Abstract
Heat stress can cause testicular damage and affect male fertility. Tanshinone IIA (TSA) is a monomer substance derived from plants, with antioxidant and anti-apoptotic effects. Whether it can repair testicular damage caused by heat stress is unclear. This study aims to construct a mouse testicular heat stress injury model and intervene with TSA. Various methods such as histopathology, high-throughput sequencing, bioinformatics analysis, and molecular biology were used to investigate whether TSA can alleviate heat stress-induced testicular injury and its mechanism. Results showed that heat stress significantly reduced the diameter of the mouse seminiferous tubules, increased cell apoptosis in the testicular tissue, and significantly decreased testosterone levels. After TSA intervention, testicular morphology and cell apoptosis improved significantly, and testosterone secretion function was restored. High-throughput transcriptome sequencing found that key differentially expressed genes between the HS group and the control and TSA groups clustered in the apoptosis and TGFβ signaling pathways. Using western blot technology, we found that the HS group upregulated TGFβ1/Smad2/Smad3 pathway protein expression, causing cell apoptosis, testicular tissue organic lesions, and affecting testicular secretion function. Through TSA intervention, we found that it can inhibit TGFβ1/Smad2/Smad3 pathway protein expression, thereby restoring testicular damage caused by heat stress. This study confirms that TSA can effectively restore testicular damage caused by heat stress in mice, possibly by inhibiting the TGFβ1/Smad2/Smad3 pathway to suppress apoptosis.
Keywords: Heat stress, Testicular damage, Tanshinone IIA, Apoptosis
Introduction
Heat stress, which results from exposure to elevated temperatures, has been demonstrated to have adverse impacts on male reproductive health (Aldahhan and Stanton 2021). Such effects include decreased fertility, impaired testicular function, alterations in reproductive hormone levels, harm to the reproductive system, and heightened risk of reproductive system diseases. To protect reproductive health, it is recommended that men limit their exposure to high temperatures and undergo routine reproductive health assessments (Gonçalves et al. 2021).
In some studies, the regulation of the Smad signaling pathway has been implicated in the effects of heat stress. Smad signaling pathways are pivotal in modulating cellular responses to extracellular signals, including those pertaining to cell growth, differentiation, and survival. Heat stress can induce the activation of the Smad signaling pathway, leading to phosphorylation and activation of specific Smad proteins (Wang et al. 2022). This activation can then result in changes in gene expression and cellular behavior and function. Additionally, some research has suggested that the Smad signaling pathway may be involved in the cellular response to heat stress, potentially by influencing the heat shock response and affording cells protection against stress-induced damage (Zhou et al. 2017).
Tanshinone IIA (TSA) is a natural diterpene quinone (Fang et al. 2020), a lipophilic component derived from the root extract of Salvia miltiorrhiza (Ansari et al. 2021).Tanshinone IIA (TSA) has been investigated for its potential as an antioxidant and a protective agent against heat shock (Chen et al. 2018, Cheng et al. 2017). Its antioxidant properties have been shown to result from its ability to scavenge reactive oxygen species (ROS), inhibit lipid peroxidation, and regulate oxidative stress-related signaling pathways (Fu et al. 2022). As a consequence, TSA has the potential to offer protection against cellular damage induced by oxidative stress (Song et al. 2022). Concerning its protective effects against heat shock, research has demonstrated that TSA can activate the heat shock response, enhance the expression of heat shock proteins (HSPs), and safeguard cells from damage caused by heat stress (Cheng et al. 2017). TSA may also exhibit protective effects against oxidative stress and inflammation, both of which are significant contributors to cellular damage caused by heat stress (Lu et al. 2022). However, prior to this study, there have been no reports on the efficacy of TSA in mitigating heat stress-induced testicular damage in mouses.
The objective of this study is to utilize rats as an experimental animal model and employ a combination of pathological examination, transcriptomic sequencing and analysis, and molecular biological techniques to examine the potential of TSA in ameliorating heat stress-induced testicular injury in rats, as well as its underlying mechanism. The results of this study will contribute to the expansion of our knowledge on the scope of TSA as an antioxidant pharmaceutical and provide experimental support for repairing heat-induced testicular damage.
Materials and methods
Ethics statement
All procedures for experiments performed on mice were reviewed and approved by Guangxi University of Chinese Medicine Institutional Welfare and Ethical Committee. (Approval No. DW2022).
Animals and experimental design
All male C57 mice of 6 weeks age used in this study were obtained from the Experimental Animal Center of Guangxi University of Chinese Medicine. The mice were housed in a specific pathogen-free environment with controlled light-dark cycle (12-h light-dark), humidity (55 ± 5%), and temperature (24 ± 2 °C) and provided with free access to food and water. A total of 60 mice were randomly assigned into five groups, namely the control group, heat stress (HS) group, heat stress with TSA at 10 μg/g/day (HS+TSA 10 μg/g/day) group, heat stress with TSA at 20 μg/g/day (HS+TSA 20 μg/g/day) group, and heat stress with TSA at 40 μg/g/day (HS+TSA 40 μg/g/day) group, with 12 mice in each group. The control group was subjected to a 25-min water bath at 24 °C, while the HS and TSA intervention groups were exposed to a 25-min water bath at 42 °C following anesthesia with pentobarbital. The experimental method of constructing the mouse testicular thermal injury model refers to the previously published literature (Liu et al. 2021, Leng et al. 2019, Shadmehr et al. 2018). After the water bath, the mice were dried and returned to their cages for normal feeding. Starting from the following day of water bath treatment, the HS and TSA intervention groups received intraperitoneal injection of TSA (Sigma, T4952) dissolved in DMSO (Sigma, D5879) at different concentrations according to the experimental dose of each group, while the HS group received an equivalent volume of DMSO solvent injection. The intervention was carried out continuously for 7 days. After 7 days of intervention, the mice were euthanized under CO2 anesthesia, and peripheral blood and testicular tissue were collected. One testis was fixed with 4% paraformaldehyde for pathological examination, and the other testis was frozen at −80 °C for molecular biology examination.
Histopathological analysis and TUNEL staining
After fixation with 4% paraformaldehyde for 48 h, testicular tissues were subjected to gradient alcohol dehydration and paraffin embedding for histopathological evaluation. Subsequently, 5-μm sections were obtained and mounted on slides, followed by drying for subsequent usage. H&E staining (G1121, Solarbio) and TUNEL staining (C1091, Beyotime) were employed to visualize the tissue sections. The diameter of the testicular seminiferous tubules was quantified and statistically analyzed using the Nikon ECLIPSE Ni-E microscope and ImageJ software. When “100 ×” was indicated in the image, it signifies that the magnification of the microscope used for capturing the image is 100 times, and the length displayed by the scale in the image corresponds to 100 μm. Conversely, when “200 ×” was indicated in the image, it signifies that the magnification of the microscope used for capturing the image is 200 times, and the length displayed by the scale in the image corresponds to 50 μm. Specifically, six non-consecutive sections were randomly selected from each mouse, and the diameter of the seminiferous tubules was measured in each section. The data were subjected to statistical analysis and presented in the form of a bar graph for further analysis.
Testosterone concentration detection
The testosterone levels in the peripheral blood serum of each group of mice were assessed using an ELISA assay kit (ab285350, Abcam). Following peripheral blood collection, the specimens were immediately centrifuged at 1200 rpm for 15 min at 4 °C, and the resultant upper serum layer was subjected to analysis as per the protocol provided with the ELISA kit. Optical density (OD) values were recorded at 450 nm utilizing an MD FilterMX microplate reader. The testosterone levels of each mouse were calculated through regression curve analysis and were subsequently subjected to statistical evaluation via comparison with the standard curve.
Quantitative real-time PCR
Six randomly selected mice from each group were subjected to testicular tissue analysis. Total RNA was extracted from the testicular tissue using Trizol® reagent (Invitrogen, 15596018), and the OD 260/280 ratio was evaluated to ensure its quality, with a value ranging between 1.8 and 2.0 considered acceptable. Each sample was then subjected to reverse transcription using a reverse transcription kit (Takara, RR047) to generate cDNA, using 100 ng of total RNA as a starting material. The qPCR reaction mixture was prepared with a total volume of 20 μL, containing the following components: converted 100 ng cDNA, 10 μm of each upstream and downstream primers, 10 μL of 2×SYBR Green Master Mix (SYBR® Premix Ex Taq™ II; Tli RNaseH Plus, Takara, Japan), and supplemented with nuclease-free water to make up the final volume. All reactions for all genes of interest were performed in triplicate and were run on an ABI 7500 real-time fluorescent quantitative PCR machine under the following conditions: 95 °C for 30 s, followed by 40 cycles at 95 °C for 5 s and 58 °C for 30 s. Relative quantification of mRNA expression was calculated using the 2−△△CT method. The housekeeping gene β-actin was used as an internal control to normalize the relative gene. The details of the selected genes and the primer pairs used in the study are provided in Table 1.
Table 1.
qPCR primers
| Name | Primer sequence (5′-3′) | Tm (°C) | Product length (bp) | Database |
|---|---|---|---|---|
| β-actin | TGTACCCAGGCATTGCTGAC | 60.32 | 238 | NM_007393.5 |
| AACGCAGCTCAGTAACAGTCC | 60.61 | |||
| HSP70 | CGAGGAGGTGGATTAGAGGC | 59.32 | 255 | NM_010478.2 |
| ACAGTGCTGCTCCCAACATT | 60.18 |
RNA extraction, library preparation, and sequencing
Random three testicular tissues of the control, HS, and HS+TSA 20 μg/g/day group were subjected to RNA extraction using TRIzol Reagent (Invitrogen, 15596018). Subsequent to RNA extraction, DNaseI was utilized to perform DNA digestion. The quality of RNA samples was assessed by NanodropTM OneC spectrophotometer (Thermo Fisher Scientific Inc.) through examination of A260/A280 ratios. In addition, RNA integrity was confirmed through electrophoresis on a 1.5% agarose gel. QubitTM RNA Broad Range Assay kit (Life Technologies, Q10210) was employed for quantification of qualified RNAs, using the Qubit3.0 instrument. For stranded RNA sequencing library preparation, KC-DigitalTM Stranded mRNA Library Prep Kit for Illumina® (Catalog No. DR08502, Wuhan Seqhealth Co., Ltd. China) was utilized according to the manufacturer’s instructions, with 2 μg of total RNAs being employed. The aforementioned kit circumvents duplication bias during PCR and sequencing steps through the application of unique molecular identifier (UMI) of 8 random bases to label pre-amplified cDNA molecules. The library products that corresponded to a length of 200–500 base pairs were enriched, quantified, and ultimately sequenced on the DNBSEQ-T7 sequencer (MGI Tech Co., Ltd. China) using the PE150 model.
RNA-Seq data analysis
The initial step of data processing involved the application of Trimmomatic (version 0.36) to filter the raw sequencing data, with low-quality reads and those contaminated with adaptor sequences being removed. Subsequently, in-house scripts were employed to mitigate the duplication bias that may have been introduced during library preparation and sequencing. Specifically, clean reads were initially clustered based on their unique molecular identifier (UMI) sequences, with those bearing identical UMIs being assigned to the same cluster. Within each cluster, pairwise alignment was performed on the reads, and those exhibiting sequence identity greater than or equal to 95% were subsequently extracted to form a new sub-cluster. Once all sub-clusters had been generated, a multiple sequence alignment was conducted to derive a consensus sequence for each sub-cluster. These steps effectively eliminated any errors or biases stemming from PCR amplification or sequencing. The consensus sequences that were free of duplicates were subjected to standard RNA-seq analysis. These sequences were then mapped onto the mouse reference genome from NCBI (https://www.ncbi.nlm.nih.gov/nuccore/NC_000067) using the STAR software (version 2.5.3a) with the default settings. The reads that mapped to each gene’s exon regions were tallied using featureCounts (Subread-1.5.1; Bioconductor), and the RPKM was calculated. Differentially expressed genes between the groups were identified using the edgeR package (version 3.12.1). A p-value cutoff of 0.05 and a fold-change cutoff of 2 were employed to determine the statistical significance of the gene expression differences. The differentially expressed genes were then subjected to gene ontology (GO) analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis. These analyses were performed using KOBAS software (version: 2.1.1), and a p-value cutoff of 0.05 was applied to determine statistically significant enrichment. The alternative splicing events were detected using rMATS (version 3.2.5), with an FDR value cutoff of 0.05 and an absolute value of Δψ of 0.05.
Western blot
Testicular tissue was lysed in RIPA-PMSF protein extraction reagent (Invitrogen, 89900), homogenized at low temperature, and centrifuged to obtain a total protein lysate. After determining the protein concentration using the BCA (bicinchoninic acid assay) method, the extracted proteins were adjusted to a concentration of 2 μg/μl in each group. During SDS-PAGE gel electrophoresis, 40 μg of protein was loaded into each well. Lysates were diluted with 6 ×protein loading buffer (Invitrogen, NP0007) and heated to 100 °C for 10 min for thermal denaturation of protein. Samples were then loaded on a 10% gradient polyacrylamide gel (P0012AC, 202 Beyotime, China) and then transferred to a PVDF membrane (ISEQ00011, Millipore). Then, the membrane was blocking in 5% (wt/vol) Difco skim milk in Tris-buffered saline containing 0.1% (vol/vol) Tween-20 (TBST) for 2 h. The primary antibody was incubated overnight at 4 °C. The detection indicators were as follows: caspase-3 (Abcam, ab184787), Smad2 (Abcam, ab33875), Smad3 (Abcam, ab40854), TGFβ1 (Abcam, ab170874), Bcl-2 (Abcam, ab182858), Bax (Abcam, ab32503), PCNA (Abcam, ab29), β-actin (Abcam, ab8227). After the primary antibody was incubated, the membrane was washed with PBST, and then the secondary antibody was incubated at 37 °C. The secondary antibody used in the experiment was Goat Anti-Rabbit IgG H&L (HRP, Abcam, ab6721, 1:10000). After 1 h of incubation, the secondary antibody was eluted with PBST, then developed with ECL exposure solution, imaged, and photographed in a multi-function imager. ImageJ 7.0 software was used to read the band gray value, and β-actin was used as the internal reference genes to normalize the target protein expression.
Statistical analysis
The statistical analysis of all data in the present study was performed utilizing SPSS 22.0 software. The obtained experimental data were expressed as standard mean deviation (mean ± SD), and a single-factor ANOVA was employed for inter-group comparisons, while the Scheffe’s post-hoc test was utilized for pairwise comparison. Statistical significance was determined at a p-value ≤ 0.05.
Results
TSA can alleviate testicular damage and testosterone disorder in mice caused by heat stress injury
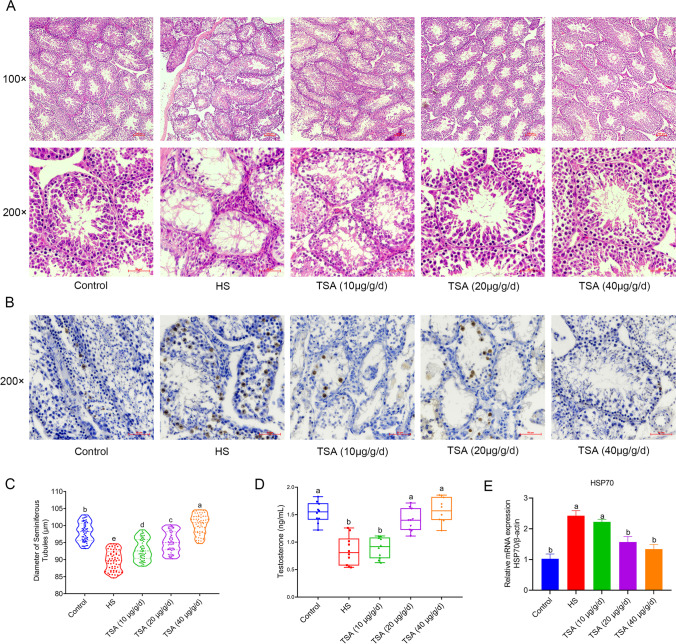
The results of H&E staining in histology showed that there were obvious cavities in the spermatozoa tubules in the heat stress group (HS) compared to the control group, with a significant decrease in the number of cells in the tubules. After treatment with different doses of TSA, the number of cells in the tubules began to increase according to the dose, and when the TSA dose reached 20 μg/g/day or higher, the cell status in the spermatozoa tubules was similar to the control group (Fig. 1A). Subsequently, we conducted TUNEL staining experiments to detect the apoptosis of cells in the testes of each group and found that the number of TUNEL-positive cells in the HS group was significantly higher than that in the control group, and after TSA intervention, the level of cell apoptosis significantly decreased (Fig. 1B). By measuring and counting the diameter of the spermatozoa tubules in the histological sections of the testes of each group, it was found that the diameter of the spermatozoa tubules in the HS group was significantly reduced compared to the control group (p < 0.05). However, following intervention with different doses of TSA, the diameter of the seminiferous tubules showed a significant increase (p < 0.05) (Fig. 1C). The testosterone content in the peripheral blood of mice in each group was detected by ELISA, and it was found that compared to the control group, the testosterone content in the HS group was significantly reduced (p < 0.05). After intervention with TSA at a dose of 20 μg/g/day or higher, the testosterone content was significantly increased compared to the HS group (p < 0.05), and there was no significant difference compared to the control group (p>0.05) (Fig. 1D). The mRNA expression level of HSP70 in the testes of mice in each group was detected by qPCR, and the results showed that compared to the control group, the expression level of HSP70 in the HS group was significantly increased (p < 0.05), and after intervention with TSA at a dose of 20 μg/g/day or higher, its expression level significantly decreased to no significant difference compared to the control group (p > 0.05) (Fig. 1E).
Fig. 1.
A Results of H&E staining and photography of mouse testes in each treatment group. B Results of TUNEL staining and photography of mouse testes in each treatment group. C Graph of the diameter sizes of the seminiferous tubules in mouse testes in each group. D Graph of testosterone levels in peripheral blood of mice in each group. E Relative expression levels of HSP70 in mouse testes of each group detected by qPCR. (100 × magnification, 100 μm; 200 × magnification, 50 μm)
RNA-sequencing results show that TSA can ameliorate heat stress injury-induced testicular injury in mice through a cytoplasmic functional pathway
Following a TSA intervention at a dose of 20 μg/g/day, the majority of testicular damage caused by heat stress was restored, leading to the selection of this dose as the treatment group for transcriptome sequencing and analysis. The reliability of the transcriptome sequencing data was determined by examining the correlation of gene expression levels between samples, with results demonstrating that the intra-group Pearson correlation coefficients were greater than 0.92 and inter-group Pearson correlation coefficients were greater than 0.8, meeting the requirements for data analysis (Fig. 2A). A volcano plot was utilized to present the distribution of differentially expressed genes between groups, with statistical results showing that compared to the control group, there were 5531 upregulated and 4101 downregulated genes in the HS group; compared to the TSA group, there were 5422 upregulated and 3981 downregulated genes in the HS group; compared to the control group, there were 199 upregulated and 357 downregulated genes in the TSA group (Fig. 2B). The clustering of all differentially expressed genes is presented in Fig. 2C. Subsequently, the differentially expressed genes were associated with the differential enrichment signals of KEGG pathway analysis through bioinformatics technology, revealing that the five most highly clustered signaling pathways were Apoptosis, TGF-beta signaling pathway, MAPK signaling pathway, NF-kappa B signaling pathway, and mTOR signaling pathway (Fig. 2D). Then, as shown in Fig. 2E, a joint analysis of the differentially expressed genes and signaling pathways was performed, resulting in a list of key differentially expressed genes. Finally, the expression levels of the most significant genes in Apoptosis (Fig. 2F) and TGF-beta signaling pathway (Fig. 2G) were listed and organized, revealing that compared to the control group, the key genes in Apoptosis and TGF-beta signaling pathway in the HS group were significantly upregulated (p < 0.05), and after TSA intervention, these upregulated genes showed a significant decline (p < 0.05).
Fig. 2.
A Hierarchical clustering of gene expression levels in the sample; the color scale ranges from green to red, representing low sample correlation and high sample correlation obtained through gene expression levels. Branch segments generated from the same node represent corresponding samples that can be clustered into the same group, and the length of the branch represents the similarity of the samples: the shorter the length, the higher the similarity between samples; the longer the length, the lower the similarity between samples. B Volcano plots and number statistics of upregulated and downregulated differentially expressed genes (DEGs) in samples between groups. The x-axis of the volcano plot represents the log2 (fold change), which is the log2-transformed fold change, and the y-axis represents the negative log10 (p-value), which is the negative logarithm of p-value using 10 as the base. Gray dots represent genes that did not show differential expression, blue dots represent downregulated DEGs, and red dots represent upregulated DEGs. C Hierarchical clustering of all DEGs in all groups based on RPKM values. Red indicates high expression genes, and blue indicates low expression genes. The x-axis represents different samples, and the y-axis represents gene names. D KEGG pathway enrichment analysis circle plot. The outermost circle on the right half with the same color represents the same classification, and the pathway classification names are shown in the legend. The second circle represents the significance of differential expression, and the different colored rectangles on the outermost circle on the left half represent the differentially expressed genes, with darker color indicating downregulation and lighter color indicating upregulation. E Based on the results of KEGG pathway enrichment analysis, the differentially expressed genes in the two most significantly enriched pathways were selected for enrichment analysis, and the expression results of 29 DEGs with the most significant differential expression were clustered and visualized in a heatmap. F Based on the results of KEGG pathway enrichment analysis, the differentially expressed genes in the top-ranked apoptosis pathway were compared between independent groups, and the results were plotted in a box plot. G Based on the results of KEGG pathway enrichment analysis, the differentially expressed genes in the second-ranked TGFβ pathway were compared between independent groups, and the results were plotted in a box plot
TSA restores testicular function mainly by inhibiting the upregulation of testicular apoptosis and TGFβ1/Smad2/Smad3 signaling pathway induced by heat stress injury in mice
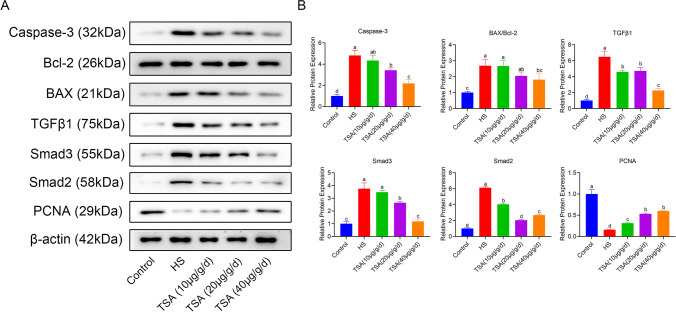
We employed Western blot technology to detect the expression of key proteins involved in cell proliferation, apoptosis, and the TGFβ1/Smad2/Smad3 signaling pathway (Fig. 3A). Results, as shown in Fig. 3B, indicate that compared to the control group, the expression of the apoptosis marker protein caspase-3 was significantly upregulated (p < 0.05) in the HS group, with a significant increase (p < 0.05) in the cell apoptosis key index of BAX/Bcl-2. On the other hand, the expression of the cell proliferation marker protein PCNA was significantly downregulated (p < 0.05). We evaluated the expression levels of TGFβ1, Smad2, and Smad3 proteins in each group and found that, compared to the control group, the expression levels of these three proteins were all significantly upregulated (p < 0.05) in the HS group. The protein expression levels underwent changes after intervention with different doses of TSA. Compared to the HS group, the expression levels of caspase-3, TGFβ1, Smad2, Smad3, and BAX/Bcl-2 were significantly downregulated in the TSA groups receiving 20 μg/g/day or more, with the expression levels of TGFβ1 and Smad2 already significantly downregulated (p < 0.05) at the 10 μg/g/day dose. Compared to the HS group, the expression levels of PCNA protein were significantly upregulated (p < 0.05) in the TSA groups receiving 10 μg/g/day or more.
Fig. 3.
A Western blot was used to detect the protein expression levels of apoptosis (Caspase-3, BAX, Bcl-2), cell proliferation (PCNA), and TGFβ1/Smad2/Smad3 pathway-related proteins. B The relative protein expression levels of each group were calculated based on the integrated optical density values of the protein expression, and a bar graph was plotted. Different letters on the bar graph indicate significant differences between groups (p < 0.05)
Discussion
Heat stress can cause cellular damage through a variety of mechanisms, including oxidative stress, inflammation, protein denaturation, membrane lipid peroxidation, DNA damage, and interference with cellular processes such as cell division, protein synthesis, and cell signaling (Zhang et al. 2023b, Zheng et al. 2022, Cao et al. 2023). These effects can harm cells and affect their function. It was crucial to avoid prolonged exposure to high temperatures to reduce the risk of cell damage and keep cells healthy. The exact signaling pathways involved in the cellular damage caused by heat stress are not yet clear, but some pathways that have been implicated include the heat shock protein (HSP) pathway (Zhang et al. 2023a), MAPK signaling pathways (Hu et al. 2023), Nrf2 pathway (Ding et al. 2023), and inflammatory pathways (Huang et al. 2023). These pathways play a role in regulating the cellular response to stress, protecting cells from oxidative damage, and producing pro-inflammatory cytokines. To further investigate the underlying mechanisms of infertility caused by heat stress, our study employed mice as a model and constructed a testicular heat injury model using local heat treatment method. By integrating various experimental approaches, we aim to elucidate the molecular mechanisms and key signaling pathways involved in heat-induced testicular injury in mice. Based on these pathways, we hope to identify more effective repair strategies or drugs.
Our study has demonstrated that heat stress induces an elevation in apoptosis of the internal cells within the seminiferous tubules of mouse testes (Fig. 1). This phenomenon is directly responsible for the reduction of the luminal diameter of the seminiferous tubules, as well as a decline in testosterone levels. There are many types of cells in the seminiferous tubules of the testis, including Sertoli cells, stromal cells, and germ cells. The increase of apoptosis will lead to the decline of male fertility and even lead to infertility (Shukla et al., 2012). Testosterone plays an important role in the process of spermatogenesis. Heat stress caused by artificial cryptorchidism model or local testicular heat treatment can lead to apoptosis of testicular cells and decrease of testosterone secretion, resulting in male infertility (Liu 2010). In addition, using qPCR to detect the expression of the HSP70 gene, we found that the expression level of the HSP70 gene in the HS group mice was significantly upregulated compared to the control group (p < 0.05), which is consistent with the results of testicular histology and cell apoptosis (Fig. 1). The above results show that it is feasible to construct a mouse testis local heat stress model by the water bath method, and it will significantly increase the level of cell apoptosis in the testis and then lead to the decrease of testosterone secretion.
Apoptosis is a controlled process of cellular suicide characterized by nuclear condensation, cell shrinkage, membrane blebbing, and DNA fragmentation (Fuchs and Steller 2011). Studies have shown that an increase in ambient temperature can cause abnormal expression of BAX and BCL-2 genes in pig testes (Fan et al. 2017). Heat stress can induce the increase of apoptosis, leading to the destruction of spermatocytes and sperm at all levels, and can effectively resist this kind of damage by inhibiting apoptosis (Shahat et al. 2020).Using high-throughput sequencing and bioinformatics analysis techniques, we performed clustering analysis and differential signal pathway enrichment analysis on differentially expressed genes in the testes of different groups of mice. The results showed that compared to the control group, the apoptosis signaling pathway was the most enriched in the HS group of mice, with significant upregulation of expression levels of apoptotic marker genes such as the caspase gene family, BAX, and Fas. In addition, we also found that the TGF-β family signaling pathway showed a similarly high degree of enrichment, with gene expression levels positively correlated (Fig. 2).
The signaling pathway of the transforming growth factor β (TGF-β) superfamily is of paramount importance in governing cell growth, differentiation, and development across a wide range of biological systems. One noteworthy study in this regard is by Wang et al. (2022) who investigated testicular heat stress-induced cryptorchidism in dogs. Their findings demonstrated increased expression levels of TGF-β family genes and their two receptors, TGF-βRI and TGF-βRII, in cryptorchidism, subsequently promoting epithelial-mesenchymal transition. In vitro, symptom improvement was observed by inhibiting the TGF-β/Smad signaling pathway (Wang et al. 2022). This study confirmed the correlation between heat stress and the TGFβ pathway. But at the same time, the environment of cryptorchidism can cause hypoxia in the local microenvironment and lead to the activation of TGF-β/Smad signaling pathway, causing Sertoli cell fibrosis, which is also one of the important factors leading to testicular damage(Xiao et al. 2022). Generally, signaling begins with ligand-induced serine/threonine receptor kinase oligomerization and cytoplasmic signaling molecule phosphorylation, which in the TGF-β/activin pathway are Smad2 and Smad3, and in the bone morphogenetic protein (BMP) pathway are Smad1/5/9(Horbelt et al. 2012). The signaling pathway involving TGFβ1/Smad2/Smad3 is a crucial pathway implicated in cellular processes such as cell growth, differentiation, and apoptosis. Studies have shown that heat stress can increase the expression level of TGF-β in the testis, and local injection of TGF-β antagonists can effectively alleviate the testicular damage caused by heat stress (Rao et al. 2019). Nevertheless, the complete comprehension of the underlying mechanisms regarding the regulation of heat stress and cell apoptosis by the TGFβ1/Smad2/Smad3 pathway is yet to be achieved, warranting additional research. We employed Western blotting techniques to detect the expression levels of key proteins involved in cell proliferation, apoptosis, and the TGFβ1/Smad2/Smad3 signaling pathway in the testes of mice from different groups. Our results demonstrate that, compared to the control group, the expression levels of apoptosis-related proteins and proteins within the TGFβ1/Smad2/Smad3 pathway were significantly upregulated (p < 0.05) in the testes of mice from the HS group. This indicates that TGFβ1/Smad2/Smad3 signaling pathway activation occurs during the testicular damage caused by heat stress, resulting in cell apoptosis, which leads to the formation of vacuoles within the seminiferous tubules and a significant decrease in the high-throughput water level (Fig. 3).
In our previous study, we found that TSA has anti-inflammatory effect (Bai et al. 2022). Some studies have suggested that TSA may have the potential to inhibit cell apoptosis through the TGFβ1/Smad2/Smad3 pathway (Feng et al. 2020). TGFβ1 serves as a growth factor responsible for modulating cell growth and differentiation while also being capable of triggering apoptosis under specific conditions. Intracellular signaling molecules known as Smad2 and Smad3, upon activation by TGFβ1, contribute to the regulation of apoptosis. Therefore, our study aimed to investigate the potential of TSA to ameliorate the testicular damage and apoptosis resulting from heat stress in mice by inhibiting the TGFβ1/Smad2/Smad3 signaling pathway. Our findings revealed that TSA treatment at various concentrations significantly attenuated the reduction in the diameters of seminiferous tubules and cell apoptosis induced by heat stress when compared to the HS group. Moreover, TSA treatment resulted in a marked upregulation of testosterone levels and a significant downregulation of HSP70 gene expression levels (p < 0.05). The differential expression of genes between groups was detected using high-throughput sequencing, and the results of the clustering analysis showed that the administration of 20 μg/g/day of TSA significantly downregulated the expression of key genes involved in cell apoptosis and the TGFβ signaling pathway (Fig. 2). Western blot analysis revealed that the expression levels of proteins related to cell apoptosis and the TGFβ1/Smad2/Smad3 pathway were significantly downregulated after treatment with different concentrations of TSA compared to the HS group (Fig. 3). These findings indicate that TSA holds promise as a potential therapeutic intervention for mitigating testicular damage induced by heat stress. The beneficial effects of TSA are likely mediated through the inhibition of the TGFβ1/Smad2/Smad3 signaling pathway.
Conclusions
The present study investigated the effects of Tanshinone IIA (TSA) on testicular injury induced by thermal stress in a murine model. Molecular biology techniques and high-throughput sequencing were employed to assess the impact of thermal stress and TSA intervention on testicular morphology, testosterone hormone secretion, and testicular transcriptome. The results demonstrated that heat stress caused a significant reduction in the diameter of seminiferous tubules, an increase in cell apoptosis within testicular tissue, as well as a marked decrease in testosterone levels in mice. However, TSA intervention effectively restored testicular morphology and reduced cell apoptosis, while also restoring testosterone secretion function. High-throughput transcriptome sequencing revealed that key differentially expressed genes were clustered in the cell apoptosis and TGFβ signaling pathways between the heat stress group (HS), the control group, and the TSA group. Western blot analysis indicated that the HS group upregulated protein expression within the TGFβ1/Smad2/Smad3 pathway, leading to cell apoptosis and testicular organic lesions, which subsequently impacted testicular secretion function. Conversely, TSA intervention inhibited protein expression within the TGFβ1/Smad2/Smad3 pathway, thereby restoring testicular damage caused by heat stress. In light of these findings, it can be inferred that TSA can effectively ameliorate testicular damage caused by heat stress, and its underlying mechanism may be attributed to its inhibitory effect on apoptosis via the TGFβ1/Smad2/Smad3 pathway. This study sheds new light on the use of TSA as a potential therapeutic strategy for treating heat-induced testicular injury.
Acknowledgements
This work was supported by the Guangxi natural science foundation of China, under grant numbers 2019JJB14021, 2020GXNSFAA159099; doctoral research start-up fund of Guangxi university of Chinese medicine under grant number 2017BS013, Nanning scientific research and technology development program under grant number 20213024, Nanning Qingxiu district science and technology plan project under grant number 2020024, 2020025, Nanning Jiangnan district science and technology plan project under grant number 202001206, 20220620-8, scientific research and technology key R&D plan of Nanning Liangqing district under grant number 202009, 202213, 202216, Guangxi young and middle-aged teachers’ scientific research basic ability improvement project under grant number 2019KY0320.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Aldahhan RA, Stanton PG. Heat stress response of somatic cells in the testis. Mol Cell Endocrinol. 2021;527:111216. doi: 10.1016/j.mce.2021.111216. [DOI] [PubMed] [Google Scholar]
- Ansari MA, Khan FB, Safdari HA, Almatroudi A, Alzohairy MA, Safdari M, Amirizadeh M, Rehman S, Equbal MJ, Hoque M. Prospective therapeutic potential of Tanshinone IIA: an updated overview. Pharmacol Res. 2021;164:105364. doi: 10.1016/j.phrs.2020.105364. [DOI] [PubMed] [Google Scholar]
- Bai L, He G, Gao C, Yang H, Li M, Huang Y, Moussa M, Xu C. Tanshinone IIA enhances the ovarian reserve and attenuates ovarian oxidative stress in aged mice. Vet Med Sci. 2022;8:1617–1625. doi: 10.1002/vms3.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Guo L, Zhou C, Huang C, Li G, Zhuang Y, Yang F, Liu P, Hu G, Gao X, Guo X. Effects of N-acetyl-l-cysteine on chronic heat stress-induced oxidative stress and inflammation in the ovaries of growing pullets. Poult Sci. 2023;102:102274. doi: 10.1016/j.psj.2022.102274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J, Bi Y, Chen L, Zhang Q, Xu L. Tanshinone IIA exerts neuroprotective effects on hippocampus-dependent cognitive impairments in diabetic rats by attenuating ER stress-induced apoptosis. Biomed Pharmacother. 2018;104:530–536. doi: 10.1016/j.biopha.2018.05.040. [DOI] [PubMed] [Google Scholar]
- Cheng Q, Zhao Y, Li J. Sodium tanshinone IIA sulfonate suppresses heat stress-induced endothelial cell apoptosis by promoting NO production through upregulating the PI3K/AKT/eNOS pathway. Mol Med Rep. 2017;16:1612–1618. doi: 10.3892/mmr.2017.6760. [DOI] [PubMed] [Google Scholar]
- Ding KN, Lu MH, Guo YN, Liang SS, Mou RW, He YM, Tang LP. Resveratrol relieves chronic heat stress-induced liver oxidative damage in broilers by activating the Nrf2-Keap1 signaling pathway. Ecotoxicol Environ Saf. 2023;249:114411. doi: 10.1016/j.ecoenv.2022.114411. [DOI] [PubMed] [Google Scholar]
- Fan X, Xi H, Zhang Z, Liang Y, Li Q, He J. Germ cell apoptosis and expression of Bcl-2 and Bax in porcine testis under normal and heat stress conditions. Acta Histochem. 2017;119:198–204. doi: 10.1016/j.acthis.2016.09.003. [DOI] [PubMed] [Google Scholar]
- Fang ZY, Zhang M, Liu JN, Zhao X, Zhang YQ, Fang L. Tanshinone IIA: a review of its anticancer effects. Front Pharmacol. 2020;11:611087. doi: 10.3389/fphar.2020.611087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng F, Li N, Cheng P, Zhang H, Wang H, Wang Y, Wang W. Tanshinone IIA attenuates silica-induced pulmonary fibrosis via inhibition of TGF-β1-Smad signaling pathway. Biomed Pharmacother. 2020;121:109586. doi: 10.1016/j.biopha.2019.109586. [DOI] [PubMed] [Google Scholar]
- Fu K, Sun Y, Wang J, Cao R. Tanshinone IIa alleviates LPS-induced oxidative stress in dairy cow mammary epithelial cells by activating the Nrf2 signalling pathway. Res Vet Sci. 2022;151:149–155. doi: 10.1016/j.rvsc.2022.08.008. [DOI] [PubMed] [Google Scholar]
- Fuchs Y, Steller H. Programmed cell death in animal development and disease. Cell. 2011;147:742–758. doi: 10.1016/j.cell.2011.10.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonçalves AA, Garcia AR, Rolim Filho ST, Silva J, Melo DN, Guimarães TC, Tavares HR, Silva TVG, Souza EB, Santos S, Ohashi OM. Scrotal thermoregulation and sequential sperm abnormalities in buffalo bulls (Bubalus bubalis) under short-term heat stress. J Therm Biol. 2021;96:102842. doi: 10.1016/j.jtherbio.2021.102842. [DOI] [PubMed] [Google Scholar]
- Horbelt D, Denkis A, Knaus P. A portrait of transforming growth factor β superfamily signalling: background matters. Int J Biochem Cell Biol. 2012;44:469–474. doi: 10.1016/j.biocel.2011.12.013. [DOI] [PubMed] [Google Scholar]
- Hu Y, Hu H, Yin L, Wang L, Luo K, Luo N. Arachidonic acid impairs the function of the blood-testis barrier via triggering mitochondrial complex-ROS-P38 MAPK axis in hyperthermal Sertoli cells. Ecotoxicol Environ Saf. 2023;252:114598. doi: 10.1016/j.ecoenv.2023.114598. [DOI] [PubMed] [Google Scholar]
- Huang P, Hu F, Yang ZB, Pan Y, Zhou R, Yan YN, Wang HZ, Wang C (2023) Matrine regulates Th1/Th2 inflammatory responses by inhibiting the Hsp90/NF-κB signaling axis to alleviate atopic dermatitis. Kaohsiung J Med Sci 39(5):501–510. 10.1002/kjm2.12655 [DOI] [PMC free article] [PubMed]
- Leng J, Hou JG, Fu CL, Ren S, Jiang S, Wang YP, Chen C, Wang Z, Li W. Platycodon grandiflorum Saponins attenuate scrotal heat-induced spermatogenic damage via inhibition of oxidative stress and apoptosis in mice. J Funct Foods. 2019;54:479–488. doi: 10.1016/j.jff.2019.01.050. [DOI] [Google Scholar]
- Liu W, Leng J, Hou JG, Jiang S, Wang Z, Liu Z, Gong XJ, Chen C, Wang YP, Li W. Saponins derived from the stems and leaves of Panax ginseng attenuate scrotal heat-induced spermatogenic damage via inhibiting the MAPK mediated oxidative stress and apoptosis in mice. Phytother Res. 2021;35:311–323. doi: 10.1002/ptr.6801. [DOI] [PubMed] [Google Scholar]
- Liu, Y. X. Temperature control of spermatogenesis and prospect of male contraception. Front Biosci (Schol Ed) 2010;2:730–755. doi: 10.2741/s97. [DOI] [PubMed] [Google Scholar]
- Lu TC, Wu YH, Chen WY, Hung YC. Targeting oxidative stress and endothelial dysfunction using tanshinone IIA for the treatment of tissue inflammation and fibrosis. Oxid Med Cell Longev. 2022;2022:2811789. doi: 10.1155/2022/2811789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao M, Ke D, Cheng G, Hu S, Wu Y, Wang Y, Zhou F, Liu H, Zhu C, Xia W. The regulation of CIRBP by transforming growth factor beta during heat shock-induced testicular injury. Andrology. 2019;7:244–250. doi: 10.1111/andr.12566. [DOI] [PubMed] [Google Scholar]
- Shadmehr S, Fatemi Tabatabaei SR, Hosseinifar S, Tabandeh MR, Amiri A. Attenuation of heat stress-induced spermatogenesis complications by betaine in mice. Theriogenology. 2018;106:117–126. doi: 10.1016/j.theriogenology.2017.10.008. [DOI] [PubMed] [Google Scholar]
- Shahat AM, Rizzoto G, Kastelic JP. Amelioration of heat stress-induced damage to testes and sperm quality. Theriogenology. 2020;158:84–96. doi: 10.1016/j.theriogenology.2020.08.034. [DOI] [PubMed] [Google Scholar]
- Shukla KK, Mahdi AA, Rajender S. Apoptosis, spermatogenesis and male infertility. Front Biosci (Elite Ed) 2012;4:746–754. doi: 10.2741/e415. [DOI] [PubMed] [Google Scholar]
- Song YC, Kuo CC, Liu CT, Wu TC, Kuo YT, Yen HR. Combined effects of tanshinone IIA and an autophagy inhibitor on the apoptosis of leukemia cells via p53, apoptosis-related proteins and oxidative stress pathways. Integr Cancer Ther. 2022;21:15347354221117776. doi: 10.1177/15347354221117776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Wang Q, Cui N, Xiao L, Wei H, Kang J, Sheng X, Qi X, Xing K, Guo Y, Ni H, Wang X. Heat stress and hypoxia inhibit the secretion of androgens and induce epithelial-to-mesenchymal transition associated with activated TGF-β/Smad signalling in canine cryptorchidism. Reprod Domest Anim. 2022;57:1046–1055. doi: 10.1111/rda.14174. [DOI] [PubMed] [Google Scholar]
- Xiao L, Wang Z, Lu N, Wei H, Kang J, Yuan M, Sheng X, Qi X, Xing K, Guo Y, Wang X, Zhao J, Gao Y, Ni H. Dihydrotestosterone through blockade of TGF-β/Smad signaling mediates the anti-fibrosis effect under hypoxia in canine Sertoli cells. J Steroid Biochem Mol Biol. 2022;216:106041. doi: 10.1016/j.jsbmb.2021.106041. [DOI] [PubMed] [Google Scholar]
- Zhang L, Xue K, Fan P, Chen C, Hu J, Huang J, Lu W, Xu J, Xu S, Ran J, Zhu S, Gan S (2023a) Geranylgeranylacetone-induced heat shock protein70 expression reduces retinal ischemia-reperfusion injury through PI3K/AKT/mTOR signaling. Exp Eye Res 229:109416. 10.1016/j.exer.2023.109416 [DOI] [PubMed]
- Zhang X, Wang D, Liu J. Hypoxia-inducible factor-1α is involved in the response to heat stress in lactating dairy cows. J Therm Biol. 2023;112:103460. doi: 10.1016/j.jtherbio.2023.103460. [DOI] [PubMed] [Google Scholar]
- Zheng Y, Zhao Y, He W, Wang Y, Cao Z, Yang H, Wang W, Li S. Novel organic selenium source hydroxy-selenomethionine counteracts the blood-milk barrier disruption and inflammatory response of mice under heat stress. Front Immunol. 2022;13:1054128. doi: 10.3389/fimmu.2022.1054128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou N, Ye Y, Wang X, Ma B, Wu J, Li L, Wang L, Wang DW, Zou Y. Heat shock transcription factor 1 protects against pressure overload-induced cardiac fibrosis via Smad3. J Mol Med (Berl) 2017;95:445–460. doi: 10.1007/s00109-016-1504-2. [DOI] [PMC free article] [PubMed] [Google Scholar]