Abstract
Introduction
The first complete long-acting antiretroviral therapy (ART) regimen, cabotegravir + rilpivirine long-acting (CAB + RPV LA) injectable, was approved in the US for HIV-1 treatment in individuals on a stable antiretroviral regimen with a viral load < 50 copies/mL, no treatment failure history, and no resistance to either cabotegravir or rilpivirine. We describe injection schedule adherence and virologic effectiveness of CAB + RPV LA in routine clinical care in the US.
Methods
From the OPERA® cohort, all adults with HIV who received their first CAB + RPV LA injection and ≥ 1 continuation injections between 21 January 2021 and 15 March 2022 were included. The injection target date was updated monthly and set to the same date of the month as the previous injection. Continuation injections administered within 7 days before or after the target date were considered on time, as per the label. Virologic undetectability (viral load < 50 copies/mL), suppression (viral load < 200 copies/mL), and confirmed virologic failure (2 consecutive viral loads ≥ 200 copies/mL or 1 viral load ≥ 200 copies/mL followed by discontinuation) were described among individuals with a viral load < 50 copies/mL at initiation and ≥ 1 follow-up viral load.
Results
Among 321 individuals on CAB + RPV LA, 90% of the continuation injections were administered on time (within ± 7 days of the target date). Of the 237 individuals with a viral load < 50 copies/mL at initiation and ≥ 1 follow-up viral load, nearly all were undetectable (95%) or suppressed (99%) at their last viral load measurement, 96% maintained virologic suppression with all measured viral loads < 200 copies/mL, and four confirmed virologic failures were observed. Injection delays were infrequent, and did not affect virologic outcomes over the short term.
Conclusion
In this large US cohort, most monthly CAB + RPV LA injections were administered on time and high levels of virologic control were achieved. These results suggest that CAB + RPB LA injectable can be administered effectively during routine clinical care.
Keywords: Real-world evidence, Adherence, Cabotegravir, HIV, Long-acting injectable, Rilpivirine, Virologic suppression
Key Summary Points
| The first complete long-acting antiretroviral therapy (ART) regimen, cabotegravir + rilpivirine long-acting injectable, was approved in the US for the treatment of HIV-1 in individuals on a stable antiretroviral regimen with a viral load < 50 copies/mL who had no history of treatment failure and no known or suspected resistance to either cabotegravir or rilpivirine. Real-world evidence is sparse and limited to small study populations or lacking clinical outcomes. |
| A total of 321 people with HIV initiated cabotegravir + rilpivirine long-acting injections and received ≥ 1 continuation injection between 21 January 2021 and 15 March 2022 during routine clinical care in the US. |
| A total of 1152 continuation cabotegravir + rilpivirine long-acting injections were administered over 13 months; 90% were administered within 7 days before or after the target date, as per the label. Only a history of AIDS-defining events was associated with an increased risk of delayed injections. |
| Among 237 individuals with a viral load < 50 copies/mL at initiation and ≥ 1 available follow-up viral load, 95% were undetectable (viral load < 50 copies/mL), 99% were virologically suppressed (viral load < 200 copies/mL) at their last viral load measurement, and 96% had sustained virologic suppression with all measured viral loads < 200 copies/mL. |
| These results suggest that CAB + RPV LA injectable can be administered effectively during routine clinical care. |
Introduction
Cabotegravir + rilpivirine long-acting (CAB + RPV LA) injectable is the first complete long-acting antiretroviral therapy (ART) regimen approved for the treatment of HIV-1 in the US. This long-acting regimen has been approved for individuals with HIV-1 who are virologically suppressed (viral load < 50 copies/mL) on a stable antiretroviral regimen, and who have no history of treatment failure and no known or suspected resistance to either cabotegravir or rilpivirine. The monthly dosing schedule was approved by the FDA in January 2021 [1], and consists of an initiation injection (600 mg CAB + 900 mg RPV), followed by continuation injections (400 mg CAB + 600 mg RPV) administered once a month. As of February 2022, the FDA expanded the label to approve every 2 months CAB + RPV LA injectable, which consists of initiation injections (600 mg CAB + 900 mg RPV) for two consecutive months followed by continuation injections (600 mg CAB + 900 mg RPV) every 2 months. As of March 2022, CAB + RPV LA can be initiated with or without an oral lead-in [2]. The CAB + RPV LA injectable treatment window is up to 7 days before or after the target date of the scheduled injection [3]. Clinical trials have demonstrated that CAB + RPV LA injectable is non-inferior to daily oral three-drug regimen [4–9], and that the every 2 months dosing is non-inferior to the monthly dosing [10–12]. However, the literature on real-world experience with CAB + RPV LA injectable is sparse, and limited to small study populations or lacking clinical outcomes [13–16]. The objective of this study was to describe adherence to the injection schedule and virologic effectiveness of CAB + RPV LA in routine clinical care in the US.
Methods
This study was conducted with data from the Observational Pharmaco-Epidemiology Research & Analysis (OPERA®) cohort, which consists of prospectively captured, routine clinical data from electronic health records from 84 clinics in 18 US states and territories. OPERA complies with all HIPAA and HITECH requirements, which expand upon the ethical principles detailed in the 1964 Declaration of Helsinki. OPERA has received annual institutional review board (IRB) approval by Advarra IRB, including a waiver of informed consent and authorization for use of protected health information (Pro00023648). The study population included all people with HIV aged 18 years or older who received their first CAB + RPV LA injection between 21 January 2021 and 28 February 2022, and at least one continuation injection by 15 March 2022.
For analysis purposes, the target date for CAB + RPV LA continuation injections was updated monthly and set to the same date of the month as the previous injection. Continuation injections administered within 7 days before or after the target date were considered on time, those administered > 7 days prior to the target date were considered early, and those administered > 7 days after the target date were considered delayed. Factors associated with delayed injections were assessed using multivariate logistic regression.
Virologic outcomes were described among individuals with viral load < 50 copies/mL at initiation and at least one follow-up viral load. Virologic undetectability (i.e., viral load < 50 copies/mL) and suppression (i.e., viral load < 200 copies/mL) were assessed at the last viral load. Maintenance of virologic suppression was achieved if all follow-up viral loads remained < 200 copies/mL. Confirmed virologic failure was defined as either two consecutive follow-up viral loads ≥ 200 copies/mL, or one follow-up viral load ≥ 200 copies/mL followed by discontinuation of CAB + RPV LA injectable.
Results
A total of 321 individuals received at least one continuation CAB + RPV LA injection during the study period. The study included 16% women, 37% Black, and 28% Hispanic PWH; 28% had a body mass index (BMI) > 30 kg/m2. CAB + RPV LA initiation occurred a median of 7.2 years after HIV diagnosis (IQR: 3.6, 14.2). At the time of the first injection, most had a CD4 cell count ≥ 500 cells/μL (69%) and a viral load < 50 copies/mL (86%). Demographic and clinical characteristics stratified by viral load at initiation are presented in Table 1.
Table 1.
Demographic and clinical characteristics at first CAB + RPV LA injections, overall and by viral load at initiation
| Overall N = 321a |
VL < 50 copies/mL N = 273 |
VL ≥ 50 copies/mL N = 43 |
|
|---|---|---|---|
| Years since HIV diagnosis, median (IQR) | 7.2 (3.6, 14.2) | 7.1 (3.5, 14.0) | 9.0 (3.8, 16.4) |
| Age, median years (IQR) | 39 (32, 52) | 39 (32, 53) | 37 (30, 44) |
| Female sex, n (%) | 53 (16) | 39 (14) | 14 (33) |
| Race, n (%) | |||
| Black | 119 (37) | 95 (35) | 24 (56) |
| White | 173 (54) | 151 (55) | 17 (40) |
| Other | 20 (6) | 18 (7) | ≤ 5b |
| Unknown | 9 (3) | 9 (3) | ≤ 5b |
| Ethnicity, n (%) | |||
| Hispanic | 91 (28) | 83 (30) | ≤ 5b |
| Not Hispanic | 220 (68) | 181 (66) | 37 (86) |
| Unknown | 10 (3) | 9 (3) | ≤ 5b |
| Men who have sex with men, n (%) | 200 (63) | 176 (64) | 20 (46) |
| Injection drug use, n (%) | 7 (2) | 7 (3) | 0 (0) |
| BMI, kg/m2 | |||
| Median (IQR) | 27 (24, 31) | 27 (24, 31) | 26 (22, 31) |
| > 30, n (%) | 91 (28) | 80 (29) | 11 (26) |
| History of AIDS-defining events, n (%) | 69 (22) | 55 (20) | 13 (30) |
| CD4 cell count, cells/μL | |||
| Median (IQR) | 638 (462, 842) | 648 (471, 855) | 539 (322, 714) |
| < 200, n (%) | 12 (4) | 7 (3) | ≤ 5b |
| ≥ 200 to < 500, n (%) | 85 (27) | 70 (26) | 14 (33) |
| ≥ 500, n (%) | 217 (69) | 194 (71) | 23 (54) |
| Viral load, copies/mL | |||
| Median (IQR) | 19 (19, 20) | 19 (19, 19) | 1110 (80, 35,550) |
| < 50, n (%) | 273 (85) | 273 (100) | NA |
| ≥ 50 to < 200, n (%) | 19 (6) | NA | 19 (44) |
| ≥ 200, n (%) | 24 (7) | NA | 24 (56) |
| Missing, n (%) | 5 (2) | NA | NA |
BMI body mass index, IQR interquartile range, Nn number, NA not applicable
aIncluded 5 individuals without viral load at initiation
bHIPAA privacy requirements preclude the reporting of 5 or fewer observations in any cell
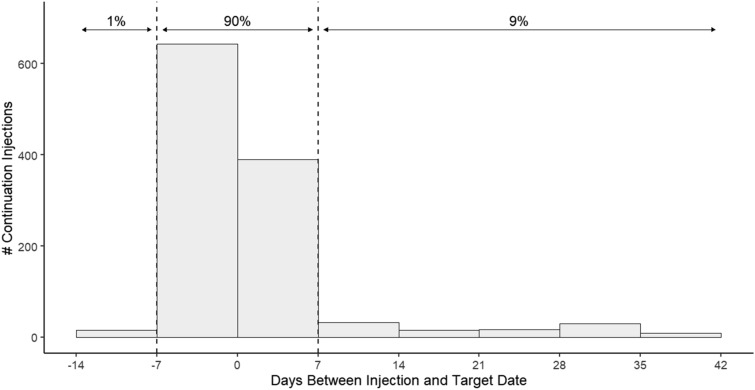
Overall, a total of 1152 continuation injections were administered over 13 months, with a median of 3 continuation injections per person (IQR: 2, 5). While 23% of individuals experienced at least one delay, few had more than one delayed injection (Table 2). Moreover, 90% of all continuation injections were administered on time (i.e., within 7 days before or after the target date) and 1% were administered early (Fig. 1). No pattern emerged when the proportion of on-time or early injections was assessed over calendar time (not shown). Similarly, the proportion of on-time or early injections remained constant across continuation injections (i.e., first, second, third, […], tenth; not shown). In a multivariate analysis, the only factor associated with the occurrence of a delayed injection was a history of AIDS-defining events, which was associated with over three times the likelihood of experiencing a delayed injection (adjusted OR: 3.10; 95% CI: 1.54, 6.22) compared to those without such history (Table 3).
Table 2.
Timing of maintenance CAB + RPV LA injections over follow-up, overall, and by viral load at initiation
| Overall N = 321 |
< 50 copies/mL N = 273 |
≥ 50 copies/mL N = 43 |
|
|---|---|---|---|
| Median months of follow-up (IQR) | 4.2 (2.7, 6.8) | 4.1 (2.5, 6.4) | 4.7 (3.0, 7.5) |
| Median number of continuation injections per person (IQR) | 3 (2, 5) | 3 (2, 5) | 3 (1, 6) |
| CAB + RPV LA discontinuation, n (%) | 42 (13) | 33 (12) | 9 (21) |
| Individuals with ≥ 1 delay, n (%) | 75 (23) | 65 (23) | 9 (21) |
| Median # delays per person (IQR) | 1 (1, 2) | 1 (1, 1) | 2 (1, 3) |
| Median days after target date, if delayed injection (IQR) | 24 (13, 31) | 22 (12, 31) | 24 (19, 31) |
| Individuals with ≥ 2 continuation injections, n (%) | 241 (75) | 205 (75) | 31 (72) |
| Individuals with ≥ 2 delays, n (%) | 19 (8) | 14 (7) | 5 (16) |
| Individuals with ≥ 2 sequential delays, n (%) | 13 (5) | 5 (2) | 4 (13) |
IQR interquartile range, Nn number
Fig. 1.
Description of continuation CAB + RPV LA injections with respect to target date, overall
Table 3.
Demographic and clinical characteristics at first injection associated with any delayed CAB + RPV LA injections
| n | # delays (%) | OR (95% CI)a | aOR (95% CI)a | |
|---|---|---|---|---|
| Age, per 10 years | 321 | 75 (23) | 0.95 (0.76, 1.18) | 0.81 (0.61, 1.09) |
| Years since HIV diagnosis, per 10 years | 321 | 75 (23) | 1.04 (0.76, 1.42) | 1.11 (0.73, 1.67) |
| Sex | ||||
| Female | 53 | 15 (28) | 1.48 (0.75, 2.90) | 1.25 (0.52, 2.98) |
| Male | 268 | 60 (22) | Ref | Ref |
| Race | ||||
| Black | 119 | 31 (26) | 1.33 (0.77, 2.28) | 1.22 (0.64, 2.32) |
| Non-Black | 193 | 42 (22) | Ref | Ref |
| Missing | 9 | 2 (22) | NA | NA |
| Ethnicity | ||||
| Hispanic | 91 | 19 (21) | 0.82 (0.44, 1.50) | 0.82 (0.41, 1.65) |
| Non- Hispanic | 220 | 53 (24) | Ref | Ref |
| Missing | 10 | 3 (30) | NA | NA |
| Men who have sex with men | ||||
| Yes | 200 | 43 (22) | 0.80 (0.46, 1.37) | 0.68 (0.32, 1.42) |
| No | 121 | 32 (26) | Ref | Ref |
| Injection drug use | ||||
| Any | 7 | 2 (28) | 1.29 (0.24, 6.81) | 1.06 (0.19, 6.08) |
| None | 314 | 73 (23) | Ref | Ref |
| History of AIDS-defining events | ||||
| Any | 69 | 25 (36) | 2.33 (1.28, 4.23) | 3.10 (1.54, 6.22) |
| None | 252 | 50 (20) | Ref | Ref |
| BMI | ||||
| > 30 kg/m2 | 91 | 21 (23) | 1.05 (0.59, 1.89) | 1.01 (0.54, 1.87) |
| ≤ 30 kg/m2 | 230 | 54 (23) | Ref | Ref |
| CD4 cell count | ||||
| ≥ 500 cells/μL | 217 | 48 (22) | 0.75 (0.43, 1.32) | 0.96 (0.51, 1.80) |
| < 500 cells/μL | 97 | 26 (27) | Ref | Ref |
| Missing | 7 | 1 (14) | NA | NA |
| Viral load | ||||
| ≥ 50 copies/mL | 43 | 9 (21) | 0.75 (0.33, 1.71) | 0.50 (0.20, 1.23) |
| < 50 copies/mL | 273 | 65 (24) | Ref | Ref |
| Missing | 5 | 1 (20) | NA | NA |
aOR adjusted odds ratio, CI confidence interval, BMI body mass index, n number, OR odds ratio
Virologic outcomes were assessed among 221 individuals with a viral load < 50 copies/mL at initiation and ≥ 1 follow-up viral load. Most were undetectable (95%) or suppressed (99%) at their last viral load measurement. Virologic suppression was maintained throughout follow-up in 96% of individuals. Four confirmed virologic failures were observed over a range of 6–36 weeks after the first injections, three of which resulted in CAB + RPV LA discontinuation; resistance data were not available. No difference in virologic outcomes were observed between individuals who experienced delays and those who did not (Table 4). Data on oral bridging were not available. Of the 36 individuals with a viral load ≥ 200 copies/mL at initiation and ≥ 1 follow-up viral load, 94% had a viral load < 200 copies/mL at their last measurement (not shown).
Table 4.
Virologic outcomes among individuals with a viral load < 50 copies/mL at initiation, overall, and by the occurrence of any injection delays during follow-up
| Overall N = 273 |
No delayed injectionsa N = 208 |
Any delayed injectionsb N = 65 |
p value | |
|---|---|---|---|---|
| ≥ 1 follow-up viral load, n (%) | 221 (81) | 165 (79) | 56 (86) | |
| Median months from 1st injection to last viral load (IQR) | 2.8 (1.1, 5.1) | 2.2 (1.1, 4.7) | 4.3 (1.8, 6.9) | |
| Last viral load < 50 copies/mL, n (%) | 210 (95) | 157 (94) | 53 (95) | 0.85 |
| Last viral load < 200 copies/mL, n (%) | 218 (99) | 163 (99) | 55 (98) | 0.74 |
| All viral load < 200 copies/mL, n (%) | 213 (96) | 160 (97) | 53 (95) | 0.44 |
| Confirmed virologic failure, n (%) | 4 (2) | 3 (2) | 1 (2) | 0.96 |
IQR interquartile range, Nn number
aAll continuation injections were administered ≤ 7 days after target date
bAt least one continuation injection was administered > 7 days after target date
Discussion
In routine clinical care in the US, 90% of monthly CAB + RPV LA continuation injections were administered on time, or within 7 days before or after the target date, as per the label. Virologic suppression was maintained throughout follow-up by 96% of individuals with a viral load < 50 copies/mL at initiation. Of note, confirmed virologic failures were infrequent.
The virologic efficacy of monthly CAB + RPV LA injectable was demonstrated in clinical trials [4–12]. In an observational study of 39 individuals with at least two continuation CAB + RPV injections, viral suppression was maintained by all 24 individuals with viral load < 30 copies/mL at initiation, and achieved by 12 (80%) of the 15 individuals with viral load ≥ 30 copies/mL at initiation [14]. Among individuals receiving CAB + RPV LA through a compassionate use program, 6/7 (86%) individuals with a viral load < 50 copies/mL maintained suppression, and 16/28 (57%) with a viral load ≥ 50 copies/mL achieved suppression [16].
The timely administration of long-acting medications such as CAB + RPV LA injectable is crucial to their success. In OPERA, 90% of continuation injections were administered on time, and only a history of AIDS-defining events was associated with an increased risk of experiencing a delay. Oral bridging should be used to maintain the therapeutic dose when missed injections are planned or when delays ranging from > 7 days to ≤ 8 weeks occur. Some of the delays observed in this study may not represent true gaps in treatment if oral bridging was used but not documented. In this study, 60% of delayed injections were administered within 28 days of the target date. However, documentation of oral bridging in the electronic health records was inconsistent and often missing. Moreover, bridging regimens and prescriptions are not uniform: some providers chose to maintain the prior ART prescription, some wrote a new prescription at CAB + RPV LA start, and a few wrote a new prescription before a gap that corresponded with the duration and timing of bridging medication use. Importantly, a population pharmacokinetic modeling and simulation study concluded that CAB LA injections could be resumed when injections had been delayed for less than 4 weeks after the target date, with or without oral bridging [17]. Similar proportions of suppression and confirmed virologic failures were observed in the minority of individuals with delays compared to the majority without any delays. Nonetheless, proper adherence to CAB + RPV LA regimen is critical and health care providers should emphasize this with individuals under their care.
While CAB + RPV LA has been approved for virologically suppressed individuals only, 13% of initiators in OPERA had a viral load ≥ 50 copies/mL at first injection. The OPERA cohort is comprised of EHR data from routine clinical care in the US, and, thus, off-label use may be observed when such use is deemed appropriate by the healthcare provider. However, reasons for regimen selection are not consistently recorded in EHRs, so justifications for off-label CAB + RPV LA use could not be investigated.
The short duration of follow-up in this study prevented the assessment of long-term adherence and virologic outcomes. In addition, while no statistical difference was observed in the likelihood of maintaining suppression or experiencing virologic failure between individuals with and without delayed injection, power to detect a difference was limited by the small number of events. However, the main objective of the study was to understand patterns of adherence. This early assessment of CAB + RPV use is important to support clinical decision making as uptake of this new treatment option increases. Recording of target dates was inconsistent in the electronic health records and implemented differently across providers and clinics. Therefore, the target date was reset every month to the same day as the previous injection and a 7-day window before and after the target was implemented in accordance with the label. Another study limitation was the unavailability of resistance testing results for analysis, which could have an impact on virologic effectiveness. The small number of confirmed virologic failures prevented the assessment of its predictors. Safety outcomes could not be assessed in this study. Finally, this study was limited to monthly injections; no inference can be made for the every 2 months dosing schedule.
This study was conducted with data from the OPERA cohort, a large, diverse cohort representative of HIV care in the US. Over 80 clinics ranging from small rural practices to large metropolitan healthcare centers contribute data to OPERA, which, at the time of this study, included EHR data from over 145,000 people with HIV, representing close to 14% of people with HIV in the US [18]. This large database of routine clinical care data provided access to information on procedures such as CAB + RPV LA injectable administration, as well as laboratory testing in a real-world population of people with HIV starting a CAB + RPV LA injectable regimen in the US. With 321 individuals receiving CAB + RPV LA injectables and 1152 continuation injections administered over 13 months, this is the largest real-world assessment of monthly CAB + RPV LA injectable administration and virologic outcomes to date.
Conclusion
In this large US cohort of routine clinical HIV care, monthly CAB + RPV LA injectable was associated with high levels of virologic control. In addition, most monthly CAB + RPV LA continuation injections were administered within the target window. These results suggest that CAB + RPB LA injectable can be administered effectively during routine clinical care.
Acknowledgements
This research would not be possible without the generosity of people living with HIV and their OPERA® caregivers. Additionally, we are grateful for the following individuals: Lito Torres and Kelly Oh (SAS programing/QA), Bernie Stooks and Lisa Lutzi (IT/data management) and Judy Johnson (medical terminology classification).
Author Contributions
Laurence Brunet and Jennifer S Fusco share the responsibility for the design of this study. Michael G Sension, Ricky K Hsu, Jennifer S Fusco, Quateka Cochran, Christine Uranaka, Lewis McCurdy, Michael B Wohlfeiler and Gregory P Fusco contributed the acquisition of data. Laurence Brunet and Jennifer S Fusco are responsible for all the analyses. All authors have contributed to the interpretation of results, have critically reviewed and approved the manuscript, and have participated sufficiently in the work to take public responsibility for its content.
Funding
This work was funded by ViiV Healthcare. The journal’s Rapid Service Fee was funded by Epividian, inc.
Data Availability
The datasets used in this study are not publicly available due to privacy concerns and the proprietary nature of the database but can be accessed upon reasonable request through the corresponding author to the OPERA Epidemiological and Clinical Advisory Board.
Declarations
Conflict of interest
Michael G Sension is on the Speakers Bureau for ViiV Healthcare and Gilead Sciences, and on the advisory board for ViiV Healthcare. Laurence Brunet, Jennifer S Fusco and Gregory P Fusco are employed by Epividian, Inc.; Epividian has had research funded by AIDS Healthcare Foundation, EMD Serono, Gilead Sciences, Janssen, Merck, Theratechnologies, and ViiV Healthcare. Ricky K Hsu has received research grants from Gilead Sciences, Janssen and ViiV Healthcare, speaker honoraria from ViiV Healthcare, Merck, Gilead Sciences and Janssen, and advisory board participation with ViiV Healthcare, Gilead Sciences, Janssen, and Epividian. Gayathri Sridhar, Vani Vannappagri and Jean van Wyk are employed by ViiV Healthcare and hold stocks and shares in GSK as part of their employment. Michael B Wohlfeiler has participated in post-conference advisory boards for the Conference on Retroviruses and Opportunistic Infections (CROI) and International AIDS Conference (IAC) and also serves as a principal investigator on ViiV Healthcare clinical trials but does not receive personal compensation for this work, which goes directly to the AIDS Healthcare Foundation. Michael B Wohlfeiler is also a member of the Epidemiology and Clinical Advisory Board for Epividian. Quateka Cochran, Christina Uranake and Lewis McCrudy have no conflicts of interest to declare.
Ethical approval
OPERA complies with all HIPAA and HITECH requirements, which expand upon the ethical principles detailed in the 1964 Declaration of Helsinki. OPERA has received annual institutional review board (IRB) approval by Advarra IRB, including a waiver of informed consent and authorization for use of protected health information (Pro00023648).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.U.S. Food & Drug Administration. FDA Approves Cabenuva and Vocabria for the Treatment of HIV-1 Infection 2021. https://www.fda.gov/drugs/human-immunodeficiency-virus-hiv/fda-approves-cabenuva-and-vocabria-treatment-hiv-1-infection. Updated 27 Jan 2021.
- 2.ViiV Healthcare. ViiV Healthcare announces label update for its long-acting HIV treatment, Cabenuva (cabotegravir, rilpivirine), to be initiated with or without an oral lead-in period 2022. https://viivhealthcare.com/en-us/media-center/news/press-releases/2022/march/viiv-healthcare-announces-label-update-for-its-long-acting-hiv/. Updated 24 Mar 2022.
- 3.ViiV Healthcare. Product monograph for Cabenuva. Laval, Quebec, Canada: ViiV Healthcare ULC; 2020.
- 4.Margolis DA, Brinson CC, Smith GHR, de Vente J, Hagins DP, Eron JJ, et al. Cabotegravir plus rilpivirine, once a day, after induction with cabotegravir plus nucleoside reverse transcriptase inhibitors in antiretroviral-naive adults with HIV-1 infection (LATTE): a randomised, phase 2b, dose-ranging trial. Lancet Infect Dis. 2015;15(10):1145–1155. doi: 10.1016/S1473-3099(15)00152-8. [DOI] [PubMed] [Google Scholar]
- 5.Orkin C, Arasteh K, Górgolas Hernández-Mora M, Pokrovsky V, Overton ET, Girard P-M, et al. Long-acting cabotegravir and rilpivirine after oral induction for HIV-1 infection. N Engl J Med. 2020;382(12):1124–1135. doi: 10.1056/NEJMoa1909512. [DOI] [PubMed] [Google Scholar]
- 6.Swindells S, Andrade-Villanueva JF, Richmond GJ, Rizzardini G, Baumgarten A, Masiá M, et al. Long-acting cabotegravir and rilpivirine for maintenance of HIV-1 suppression. N Engl J Med. 2020;382(12):1112–1123. doi: 10.1056/NEJMoa1904398. [DOI] [PubMed] [Google Scholar]
- 7.Orkin C, Oka S, Philibert P, Brinson C, Bassa A, Gusev D, et al. Long-acting cabotegravir plus rilpivirine for treatment in adults with HIV-1 infection: 96-week results of the randomised, open-label, phase 3 FLAIR study. Lancet HIV. 2021;8(4):e185–e196. doi: 10.1016/S2352-3018(20)30340-4. [DOI] [PubMed] [Google Scholar]
- 8.Swindells S, Lutz T, Van Zyl L, Porteiro N, Stoll M, Mitha E, et al. Week 96 extension results of a Phase 3 study evaluating long-acting cabotegravir with rilpivirine for HIV-1 treatment. AIDS. 2022;36(2):185–194. doi: 10.1097/QAD.0000000000003025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ramgopal MN, Castagna A, Cazanave C, Diaz-Brito V, Dretler R, Oka S, et al. Efficacy, safety, and tolerability of switching to long-acting cabotegravir plus rilpivirine versus continuing fixed-dose bictegravir, emtricitabine, and tenofovir alafenamide in virologically suppressed adults with HIV, 12-month results (SOLAR): a randomised, open-label, phase 3b, non-inferiority trial. Lancet HIV. 2023;10(9):e566–e577. doi: 10.1016/S2352-3018(23)00136-4. [DOI] [PubMed] [Google Scholar]
- 10.Margolis DA, Gonzalez-Garcia J, Stellbrink H-J, Eron JJ, Yazdanpanah Y, Podzamczer D, et al. Long-acting intramuscular cabotegravir and rilpivirine in adults with HIV-1 infection (LATTE-2): 96-week results of a randomised, open-label, phase 2b, non-inferiority trial. Lancet. 2017;390(10101):1499–1510. doi: 10.1016/S0140-6736(17)31917-7. [DOI] [PubMed] [Google Scholar]
- 11.Smith GHR, Henry WK, Podzamczer D, Masiá MDM, Bettacchi CJ, Arasteh K, et al. Efficacy, safety, and durability of long-acting cabotegravir and rilpivirine in adults with human immunodeficiency virus type 1 infection: 5-year results from the LATTE-2 study. Open Forum Infect Dis. 2021;8(9). [DOI] [PMC free article] [PubMed]
- 12.Overton ET, Richmond G, Rizzardini G, Thalme A, Girard P-M, Wong A, et al. Long-acting cabotegravir and rilpivirine dosed every 2 months in adults with human immunodeficiency virus 1 type 1 infection: 152-week results from ATLAS-2M, a randomized, open-label, phase 3b, noninferiority study. Clin Infect Dis. 2023;76(9):1646–1654. doi: 10.1093/cid/ciad020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Collins LF, Corbin-Johnson D, Asrat M, Morton ZP, Dance K, Condra A, et al. Early experience implementing long-acting injectable cabotegravir/rilpivirine for human immunodeficiency virus-1 treatment at a Ryan white-funded Clinic in the US south. Open Forum Infect Dis. 2022;9(9). [DOI] [PMC free article] [PubMed]
- 14.Christopoulos KA, Grochowski J, Mayorga-Munoz F, Hickey MD, Imbert E, Szumowski JD, et al. First demonstration project of long-acting injectable antiretroviral therapy for persons with and without detectable HIV viremia in an urban HIV clinic. Clin Infect Dis. 2022. [DOI] [PMC free article] [PubMed]
- 15.Hill LA, Abulhosn KK, Yin JF, Bamford LP. Single-center experience evaluating and initiating people with HIV on long-acting cabotegravir/rilpivirine. AIDS. 2023;37(4):605–609. doi: 10.1097/QAD.0000000000003446. [DOI] [PubMed] [Google Scholar]
- 16.D'Amico R, Cenoz Gomis S, Moodley R, Van Solingen-Ristea R, Baugh B, Van Landuyt E, et al. Compassionate use of long-acting cabotegravir plus rilpivirine for people living with HIV-1 in need of parenteral antiretroviral therapy. HIV Med. 2023;24(2):202–211. doi: 10.1111/hiv.13370. [DOI] [PubMed] [Google Scholar]
- 17.Han K, Baker M, Patel P, Margolis D, Spreen W, Moore KP, et al. (1532) Population pharmacokinetic (PPK) modeling and simulation of long-acting (LA) cabotegravir (CAB) to inform strategies following dosing interruptions in HIV-1-infected subjects. Open Forum Infect Dis. 2019;6(Supplement_2):S558-S.
- 18.Centers for Disease Control and Prevention. HIV surveillance report, 2020; vol. 33 2022. 2022. https://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this study are not publicly available due to privacy concerns and the proprietary nature of the database but can be accessed upon reasonable request through the corresponding author to the OPERA Epidemiological and Clinical Advisory Board.