Abstract
Maintenance of calcium and phosphorus homeostasis in laying hens is crucial for preservation of skeletal integrity and eggshell quality, though physiological regulation of these systems is incompletely defined. To investigate changes in mineral and vitamin D3 homeostasis during the 24-h egg formation cycle, 32-wk-old commercial laying hens were sampled at 1, 3, 4, 6, 7, 8, 12, 15, 18, 21, 23, and 24 h post-oviposition (HPOP; n ≥ 4). Ovum location and egg calcification stage were recorded, and blood chemistry, plasma vitamin D3 metabolites, circulating parathyroid hormone (PTH), and expression of genes mediating uptake and utilization of calcium and phosphorus were evaluated. Elevated levels of renal 25-hydroxylase from 12 to 23 HPOP suggest this tissue might play a role in vitamin D3 25-hydroxylation during eggshell calcification. In shell gland, retinoid-x-receptor gamma upregulation between 6 and 8 HPOP followed by subsequently increased vitamin D receptor indicate that vitamin D3 signaling is important for eggshell calcification. Increased expression of PTH, calcitonin, and fibroblast growth factor 23 (FGF23) receptors in the shell gland between 18 and 24 HPOP suggest elevated sensitivity to these hormones toward the end of eggshell calcification. Shell gland sodium-calcium exchanger 1 was upregulated between 4 and 7 HPOP and plasma membrane calcium ATPase 1 increased throughout eggshell calcification, suggesting the primary calcium transporter may differ according to eggshell calcification stage. Expression in shell gland further indicated that bicarbonate synthesis precedes transport, where genes peaked at 6 to 7 and 12 to 18 HPOP, respectively. Inorganic phosphorus transporter 1 (PiT-1) expression peaked in kidney between 12 and 15 HPOP, likely to excrete excess circulating phosphorus, and in shell gland between 18 and 21 HPOP. Upregulation of FGF23 receptors and PiT-1 during late eggshell calcification suggest shell gland phosphorus uptake is important at this time. Together, these findings identified potentially novel hormonal pathways involved in calcium and phosphorus homeostasis along with associated circadian patterns in gene expression that can be used to devise strategies aimed at improving eggshell and skeletal strength in laying hens.
Key words: mineral homeostasis, vitamin D3, shell gland, kidney, hormonal signaling
INTRODUCTION
Eggs produced by commercial laying hens are a source of affordable, high-quality protein. Modern layers produce an egg approximately every 24 h (Bell, 2002), and during this process there is a continuous and substantial demand for calcium and phosphorus for eggshell calcification and bone remodeling. In recent decades, the number of eggs produced per hen housed has substantially increased. These increases in egg number are largely due to lengthened production cycles that have been achieved through both genetic selection and improved management practices (Bain et al., 2016). As hens age, a disequilibrium often occurs in which calcium and phosphorus uptake and utilization becomes disrupted, leading to a reduction in bone integrity and eggshell quality (Al-Batshan et al., 1994; Alfonso-Carrillo et al., 2021; Benavides-Reyes et al., 2021). However, the physiological pathways that become perturbed are incompletely defined. This makes a deeper understanding of processes regulating calcium and phosphorus homeostasis during the 24-h oviposition cycle in peak production essential in order to facilitate effective investigation into how they change in older hens.
Regulation of calcium and phosphorus metabolism is influenced by hormonally active 1α,25-dihydroxycholecalciferol [1α,25(OH)2D3], which undergoes 2 sequential hydroxylation reactions in the liver and kidney before becoming biologically active (Ponchon and DeLuca, 1969; Fraser and Kodicek, 1970). In both mammalian (Cheng et al., 2003) and avian species (Watanabe et al., 2013), hepatic formation of 25-hydroxycholecalciferol [25(OH)D3] is modulated by 25-hydroxylase that is encoded by the gene cytochrome P450 2R1 (CYP2R1). In mammals, the second 1α-hydroxylation is catalyzed by cytochrome P450 27 B1 (CYP27B1) (Meyer and Pike, 2020); however, this gene has not been identified in avian species (Sinclair-Black et al., 2023). Alternatively, 25(OH)D3 may undergo renal 24-hydroxylation that is modulated by the gene cytochrome P450 24 A1 (CYP24A1), to form inactive 24,25-dihydroxycholecalciferol [24,25(OH)2D3]. The impact of 1α,25(OH)2D3 on calcium and phosphorus homeostasis occurs primarily through its genomic actions on vitamin D3 responsive genes such as calbindin (CALB1), an intracellular calcium chaperone (Jande et al., 1981) that facilitates its cytosolic movement following cellular uptake via transporters such as transient receptor potential cation channel subfamily V member 6 (TRPV6), sodium-calcium exchanger 1 (NCX1), and ATPase plasma membrane calcium transporting 1 (PMCA1) (Bar, 2009; Gloux et al., 2019). A study conducted in broilers (Han et al., 2023) suggests that 1α,25(OH)2D3 may influence expression of NCX1 and PMCA1, while findings in mice also indicate that TRPV6 may be regulated by 1α,25(OH)2D3 (Nijenhuis et al., 2003). As such, increases in 1α,25(OH)2D3 can enhance calcium uptake in the laying hen at tissues such as intestine and kidney and influence ionized calcium (iCa2+) levels.
Circulating iCa2+ concentrations are monitored by calcium-sensing receptor (CASR) (Diaz et al., 1997), and high levels lead to production of calcitonin (CALC) from the ultimobranchial glands in chickens (Ziegler et al., 1969). In mammals, the secretion of CALC serves to decrease iCa2+ by increasing renal calcium excretion; however, the overall impact of CALC on plasma iCa2+ in poultry is negligible (Sommerville and Fox, 1987), and its influence on avian calcium and phosphorus homeostasis remains unclear. Periods of low iCa2+ stimulate parathyroid hormone (PTH) excretion by the parathyroid glands (Singh et al., 1986). Circulating PTH binds parathyroid 1 receptor (PTH1R) in tissues such as the small intestine and kidney to enhance calcium uptake and reabsorption both in chickens (Pinheiro et al., 2012) and mammals (Sato et al., 2017). Further influences of PTH in chickens are elevated bone remodeling through increased osteoclast activity (Taylor and Belanger, 1969; Miller, 1978), increased 1α,25(OH)2D3 formation, and enhanced renal phosphorus excretion (Sommerville and Fox, 1987).
Further regulation of renal phosphorus homeostasis is mediated by bone-derived fibroblast growth factor 23 (FGF23) signaling through fibroblast growth factor receptors 1 (FGFR1), 2 (FGFR2), 3 (FGFR3), and 4 (FGFR4) and their coreceptor klotho (KL). It is released in response to elevated circulating phosphorus, and studies in mammals show that FGF23 increases renal phosphorus elimination (Shimada et al., 2004), decreases production of 1α,25(OH)2D3 (Bai et al., 2003), and inhibits PTH secretion (Ben-Dov et al., 2007; Carpenter et al., 2020). In laying hens, hyperphosphatemia elevates expression of FGF23 mRNA in bone (Hadley et al., 2016; Wang et al., 2018; Ren et al., 2020). A study by Ren et al. (2017) demonstrated that immunoneutralization of FGF23 decreased phosphorus excretion in laying hens, which suggests that FGF23 may play a similar phosphaturic role to that observed in mammals; however, the mechanisms through which FGF23 alters avian phosphorus homeostasis is not fully characterized, and its effects on vitamin D3 metabolism and PTH production are unclear (Ren et al., 2017, 2020).
Several tissues are involved in regulating calcium and phosphorus homeostasis in laying hens, such as the small intestine, bone, and kidney (Bar, 2009). Hormonal action at bone and small intestine elicits similar changes in circulating calcium and phosphorus levels due to increased or decreased uptake of both minerals simultaneously. In contrast, the kidney is able to independently regulate calcium and phosphorus reabsorption and excretion so that levels in the blood can be differentially regulated. Differential excretion of calcium and phosphorus in the chicken kidney is regulated by the actions of PTH, 1α,25(OH)2D3, and FGF23 (Wideman, 1986; Wang et al., 2018). In this way, the kidney is a key tissue mediating dynamic changes in mineral homeostasis during the oviposition cycle.
An additional tissue that places high demand upon calcium and phosphorus utilization in laying hens is the shell gland. During the oviposition cycle, this tissue rapidly delivers minerals and bicarbonate ions into the shell gland lumen for deposition onto the developing eggshell. The eggshell is composed of 96% calcium carbonate (Hincke et al., 2012), and the calcium required for its formation originates from circulation and is transported across shell gland epithelial cells into the lumen (Nys et al., 1999). Circulating calcium is either obtained from medullary bone that serves as a labile calcium source or the diet (Dacke et al., 1993). As the bulk of eggshell calcification takes place during the dark phase (Hertelendy and Taylor, 1960; Nys and Guyot, 2011), bone-derived calcium may contribute up to 40% of that in the eggshell (Comar and Driggers, 1949). The developing eggshell also requires bicarbonate ions, which precipitate with calcium in the shell gland lumen to form the calcium carbonate (Nys et al., 1999). Bicarbonate is formed by the intracellular hydration of carbon dioxide by carbonic anhydrase 2 (CA2) and transported into the shell gland lumen by solute carrier family 26 member 9 (SLC26A9) (Hodges and LöRcher, 1967; Nys and Le Roy, 2018). Low amounts of phosphorus (0.1–0.4%) are incorporated into the eggshell at increasing levels toward the outer layers of the inorganic component and into the cuticle (Cusack et al., 2003) that prevents microbial contamination.
As the length of commercial egg production cycles continues to increase, close attention must be paid to the physiological systems that govern calcium and phosphorus utilization in modern laying hens. However, limited knowledge pertaining to the influence of 1α,25(OH)2D3, CALC, PTH, and FGF23 on local and systemic calcium and phosphorus homeostasis exists in avian species, particularly in relation to calcium and phosphorus transporter expression in shell gland and kidney. Furthermore, physiological changes that occur during the daily oviposition cycle are not well understood. Therefore, the objective of this study was to investigate the dynamic nature of calcium and phosphorus homeostasis during the 24-h oviposition cycle in laying hens in order to identify systems and transporters that may regulate key processes in calcium and phosphorus utilization during stages of eggshell calcification, medullary bone repletion, and transition between the 2.
MATERIALS AND METHODS
Animals and Tissue Collection
A total of 80 Hy-Line W-36 hens (32 wk of age) were housed in an environmentally controlled room under a 16H light:8H dark lighting program in individual cages so oviposition time of each hen could be monitored. Hens had free access to water and were fed a commercially available pelleted diet [Country Feeds, Layer Feed 16%, Nutrena] with the guaranteed analysis listed in Table 1. All animal procedures used in this study were approved by The Institutional Animal Care and Use Committee of the University of Georgia.
Table 1.
Guaranteed analysis for Country Feeds, Layer Feed 16% Pellet, Nutrena.
| Nutrient | Min % | Max % |
|---|---|---|
| Crude protein | 16 | |
| Crude fat | 2.5 | |
| Crude fiber | 8 | |
| Calcium | 3.4 | 3.9 |
| Total phosphorus | 0.45 | |
| Sodium | 0.15 | 0.23 |
| Lysine | 0.7 | |
| Methionine | 0.3 |
During the experiment, hens monitored and subsequently selected for sampling were laying at 92.3% (±2.8% SEM) hen day egg production. Individual oviposition times of each hen were determined for 3 d, and they were allocated into 12 groups that were sampled at the following hours post-oviposition (HPOP): 1, 3, 4, 6, 7, 8, 12, 15, 18, 21, 23, and 24. These time points were selected to investigate in detail the dynamics of calcium and phosphorus utilization related to bone remodeling and eggshell calcification in order to define physiological signatures associated with mineral homeostasis during periods of bone deposition, shell deposition, and transitions between these states (Etches, 1986; Wilson and Duff, 1990; Sugiyama and Kusuhara, 1993; Clunies and Leeson, 1995; Kebreab et al., 2009; Rodriguez-Navarro et al., 2015). At the allocated HPOP, 4 mL of blood were collected from the brachial wing vein into a lithium heparinized syringe. Whole blood (100 µL) was immediately analyzed using the iSTAT hand-held blood analyzer with CG8+ cartridges (Zoetis, Parsippany-Troy Hills, NJ), and remaining blood was centrifuged at 1,500 relative centrifugal force at 4°C for 10 min for plasma collection. Plasma was stored at −20°C until analysis of circulating vitamin D3 metabolites, total phosphorus (tP), and PTH was conducted as described below. Following blood collection, hens were euthanized by injection of 1 mL sodium pentobarbital (Euthasol, Virbac, Westlake, TX) into the alternate brachial wing vein. The following tissues were collected from each hen (approximately 500 mg each): right lobe of the liver, caudal lobe of the right kidney, and central shell gland. Tissues were immediately snap-frozen in liquid nitrogen and stored at −80°C until RNA extraction and reverse transcription reactions were conducted.
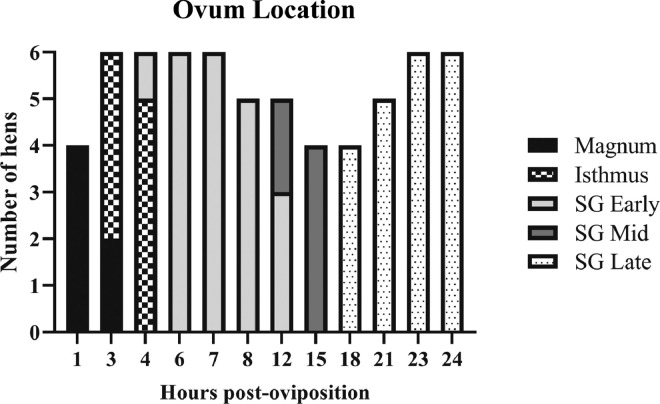
The location of the ovum within the reproductive tract and calcification stage of those in the shell gland were recorded following tissue collection (Figure 1). Eggshell calcification progress was described as early, mid, or late using the following parameters. Early calcification was described as the presence of a weak, thin eggshell with a rough texture, mid-calcification was delineated by a thick eggshell with a rough texture that crackled when mild pressure was applied by hand, and late eggshell calcification was used to describe a smooth, thick-shelled egg similar to a fully formed egg that cracked only after strong pressure was applied by hand. Hens that had not ovulated or exhibited an ovum that was located at an unexpected location for the time point at which they were sampled were excluded from further analysis. As a result, the number of hens that were utilized for further analysis were as follows (Figure 1): n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP.
Figure 1.
Ovum position and eggshell calcification stage across the 24-h oviposition cycle. Ovum location within the oviduct and state of eggshell calcification when the ovum was in the shell gland (SG) are shown for hens used at indicated hours post-oviposition (HPOP).
RNA Extraction and Reverse Transcription-Quantitative PCR (RT-qPCR)
Total RNA isolation was completed using QIAzol lysis reagent (Qiagen, Germantown, MD) according to the manufacturer's protocol. In brief, 1 mL of QIAzol was added to the sample tubes followed by homogenization using a Mini-Bead Beater (Biospec Products, Bartlesville, OK). Tissues were homogenized over three 45-s bursts and allowed to cool on ice for 1 min between bursts. Homogenate was extracted with chloroform, and total RNA was precipitated with isopropanol at 4°C for 10 min. Pellets were washed twice with 70% ethanol, allowed to dry, and reconstituted with 200 µL RNase-free water. Total RNA concentration was quantified using a NanoDrop-1000 spectrophotometer (Thermo Scientific, Waltham, MA). Following quantification, 1 µg total RNA was analyzed on a 1% agarose gel and visualized using a UV imaging system (BioSpectrum, Upland, CA) in conjunction with Visionworks LS software (Wasserburg, Germany) to verify the integrity of the RNA.
One µg of total RNA was reverse transcribed into cDNA in 20 µL reactions. Each reaction contained 4 µL of total RNA (250 ng/µL), 5 µM random hexamers (Invitrogen, Carlsbad, CA), 5 µM anchored-dT primer (Integrated DNA Technologies, Coralville, IA), 0.5 mM dNTPs (Thermo Scientific), 200 U M-MuLV reverse transcriptase (New England Biolabs, Ipswich, MA), 2 µL 10× M-MuLV buffer (New England Biolabs), and 8 U of RNaseOUT (Invitrogen). Initially, total RNA, primers, and dNTPs were combined and incubated at 65°C for 5 min. Following this, samples were placed on ice for at least 1 min before the addition of the M-MuLV enzyme, M-MuLV buffer, and RNaseOUT. The samples were then further incubated at 25°C for 5 min, 42°C for 60 min, and finally 65°C for 20 min. A negative no RT reaction to control for genomic DNA contamination was created using an RNA pool from all samples but the reverse transcriptase enzyme was not included. All reactions were diluted 1:10 with nuclease-free water, with the exception of cDNA used for 18S ribosomal RNA (18S) amplification, which was diluted 1:500.
Primers (Table 2) for measuring levels of each transcript were designed using Primer3 Plus software (Untergasser et al., 2012) to span introns with the following characteristics: amplicon length of 100 to 125 base pairs, primer length between 18 and 30 nucleotides, primer melting temperature between 58°C and 60°C, and GC content between 40 to 60%. Expression of mRNA was evaluated for the indicated genes using duplicate 10 µL reactions that consisted of 2 µL diluted cDNA, 5 µL PowerUp SYBR Green Master Mix (Applied Biosystems, Foster City, CA), and 400 nM of each forward and reverse primer. Thermal cycling was conducted on a StepOnePlus real-time PCR instrument (Applied Biosystems), with cycling conditions as follows: 95°C for 5 min, 40 cycles of 95°C for 15 s, 58°C for 30 s, 72°C for 30 s, and a postamplification dissociation curve to ensure that a single product was amplified. Levels of target mRNA expression for the kidney were normalized to glyceraldehyde-3-phosphate (GAPDH) mRNA, and expression of target genes in the liver and shell gland were normalized to 18S ribosomal RNA (18S). Levels of mRNA were normalized using the following equation: ΔCT = CTTarget gene − (CT18S or CTGAPDH) and then transformed as 2−ΔCT. To express these data relative to 1 HPOP, each transformed value was divided by the average transformed value at 1 HPOP using the following equation [(2−ΔCT) Sample]/[Average (2−ΔCT) 1 HPOP]. As a result, the value for 1 HPOP is equal 1 in all cases.
Table 2.
Primers used for reverse transcription-quantitative PCR.
| Target | Forward primer (5′–3′) | Reverse primer (5′–3′) | Transcript ID1 |
|---|---|---|---|
| Vitamin D3 metabolism and action | |||
| CYP2R1 | GGACAGCAATGGACAGTTTG | AGGAAAACGCAGGTGAAATC | 09745 |
| CYP24A1 | TGGTGACACCTGTGGAACTT | CTCCTGAGGGTTTGCAGAGT | 59161 |
| VDR | CTGCAAAATCACCAAGGACA | CATCTCACGCTTCCTCTGC | 96121 |
| RXRA | ACTGCCGCTACCAGAAGTGT | GACTCCACCTCGTTCTCGTT | 59924 |
| RXRG | GAAGCCTACACGAAGCAGAA | CCGATCAGCTTGAAGAAGAA | 49224 |
| Calcium homeostasis and eggshell calcification | |||
| CASR | CTGCTTCGAGTGTGTGGACT | GATGCAGGATGTGTGGTTCT | 55986 |
| PTH1R | CCAAGCTACGGGAAACAAAT | ATGGCATAGCCATGAAAACA | 08796 |
| CALCR | GCAGTTGCAAGAGCCAAATA | AGCTTTGTCACCAACACTCG | 15478 |
| CALB1 | AAGCAGATTGAAGACTCAAAGC | CTGGCCAGTTCAGTAAGCTC | 74265 |
| NCX1 | TCACTGCAGTCGTGTTTGTG | AAGAAAACGTTCACGGCATT | 13920 |
| PMCA1 | TTAATGCCCGGAAAATTCAC | TCCACCAAACTGCACGATAA | 80355 |
| TRPV6 | TATGCTGGAACGAAAACTGC | TTGTGCTTGTTGGGATCAAT | 23779 |
| CA2 | CCTGACTACTCCACCACTGC | TCTCAGCACTGAAGCAAAGG | 52439 |
| SLC26A9 | TCCACGATGCTGTTTTGTTT | GAGCTGCTTTCATCCACAGA | 01070 |
| Phosphorus homeostasis | |||
| FGFR1 | GACAGACTTCAACAGCCAGC | CCAACATCACACCCGAGTTC | 66938 |
| FGFR2 | CAGGGGTCTCGGAATATGAA | GCTTCAGCCATCACCACTT | 38732 |
| FGFR3 | GGAGTACTTGGCGTCACAGA | TCTAGCAAGGCCAAAATCAG | 01712 |
| FGFR4 | CTTGCCCGTCAAGTGGAT | TGAAGATCTCCCACATCAGAA | 28231 |
| KL | CCAAGAGAGATGATGCCAAA | CATCCAGAAGGGACCAGACT | 157852 |
| PiT-1 | TATCCTCCTCATTTCGGCGG | CTCTTCTCCATCAGCGGACT | 94781 |
| PiT-2 | CCATCCCCGTGTACCTTATG | AGACATGGCCATCACTCCTC | 51992 |
| NaPiIIa | ATCGGCTTGGGGGTGATC | GAGGGCGATCTGGAAGGAG | 58978 |
| Housekeeping genes | |||
| GAPDH | AGCCATTCCTCCACCTTTGAT | AGTCCACAACACGGTTGCTGTAT | 23323 |
| 18S3 | AGCCTGCGGCTTAATTTGAC | CAACTAAGAACGGCCATGCA | |
Transcript identification from Ensemble chicken genome assembly (GRCg6a; (http://www.ensembl.org/Gallus_gallus/Info/Index) preceded by ENSGALT000000.
Transcript identification from Ensemble chicken genome assembly (GRCg7b; (http://www.ensembl.org/Gallus_gallus/Info/Index) preceded by ENSGALT000100.
Sequence for 18S rRNA is not on the assembled chicken genome, and primers were designed based on the sequence in GenBank (accession numbers AF17361).
Vitamin D3 Metabolites
Plasma was analyzed for 25(OH)D3, 1α,25(OH)2D3, and 24,25(OH)2D3 levels. Analysis was completed by liquid chromatography-tandem mass spectrometry (Heartland Assays, Ames, IA) with the following lower limits of detection (LLD) and lower limits of quantification (LLQ): 25(OH)2D3—0.5 ng/mL (LLD) and 1.5 ng/mL (LLQ); 24,25(OH)2D3—0.1 ng/mL (LLD) and 0.3 ng/mL (LLQ); and 1α,25(OH)2D3—5.0 pg/mL (LLD) and 10 pg/mL (LLQ).
Parathyroid Hormone
A competitive enzyme-linked immunosorbent assay (ELISA) for chicken PTH (CUSABIO, Wuhan, China) was utilized according to a modified version of the manufacturer's instructions. In brief, plasma samples were added to the ELISA plate in 50 µL duplicates along with PTH standards (0, 6.25, 12.5, 25, 50, and 100 pg/mL) in 50 µL triplicates. After addition of the samples and standards, biotin-conjugated PTH was added, followed by overnight incubation at 4°C for 18 h. After incubation, the plate was rinsed using wash buffer, followed by the addition of horseradish peroxidase-avidin, and a second incubation at 37°C for 30 min was conducted. The plate was washed a final time, followed by the addition of enzyme substrate, and incubated at 37°C for 15 min. Finally, the stop solution was applied, and the optical density of each well was read at a 450 nm wavelength using a Victor Nivo Multimode microplate reader (Perkin Elmer, Waltham, MA). This assay has been previously validated for parallelism and sensitivity using a series of four 2-fold dilutions of 5 independent playing hen plasma samples with starting concentrations of 40 to 60 pg/mL. All samples exhibited parallelism at least through the manufacturer's reported sensitivity of 6.25 pg/mL. Intra- and interassay coefficient of variations (CVs) for the ELISA were determined to be 9.9 and 12.4%, respectively, and fall under the manufacturer's value of <15% for each measurement of precision.
Blood Chemistry
Immediately following collection, 100 µL of whole blood was analyzed using an iSTAT hand-held blood analyzer with CG8+ cartridges (Zoetis). Additionally, 100 µL of plasma was analyzed using a VetScan2 equipped with Avian/Reptilian Profile Plus cartridges (Zoetis). Parameters measured by CG8+ cartridges included pH, partial pressure of carbon dioxide (pCO2), partial pressure of oxygen (pO2), base excess (BEecf), bicarbonate, total carbon dioxide (TCO2), oxygen saturation (sO2), sodium (Na+), potassium (K+), iCa2+, glucose (GLU), hematocrit (Hct), and hemoglobin (Hb) (Supplementary Table S1). Parameters measured using the Avian/Reptilian Profile Plus cartridges included aspartate amino transferase (AST), creatinine kinase (CK), uric acid (UA), GLU, tP, total protein (tPr), albumin (ALB), globulin (GLOB), K+, and Na+ (Supplementary Table S2). Values for bile acid and total calcium were also measured using VetScan2 device but fell outside the upper detection limit.
Statistical Analysis
Each hen was considered the experimental unit, and the level of replication for each parameter is indicated in the appropriate figure legend. All data were analyzed with JMP Pro 16 software (SAS Institute, Inc., Cary, NC) using a 1-way analysis of variance (ANOVA), with HPOP as the model effect. Fishers’ test of least significant difference was utilized to separate means. All differences were considered significant at P ≤ 0.05. Normalized CT values (ΔCT) were analyzed for gene expression data to correct for non-normality of transformed and relative data. Absolute concentration of other parameters followed a normal distribution, and these values were directly analyzed.
RESULTS
Ovum Location
Following ovulation, progression of the ovum through the reproductive tract ensures the sequential development of the follicle into a calcified egg over approximately 24-h. Establishing the times in which the ovum resides at each location within the oviduct and evaluating eggshell calcification state once it reaches the shell gland is crucial for understanding stages of mineral utilization for bone or shell formation during the daily oviposition cycle. As such, prior to analysis, both ovum location and stage of eggshell calcification were recorded for all hens used in further analysis is shown in Figure 1. One hour following oviposition, all hens that had ovulated were found to have the ovum present in the magnum. The ovum then transitioned into the isthmus between 3 and 4 HPOP, where the eggshell membrane was added to the follicle. Entry of the developing egg into the shell gland took place between 4 and 6 HPOP, and from this point onward calcification on the shell membranes took place. Early calcification of the eggshell occurred between 6 and 12 HPOP, while mid-calcification was observed between 12 and 18 HPOP, and finally, late eggshell calcification took place between 18 and 24 HPOP. Together, this information was used to ensure that ovum location and stage of calcification was as expected for all hens used for physiological analyses.
Vitamin D3 Metabolism and Action
Enzymatic Conversion
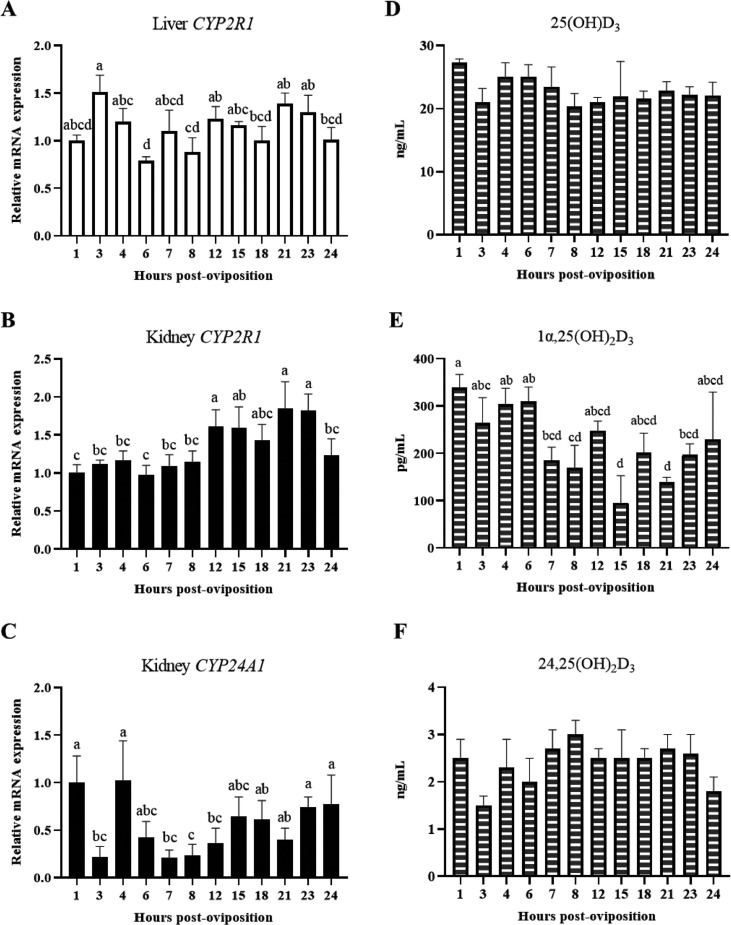
Vitamin D3 is a crucial regulatory component of calcium and phosphorus homeostasis; however, dietary vitamin D3 must undergo a series of hydroxylation reactions in the liver and kidney before biologically active 1α,25(OH)2D3 is formed. As such, relative mRNA expression of vitamin D3 hydroxylase enzymes were measured in the liver and kidney in conjunction with circulating vitamin D3 metabolites throughout the oviposition cycle. Hepatic expression of the vitamin D3 25-hydroxylase enzyme CYP2R1 initially exhibited a decrease between 3 and 6 HPOP before levels were re-established at 7 HPOP. Thereafter, hepatic CYP2R1 remained relatively stable through 24 HPOP (Figure 2A; P ≤ 0.05) and, overall, it did not exhibit consistent changes throughout the egg-laying cycle. Renal expression of CYP2R1 was lowest between 1 and 8 HPOP and again at 24 HPOP when compared to expression between 12 and 23 HPOP (Figure 2B; P ≤ 0.05), a period of high calcium demand. Levels of renal CYP24A1 that catabolizes bioactive metabolites decreased considerably between 1 and 7 HPOP, including a transient drop that occurred at 3 HPOP. Levels then remained low between 7 and 12 HPOP, followed by heightened expression between 15 and 24 HPOP (Figure 2C; P ≤ 0.05). While differences in renal and hepatic mRNA expression of CYP2R1 were observed across different HPOP, circulating levels of plasma 25(OH)D3 were not influenced by time post-oviposition (Figure 2D; P > 0.05). However, bioactive 1α,25(OH)2D3 concentrations were elevated between 1 and 6 HPOP, and decreased between 7 and 21 HPOP, except for peaks occurring at 12 and 18 HPOP. Finally, 1α,25(OH)2D3 increased between 21 and 24 HPOP (Figure 2E; P ≤ 0.05). Levels of 24,25(OH)2D3 in the plasma did not change significantly over time (Figure 2F; P > 0.05), which was in contrast to observed changes in CYP24A1 mRNA expression. Given the expression patterns of renal CYP2R1 and the lack of changes in circulating 25(OH)2D3, it is possible that kidney could play a role in local production of this metabolite to facilitate renal mineral homeostasis during eggshell calcification.
Figure 2.
Expression of genes for enzymes that metabolize vitamin D3 and levels of circulating vitamin D3 metabolites. Relative levels of mRNA were measured at indicated hours post-oviposition (HPOP) for (A) liver CYP2R1, (B) renal CYP2R1, and (C) renal CYP24A1. Expression of target genes was determined by RT-qPCR and normalized to GAPDH mRNA for kidney and liver. All values (mean + SEM; n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP) are expressed relative to 1 HPOP (equivalent to 1). Circulating levels of (D) 25(OH)D3, (E) 1α,25(OH)2D3, and (F) 24,25(OH)2D3 were analyzed by liquid chromatography-tandem mass spectrometry (mean + SEM; n = 6 hens at 23 HPOP; n = 5 hens at 3, 7, 8, 21, and 24 HPOP; n = 4 hens at 4, 6, 12, and 18 HPOP; n = 3 hens at 1 HPOP; n = 2 hens at 15 HPOP). Differences in available plasma volumes resulted in fewer hens analyzed at some time points. Data were analyzed by 1-way ANOVA followed by Fisher's test of least significant difference when ANOVA indicated significance, and bars not sharing a common letter differ significantly (P ≤ 0.05). Expression of CYP27B1 was not measured, as the gene has not been identified in chickens.
1α,25(OH)2D3 Signaling
Hormonally active 1α,25(OH)2D3 imparts effects on calcium and phosphorus metabolism through transcriptional regulation of vitamin D3-responsive genes in tissues that participate in mineral uptake and utilization. Expression of vitamin D receptor (VDR) and its associated heterodimeric partners, retinoid-X-receptors alpha (RXRA) and gamma (RXRG), were measured in the shell gland and kidney to evaluate 1α,25(OH)2D3 responsiveness in these tissues. Renal expression of VDR exhibited minimal variation across the 24-h period, except for a peak that occurred at 1 HPOP (Figure 3A; P ≤ 0.05). Levels of renal RXRA remained consistent between 1 and 12 HPOP, then declined gradually between 12 and 21 HPOP before increasing again between 21 and 24 HPOP (Figure 3B; P ≤ 0.05). Expression of RXRG in the kidney was unaltered across time points (Figure 3C; P > 0.05). Shell gland VDR increased between 1 and 12 HPOP before gradually declining again between 15 and 24 HPOP (Figure 3D; P ≤ 0.05). Though mRNA expression of RXRA in shell gland followed a similar pattern to that of renal RXRA, differences were not statistically significant (Figure 3E; P > 0.05). Lastly, levels of RXRG in the shell gland increased 6-fold between 1 and 6 HPOP, and this upregulated expression was maintained through 8 HPOP before substantially dropping again at 12 HPOP and remaining low through 24 HPOP (Figure 3F; P ≤ 0.05). These results demonstrate the dynamic responsiveness of these tissues, particularly the shell gland, during the oviposition cycle and indicate that sensitivity of the shell gland to 1α,25(OH)2D3 could increase as the eggshell enters the shell gland.
Figure 3.
Expression of genes for vitamin D3 receptor and its heterodimeric partners in kidney and shell gland. Relative levels of mRNA were measured at indicated hours post-oviposition (HPOP) for renal (A) VDR, (B) RXRA, (C) RXRG, and shell gland (D) VDR, (E) RXRA, (F) RXRG. Expression of target genes was determined by RT-qPCR and normalized to GAPDH mRNA for the kidney and 18S rRNA for shell gland. All values (mean + SEM; n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP) are expressed relative to 1 HPOP (equivalent to 1). Data were analyzed by 1-way ANOVA followed by Fisher's test of least significant difference when ANOVA indicated significance, and bars not sharing a common letter differ significantly (P ≤ 0.05).
Circulating Factors Associated With Calcium and Phosphorus Homeostasis
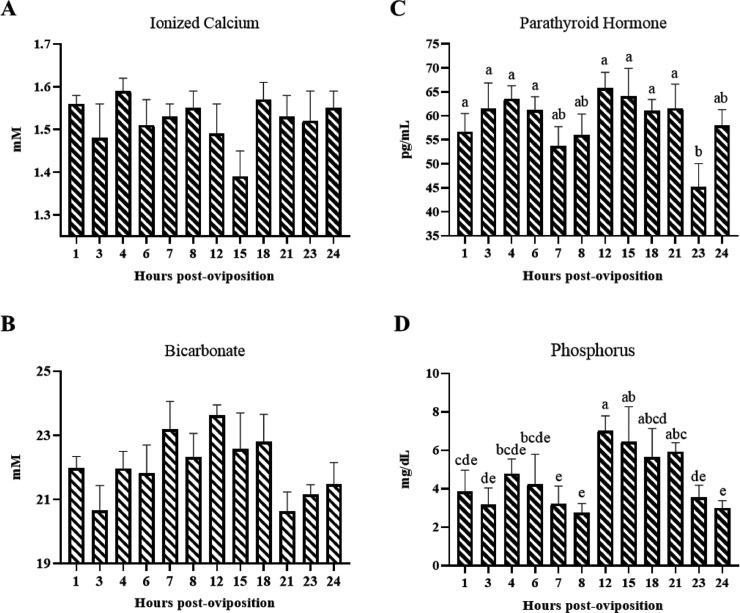
Measuring fluctuations in circulating calcium, phosphorus, and factors that influence their homeostasis and utilization for eggshell and bone formation is integral for understanding both mineral status and metabolic responses of layers. To evaluate this, circulating levels of iCa2+, bicarbonate, PTH, and tP were measured. Circulating iCa2+ was not significantly influenced by HPOP (Figure 4A; P > 0.05); however, a transient drop was noted at 3 HPOP, as well as a notable decrease that occurred between 8 and 15 HPOP. Circulating bicarbonate levels tended to increase between 3 and 7 HPOP and remained high until 18 HPOP before dropping again at 21 HPOP (Figure 4B; P = 0.0752). Plasma PTH concentrations remained relatively stable throughout the oviposition cycle, except for significant decreases between 21 and 23 HPOP, after which levels increased again at 24 HPOP (Figure 4C; P ≤ 0.05). Lastly, tP was initially low between 1 and 8 HPOP before increasing significantly at 12 HPOP. It remained elevated through 21 HPOP and then dropped at 23 and 24 HPOP to levels similar to those observed between 1 and 8 HPOP (Figure 4D; P ≤ 0.05).This pattern in tP is likely reflective of medullary bone resorption that occurs to provide calcium necessary for rapid eggshell formation between 12 and 18 HPOP. Additional whole blood parameters measured (PH, pCO2, pO2, TCO2, K+, Glu, Hct, and Hb) did not significantly differ across time points (Supplementary Table S1; P > 0.05); however, BEecf generally increased between 1 and 12 HPOP before gradually declining at subsequent time points. Levels of sO2 were stable between 1 and 7 HPOP before gradually declining through 18 HPOP and remained low thereafter (Supplementary Table S1; P ≤ 0.05). Whole blood Na+ decreased between 6 and 12 HPOP and exhibited a transient increase at 15 HPOP. Thereafter, Na+ concentrations increased between 18 and 23 HPOP (Supplementary Table S1; P ≤ 0.05). Several plasma parameters exhibited no significant differences across time points such as AST, CK, tPr, ALB, GLOB, K+, and Na+ (Supplementary Table S2; P > 0.05). Contrastingly, UA and GLU exhibited differences across time points wherein both showed similar drops in concentration between 8 and 15 HPOP; however, prior to this decrease, GLU significantly increased between 3 and 7 HPOP (Supplementary Table S2; ≤0.05).
Figure 4.
Circulating factors associated with mineral homeostasis. Circulating (A) ionized calcium and (B) bicarbonate were measured using CG8+ cartridges and an iSTAT hand-held blood analyzer at indicated hours post-oviposition (HPOP) (mean + SEM; n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP). (C) Plasma parathyroid hormone was measured using an ELISA (mean + SEM; n = 6 hens at 3, 4, 6, and 23 HPOP; n = 5 hens at 7, 8, 12, 21, and 24 HPOP; n = 4 hens at 1, 15, and 18 HPOP). (D) Plasma phosphorus was measured using Avian/Reptile Profile Plus cartridges and a VetScan2 (mean + SEM; n = 6 hens at 7, 23, and 24 HPOP; n = 5 hens at 12 HPOP; n = 4 hens at 1, 4, 8, 15, and 21 HPOP; n = 3 hens at 3,6, and 18 HPOP). Differences in available plasma volumes resulted in fewer hens analyzed at some time points. Data were analyzed by 1-way ANOVA followed by Fisher's test of least significant difference when ANOVA indicated significance, and bars not sharing a common letter differ significantly (P ≤ 0.05).
Calcium Homeostasis
Hormonal Signaling
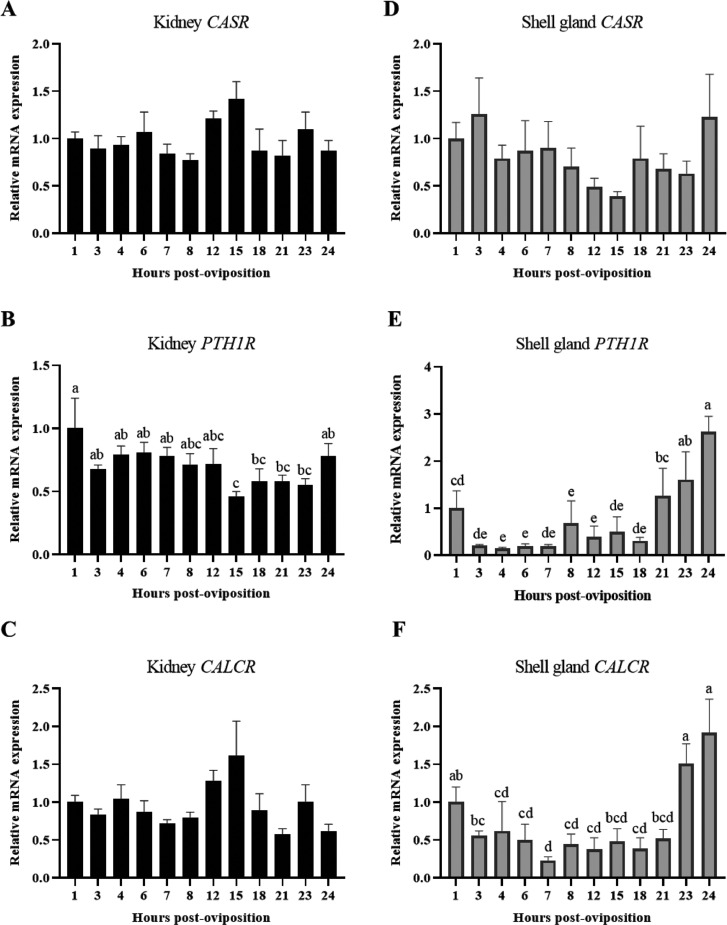
Calcium homeostasis is modulated, in large part, by the hormonal actions of PTH and CALC at tissues that express their receptors in response to CASR. Therefore, expression of CASR, PTH1R, and CALCR were measured in the kidney and shell gland. Though renal expression of CASR and CALCR were not significantly influenced by time post-oviposition (Figure 5A, C; P > 0.05), changes in CALCR approached significance (P = 0.0596). Levels of renal CALCR remained stable between 1 and 8 HPOP, increased gradually through 15 HPOP, and then decreased at 18 HPOP and remained low for the remaining time points. Expression of PTH1R in kidney was impacted by time within the oviposition cycle, wherein levels were lowest between 15 and 23 HPOP (Figure 5B; P ≤ 0.05). While expression of CASR in the shell gland was not significantly altered by time post-oviposition (Figure 5D; P > 0.05), significant changes in PTH1R (Figure 5E; P ≤ 0.05) and CALCR (Figure 5F; P ≤ 0.05) were observed, though differences were more apparent for PTH1R. Both genes exhibited similar patterns, wherein their expression decreased from intermediate levels at 1 HPOP to low levels between 3 and 18 (PTH1R) or 21 (CALCR) HPOP before markedly increasing again through 24 HPOP. These results suggest that both CALC and PTH are important mediators of mineral homeostasis during the oviposition cycle, particularly within the shell gland.
Figure 5.
Expression of hormonal mediators of calcium homeostasis in kidney and shell gland. Relative levels of mRNA were measured at indicated hours post-oviposition (HPOP) for kidney (A) CASR, (B) PTH1R, (C) CALCR, and shell gland (D) CASR, (E) PTH1R, and (F) CALCR. Expression of target genes was determined by RT-qPCR and normalized to GAPDH mRNA for the kidney and 18S rRNA for shell gland. All values (mean + SEM; n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP) are expressed relative to 1 HPOP (equivalent to 1). Data were analyzed by 1-way ANOVA followed by Fisher's test of least significant difference when ANOVA indicated significance, and bars not sharing a common letter differ significantly (P ≤ 0.05).
Calcium Transport and Utilization
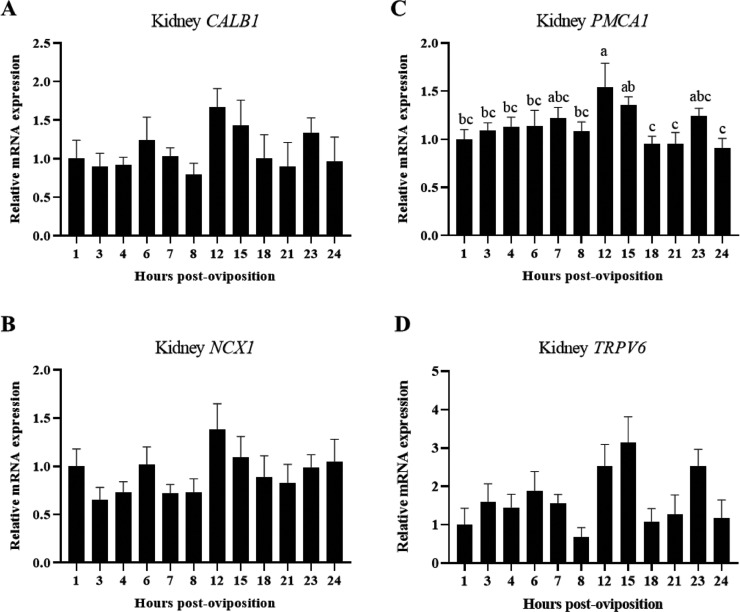
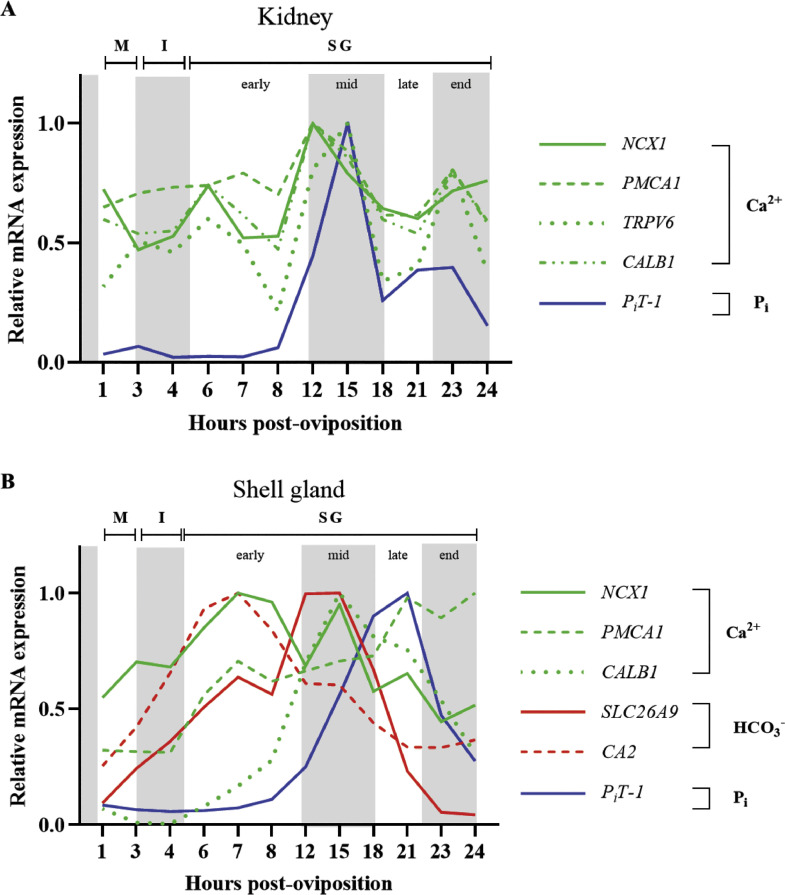
In order to determine which transporters are important for mediating calcium movement in the kidney and shell gland throughout the oviposition cycle, expression of CALB1, NCX1, PMCA1, and TRPV6 were evaluated. Levels of renal PMCA1 remained consistent between 1 and 8 HPOP, elevated at 12 HPOP, and then gradually declined until 18 HPOP (Figure 6C; P ≤ 0.05). Unlike PMCA1, renal CALB1 (Figure 6A; P > 0.05), NCX1 (Figure 6B; P > 0.05), and TRPV6 (Figure 6D; P > 0.05) expression was not significantly influenced by time post-oviposition; however, they all showed similar patterns, with the highest levels around 12 and 15 HPOP during peak eggshell calcification.
Figure 6.
Expression of genes associated with calcium transport in kidney. Relative levels of mRNA were measured at indicated hours post-oviposition (HPOP) for kidney (A) CALB1, (B) NCX1, (C) PMCA1, and (D) TRPV6. Expression of target genes was determined by RT-qPCR and normalized to GAPDH mRNA. All values (mean + SEM; n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP) are expressed relative to 1 HPOP (equivalent to 1). Data were analyzed by 1-way ANOVA followed by Fisher's test of least significant difference when ANOVA indicated significance, and bars not sharing a common letter differ significantly (P ≤ 0.05).
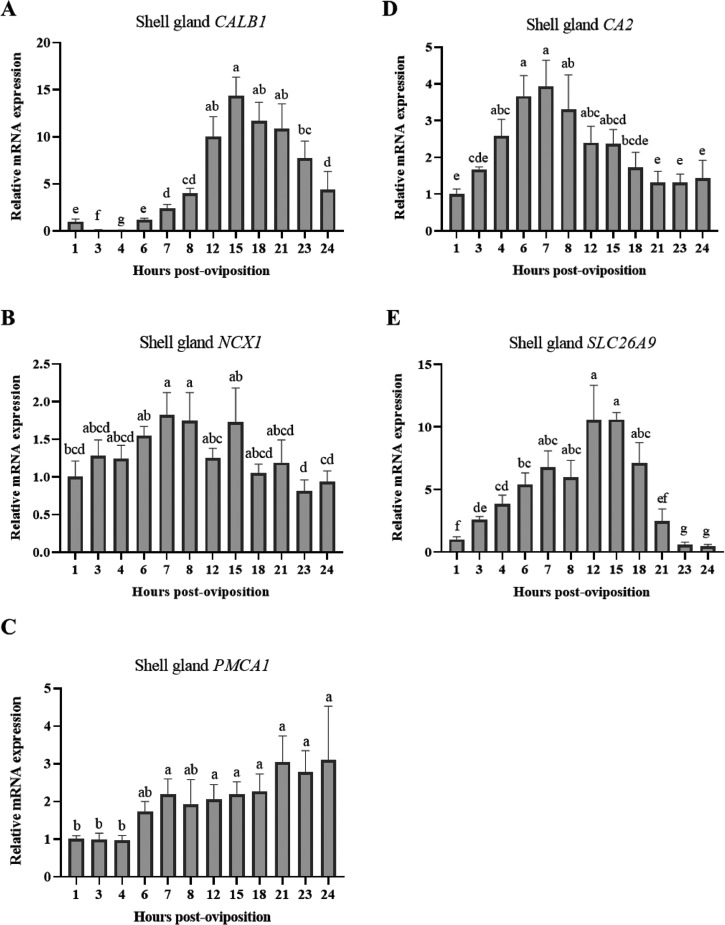
Eggshell is primarily composed of calcium carbonate crystals, requiring both calcium and bicarbonate ions for its formation. To investigate changes in the shell gland associated with eggshell calcification, levels of CALB1, NCX1, PMCA1, CA2, and SLC26A9 were evaluated. The calcium chaperone CALB1 exhibited considerable differences in expression throughout the 24-h egg formation cycle, wherein low levels between 1 and 6 HPOP were followed by a 14-fold increase between 6 and 15 HPOP and a subsequent steady decline through 24 HPOP (Figure 7A; P ≤ 0.05). Shell gland NCX1 also showed significant effects of HPOP, with levels rising between 1 and 8 HPOP before gradually dropping until 24 HPOP (Figure 7B; P ≤ 0.05). Expression of PMCA1 was found to be low from 1 to 4 HPOP, after which it steadily increased through 24 HPOP (Figure 7C; P ≤ 0.05). Expression of another membrane calcium transporter, TRPV6, was not detected in this tissue. Levels of CA2 increased gradually between 1 and 8 HPOP and declined thereafter (Figure 7D; P ≤ 0.05), while the bicarbonate transporter SLC26A9 exhibited a 10-fold increase in expression between 1 and 12 HPOP before declining between 12 and 24 HPOP (Figure 7E; P ≤ 0.05). Overall, findings from the kidney and shell gland indicate which transporters play important roles in retaining calcium at the kidney and utilizing it for eggshell formation during times of peak mineralization, as well as define the timing of bicarbonate synthesis and transport.
Figure 7.
Expression of genes associated with eggshell calcification in shell gland. Relative levels of mRNA were measured at indicated hours post-oviposition (HPOP) for shell gland (A) CALB1, (B) NCX1, (C) PMCA1, (D) CA2, (E) SLC26A9. Expression of target genes was determined by RT-qPCR and normalized to 18S rRNA. All values (mean + SEM; n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP) are expressed relative to 1 HPOP (equivalent to 1). Data were analyzed by 1-way ANOVA followed by Fisher's test of least significant difference when ANOVA indicated significance, and bars not sharing a common letter differ significantly (P ≤ 0.05).
Phosphorus Homeostasis
Hormonal Signaling
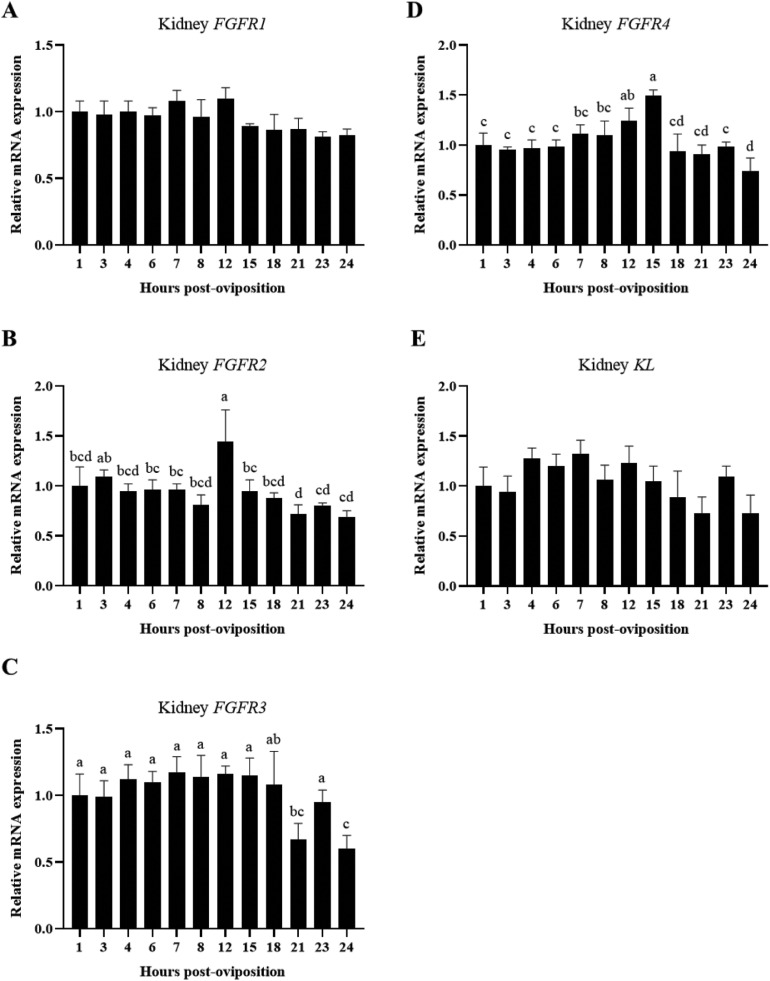
Phosphorus homeostasis is mediated by the interactions of FGF23 with its receptors; therefore, kidney and shell gland were evaluated as potential targets of FGF23 signaling by measuring mRNA expression of FGFR1–4 and KL throughout the oviposition cycle. Renal expression of FGFR1 (Figure 8A; P > 0.05) and KL (Figure 8E; P > 0.05) were not significantly influenced by time post-oviposition. Contrastingly, other FGFRs did change in the kidney throughout the oviposition cycle. Levels of FGFR2 exhibited a peak in expression at 12 HPOP (Figure 8B; P ≤ 0.05), FGFR3 declined toward the end of eggshell calcification at 21 and 24 HPOP (Figure 8C; P ≤ 0.05), FGFR4 increased gradually between 6 and 15 HPOP before declining again at subsequent time points (Figure 8D; P ≤ 0.05). Changes in expression of FGFR2 and FGFR4 suggest these receptors could be important for increasing renal sensitivity to FGF23 during periods of elevated bone breakdown to support rapid eggshell calcification.
Figure 8.
Expression of genes for FGF23 receptors in kidney. Relative levels of mRNA were measured at indicated hours post-oviposition (HPOP) for kidney (A) FGFR1, (B) FGFR2, (C) FGFR3, and (D) FGFR4, and (E) KL. Expression of target genes was determined by RT-qPCR and normalized to GAPDH mRNA. All (mean + SEM; n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP) are expressed relative to 1 HPOP (equivalent to 1). Data were analyzed by 1-way ANOVA followed by Fisher's test of least significant difference when ANOVA indicated significance, and bars not sharing a common letter differ significantly (P ≤ 0.05).
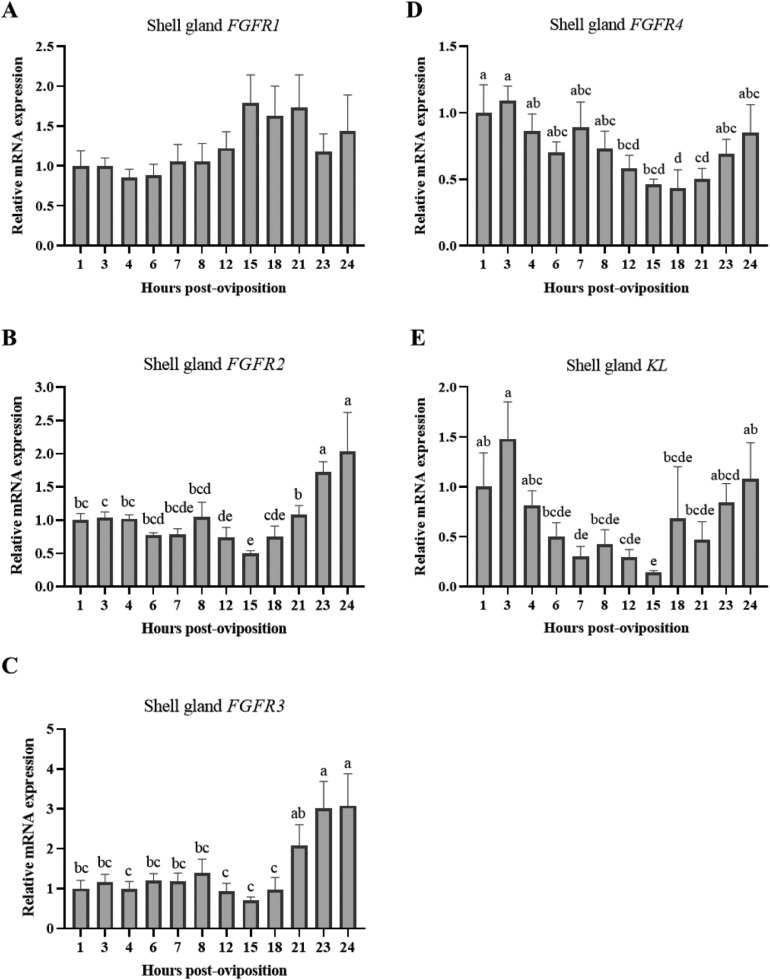
Phosphorus within the eggshell is primarily deposited as a hydroxyapatite layer beneath the cuticle and in phosphate-containing organic compounds within the cuticle. As such, temporal regulation of FGFR1–4 and KL expression was examined in the shell gland. As seen in the kidney, FGFR1 expression in the shell gland was not significantly influenced by time post-oviposition (Figure 9A; P > 0.05), though mRNA for other FGF23 receptors did vary in this tissue. Levels of shell gland FGFR2 (Figure 9B; P ≤ 0.05) and FGFR3 (Figure 9C; P ≤ 0.05) exhibited similar patterns, where expression remained low until 18 HPOP and then increased sharply between 18 and 24 HPOP, as eggshell calcification was concluding. Additionally, FGFR2 exhibited a gradual decline between 8 and 15 HPOP, when the eggshell begins rapidly calcifying. Expression of FGFR4 in the shell gland gradually declined between 7 and 18 HPOP before re-establishing expression levels between 21 and 24 HPOP (Figure 9D; P ≤ 0.05). Unlike renal expression of KL, shell gland KL showed significant changes in expression across time points. Levels were highest between 1 and 3 HPOP, then dropped through 15 HPOP and increased again between 18 and 24 HPOP (Figure 9E; P ≤ 0.05). Dynamic changes observed in the expression of FGF23 receptors in the shell gland indicate that this tissue could be a novel target for FGF23 signaling, which likely increases in sensitivity toward the end of eggshell calcification.
Figure 9.
Expression of genes for FGF23 receptors in shell gland. Relative levels of mRNA were measured at indicated hours post-oviposition (HPOP) for shell gland (A) FGFR1, (B) FGFR2, (C) FGFR3, and (D) FGFR4, and (E) KL. Expression of target genes was determined by RT-qPCR and normalized to 18S rRNA. All values (mean + SEM; n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP) are expressed relative to 1 HPOP (equivalent to 1). Data were analyzed by 1-way ANOVA followed by Fisher's test of least significant difference when ANOVA indicated significance, and bars not sharing a common letter differ significantly (P ≤ 0.05).
Phosphorus Transport and Utilization
Phosphorus transporters are crucial for modulating renal uptake and excretion of this mineral during the oviposition cycle. Renal expression of inorganic phosphorus transporter 1 (PiT-1) remained very low from 1 to 8 HPOP, and this was followed by a stark almost 30-fold increase between 8 and 15 HPOP before levels dropped to intermediate values thereafter (Figure 10A; P ≤ 0.05). Renal inorganic phosphorus transporter 2 (PiT-2) also remained relatively low between 1 and 8 HPOP, increased at 12 and 15 HPOP, and then dropped to intermediate levels between 18 and 24 HPOP (Figure 10B; P ≤ 0.05), though the fold changes were not as large. Renal sodium-dependent phosphate transporter IIa (NaPiIIa) expression was not affected by time post-oviposition (Figure 10C; P > 0.05), and kidney expression of sodium-dependent phosphate transporter IIb (NaPiIIb) was not detected. These results highlight the need for renal excretion of excess phosphorus resulting from medullary bone break down necessary to support eggshell calcification and identify PiT-1, and to a lesser extent PiT-2, as key transporters involved in this process.
Figure 10.
Expression of genes for phosphorus transporters in kidney and shell gland. Relative levels of mRNA were measured at indicated hours post-oviposition (HPOP) for kidney (A) PiT-1, (B) PiT-2, (C) NaPiIIa, and shell gland (D) PiT-1, (E) PiT-2. Expression of target genes was determined by RT-qPCR and normalized to GAPDH mRNA for the kidney and 18S rRNA for shell gland. All values (mean + SEM; n = 6 hens at 3, 4, 6, 7, 23, and 24 HPOP; n = 5 hens at 8, 12, and 21 HPOP; n = 4 hens at 1, 15, and 18 HPOP) are expressed relative to 1 HPOP (equivalent to 1). Data were analyzed by 1-way ANOVA followed by Fisher's test of least significant difference when ANOVA indicated significance, and bars not sharing a common letter differ significantly (P ≤ 0.05).
Expression patterns of the phosphorus transporters PiT-1, PiT-2, and NaPiIIa were examined in the shell gland to evaluate if they changed throughout the daily oviposition cycle Expression of PiT-1 remained low from 1 to 8 HPOP, and then it steadily increased over 10-fold by 21 HPOP before declining at 23 and 24 HPOP (Figure 10D; P ≤ 0.05). In contrast, expression of PiT-2 in the shell gland was not significantly impacted by time post-oviposition (Figure 10E; P > 0.05). As in kidney, expression of NaPiIIb was not detected in the shell gland, nor was NaPiIIa. As in the kidney, PiT-1 likely plays a major role in transporting phosphorus within the shell gland during late eggshell calcification and cuticle formation.
DISCUSSION
Modern laying hens develop an egg approximately every 24 h (Nys and Guyot, 2011), during which calcium and phosphorus homeostasis is influenced by bone remodeling and eggshell calcification. This study set out to identify circadian fluctuations in pathways that mediate regulation of calcium and phosphorus utilization during the 24-h oviposition cycle. Findings suggest a possible role of the kidney in 25-hydroxylation of vitamin D3 and highlighted key transporters involved in mineral utilization during egg formation in both shell gland and kidney. Additionally, the shell gland was identified as a novel target for FGF23 signaling, and expression patterns suggest that the shell gland may be responsive to other hormonal regulators of calcium and phosphorus homeostasis such as 1α,25(OH)2D3, PTH, and CALC.
Canonically, vitamin D3 undergoes 2 independent hydroxylation reactions in the liver and kidney, respectively, before it can be transformed into biologically active 1α,25(OH)2D3 (Bikle, 2018). In mammalian (Ponchon and DeLuca, 1969; Ponchon et al., 1969) and avian species (Watanabe et al., 2013), the first reaction occurs in the liver wherein 25-hydroxylase, encoded by the CYP2R1 gene, converts vitamin D3 into 25(OH)D3. Under physiological conditions, this process occurs in an unregulated and constitutive fashion (Tucker et al., 1972; Heaney et al., 2008). Interestingly, findings by Tucker et al. (1972) identified 25-hydroxylase in the kidney of white leghorn chicks that was capable of forming 25(OH)D3 when vitamin D3 was added in vitro. Results from our study showed that hepatic expression of CYP2R1 exhibited minor changes during the oviposition cycle; however, renal mRNA expression of CYP2R1 was generally upregulated between 12 and 23 HPOP, which coincides with observed stages of mid and late eggshell calcification. In contrast, circulating levels of 1α,25(OH)2D3 were generally lower during these time points, suggesting its rapid utilization by the kidney, shell gland, and small intestine to facilitate eggshell calcification. Together, these results suggest that the kidney may play a supporting role in the 25-hydroxylation of vitamin D3, particularly during periods of high calcium demand when the eggshell is most rapidly calcifying. This 25(OH)D3 may be utilized locally to enhance the efficacy of conversion of dietary vitamin D3 into 1α,25(OH)2D3 and may not contribute to circulating levels of 25(OH)D3. Since 1α,25(OH)2D3 is involved in regulating calcium and phosphorus uptake and excretion in the kidney of chickens (Taylor and Wasserman, 1972), the presence of both 25-hydroxylase and 1α-hydroxylase enzymes in the kidney would likely improve efficiency of renal calcium and phosphorus handling.
When 1α,25(OH)2D3 binds VDR, it functions as a ligand-activated transcription factor that binds with its heterodimeric partners RXRA and RXRG (Kliewer et al., 1992). Together, this complex binds vitamin D3 response elements (VDRE) in the promotor regions of vitamin D3 responsive genes to regulate their transcription. Studies conducted in chickens show that CALB1 is transcriptionally upregulated by VDR/RXR complexes in both kidney and small intestine (Bar et al., 1990; Norman, 1990; Nys et al., 1992). However, evidence that CALB1 is similarly responsive in the shell gland is limited (Bar et al., 1977; Bar et al., 1984). Despite this, Yoshimura et al. (1997) showed strong immunohistochemical staining of VDR in the shell gland of egg-producing hens, and RT-qPCR results from this study confirm the presence of VDR mRNA in this tissue. In the current study, expression of shell gland VDR gradually increased from the time of oviposition through 12 HPOP, when peak eggshell calcification begins, before gradually declining through 24 HPOP as calcification slowed. Additionally, RXRG levels increased 6-fold from 6 to 8 HPOP and rapidly decreased thereafter, which coincided with observed early stages of eggshell calcification. Contrastingly, RXRA exhibited minimal variation across time points. Together, these results suggest that the shell gland is a likely target tissue for 1α,25(OH)2D3 and that RXRG may be of greater importance in eliciting dynamic genomic responses to this hormone than RXRA during the oviposition cycle. Furthermore, differences in the availability of RXRG may alter the hormonal responsiveness of the shell gland. These temporal changes in the transcriptional machinery during eggshell calcification indicate that 1α,25(OH)2D3 plays a role in facilitating eggshell development.
In addition to potentially mediating uptake and utilization of calcium by the shell gland, 1α,25(OH)2D3 may also regulate bicarbonate production in shell gland epithelial cells. Bicarbonate formation by CA2 is crucial for precipitation of calcium carbonate crystals that form the bulk of the eggshell (Gutowska and Mitchell, 1945; Robinson and King, 1963), and a functional VDRE has been identified in the promotor region of chicken CA2 (Quelo et al., 1994; Quelo et al., 1998). Additionally, studies conducted by Narbaitz et al. (1981) and Grunder et al. (1990) demonstrated that differences in 1α,25(OH)2D3 supply influence CA2 activity in chickens. In the current study, expression of CA2 mRNA in shell gland tissue increased 4-fold from oviposition through early eggshell calcification and remained elevated until 8 HPOP. This closely mirrors expression of RXRG, suggesting that differences in CA2 expression may be modulated by RXRG availability to dimerize with existing VDR in the shell gland. Bicarbonate ions are transferred into the shell gland lumen by the SLC26A9 transporter (Nys and Le Roy, 2018). Our findings demonstrated a considerable 10-fold increase in expression of this transporter between 1 and 12 HPOP before levels declined from 15 to 21 HPOP, as eggshell calcification slowed. Interestingly, the expression profile of SLC26A9 also closely resembled that of VDR, thereby illustrating a further mechanism by which 1α,25(OH)2D3 genomic actions may regulate bicarbonate production and transport in the shell gland. Additional bicarbonate for eggshell calcification is also supplied from circulation (Nys and Le Roy, 2018) and results from this study show that circulating bicarbonate levels were generally higher during the periods of eggshell calcification, between 7 and 18 HPOP. In this way, both local and systemic bicarbonate formation is increased to support eggshell calcification.
Calcium levels in both circulation and tissues are closely monitored by CASR, which can influence the expression of calcium and phosphorus transporters, either directly (Kantham et al., 2009; Loupy et al., 2012) or indirectly through its stimulatory influence on PTH secretion (Ba et al., 2003). Circulating PTH binds PTH1R located in the plasma membrane of target tissues, invoking hormonal signaling that facilitates increases in circulating iCa2+ levels. In contrast, binding of CALC to its receptor, CALCR, generally serves to decrease blood iCa2+ levels. Together, these hormonal signaling pathways act upon tissues such as the kidney to facilitate changes in calcium and phosphorus transporter expression and mediate uptake or excretion of these minerals. Findings from our study showed that renal CASR expression increased numerically between 8 and 15 HPOP, which was inversely related to circulating iCa2+ during this time. The period of increased renal CASR also coincided with upregulated expression of the calcium transporters NCX1, PMCA1, TRPV6, as well as the chaperone protein CALB1. Upregulation of these renal calcium transporters likely served to increase calcium reabsorption necessary for eggshell calcification.
Renal CALCR expression was also increased from 8 to 15 HPOP, though the change only approached statistical significance. While this increase seems counterintuitive to calcium reabsorption in the kidney, it is possible that CALC signaling is also involved in mediating the renal phosphaturic response, as levels of PiT-1, PiT-2, FGFR2, and FGFR4 show similar increases. However, it should be noted the role of CALC signaling in avian renal function is incompletely understood. In chickens, CALC does not appear to stimulate the production of the intracellular second messenger, cyclic AMP, which is in contrast to the response noted in rats (Dousa, 1974). Additionally, administration of exogenous CALC to hens was shown to only alter renal function in the absence of PTH (Sommerville and Fox, 1987). Lastly, there is conflicting evidence as to whether CALC influences renal production of 1α,25(OH)2D3. In vitro findings by Rasmussen et al. (1972) suggest that CALC may inhibit 1α,25(OH)2D3 production in cultured chick renal cells, while a similar study conducted by Larkins et al. (1973) showed opposing results. Findings from the aforementioned studies suggest that avian CALC may utilize alternative cellular signaling pathways or function secondarily to PTH to alter renal vitamin D3 metabolism. Since the role of CALC in avian species is not well defined, understanding how it mediates calcium and phosphorus homeostasis in the kidney and other organs is important to gain a holistic view of these physiological systems.
Results from the shell gland showed substantial increases in both PTH1R and CALCR as eggshell calcification slows and shortly after ovulation, thereby suggesting that the shell gland has increased hormonal sensitivity to PTH and CALC during these times. Interestingly, previous studies have also found an increased binding affinity of CALC to the plasma membrane fraction of shell gland mucosal tissue in chickens (Ieda et al., 2001) and guineafowl (Ogawa et al., 2003) during late shell calcification. The observed differences in binding affinity in these studies are likely a result of an increased number of receptors in the shell gland, which is supported by the RT-qPCR expression data from the present study. Furthermore, large differences in expression of CALCR and PTH1R between stages of the oviposition cycle indicate that CALC and PTH may elicit greater dynamic effects in the shell gland than in other tissues examined. Upregulation of these receptors in the shell gland late in the oviposition cycle suggests that PTH and CALC hormonal signaling may contribute to processes involved in cessation of eggshell calcification.
Outside of periods when eggshell calcification is occurring, phosphorus in the blood is used to replenish hydroxyapatite crystals in medullary bone (Dacke et al., 2015). However, once eggshell calcification is initiated, calcium demands increase and medullary bone is resorbed to support eggshell calcification (Sinclair-Black et al., 2023). As a result, phosphorus originating from this hydroxyapatite is released into the blood (Nys et al., 1986). Since elevated phosphorus levels in the blood can be toxic, it must be either eliminated at the kidney or utilized for physiological processes. One of the key hormones regulating phosphorus homeostasis is FGF23. It is released from bone in response to elevated circulating phosphorus and signals through 1 of 4 receptors in combination with their coreceptor, KL, to decrease circulating phosphorus. In mammals, it does so by inhibiting renal phosphorus absorption and decreasing 1α-hydroxylation of vitamin D3 and PTH production (Razzaque, 2009; Donate-Correa et al., 2012); however, the extent of FGF23’s effects in chickens has not been fully elucidated. In chickens, FGF23 is expressed in both structural (Hadley et al., 2016; Lyu et al., 2023) and medullary (Hadley et al., 2016) bone, its expression in medullary bone increases with age (Gloux et al., 2020), and its receptors have been identified in kidney, small intestine, and bone (Ren et al., 2020). Hyperphosphatemia has been shown to increase bone-derived FGF23 expression (Wang et al., 2018), and immunoneutralization of FGF23 in chickens reduced phosphorus excretion (Bobeck et al., 2012; Ren et al., 2017). Functionally, it appears that avian FGF23 responds to high phosphorus levels and increases its excretion; however, effects on 1α-hydroxylation of vitamin D3 and its influence on PTH activity are unclear. It has been shown to either have no effect on levels of 1α,25(OH)2D3 and PTH (Ren et al., 2020) or increase PTH while reducing 1α,25(OH)2D3 (Ren et al., 2017).
Findings herein showed that circulating tP was highest from 12 to 21 HPOP, which corresponded with mid and late eggshell calcification stages in this study. As such, increases in blood phosphorus were likely due to medullary bone breakdown to support eggshell calcification. In a possible response to these elevated phosphorus levels, expression of renal FGFR2 and FGFR4 were upregulated at 12 and 15 HPOP, respectively, and they could be at least partially responsible for the considerable 20- to 30-fold increase in PiT-1 levels and 3-fold increase in PiT-2 at these same time points. Furthermore, while Ren et al. (2020) noted differences in renal expression of NaPiIIa during phosphorus restriction, no notable differences were observed in the current study. Taken together, it appears that FGF23 signaling may influence renal phosphorus excretion by acting through select FGF23 receptors to modulate expression of phosphorus transporters during the oviposition cycle. Based on the fold-changes observed, PiT-1 appears to have the greatest influence on phosphorus dynamics in the kidney during egg formation.
Evidence from this study also revealed a potentially novel role for FGF23 in regulating phosphorus dynamics in the shell gland. Phosphorus concentrations increase in the outer quarter of the eggshell and the cuticle (Cusack et al., 2003), and it is thought that this may be involved in the cessation of eggshell calcification due to its inhibition of calcite formation (Bachra et al., 1963; Simkiss, 1964) or as a result of an increase in phosphate-containing organic components in the cuticle layers (Nys et al., 1991). Since FGF23 appears to alter phosphorus transporter expression in the kidney (Ren et al., 2020), it is possible that similar effects may occur in the shell gland. Findings from this study showed notable decreases in expression of shell gland FGF2, FGF3, FGF4, and KL between 8 and 15 HPOP, a period of rapid eggshell calcification, followed by increases in expression of shell gland FGFR2, FGFR3, and KL between 18 and 24 HPOP, as eggshell calcification slows. Furthermore, levels of PiT-1 increased approximately 10-fold between 8 and 21 HPOP. Together, it appears that sensitivity to FGF23 and levels of PiT-1 transporters remain low during active eggshell calcification, likely to prevent the premature termination of eggshell calcification. Following this, levels of both FGF23 receptors and PiT-1 rise, which may facilitate cessation of the eggshell mineralization process.
Together, it was shown that several genes exhibited dynamic changes in expression during the daily oviposition cycle in both the kidney (Figure 11 A) and shell gland (Figure 11B). Select renal plasma membrane calcium transporters and the intracellular calcium chaperone CALB1 maintained intermediate levels prior to ovum entry into the shell gland and during early eggshell calcification stages; however, during rapid eggshell calcification, expression of these transporters in the kidney peaked. This peak in expression likely resulted in enhanced renal calcium reabsorption to supply the developing eggshell. Renal phosphorus transporters followed a similar pattern to that of calcium transporters; however, initial expression from 1 to 8 HPOP was substantially lower. Unlike elevated calcium transporters, which serve to retain calcium for eggshell formation, increased phosphorus transporter expression during mid eggshell calcification was likely a result of the need to excrete excess circulating phosphorus originating from medullary bone breakdown during this period. Shell gland plasma membrane calcium transporters were either increasingly upregulated for the duration that the eggshell was present in the shell gland (PMCA1) or peaked during early eggshell calcification (NCX1), while CALB1 levels increased through mid-calcification before rapidly declining. These results demonstrate how differences in expression of calcium transporters and CALB1 may regulate the rate of calcium transfer to the developing eggshell. Bicarbonate formation in the shell gland epithelial cells appears to peak prior to its transfer into the shell gland lumen during mid-calcification, based on the expression patterns of CA2 and SLC26A9. Lastly, as calcification of the eggshell slows, phosphorus transporter expression increases and likely influences the termination of eggshell calcification and facilitates cuticle formation.
Figure 11.
Expression profiles of key genes mediating calcium and phosphorus utilization by laying hens in the kidney and shell gland during the 24-h oviposition cycle. (A) Relative expression of genes involved in calcium (NCX1, PMCA1, TRPV6, CALB1) and phosphorus (PiT-1) transport in the kidney. (B) Relative expression of genes involved in calcium transport (NCX1, PMCA1, CALB1), bicarbonate synthesis and transport (CA2, SLC26A9), and phosphorus transport (PiT-1) in the shell gland. Location of ovum within the reproductive tract [magnum (M), isthmus (I), shell gland (SG)] and stage of eggshell calcification [early, mid, late] for each time point are also shown. For each gene, expression is plotted relative to the maximum value (equivalent to 100%).
In this study, hens were fed ad libitum and sampled during both the light (1, 3, 4, 6, 7, 8, 21, 23, and 24 HPOP) and dark (12, 15, and 18 HPOP) periods. Since there was not a period of feed withdrawal prior to euthanasia, timing of sample collection relative to the last meal and level of digesta within the intestinal tract could have influenced our findings. This effect would likely be greatest during the light period, as hen-to-hen variations in feeding patterns could affect the amount of dietary calcium and phosphorus that would be available for bone remodeling and eggshell calcification at the time of sampling. In contrast, there is a nocturnal fast during the dark period (Scanes et al., 1987), and hens sampled during this time should have a more consistent, albeit lower, level of feed within the intestinal tract that would reduce variability during the 12 to 18 HPOP timepoints. However, during these times the egg had entered the shell gland and was at varying levels of mineralization. As the gross level of eggshell calcification was determined by hand, more subtle differences in calcification state could not be determined and could have been an additional source of variation during these times.
In conclusion, this study investigated physiological processes regulating calcium and phosphorus homeostasis that occur during the daily oviposition cycle in commercial laying hens. In doing so, key genes that exhibited dynamic changes during the oviposition cycle were identified, and these likely play a key role in mediating mineral homeostasis by driving uptake or excretion in the kidney and utilization by the shell gland. In addition, a possible role for the kidney in local 25-hydroxylation of vitamin D3 was uncovered. It was also shown that 1α,25(OH)2D3 could facilitate the shell gland's ability to utilize calcium for eggshell formation through VDR/RXRG transcriptional regulation of select transport proteins. Furthermore, the shell gland appears to be a novel target for FGF23 signaling, and this hormone could regulate its ability to incorporate phosphorus into outer layers of the shell. Together, this new fundamental information can be used both to develop additional studies aimed at understanding dynamics of calcium and phosphorus homeostasis in layers and to identify disturbances in these processes that occur as hens age. This knowledge is crucial in the development of effective strategies aimed at improving the calcium and phosphorus balance in laying hens, which would ultimately improve egg quality and skeletal health.
ACKNOWLEDGMENTS
Funding for this project was provided by a competitive Faculty Seed Grant in the Sciences and Engineering from the Office of Research at the University of Georgia to L.E. Ellestad (Award #000338). The authors would like to thank Brett Marshall, Shailes Bhattrai, Manuel Arango, Ky Meeks, Lauren Vaccaro, Grant Bennett, and Cory Yarbrough for assistance with sample collection, and Brett Marshall, Shailes Bhattrai, Grant Bennet, Lauren Vaccaro, and Ky Meeks for constructive feedback during preparation of the manuscript.
DISCLOSURES
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in the present study.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.psj.2023.103209.
Appendix. Supplementary materials
REFERENCES
- Al-Batshan H.A., Scheideler S.E., Black B.L., Garlich J.D., Anderson K.E. Duodenal calcium uptake, femur ash, and eggshell quality decline with age and increase following molt. Poult. Sci. 1994;73:1590–1596. doi: 10.3382/ps.0731590. [DOI] [PubMed] [Google Scholar]
- Alfonso-Carrillo C., Benavides-Reyes C., de Los Mozos J., Dominguez-Gasca N., Sanchez-Rodriguez E., Garcia-Ruiz A.I., Rodriguez-Navarro A.B. Relationship between bone quality, egg production and eggshell quality in laying hens at the end of an extended production cycle (105 weeks) Animals. 2021;11:1–12. doi: 10.3390/ani11030623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ba J., Brown D., Friedman P.A. Calcium-sensing receptor regulation of PTH-inhibitable proximal tubule phosphate transport. Am. J. Physiol.-Renal. 2003;285:1233–1243. doi: 10.1152/ajprenal.00249.2003. [DOI] [PubMed] [Google Scholar]
- Bachra B.N., Trautz O.R., Simon S.Lawrence. Precipitation of calcium carbonates and phosphates. I. Spontaneous precipitation of calcium carbonates and phosphates under physiological conditions. Biochem. Biophys. 1963;103:124–138. doi: 10.1016/0003-9861(63)90018-3. [DOI] [PubMed] [Google Scholar]
- Bai X., Miao D., Goltzman D., Karaplis A.C. The autosomal dominant hypophosphatemic rickets R176Q mutation in fibroblast growth factor 23 resists proteolytic cleavage and enhances in vivo biological potency. J. Biol. Chem. 2003;278:9843–9849. doi: 10.1074/jbc.M210490200. [DOI] [PubMed] [Google Scholar]
- Bain M.M., Nys Y., Dunn I.C. Increasing persistency in lay and stabilizing egg quality in longer laying cycles. What are the challenges? Br. Poult. Sci. 2016;57:330–338. doi: 10.1080/00071668.2016.1161727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar A. Calcium transport in strongly calcifying laying birds: mechanisms and regulation. Comp. Biochem. Physiol. 2009;152:447–469. doi: 10.1016/j.cbpa.2008.11.020. [DOI] [PubMed] [Google Scholar]
- Bar A., Cohen A., Eisner U., Riesenfeld G., Hurwitz S. Differential response of calcium transport systems in laying hens to exogenous and endogenous changes in vitamin D status. J. Nutr. 1977;108:1322–1328. doi: 10.1093/jn/108.8.1322. [DOI] [PubMed] [Google Scholar]
- Bar A., Rosenberg J., Hurwitz S. The lack of relationships between vitamin D3 metabolites and calcium-binding protein in the eggshell gland of laying birds. Comp. Biochem. Physiol. 1984;78B:75–79. doi: 10.1016/0305-0491(84)90148-2. [DOI] [PubMed] [Google Scholar]
- Bar A., Shani M., Fullmer C.S., Brindak M.E., Striem S. Modulation of chick intestinal and renal calbindin gene expression by dietary vitamin D3, 1,25-dihydroxyvitamin D3, calcium, and phosphorus. Mol. Cell. Endocrinol. 1990;72:23–31. doi: 10.1016/0303-7207(90)90236-2. [DOI] [PubMed] [Google Scholar]
- Bell D.D. Formation of the egg, Pages 59–69 in Commercial Chicken Meat and Egg Production. Springer Science; New York, NY: 2002. [Google Scholar]
- Benavides-Reyes C., Folegatti E., Dominguez-Gasca N., Litta G., Sanchez-Rodriguez E., Rodriguez-Navarro A.B., Umar Faruk M. Research note: changes in eggshell quality and microstructure related to hen age during a production cycle. Poult. Sci. 2021;100 doi: 10.1016/j.psj.2021.101287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Dov I.Z., Galitzer H., Lavi-Moshayoff V., Goetz R., Kuro-o M., Mohammadi M., Sirkis R., Naveh-Many T., Silver J. The parathyroid is a target organ for FGF23 in rats. J. Clin. Invest. 2007;117:4003–4008. doi: 10.1172/JCI32409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bikle D.D. In: Pages 1–40 in Extra-Skeletal Effects of Vitamin. Liao D.E.P., editor. Humana Press; New York, NY: 2018. Vitamin D biochemistry and physiology. [Google Scholar]
- Bobeck E.A., Burgess K.S., Jarmes T.R., Piccione M.L., Cook M.E. Maternally-derived antibody to fibroblast growth factor-23 reduced dietary phosphate requirements in growing chicks. Biochem. Biophys. Res. Co. 2012;420:666–670. doi: 10.1016/j.bbrc.2012.03.063. [DOI] [PubMed] [Google Scholar]
- Carpenter T.O., Bergwitz C., Insogna K.L. In: Principles of Bone Biology. Bilezikian J.P., Martin T.J., Clemens T.L., Rosen C.J, editors. Academic Press; Cambridge, MA: 2020. Phosphorus homeostasis and related disorders; pp. 469–507. [Google Scholar]
- Cheng J.B., Motola D.L., Mangelsdorf D.J., Russell D.W. De-orphanization of cytochrome P450 2R1. J. Biol. Chem. 2003;278:38084–38093. doi: 10.1074/jbc.M307028200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clunies M., Leeson S. Effect of dietary calcium level on plasma proteins and calcium flux occurring during a 24 h ovulatory cycle. Can. J. Anim. Sci. 1995;75:439–444. [Google Scholar]
- Comar C.L., Driggers J.C. Secretion of radioactive calcium in the hen's egg. Science. 1949;109:282. doi: 10.1126/science.109.2829.282. [DOI] [PubMed] [Google Scholar]
- Cusack M., Fraser A.C., Stachel T. Magnesium and phosphorus distribution in the avian eggshell. Comp. Biochem. Physiol. 2003;134:63–69. doi: 10.1016/s1096-4959(02)00185-9. [DOI] [PubMed] [Google Scholar]
- Dacke C.G., Arkle S., Cook D.J., Wormstone I.M., Jones S., Zaidi M., Bascal Z.A. Medullary bone and avian calcium regulation. J. Exp. Biol. 1993;184:63–88. [Google Scholar]
- Dacke C.G., Sugiyama T., Gay C.V. In: Sturkie’s Avian Physiology. Scanes C.G., editor. Academic Press; Cambridge, MA: 2015. The role of hormones in the regulation of bone turnover and eggshell calcification; pp. 549–575. [Google Scholar]
- Diaz R., Hurwitz S., Chattopadhyay N., Pines M., Yang Y., Kifor O., Einat M., Butters R., Herbert S.C., Brown E.M. Cloning, expression and tissue localization of the calcium-sensing receptor in chicken (Gallus domesticus) Am. J. Physiol. 1997;273:R1008–R1016. doi: 10.1152/ajpregu.1997.273.3.R1008. [DOI] [PubMed] [Google Scholar]
- Donate-Correa J., Muros-de-Fuentes M., Mora-Fernandez C., Navarro-Gonzalez J.F. FGF23/Klotho axis: phosphorus, mineral metabolism, and beyond. Cytokine Growth Factor Rev. 2012;23:37–46. doi: 10.1016/j.cytogfr.2012.01.004. [DOI] [PubMed] [Google Scholar]
- Dousa T.P. Effects of hormones on cyclic AMP formation in kidneys of nonmammalian vertebrates. Am. J. Physiol. 1974;226:1193–1197. doi: 10.1152/ajplegacy.1974.226.5.1193. [DOI] [PubMed] [Google Scholar]
- Etches J.R. Calcium logistics in the laying hen. J. Am. Coll. Nutr. 1986;117:619–628. doi: 10.1093/jn/117.3.619. [DOI] [PubMed] [Google Scholar]
- Fraser D.R., Kodicek E. Unique biosynthesis by kidney of a biologically active vitamin D metabolite. Nature. 1970;228:764–766. doi: 10.1038/228764a0. [DOI] [PubMed] [Google Scholar]
- Gloux A., Le Roy N., Brionne A., Bonin E., Juanchich A., Benzoni G., Piketty M.L., Prie D., Nys Y., Gautron J., Narcy A., Duclos M.J. Candidate genes of the transcellular and paracellular calcium absorption pathways in the small intestine of laying hens. Poult. Sci. 2019;98:6005–6018. doi: 10.3382/ps/pez407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloux A., Le Roy N., Meme N., Piketty M.L., Prie D., Benzoni G., Gautron J., Nys Y., Narcy A., Duclos M.J. Increased expression of fibroblast growth factor 23 is the signature of a deteriorated Ca/P balance in ageing laying hens. Sci. Rep. 2020;10:1–13. doi: 10.1038/s41598-020-78106-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grunder A.A., Tsang C.P., Narbaitz R. Effects of vitamin D3 metabolites on physiological traits of white leghorn hens. Poult. Sci. 1990;69:1204–1208. doi: 10.3382/ps.0691204. [DOI] [PubMed] [Google Scholar]
- Gutowska M.S., Mitchell C.A. Carbonic anhydrase in the calcification of the egg shell. Poult. Sci. 1945;24:159–167. [Google Scholar]
- Hadley J.A., Horvat-Gordon M., Kim W.K., Praul C.A., Burns D., Leach Jr R.M. Bone sialoprotein keratan sulfate proteoglycan (BSP-KSPG) and FGF-23 are important physiological components of medullary bone. Comp. Biochem. Physiol. 2016;194:1–7. doi: 10.1016/j.cbpa.2015.12.009. [DOI] [PubMed] [Google Scholar]
- Han J., Wu L., LV X., Liu M., Zhang Y., He L., Hao J., Xi L., Qu H., Shi C., Li Z., Wang Z., Tang F., Qiao Y. Intestinal segment and vitamin D3 concentration affect gene expression levels of calcium and phosphorus transporters in broiler chickens. J. Anim. Sci. Technol. 2023;65:336–350. doi: 10.5187/jast.2022.e78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heaney R.P., Armas L.A., Shary J.R., Bell N.H., Binkley N., Hollis B.W. 25-Hydroxylation of vitamin D3: relation to circulating vitamin D3 under various input conditions. Am. J. Clin. Nutr. 2008;87:1738–1742. doi: 10.1093/ajcn/87.6.1738. [DOI] [PubMed] [Google Scholar]
- Hertelendy F., Taylor T.G. Changes in blood calcium associated with eggshell calcification in the domestic fowl. Poult. Sci. 1960;40:108–114. [Google Scholar]
- Hincke M.T., Nys Y., Gautron J., Mann K., Rodriguez-Navarro A.B., McKee M.D. The eggshell: structure, composition and mineralization. Front. Biosci. 2012;17:1266–1280. doi: 10.2741/3985. [DOI] [PubMed] [Google Scholar]
- Hodges R.D., LöRcher K. Possible source of the carbonate fraction of eggshell calcium carbonate. Nature. 1967;216:609–610. [Google Scholar]
- Ieda T., Takahasi T., Saito N., Yasuoka T., Kawashima M., Izumi T., Shimada K. Changes in calcitonin receptor binding in the shell gland of laying hens (Gallus domesticus) during the oviposition cycle. J. Poult. Sci. 2001;38:203–212. doi: 10.1006/gcen.1999.7395. [DOI] [PubMed] [Google Scholar]
- Jande S.S., Tolnai S., Lawson D.E.M. Immunohistochemical localization of vitamin D-dependent calcium binding protein in duodenum, kidney, uterus, and cerebellum of chickens. Histochemistry. 1981;71:99–116. doi: 10.1007/BF00592574. [DOI] [PubMed] [Google Scholar]
- Kantham L., Quinn S.J., Egbuna O.I., Baxi K., Butters R., Pang J.L., Pollak M.R., Goltzman D., Brown E.M. The calcium-sensing receptor (CaSR) defends against hypercalcemia independently of its regulation of parathyroid hormone secretion. Am. J. Physiol-Endocr. Metab. 2009;297:915–923. doi: 10.1152/ajpendo.00315.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kebreab E., France J., Kwakkel R.P., Leeson S., Darmani Kuhi H.D., Dijkstra J. Development and evaluation of a dynamic model of calcium and phosphorus flows in layers. Poult. Sci. 2009;88:680–689. doi: 10.3382/ps.2008-00157. [DOI] [PubMed] [Google Scholar]
- Kliewer S.A., Kazuhiko U., Mangelsdorf D.J., Evans R.M. Retinoid X receptor interacts with nuclear receptors in retinoic acid, thyroid hormone and vitamin D3 signaling. Nature. 1992;30:446–449. doi: 10.1038/355446a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins R.G., MacAuley S.J., Rapoport A., Martin T.J., Tulloch B.R., Byfield P.G., Matthews E.W., MacIntyre I. Effects of nucelotides, hormones, ions, and 1,25-dihydroxycholecalciferol on 1,25-dihydroxychoelcalciferol production in isolated chick renal tubules. Clin. Sci. Mol. Med. 1973;46:569–582. doi: 10.1042/cs0460569. [DOI] [PubMed] [Google Scholar]
- Loupy A., Ramakrishnan S.K., Wootla B., Chambrey R., de la Faille R., Bourgeois S., Bruneval P., Mandet C., Christensen E.I., Faure H., Cheval L., Laghmani K., Collet C., Eladari D., Dodd R.H., Ruat M., Houillier P. PTH-independent regulation of blood calcium concentration by the calcium-sensing receptor. J. Clin. Invest. 2012;122:3355–3367. doi: 10.1172/JCI57407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyu Z., Li H., Li X., Wang H., Jiao H.C., Wang X.J., Zhao J.P., Lin H. Fibroblast growth factor 23 inhibits osteogenic differentation and mineralization of chicken bone marrow mesenchymal stem cells, Poult. Sci. 2023;102:1–12. doi: 10.1016/j.psj.2022.102287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer M.B., Pike J.W. Mechanistic homeostasis of vitamin D metabolism in the kidney through reciprocal modulation of CYP27B1 and CYP24A1 expression. J. Steroid Biochem. 2020;196:1–6. doi: 10.1016/j.jsbmb.2019.105500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S.C. Rapid activation of the medullary bone osteoclast cell surface by parathyroid hormone. J. Cell Biol. 1978;76:615–618. doi: 10.1083/jcb.76.3.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narbaitz R., Kacew S., Sitwell L. Carbonic anhydrase activity in the chick embryo chorioallantois: regional distribution and vitamin D regulation. J. Embryol. Exp. Morph. 1981;65:127–137. [PubMed] [Google Scholar]
- Nijenhuis T., Hoenderop J.G., Van der Kemp A.W., Bindels R.J. Localization and regulation of the epithelial Ca2+ channel TRPV6 in the kidney. J. Am. Soc. Nephrol. 2003;14:2731–2740. doi: 10.1097/01.asn.0000094081.78893.e8. [DOI] [PubMed] [Google Scholar]
- Norman A.W. Intestinal calcium absorption: a vitamin D-hormone-mediated adaptive response. Am. J. Clin. Nutr. 1990;51:290–300. doi: 10.1093/ajcn/51.2.290. [DOI] [PubMed] [Google Scholar]
- Nys Y., Baker K., Bouillon H., Van Baelen H., Lawson D.E.M. Regulation of calbindin D28k and its mRNA in the intestine of the domestic hen. Gen. Comp. Endocr. 1992;86:460–468. doi: 10.1016/0016-6480(92)90071-q. [DOI] [PubMed] [Google Scholar]
- Nys Y., Guyot N. In: Improving the Safety and Quality of Eggs and Egg Products. Nys Y., Bain M.M., Van Immerseel F., editors. Woodhead Publishing; Sawston, Cambridge: 2011. Egg formation and chemistry; pp. 83–132. [Google Scholar]
- Nys Y., Hincke M.T., Arias J.L., Garcia-Ruiz J.M., Solomon S.E. Avian eggshell mineralization. Poult. Avian Biol. Rev. 1999;10:143–166. [Google Scholar]
- Nys Y., Le Roy N. In: InVitamin D. Feldman D., editor. Academic press; Cambridge, MA: 2018. Calcium homeostasis and eggshell biomineralization in female chicken; pp. 361–382. [Google Scholar]
- Nys Y., N'Guyen T.M., Williams J., Etches J.R. Blood levels of ionized calcium, inorganic phosphorus, 1,25-dihydroxycholecalciferol and gonadal hormones in hens laying hard-shelled or shell-less eggs. J. Endocrinol. 1986;111:151–157. doi: 10.1677/joe.0.1110151. [DOI] [PubMed] [Google Scholar]
- Nys Y., Zawadzki J., Gautron J., Mills A.D. Whitening of brown-shelled eggs: mineral composition of uterine fluid and rate of protoporphyrin deposition. Poult. Sci. 1991;70:1236–1245. doi: 10.3382/ps.0701236. [DOI] [PubMed] [Google Scholar]
- Ogawa H., Takahashi T., Kuwayama T., Kawashima M. Presence of calcitonin receptors in shell gland of the guineafowl and changes in binding property during an oviposition cycle. Poult. Sci. 2003;82:1302–1306. doi: 10.1093/ps/82.8.1302. [DOI] [PubMed] [Google Scholar]
- Pinheiro P.L.C., Cardoso J.C.R., Power D.M., Canario A.V.M. Functional characterization and evolution of PTH/PTHrP receptors: insights from the chicken. BMC Evol. Biol. 2012;12:1–15. doi: 10.1186/1471-2148-12-110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponchon G., DeLuca H.F. The role of the liver in the metabolism of vitamin D. J. Clin. Invest. 1969;48:1273–1279. doi: 10.1172/JCI106093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponchon G., Kennan A.L., DeLuca H.F. Activation of vitamin D by the liver. J. Clin. Invest. 1969;48:2032–2037. doi: 10.1172/JCI106168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quelo I., Kahlen J.P., Rascle A., Jurdic P., Carlberg C. Identification and characterization of a vitamin D3 response element of chicken carbonic anhydrase-II. DNA Cell Biol. 1994;13:1181–1187. doi: 10.1089/dna.1994.13.1181. [DOI] [PubMed] [Google Scholar]
- Quelo I., Machuca I., Jurdic P. Identification of a vitamin D response element in the proximal promoter of the chicken carbonic anhydrase II gene. J. Biol. Chem. 1998;273:10638–10646. doi: 10.1074/jbc.273.17.10638. [DOI] [PubMed] [Google Scholar]
- Rasmussen H., Wong M., Bikle D.D., Goodman D.B.P. Hormonal control of the renal conversion of 25-hydroxycholecalciferol to 1,25-dihydroxychoelcalciferol. J. Clin. Invest. 1972;51:2502–2504. doi: 10.1172/JCI107065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Razzaque M.S. The FGF23-Klotho axis: endocrine regulation of phosphate homeostasis. Nat. Rev. Endocrinol. 2009;5:611–619. doi: 10.1038/nrendo.2009.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren Z., Ebrahimi M., Butz D.E., Sand J.M., Zhang K., Cook M.E. Antibody to fibroblast growth factor 23-peptide reduces excreta phosphorus of laying hens. Poult. Sci. 2017;96:127–134. doi: 10.3382/ps/pew189. [DOI] [PubMed] [Google Scholar]
- Ren Z., Yan J., Hu Q., Liu X., Pan C., Liu Y., Zhang X., Yang X., Yang X. Phosphorus restriction changes the expression of fibroblast growth factor 23 and its receptors in laying hens. Front. Physiol. 2020;11:1–12. doi: 10.3389/fphys.2020.00085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson D.S., King N.R. Carbonic anhydrase and formation of the hen's egg shell. Nature. 1963;199:497–498. doi: 10.1038/199497a0. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Navarro A.B., Marie P., Nys Y., Hincke M.T., Gautron J. Amorphous calcium carbonate controls avian eggshell mineralization: a new paradigm for understanding rapid eggshell calcification. J. Struct. Biol. 2015;190:291–303. doi: 10.1016/j.jsb.2015.04.014. [DOI] [PubMed] [Google Scholar]
- Sato T., Courbebaisse M., Ide N., Fan Y., Hanai J.I., Kaludjerovic J., Densmore M.J., Yuan Q., Toka H.R., Pollak M.R., Hou J., Lanske B. Parathyroid hormone controls paracellular Ca2+ transport in the thick ascending limb by regulating the tight-junction protein Claudin 14. Proc. Natl. Acad. Sci. USA. 2017;114:3344–3353. doi: 10.1073/pnas.1616733114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scanes C.G., Campbell R., Griminger P. Control of energy balance during egg production in the laying hen. J. Nutr. 1987;117:605–611. doi: 10.1093/jn/117.3.605. [DOI] [PubMed] [Google Scholar]
- Shimada T., Kakitani M., Yamazaki Y., Hasegawa H., Takeuchi Y., Fujita T., Fukumoto S., Tomizuka K., Yamashita T. Targeted abalation of FGF23 demonstrates an essential physiological role of FGF23 in phosphate and vitamin D3 metabolism. J. Clin. Invest. 2004;113:561–568. doi: 10.1172/JCI19081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simkiss K. Phosphates as crystal poisons of calcification. Biol. Rev. 1964;39:487–504. doi: 10.1111/j.1469-185x.1964.tb01166.x. [DOI] [PubMed] [Google Scholar]
- Sinclair-Black M., Garcia R.A., Ellestad L.E. Physiological regulation of calcium and phosphorus utilization in laying hens. Front. Physiol. 2023;14:1–8. doi: 10.3389/fphys.2023.1112499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh R., Joyner C.J., Peddie M.J., Taylor T.Geoffrey. Changes in the concentrations of parathyroid hormone and ionic calcium in the plasma of laying hens during the egg cycle in relation to dietary deficiencies of calcium and vitamin D. Gen. Comp. Endocr. 1986;61:20–28. doi: 10.1016/0016-6480(86)90245-5. [DOI] [PubMed] [Google Scholar]
- Sommerville B.A., Fox J. Changes in renal function of the chicken associated with calcitonin and parathyroid hormone. Gen. Comp. Endocr. 1987;66:381–386. doi: 10.1016/0016-6480(87)90248-6. [DOI] [PubMed] [Google Scholar]
- Sugiyama T., Kusuhara S. Ultrastructural changes of osteoclasts on hen medullary bone during the egg-laying cycle. Br. Poult. Sci. 1993;34:471–477. doi: 10.1080/00071669308417602. [DOI] [PubMed] [Google Scholar]
- Taylor T.G., Belanger L.F. The mechanism of bone resorption in laying hens. Calcified Tissue Res. 1969;4:162–173. doi: 10.1007/BF02279117. [DOI] [PubMed] [Google Scholar]
- Taylor T.G., Wasserman R.H. Vitamin D-induced calcium-binding protein: comparative aspects in kidney and intestine. Am. J. Physiol. 1972;223:110–114. doi: 10.1152/ajplegacy.1972.223.1.110. [DOI] [PubMed] [Google Scholar]
- Tucker G., Gagnon R.E., Haussler M.R. Vitamin D3-25 hydroxylase: tissue occurrence and apparent lack of regulation. Arch. Biochem. Biophys. 1972;155:47–57. doi: 10.1016/s0003-9861(73)80008-6. [DOI] [PubMed] [Google Scholar]
- Untergasser A., Cutcutache I., Koressaar T., Ye J., Faircloth B.C., Remm M., Rozen S.G. Primer3—new capabilities and interfaces. Nucleic Acids Res. 2012;40:1–12. doi: 10.1093/nar/gks596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang R.M., Zhao J.P., Wang X.J., Jiao H.C., Wu J.M., Lin H. Fibroblast growth factor 23 mRNA expression profile in chickens and its response to dietary phosphorus. Poult. Sci. 2018;97:2258–2266. doi: 10.3382/ps/pey092. [DOI] [PubMed] [Google Scholar]
- Watanabe K.P., Kawai Y.K., Ikenaka Y., Kawata M., Ikushiro S., Sakaki T., Ishizuka M. Avian cytochrome P450 (CYP) 1-3 family genes: isoforms, evolutionary relationships, and mRNA expression in chicken liver. PLoS One. 2013;8:1–11. doi: 10.1371/journal.pone.0075689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wideman R.F. Renal regulation of avian calcium and phosphorus metabolism. J. Nutr. 1986;117:808–815. doi: 10.1093/jn/117.4.808. [DOI] [PubMed] [Google Scholar]
- Wilson S., Duff S.R. Morphology of medullary bone during the egg formation cycle. Res. Vet. Sci. 1990;48:216–220. [PubMed] [Google Scholar]
- Yoshimura Y., Ohira H., Tamura T. Immunocytochemical localization of vitamin D receptors in the shell gland of immature, laying and molting hens. Gen. Comp. Endocr. 1997;108:282–289. doi: 10.1006/gcen.1997.6972. [DOI] [PubMed] [Google Scholar]
- Ziegler R., Telib M., Pfeiffer E.F. The secretion of calcitonin by the perfused ultimobranchial gland of the hen. Horm. Metab. Res. 1969;1:39–40. doi: 10.1055/s-0028-1096824. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.