Walker B., Trimble E.R. High glucose decreases intracellular glutathione concentrations and upregulates inducible nitric oxide synthase gene expression in intestinal epithelial cells. J. Mol. Endocrinol. 2004;33:797–803. doi: 10.1677/jme.1.01671. [DOI] [PubMed] [Google Scholar]
- Prajapati P., Dalwadi P., Gohel D., Singh K., Sripada L., Bhatelia K., Joshi B., Roy M., Wang W.X., Springer J.E., Singh R., Singh R. Enforced lysosomal biogenesis rescues erythromycin- and clindamycin-induced mitochondria-mediated cell death in human cells. Mol. Cell. Biochem. 2019;461:23–36. doi: 10.1007/s11010-019-03585-w. [DOI] [PubMed] [Google Scholar]
- Pratt R., Dufrenoy J. Cytochemical interpretation of the mechanism of penicillin action. Bacteriol. Rev. 1948;12:79–103. doi: 10.1128/br.12.1.79-103.1948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirós P.M., Mottis A., Auwerx J. Mitonuclear communication in homeostasis and stress. Nat. Rev. Mol. Cell. Biol. 2016;17:213–226. doi: 10.1038/nrm.2016.23. [DOI] [PubMed] [Google Scholar]
- Råberg L., Graham A.L., Read A.F. Decomposing health: tolerance and resistance to parasites in animals. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2009;364:37–49. doi: 10.1098/rstb.2008.0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Råberg L., Sim D., Read A.F. Disentangling genetic variation for resistance and tolerance to infectious diseases in animals. Science. 2007;318:812–814. doi: 10.1126/science.1148526. [DOI] [PubMed] [Google Scholar]
- Råberg L., Vestberg M., Hasselquist D., Holmdahl R., Svensson E., Nilsson J.A. Basal metabolic rate and the evolution of the adaptive immune system. Proc. Biol. Sci. 2002;269(1493):817–821. doi: 10.1098/rspb.2001.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahnamaeian M., Cytryńska M., Zdybicka-Barabas A., Dobslaff K., Wiesner J., Twyman R., Zuchner T., Sadd B.M., Regoes R.R., Schmid-Hempel P., Vilcinskas A. Insect antimicrobial peptides show potentiating functional interactions against Gram-negative bacteria. Proc. Biol. Sci. 2015;282 doi: 10.1098/rspb.2015.0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rake G. Streptomycin as an essential niutrilite. Proc. Soc. Exp. Biol. Med. 1948;67:249–253. doi: 10.3181/00379727-67-16268. [DOI] [PubMed] [Google Scholar]
- Randall W.A., Price C.W., Welch H. Present specifications for commercial streptomycin. Am. J. Publ. Health. 1947;37:421. [PubMed] [Google Scholar]
- Rattan S.I. Mechanisms of hormesis through mild heat stress on human cells. Ann. N. Y. Acad. Sci. 2004;1019:554–558. doi: 10.1196/annals.1297.103. [DOI] [PubMed] [Google Scholar]
- Riboulet E., Verneuil N., La Carbona S., Sauvageot N., Auffray Y., Hartke A., Giard J.C. Relationships between oxidative stress response and virulence in Enterococcus faecalis. J. Mol. Microbiol. Biotechnol. 2007;13:140–146. doi: 10.1159/000103605. [DOI] [PubMed] [Google Scholar]
- Robbins W.J., Hervey A., Stebbins M.E. Bull. Torrey Botan. Club. 1951;77:423. 78,363. [Google Scholar]
- Rodrigue-Gervais I.G., Labbé K., Dagenais M., Dupaul-Chicoine J., Champagne C., Morizot A., Skeldon A., Brincks E.L., Vidal S.M., Griffith T.S., Saleh M. Cellular inhibitor of apoptosis protein cIAP2 protects against pulmonary tissue necrosis during influenza virus infection to promote host survival. Cell Host Microbe. 2014;15:23–35. doi: 10.1016/j.chom.2013.12.003. [DOI] [PubMed] [Google Scholar]
- Rodríguez-Ramiro I., Ramos S., Bravo L., Goya L., Martín M.A. Procyanidin B2 induces Nrf2 translocation and glutathione S-transferase P1 expression via ERKs and p38-MAPK pathways and protect human colonic cells against oxidative stress. Eur. J. Nutr. 2012;51:881–892. doi: 10.1007/s00394-011-0269-1. [DOI] [PubMed] [Google Scholar]
- Roessler W.G., Herbst E.J. Studies with Coccidioides immitis; submerged growth in liquid mediums. J. Infect. Dis. 1946;79:12–22. [PubMed] [Google Scholar]
- Romano K.A., Vivas E.I., Amador-Noguez D., Rey F.E. Intestinal microbiota composition modulates choline bioavailability from diet and accumulation of the proatherogenic metabolite trimethylamine-N-oxide. mBio. 2015;6:e02481. doi: 10.1128/mBio.02481-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G.D. In: Wallace R.J., Chesson A., editors. Wiley; Weinhein, Germany/New York, NY, 172: 1995. Antibacterials in poultry and pig nutrition. (Biotechnology in Animal Feeds and Animal Feeding). [Google Scholar]
- Saeedi B.J., Liu K.H., Owens J.A., Hunter-Chang S., Camacho M.C., Eboka R.U., Chandrasekharan B., Baker N.F., Darby T.M., Robinson B.S., Jones R.M., Jones D.P., Neish A.S. Gut-resident lactobacilli activate hepatic Nrf2 and protect against oxidative liver injury. Cell Metabol. 2020;31:956–968.e5. doi: 10.1016/j.cmet.2020.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawa T., Naito Y., Kato H., Amaya F. Cellular stress responses and monitored cellular activities. Shock. 2016;46:113–121. doi: 10.1097/SHK.0000000000000603. [DOI] [PubMed] [Google Scholar]
- Schmeisser S., Schmeisser K., Weimer S., Groth M., Priebe S., Fazius E., Kuhlow D., Pick D., Einax J.W., Guthke R., Platzer M., Zarse K., Ristow M. Mitochondrial hormesis links low-dose arsenite exposure to lifespan extension. Aging Cell. 2013;12:508–517. doi: 10.1111/acel.12076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider D.S., Ayres J.S. Two ways to survive infection: what resistance and tolerance can teach us about treating infectious diseases. Nat. Rev. Immunol. 2008;8:889–895. doi: 10.1038/nri2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schugar R.C., Willard B., Wang Z., Brown J.M. Postprandial gut microbiota-driven choline metabolism links dietary cues to adipose tissue dysfunction. Adipocyte. 2018;7:49–56. doi: 10.1080/21623945.2017.1398295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulz H., Uber Hefegifte. Pfligers Arch. Ges. Physiol., 42, 1888, 517-541.
- Shao L.-W., Niu R., Liu Y. Neuropeptide signals cell non-autonomous mitochondrial unfolded protein response. Cell Res. 2016;26:1182–1196. doi: 10.1038/cr.2016.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheldon B.C., Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol. Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Shen K., Pender C.L., Bar-Ziv R., Zhang H., Wickham K., Willey E., Durieux J., Ahmad Q., Dillin A. Mitochondria as cellular and organismal signaling hubs. Ann. Rev. Cell Dev. Biol. 2022;38:179–218. doi: 10.1146/annurev-cellbio-120420-015303. [DOI] [PubMed] [Google Scholar]
- Shore D.E., Ruvkun G. A cytoprotective perspective on longevity regulation. Trends Cell Biol. 2013;23:409–420. doi: 10.1016/j.tcb.2013.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shpilka T., Haynes C.M. The mitochondrial UPR: mechanisms, physiological functions and implications in ageing. Nat. Rev. Mol. Cell Biol. 2018;19:109–120. doi: 10.1038/nrm.2017.110. [DOI] [PubMed] [Google Scholar]
- Sighel D., Notarangelo M., Aibara S., Re A., Ricci G., Guida M., Soldano A., Adami V., Ambrosini C., Broso F., Rosatti E.F., Longhi S., Buccarelli M., D'Alessandris Q.G., Giannetti S., Pacioni S., Ricci-Vitiani L., Rorbach J., Pallini R., Roulland S., Amunts A., Mancini I., Modelska A., Quattrone A. Inhibition of mitochondrial translation suppresses glioblastoma stem cell growth. Cell Rep. 2021;35 doi: 10.1016/j.celrep.2021.109024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siva-Jothy M.T., Thompson J.W. Short-term nutrient deprivation affects immune function. Physiol. Entomol. 2002;27:206–212. [Google Scholar]
- Soares M.P., Gozzelino R., Weis S. Tissue damage control in disease tolerance. Trends Immunol. 2014;35:483–494. doi: 10.1016/j.it.2014.08.001. [DOI] [PubMed] [Google Scholar]
- Soares M.P., Teixeira L., Moita L.F. Disease tolerance and immunity in host protection against infection. Nat. Rev. Immunol. 2017;2:83–96. doi: 10.1038/nri.2016.136. [DOI] [PubMed] [Google Scholar]
- Southam C.M., Ehrlich J. Effects of extracts of western red-cedar heartwood on certain wood-decaying fungi in culture. Phytopathology. 1943;33:517-524. [Google Scholar]
- Spendlove G.A., Cummings M.M., Fackler W.B., Jr., Michael M., Jr Enhancement of growth of a strain of M. tuberculosis s (var. homiinis) by streptomycin. Publ. Health Repts. 1948;63:1177–1179. [PubMed] [Google Scholar]
- Stebbing A. Hormesis—the stimulation of growth by low levels of inhibitors. Sci. Total Environ. 1982;22:213–234. doi: 10.1016/0048-9697(82)90066-3. [DOI] [PubMed] [Google Scholar]
- Stebbing A.R. A theory for growth hormesis. Mutat. Res. 1998;403:249–258. doi: 10.1016/s0027-5107(98)00014-1. [DOI] [PubMed] [Google Scholar]
- Stewart G.S., Smith C.P. Urea nitrogen salvage mechanisms and their relevance to ruminants, non-ruminants and man. Nutr. Res. Rev. 2005;18:49–62. doi: 10.1079/NRR200498. [DOI] [PubMed] [Google Scholar]
- Storelli G., Defaye A., Erkosar B., Hols P., Royet J., Leulier F. Lactobacillus plantarum promotes Drosophila systemic growth by modulating hormonal signals through TOR-dependent nutrient sensing. Cell Metabol. 2011;14:403–414. doi: 10.1016/j.cmet.2011.07.012. [DOI] [PubMed] [Google Scholar]
- Stowe K.A., Marquis R.J., Hochwender C.G., Simms E.L. The evolutionary ecology of tolerance to consumer damage. Ann. Rev. Ecol. Systemat. 2000;31:565–595. [Google Scholar]
- Suárez-Rivero J.M., Pastor-Maldonado C.J., Povea-Cabello S., Álvarez-Córdoba M., Villalón-García I., Talaverón-Rey M., Suárez-Carrillo A., Munuera-Cabeza M., Sánchez-Alcázar J.A. Mitochondria and antibiotics: for good or for evil? Biomolecules. 2021;11:1050. doi: 10.3390/biom11071050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suntur B.M., Yurtseven T., Sipahi O.R., Buke C., Buke M. Rifampicin+ceftriaxone versus vancomycin+ceftriaxone in the treatment of penicillin- and cephalosporin-resistant pneumococcal meningitis in an experimental rabbit model. Int. J. Antimicrob. Agents. 2005;26:258–260. doi: 10.1016/j.ijantimicag.2005.06.010. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Motohashi H., Yamamoto M. Toward clinical application of the Keap1-Nrf2 pathway. Trends Pharmacol. Sci. 2013;34:340–346. doi: 10.1016/j.tips.2013.04.005. [DOI] [PubMed] [Google Scholar]
- Swanson P.A., 2nd, Kumar A., Samarin S., Vijay-Kumar M., Kundu K., Murhty N., Hansen J., Nusrat A., Neish A.S. Enteric commensal bacteria potentiate epithelial restitution via reactive oxygen species-mediated inactivation of focal adhesion kinase phosphatases. Proc. Natl. Acad. Sci. 2011;108:8803–8808. doi: 10.1073/pnas.1010042108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan B.L., Norhaizan M.E., Liew W.P. Nutrients and oxidative stress: friend or foe? Oxid. Med. Cell. Longev. 2018;2018 doi: 10.1155/2018/9719584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber S.C., Nau R. Immunomodulatory properties of antibiotics. Curr. Mol. Pharmacol. 2008;1:68–79. [PubMed] [Google Scholar]
- Teirlynck E., Bjerrum L., Eeckhaut V., Huygebaert G., Pasmans F., Haesebrouck F., Dewulf J., Ducatelle R., Van Immerseel F. The cereal type in feed influences gut wall morphology and intestinal immune cell infiltration in broiler chickens. Br. J. Nutr. 2009;102:1453–1461. doi: 10.1017/S0007114509990407. [DOI] [PubMed] [Google Scholar]
- Thanner S., Drissner D., Walsh F. Antimicrobial resistance in agriculture. mBio. 2016;7 doi: 10.1128/mBio.02227-15. e02227-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiss A.L., Vijay-Kumar M., Obertone T.S., Jones D.P., Hansen J.M., Gewirtz A.T., Merlin D., Sitaraman S.V. Prohibitin is a novel regulator of antioxidant response that attenuates colonic inflammation in mice. Gastroenterology. 2009;137:199–208. doi: 10.1053/j.gastro.2009.03.033. 208.e1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Troha K., Ayres J.S. Metabolic adaptations to infections at the organismal level. Trends Immunol. 2020;41:113–125. doi: 10.1016/j.it.2019.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Immerseel F., De Buck J., Pasmans F., Huyghebaert G., Haesebrouck F., Ducatelle R. Clostridium perfringens in poultry: an emerging threat for animal and public health. Avian Pathol. 2004;33:537–549. doi: 10.1080/03079450400013162. [DOI] [PubMed] [Google Scholar]
- Wang Z., Klipfell E., Bennett B.J., Koeth R., Levison B.S., Dugar B., Feldstein A.E., Britt E.B., Fu X., Chung Y.M., Wu Y., Schauer P., Smith J.D., Allayee H., Tang W.H., DiDonato J.A., Lusis A.J., Hazen S.L. Gut flora metabolism of phosphatidylcholine promotes cardiovascular disease. Nature. 2011;472:57–63. doi: 10.1038/nature09922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Liu F., Liu M., Zhou X., Wang M., Cao K., Jin S., Shan A., Feng X. Curcumin mitigates aflatoxin B1-induced liver injury via regulating the NLRP3 inflammasome and Nrf2 signaling pathway. Food Chem. Toxicol. 2022;161 doi: 10.1016/j.fct.2022.112823. [DOI] [PubMed] [Google Scholar]
- Wang X., Ryu D., Houtkooper R.H., Auwerx J. Antibiotic use and abuse: a threat to mitochondria and chloroplasts with impact on research, health, and environment. Bioessays. 2015;37:1045–1053. doi: 10.1002/bies.201500071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch H., Price C.W., Randall W.A. Increase in fatality rate of E. typhosa for white mice by streptomycin. J. Am. Pharm. A. 1946;35:155. doi: 10.1002/jps.3030350505. [DOI] [PubMed] [Google Scholar]
- Wen Z., Liu W., Li X., Chen W., Liu Z., Wen J., Liu Z. A protective role of the NRF2-Keap1 pathway in maintaining intestinal barrier function. Oxid. Med. Cell. Longev. 2019;2019 doi: 10.1155/2019/1759149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth C.C., Alam A., Jones R.M., Nusrat A., Neish A.S. Enteric commensal bacteria induce ERK pathway signaling via formyl peptide receptor (FPR)-dependent redox modulation of dual specific phosphatase 3 (DUSP3) J. Biol. Chem. 2011;286:38448–38455. doi: 10.1074/jbc.M111.268938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wentworth C.C., Jones R.M., Kwon Y.M., Nusrat A., Neish A.S. Commensal-epithelial signaling mediated via formyl peptide receptors. Am. J. Pathol. 2010;177:2782–2790. doi: 10.2353/ajpath.2010.100529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witzig M., Camarinha-Silva A., Green-Engert R., Hoelzle K., Zeller E., Seifert J., Hoelzle L.E., Rodehutscord M. Correction: spatial variation of the gut microbiota in broiler chickens as affected by dietary available phosphorus and assessed by T-RFLP analysis and 454 pyrosequencing. PLoS One. 2015;10 doi: 10.1371/journal.pone.0143442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu S.R., Reddy P. Regulating damage from sterile inflammation: a tale of two tolerances. Trends Immunol. 2017;38:231–235. doi: 10.1016/j.it.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu R., Wang T., Ding F.F., Zhou N.N., Qiao F., Chen L.Q., Du Z.Y., Zhang M.L. Lactobacillus plantarum ameliorates high-carbohydrate diet-induced hepatic lipid accumulation and oxidative stress by upregulating uridine synthesis. Antioxidants. 2022;11:1238. doi: 10.3390/antiox11071238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada M., Suzuki M., Noguchi T., Yokosawa T., Sekiguchi Y., Mutoh N., Toyama T., Hirata Y., Hwang G.W., Matsuzawa A. The antibiotic cefotaxime works as both an activator of Nrf2 and an inducer of HSP70 in mammalian cells. BPB Rep. 2020;3:16–21. [Google Scholar]
- Yang H., Park S.H., Choi H.J., Moon Y. The integrated stress response-associated signals modulates intestinal tumor cell growth by NSAID-activated gene 1 (NAG-1/MIC-1/PTGF-β) Carcinogenesis. 2010;31:703–711. doi: 10.1093/carcin/bgq008. [DOI] [PubMed] [Google Scholar]
- Yu Y., Yang Y., Bian Y., Li Y., Liu L., Zhang H., Xie K., Wang G., Yu Y. Hydrogen gas protects against intestinal injury in wild type but not NRF2 knockout mice with severe sepsis by regulating HO-1 and HMGB1 release. Shock. 2017;48:364–370. doi: 10.1097/SHK.0000000000000856. [DOI] [PubMed] [Google Scholar]
- Yun J., Finkel T. Mitohormesis. Cell Metabol. 2014;19:757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yunis R., Ben-David A., Heller E.D., Cahaner A. Immunocompetence and viability under commercial conditions of broiler groups differing in growth rate and in antibody response to Escherichia coli vaccine. Poult. Sci. 2000;79:810–816. doi: 10.1093/ps/79.6.810. [DOI] [PubMed] [Google Scholar]