Abstract
Aims and Objectives
To determine utilization of spot urinary albumin/creatinine ratio (UACR) to predict subsequent development of preeclampsia, measured between 17 and 24 weeks of gestational age in asymptomatic antenatal woman and determine their maternal and neonatal outcomes.
Introduction
In preeclampsia the basic pathology is generalized endothelial dysfunction. It causes glomerular endotheliosis which leads to proteinuria, decreased glomerular filtration rate and renal blood flow. Thus microalbuminuria is an early marker which can measured to predict preeclampsia.
Materials and Methods
It is a prospective observational study, carried out for one year in a cohort of asymptomatic antenatal women at 17–24 weeks of gestational age, attending hospital for routine antenatal check-up with a singleton pregnancy and no associated complications. Urine albumin and creatinine ratio (UACR) is measured at first visit, and women were followed till delivery and the maternal and foetal outcomes were recorded.
Results
Out of 81 pregnant women enrolled in the study, 58% belonged to 18–25 years, 54.3% belonged to lower middle class. There was a significant difference in mean UACR among women who developed preeclampsia (PE) and gestational diabetes mellitus (GDM) with p value < 0.05. In the study there was significant association between severe PE, PE and GDM with UACR at 22 as cut-off, with p value < 0.05. In the study among those with UACR > 22, 2.5% had IUFD, 12.5% had LBW, and 7.5% were admitted to NICU.
Conclusion
With the measurement of spot UACR in mid-trimester we can predict the development of preeclampsia before the onset of clinical manifestations. UACR > = 171 mg/g predicted preeclampsia well before the onset of clinical manifestations with high sensitivity of 83.3% and specificity of 98.6%.
Supplementary Information
The online version contains supplementary material available at 10.1007/s13224-023-01862-9.
Keywords: Preeclampsia, Spot urine albumin/creatinine ratio, Antenatal women, Proteinuria, Gestational hypertension
Introduction
Preeclampsia (PE) is a pregnancy-specific disorder affecting all the organ systems. Research is going on for a cost-effective, rapid and convenient test to predict preeclampsia to prevent complications. ACOG Task Force on Hypertension in Pregnancy (2013) in its report described it as a constantly evolving multisystemic disorder with a dynamic process. Preeclampsia complicates 4–5% of all pregnancies [1].
The two-stage theory of pathophysiology of preeclampsia explained by inadequate trophoblastic invasion and abnormal placentation subsequently leading to uteroplacental insufficiency provides a window period to screen for it [2].
There are various predictive tests available for detecting preeclampsia, but none is reliable. There is a systemwide endothelial leak, causing glomerular endotheliosis in preeclampsia which is characterized by proteinuria [3]. There is general endothelial dysfunction vascular insufficiency [4]. Though the exact etiology is not known, there is abnormal placentation leading to synthesis and release of various factors which disrupt angiogenesis, causing wide spread endothelial damage effecting multiple organ systems [5]. This systemic endothelial dysfunction leads to proteinuria. Microalbuminuria is a manifestation of renal involvement. It is seen as subclinical elevation of urinary albumin excretion. But urinary microalbuminuria is seen in various other medical conditions; hence, it needs a quantification process, like the 24-h urine protein estimation [6, 7]. As 24-h urine protein estimation is practically inconvenient to screen in normal pregnant women who come for routine check-up, hence the need for a test like spot urine/creatinine ratio which is rapid and quick to detect and quantify proteinuria. This study was advocated on this basis to predict preeclampsia by doing mid-trimester albumin creatinine ratio and also to find out its role in other pregnancy outcomes.
Materials and Methods
The study was conducted in the Department of Obstetrics and Gynecology, Kamineni Hospitals, LB Nagar, Hyderabad. The study population included all pregnant women who are registered and attending antenatal clinic during the study period at Kamineni Hospitals, L B Nagar. These women were enrolled after an informed written consent. It is a prospective observational study conducted for a period of one year (1st June 2018 and 30th May 2019).
The inclusion criteria were 1. pregnant women of 17–24 weeks of gestation by last menstrual period verified by USG, 2. singleton pregnancy, 3. normal renal function.
The exclusion criteria were: 1. hematuria, 2.UTI—urinary tract infections, 3. multiple pregnancy, 4. acute renal failure, 5. chronic kidney disease, 6. chronic hypertension, 7. diabetes, 8. known major foetal anomaly/foetal demise.
Pregnant women attending the antenatal clinic who fulfilled the inclusion criteria were enrolled for the study. The study was approved by the institutional ethical committee, and informed written consent was obtained from all participating women. Data regarding demographic profile, blood pressure, BMI (body mass index) at the first antenatal visit, past medical and surgical history and family history were documented. All women were clinically evaluated at the booking visit to rule out any risk factor for the development of pre-eclampsia. Obstetric history was documented regarding gravida, parity, past h/o preeclampsia, prematurity, SGA (small for gestational age) and miscarriage. All women were given a sterile plastic container without preservatives and instructed to collect a random mid-stream urinary sample. The collected urine sample was sent for estimation of albumin by turbidimetric immunoassay method through fully automated biochemistry analyser and estimation of creatinine by modified Jaffe's method. Then spot urinary ACR (albumin creatinine ratio) was calculated.
UACR is expressed in mg/gm. Participants were followed until delivery. The primary outcome measure was preeclampsia; secondary outcomes measured were gestational hypertension, gestational diabetes, intrauterine growth retardation (IUGR) and preterm labour. At each visit, their blood pressure was recorded and they were assessed for any signs and symptoms of pre-eclampsia such as oedema, nausea, vomiting, epigastric pain, decreased urine output and visual disturbances. Foetal outcomes were measured like live birth/ still born, birth weight, APGAR at 5 min, need for NICU admission.
For statistical analysis data were entered into Microsoft excel data sheet and were analysed using SPSS 22 version software. Categorical data were represented in the form of frequencies and proportions. Chi-square test was used as test of significance for qualitative data. Continuous data were represented as mean and standard deviation. Graphical representation of data was done by using MS Excel and MS word to obtain various types of graphs such as bar diagram, pie diagram and scatter plots. The p value (probability that the result is true) of < 0.05 was considered as statistically significant after assuming all the rules of statistical tests. Statistical software like MS Excel, SPSS version 22 (IBM SPSS Statistics, Somers NY, USA) was used to analyse data.
Results
In the study 58% (47) were in the age group 18–25 years, 38.3% (31) were in the age group 26 to 35 years, and 3.7% (3) were in the age group 36–45 years (Table 1). Majority of pregnant women belonged to age group 18–25 years (58%). In the study 8.6% (7) belonged to upper class, 21% (17) belonged to upper middle class, 8.6% (7) belonged to upper lower, 54.3% (44) belonged to lower middle, and 7.4% (6) belonged to lower class. Majority of the pregnant women belongs to lower middle class. In the study 43.2% (35) had normal BMI, 39.5% (32) were overweight, and 17.3% (14) were obese. In the study 54.3% (44) were multigravida and 45.7% (37) were primigravida.
Table 1.
Demographic distribution
| No. of women | % | |
|---|---|---|
| Age | ||
| 18 to 25 years | 47 | 58.0 |
| 26 to 35 years | 31 | 38.3 |
| 36 to 45 years | 3 | 3.7 |
| Total | 81 | 100.0 |
| Socioeconomic class | ||
| Upper | 7 | 8.6 |
| Upper middle | 17 | 21.0 |
| Upper lower | 7 | 8.6 |
| Lower middle | 44 | 54.3 |
| Lower | 6 | 7.4 |
| Total | 81 | 100.0 |
| BMI | ||
| < 18.5 | 0 | 0.0 |
| 18.5 to 24.9 | 35 | 43.2 |
| 25 to 29.9 | 32 | 39.5 |
| > 30 | 14 | 17.3 |
| Total | 81 | 100.0 |
| Parity | ||
| Multigravida | 44 | 54.3 |
| Primigravida | 37 | 45.7 |
| Total | 81 | 100.0 |
Mean UACR was 49.79 ± 72.67. It shows skewed distribution for UACR in the study; hence, median value is better to be considered in statistical analysis. In the study there was no significant correlation between UACR and urine albumin and UACR and urine creatinine. There was positive correlation between UACR and BMI, i.e. with increase in BMI there was increase in UACR and vice versa; however, the correlation was not statistically significant.
In the study there was significant difference in mean UACR among those without complications and those with complication (Fig. 1). There was significant difference in mean UACR among subjects who developed preeclampsia and GDM with p value < 0.05. Among those without complications mean UACR was low compared to those with complications. Mean UACR was highest in preeclampsia subjects among all other complications in the study (195.29 ± 79.29) (Fig. 1).
Fig. 1.
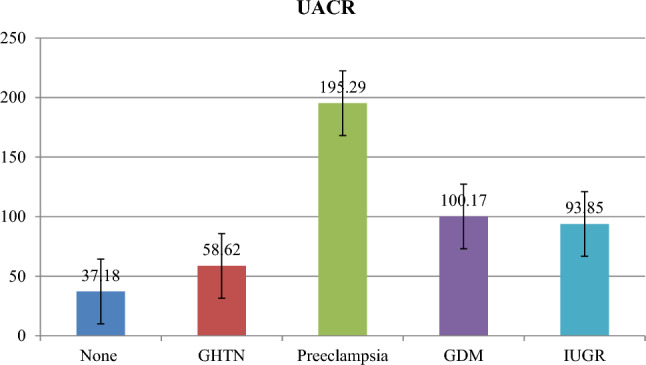
Bar diagram showing UACR comparison with respect to maternal complications
In the study there was significant association between severe PE, PE and GDM with UACR at 22 as cut-off (Table 2), with p value < 0.05. Among those with UACR > 22, 5 had GHTN, 2 had nonsevere preeclampsia, 4 had severe PE, 6 had PE, 10 had GDM, 8 had IUGR, and 13 had preterm labour.
Table 2.
Association between UACR (at cut-off of median value, i.e. 22) with maternal complications
| Maternal complications | UACR (22 cut-off) | p value | ||
|---|---|---|---|---|
| < 22 | > 22 | |||
| No. | No. | |||
| GHTN | Present | 2 | 5 | 0.222 |
| Nonsevere PE | Present | 0 | 2 | 0.147 |
| Severe PE | Present | 0 | 4 | 0.038* |
| PE | Present | 0 | 6 | 0.001* |
| GDM | Present | 2 | 8 | 0.039* |
| IUGR | Present | 5 | 3 | 0.479 |
| Preterm labour | Present | 4 | 9 | 0.118 |
Statistically significant p value < 0.05
In the study there was no significant association between mode of delivery and complications (Table 3). There was significant association between prematurity and complications. Those with preeclampsia had higher incidence of prematurity compared to other complications. In the study there was significant difference in mean UACR levels with respect to complications. Subjects with preeclampsia had higher levels of UACR as compared to those with other complications. There was significant difference in mean GA at delivery with respect to complications. Subjects with preeclampsia were in gestational age (33.37 ± 6.31Weeks) which was low compared to those with other complications. There was significant difference in mean birth weight with respect to complications. Subjects with preeclampsia had mean birth weight (1.91 ± 1.01 Kgs) which was low compared to those with other complications.
Table 3.
Association of UACR with mode of delivery and complications
| None (n = 50) | GHTN (n = 7) | Preeclampsia (n = 6) | GDM (n = 10) | IUGR (n = 8) | p value | ||
|---|---|---|---|---|---|---|---|
| UACR(mg/g) | Median | 15.40 | 41.70 | 224.80 | 71.40 | 15 | < 0.001* |
| Mode of delivery | LSCS | 22 (44%) | 4 (57.1%) | 5 (83.3%) | 7 (70%) | 2 (25%) | 0.129 |
| Vaginal | 28 (56%) | 3 (42.9%) | 1 (16.7%) | 3 (30%) | 6 (75%) | ||
| Prematurity (< 37 weeks) | 0 (0%) | 2 (28.6%) | 2 (33.3%) | 2 (20%) | 2 (25%) | 0.004* | |
| GA at delivery wks | 36.429 ± 3.25 (37.10) | 37.26 ± 1.51 (37.50) | 33.37 ± 6.31 (35.70) | 36.85 ± 2.65 (37.20) | 34.69 ± 5.79 (37.15) | < 0.001* | |
| Birth weight | 2.59 ± 0.67 (2.60) | 2.98 ± 0.43 (3.01) | 1.91 ± 1.01 (2.14) | 2.85 ± .70 (3.07) | 2.30 ± .72 (2.39) | < 0.001* | |
Statistically significant p value < 0.05
UACR at > 171 had highest sensitivity (83.33%) and specificity (98.67%) in diagnosis of preeclampsia. PPV was 83.3 and NPV was 98.7(Fig. 2).
Fig. 2.
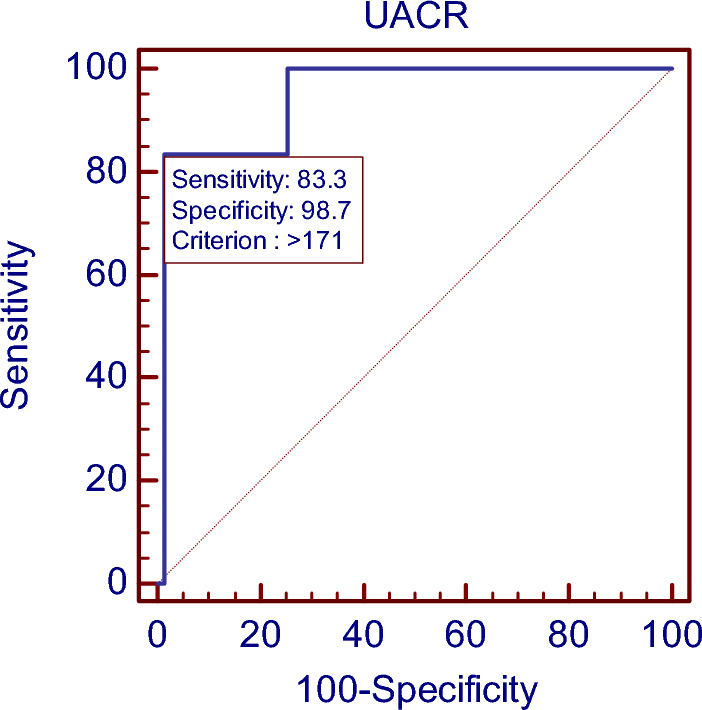
ROC curve showing area under the ROC curve (AUC) for UACR in diagnosis of preeclampsia
In the study there was significant difference in mean gestational age at complications with respect to UACR levels for IUGR, i.e. among those with UACR < 22 mean GA at complications among those with IUGR was 36.00 ± 0.82 weeks and among those with UACR > 22 mean GA at complications among those with IUGR was 27.50 ± 6.36 weeks (Table 4). In the study among those with UACR > 22, 2.5% had IUFD, 12.5% had LBW and 7.5% were admitted to NICU.
Table 4.
Mean gestational age at complications among those with maternal complications with respect to UACR
| UACR | p value | ||||||
|---|---|---|---|---|---|---|---|
| < 22 | > 22 | ||||||
| GA at Complications | GA at Complications | ||||||
| Mean | SD | Median | Mean | SD | Median | ||
| GHTN (n = 7) | 31.00 | 7.07 | 31.00 | 34.24 | 2.99 | 35.20 | 0.392 |
| Preeclampsia (n = 6) | – | – | – | 31.20 | 4.87 | 32.00 | – |
| GDM (n = 10) | 25.00 | 1.41 | 25.00 | 31.53 | 4.62 | 33.00 | 0.109 |
| IUGR (n = 8) | 36.00 | .82 | 36.00 | 27.50 | 6.36 | 27.50 | 0.04* |
Statistically significant p value < 0.05
Discussion
In our study, a total of 81 pregnant women were enrolled. In previous studies there is significant association of microalbuminuria with various conditions like PE, GDM, IUGR and preterm labour. In our study, majority of the pregnant women belonged to age group of 18–25 years, i.e. 58% (47 respondents). Majority of the pregnant women belonged to lower middle class i.e. 54.3% (44 respondents). Majority of women fall under normal BMI (18.5–24.9 kg/m2), i.e. 43%. In our study group 54.3% (44 respondents) were multigravida and 45.7% (37 respondents) were primigravida. The median value of UACR among the subjects who did not develop any maternal complications was 15.4 mg/g that is statistically significant (p value of 0.047*) in comparison with the subjects who developed maternal complications. Among those without complications mean UACR was low compared to those with complications. Mean UACR was highest among all other complications in the study (195.29 ± 79.29). Spot UACR at 17–24 weeks of gestation was significantly higher in women who subsequently developed maternal complications like preeclampsia and GDM.
The number of women who developed preeclampsia in our study was 7.4% (6/81) which is comparable to the incidence of 4–18% in developing countries as studied by Kozer et al. [8]. The overall median spot urine ACR measured between 17 and 24 weeks of gestation among the subjects who developed preeclampsia was 224.8 mg/g. We found that spot urine ACR (mean—195.2 mg/g) was statistically significant (p value—0.01*) in women who developed preeclampsia. Similar observations were noted in the previous studies conducted by Gupta et al. [9] and Baweja et al. [10]. Preeclampsia was subsequently developed in most of the pregnant ladies with high UACR that is 171 mg/g with a sensitivity of 83.33% (calculated by AUC) which is comparable to similar studies by Mishra et al. [7] with a sensitivity of 87.5% and Baweja et al. [10] with a sensitivity of 83.3%. The specificity of 98.67% and a positive predictive value (PPV) of 83.3% and negative predictive value (NPV) of 98.7% derived in our study are high and can be compared to earlier studies conducted by Vineeth et al. [7] and Nisell et al. [11]. Conversely 95% of the women, who did not demonstrate UACR more than 22 mg/g, remained normotensive at the time of delivery in our study like in other studies conducted by Sibai et al. [12], Rodriguiz et al. [13], where 94% women without microalbuminuria were normotensive.
In our study, the mean gestational age at the time of development of complication that is preeclampsia was 31 weeks. There was a significant difference in the mean GA at delivery to complications. Subjects with preeclampsia were in gestational age (33.37 ± 6.31Weeks) which was low as compared to those with other complications [14].
The incidence of GDM in our study population was 12.3% (10/81). There was a significant association between GDM with UACR at 22 as cut-off (p value 0.018*). Among those with UACR > 22, 20% had GDM. Similar to a study conducted by Rachitha et al. [15] there is higher incidence of GDM among women with microalbuminuria. The incidence of GDM was higher among the subjects with median UACR value 71.4 mg/g, like the study by Bomback AS et al. [15]. At UACR > 25, sensitivity and specificity of detecting GDM were 80% and 63.38% with a PPV of 23.5 and NPV of 95.7 which is comparable with the study conducted by Rachitha et al. [16] where the sensitivity—80%, specificity—56% and PPV—26.6%, NPV of 93.30%.
Incidence of IUGR was 9.9% (8/81). Among the subjects whose UACR value was more than 22 mg/g, (3/81) that is 3.7% had IUGR. The Chi-square test is not statistically significant among the subjects with p value 0.479. Contradicting to study by Gupta et al. [9] and Baweja et al. [10] which showed that UACR predicts IUGR along with preeclampsia, Bar et al. [17] have reported that it cannot predict IUGR and neonatal outcome as in our study.
In our study, 13 subjects out of 81(16.0%) delivered prior to 37 weeks of gestational age. Of them, 9 subjects show UACR > 22 mg/g and 4 subjects showed UACR < 22 mg/g. But there is no statistical significance between UACR and preterm labour (p value 0.118). Similar results were given by Masse et al. [18] and concluded no association between microalbuminuria and preterm labour. Ekbom et al. [19] have reported increased incidence of preterm labour in pregnant women with microalbuminuria, but mainly caused by preeclampsia, and also in a study conducted by Singh et al. [20] there was increased incidence of preterm labour. In our study among those with UACR > 22, 2.5% had IUFD, 12.5% had LBW, and 7.5% were admitted to NICU.
Summary and Conclusion
Our study inferred that single random spot urinary albumin creatinine values were higher in mid-trimester of pregnancy in women without any symptoms, who developed preeclampsia and gestational diabetes later on. When UACR is measured during 17–24 weeks of gestation, UACR > = 171 mg/g predicted preeclampsia well before the onset of any signs and symptoms with high sensitivity of 83.3% and specificity of 98.6%, indicating the role of UACR for predicting preeclampsia in early pregnancy and preventing further complications of preeclampsia. The limitations in our study are we need a larger cohort of normal pregnant women as Indian women are more prone to preeclampsia.
Supplementary Information
Below is the link to the electronic supplementary material.
Funding
There is no funding for the study.
Declaration
Conflicts of interest
The authors declare that there are no conflicts of interest for the present study.
Ethical standards statement
Ethical standards maintained according to WMA Helinski declaration.
Informed consent
This study was approved by institutional scientific committee and ethical committee. Informed consent was taken from all the participants before enrolment after explaining the study protocol and confidentiality maintained.
Footnotes
Akkenapally Prasanna Latha is an Associate Professor; V. Haripriya is an Associate Professor; P. Ramya Raj is a Resident.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Martin JN, Jr, My O, Keiser SD, et al. Standardized Mississippi protocol treatment of 190 patients with HELLP syndrome: slowing disease progression and preventing new major maternal morbidity. Hypertens Pregnancy. 2012;31(1):79–90. doi: 10.3109/10641955.2010.525277. [DOI] [PubMed] [Google Scholar]
- 2.Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl. A):S32–S37. doi: 10.1016/j.placenta.2008.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Macdonald-Wallis C, Silberwood RJ, de Stavola BL, et al. Antenatal blood pressure for prediction of preeclampsia, preterm birth, and small for gestational age babies: development and validation in two general population cohorts. BMJ. 2015;351:h5948. doi: 10.1136/bmj.h5948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ohkuchi A, Hirashima C, Takahashi K, et al. Prediction and prevention of hypertensive disorders of pregnancy. Hypertens Res. 2017;40:5–14. doi: 10.1038/hr.2016.107. [DOI] [PubMed] [Google Scholar]
- 5.Arias F, Daftary SN, Bhide AG, et al. Hypertensive Disorders in Pregnancy. In: Arias F, Bhide AG, Arulkumaran S, Damania K, Daftary SN, et al., editors. Practical guide to high risk pregnancy and delivery. A South Asian perspective. New Delhi: Elsevier India; 2015. pp. 185–230. [Google Scholar]
- 6.Huang Q, Gao Y, Yu Y, et al. Urinary spot albumin:creatinine ratio for documenting proteinuria in women with preeclampsia. Rev Obstet Gynecol. 2012;5(1):9–15. [PMC free article] [PubMed] [Google Scholar]
- 7.Mishra VV, Goyal PA, Priyankur R, et al. Evaluation of spot urinary albumin-creatinine ratio as screening tool in prediction of pre-eclampsia in early pregnancy. J Obstet Gynaecol India. 2017;67(6):405–408. doi: 10.1007/s13224-016-0950-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kozer E, Costei AM, Boskovic R, et al. Effects of aspirin consumption during pregnancy on pregnancy outcomes: meta-analysis. Birth Defects Res Part B Dev Reprod Toxicol. 2013;68:70–84. doi: 10.1002/bdrb.10002. [DOI] [PubMed] [Google Scholar]
- 9.Gupta N, Gupta T, Asthana D. Prediction of preeclampsia in early pregnancy by estimating the spot urinary albumin/creatinine ratio. J Obstet Gynecol India. 2017;67(4):258–262. doi: 10.1007/s13224-016-0958-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baweja S, Kent A, Masterson R, et al. Prediction of pre-eclampsia in early pregnancy by estimating the spot urinary albumin: creatinine ratio using high-performance liquid chromatography. BJOG. 2011;118(9):1126–1132. doi: 10.1111/j.1471-0528.2011.02960.x. [DOI] [PubMed] [Google Scholar]
- 11.Nisell H, Trygg M, Back R. Urine albumin/creatinine ratio for the assessment of albuminuria in pregnancy hypertension. Acta Obstet Gynecol. 2012;85:1327–1330. doi: 10.1080/00016340600808747. [DOI] [PubMed] [Google Scholar]
- 12.Sibai BM, Caritis S, Hauth J, et al. Risks of preeclampsia and adverse neonatal outcomes amongwomen with pregestational diabetes mellitus. Am J Obstet Gynaecol. 2000;182:364–369. doi: 10.1016/S0002-9378(00)70225-0. [DOI] [PubMed] [Google Scholar]
- 13.Rodriguez MH, Masaki DI, Mestman J, et al. Calcium/creatinine ratio and microalbuminuria in the prediction of preeclampsia. Am J Obstet Gynecol. 1988;159(6):1452–1455. doi: 10.1016/0002-9378(88)90573-X. [DOI] [PubMed] [Google Scholar]
- 14.Shaarawy M, Salem ME. The clinical value of microtransferrinuria and microalbuminuria in the prediction of preeclampsia. Clin Chem Lab Med. 2001;39(1):29–34. doi: 10.1515/CCLM.2001.008. [DOI] [PubMed] [Google Scholar]
- 15.Chawla R, Malik S. Microalbuminuria detected at Mid Term as a marker for adverse pregnancy outcome. Int J Health Sci Res. 2018;8(2):41–52. [Google Scholar]
- 16.Bomback AS, Rekhtman Y, Whaley-Connell AT, et al. Gestational diabetes mellitus alone in the absence of subsequent diabetes is associated with microalbuminuria: results from the kidney early evaluation program (KEEP) Diabetes Care. 2010;33(12):2586–2591. doi: 10.2337/dc10-1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bar J, Hod M, Erman A, et al. Microalbuminuria as an early predictor of hypertensive complications in pregnant women at high risk. Am J Kidney Dis. 1996;28:220–225. doi: 10.1016/S0272-6386(96)90305-4. [DOI] [PubMed] [Google Scholar]
- 18.Massé J, Forest JC, Moutquin JM. Microalbumin as a marker of premature delivery. Obstet Gynecol. 1996;87:661–663. doi: 10.1016/0029-7844(96)00038-5. [DOI] [PubMed] [Google Scholar]
- 19.Ekbom P, Damm P, Feldt-Rasmussen B, et al. Pregnancy outcome in type 1 diabetic women with microalbuminuria. Diabetes Care. 2001;24:1739–1744. doi: 10.2337/diacare.24.10.1739. [DOI] [PubMed] [Google Scholar]
- 20.Singh H, Samal S, Mahapatro A, et al. Comparison of obstetric outcome in pregnant women with and without microalbuminuria. J Nat Sci Biol Med. 2015;6(1):120–124. doi: 10.4103/0976-9668.149106. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


