Introduction
Plasma lipid concentrations get altered physiologically during pregnancy, albeit with minimal clinical consequences. Pregnancy-related hypertriglyceridemia is one such alteration wherein plasma triglycerides increase 2–3 folds, especially during the third trimester. These changes are usually well tolerated by a normal pregnant woman. However, very rarely, generally associated with genetic modifications can a pregnant lady have severe hypertriglyceridemia (> 1000 mg/dl). Severe hypertriglyceridemia in a pregnant lady can have serious complications like pancreatitis, pre-eclampsia and hyperviscosity syndrome. Considering the rarity of the condition, the management recommendations are limited to case reports and personal experiences [1]. Here, we describe the complete clinical course of severe hypertriglyceridemia in a pregnant woman due to lipoprotein lipase deficiency who developed pancreatitis.
Case Presentation
A 24-year-old lady, Gravida 2, Para 2 with 31 weeks of gestation presented to us for the evaluation of a deranged lipid profile. She had a history of pancreatitis during her first pregnancy a couple of years back. She was born out of second-degree consanguineous marriage. There was no history of uncontrolled diabetes, uncontrolled hypothyroidism, alcohol abuse or gallstones. There was no history of pancreatitis or atherosclerotic cardiovascular event at a young age in her family. Her BMI was 23.2 kg/m2. She did not have eruptive xanthomas, tendon xanthomas, xanthelasma, acanthosis nigricans, acrochordons or goiter. She had lipemia retinalis on fundus examination (Fig. 1). The review of systems was non-contributory.
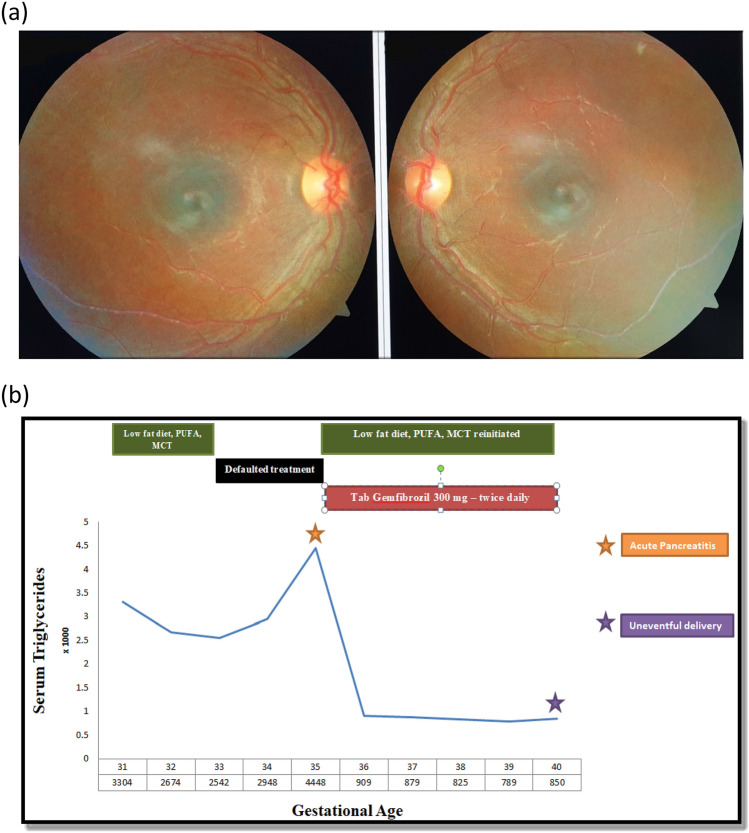
Figure 1.
a Fundus image showing lipemia retinalis, b Clinical, biochemical course and response to therapy during the course of gestation.
On investigation, her fasting triglyceride values were 3304 mg/dl with total cholesterol of 425 mg/dl. Thyroid function test, liver function test, renal function test, complete blood count and fasting blood glucose were within normal limits. The family screening revealed similar levels of dyslipidemia in her younger sister (Table 1). With this presentation, familial hypertriglyceridemia due to a genetic mutation was suspected. Next-generation sequencing revealed a homozygous missense variation in exon 6 of the lipoprotein lipase—LPL gene (chr8:g.19813385G > A; Depth: 275x) that results in the amino acid substitution of histidine for arginine at codon 270 (p.Arg270His; ENST00000311322.8). This variant was classified as pathogenic as per ACMG guidelines to cause lipoprotein lipase deficiency.
Table 1.
Family screening for dyslipidemia
| Index patient (at presentation) | Child-1 (3 year) | Child-2 (neonate) | Father | Mother | Sister | |
|---|---|---|---|---|---|---|
| Total Cholesterol (mg/dl) | 585 | 210 | 158 | 206 | Deceased | 452 |
| Triglycerides (mg/dl) | 4448 | 124 | 118 | 189 | 3200 | |
| LDL (mg/dl) | 23 | 100 | 98 | 154 | 160 | |
| VLDL (mg/dl) | 22 | 29 | 22 | 29 | 34 | |
| HDL (mg/dl) | 25 | 39 | 32 | 45 | 56 |
A multidisciplinary team (MDT) comprising an obstetrician, an endocrinologist and a dietician was formed for further management. She was admitted for the initiation of dietary intervention under observation. A customized diet with low fat (< 20% of total calories) was initiated. She was also started on 2 g of polyunsaturated fatty acid (PUFA) and 15 g of medium chain triglycerides (MCT oil) per day. With these interventions, her triglycerides decreased to 2674 mg/dl, a reduction of around 20% from baseline. There is a paucity of the literature on the safety of pharmacological interventions for hypertriglyceridemia in pregnancy. As the patient was currently asymptomatic, it was decided by the MDT to follow her up closely on an outpatient basis.
After discharge, the patient failed to comply with the stringent dietary recommendations. At around 35 weeks of gestation, she presented to the emergency room with another episode of pancreatitis. On evaluation, she was mildly dehydrated, had tachycardia and had severe epigastric-tenderness. The rest of the vital examination and the review of systems were non-contributary. Serum triglyceride values were 4448 mg/dl with total cholesterol of 585 mg/dl. Serum amylase and lipase were 884 IU/L (< 85 IU/L) and 1018 IU/L (< 160 IU/L) respectively. USG abdomen showed diffusely edematous pancreas with peri-pancreatic fluid collections. There were no gallstones, and the biliary tract was normal. An obstetric evaluation ruled out fetal distress. With this clinical, biochemical and radiological profile, a diagnosis of mild-acute pancreatitis was considered. She was kept nil per oral and started on intravenous dextrose-containing fluids and intravenous analgesics. Enteral feeds were restarted after the first 24–48 h in a graded manner. She was restarted on the dietary modifications—a low-fat diet (LFD) with PUFA and MCT oil. Considering the severity of hypertriglyceridemia and advanced pregnancy, an informed decision was taken to start the patient on oral gemfibrozil (300 mg twice a day). Also, a consultation with a transfusion medicine physician was sought to decide on therapeutic plasma exchange (TPE). However, as the patient was improving clinically with a significant drop in triglycerides, MDT decided against initiating TPE. The rest of the gestation period was uneventful, and she delivered a healthy neonate at 40 weeks of gestation with normal vaginal delivery (Fig. 1).
Discussion
Our patient had a homozygous missense variation causing LPL deficiency. She developed HTG-related pancreatitis during her both conceptions during the third trimester. The principles of management included: (1) MDT approach, (2) hospitalization for initiation and intensification of LFD, (3) supplementation with MCT oil and PUFA, (4) judicious use of lipid-lowering agents like fibrate, (5) coordinated delivery at term gestation and (6) family screening and proactive treatment of the relative (sister in our case).
All lipoprotein fractions increase normally during pregnancy as a physiological adaptation. These changes are predominantly dependent on the changes in the maternal hormonal milieu. Estrogen stimulates hepatic lipogenesis causing an increase in LDL and VLDL. It also decreases the effect of LPL, causing reduced catabolism of the triglycerides by the liver and fat. Also, exogenous triglycerides related to increased appetite add to this endogenous lipid pool. Normally, these changes do not have any clinical connotations. However, in the background of either increased endogenous triglyceride production or decreased triglyceride catabolism, these changes may cause severe HTG [2]. Also, clinicians need to be wary of the in vitro assay interference in the background of severe HTG, which may cause fallacious results for amylase, glucose, etc.—based on the analytical platform used.
Considering the rarity of the condition, we do not have any clinical guidelines for the management of HTG in pregnancy. Most of the literature is limited to personal experiences and case reports. However, with a better understanding of the condition, the maternal and neonatal morbidity due to this condition is on a decreasing trend (which in the remote past was 20% and 21%, respectively). LFD, MCT oil and PUFA are the earliest interventions for the management of HTG, with minuscule effects on the fetus. However, these interventions seldom reduce triglycerides by more than 20% from the baseline. During the third trimester (when the HTG peaks) or during the complications like pancreatitis—an informed decision on the use of fibrates and niacin becomes imperative to quickly reduce the triglyceride levels. Unfortunately, we do not have safety data on these drugs during pregnancy [3]. Nonetheless, teratogenicity has not been reported in the cases where these drugs were used. There are reports of successful management of HTG with TPE. However, its effects are short-lived—necessitating multiple sessions of TPE [4]. Gene therapy, APOC III inhibitors and ANGPTL3 inhibitors are the exciting newer additions to the therapeutic armamentarium targeting HTG. However, their safety in pregnancy remains to be proven.
Most of the complications due to HTG in pregnancy have been reported during the third trimester. Clinically stable condition of our patient and the decreasing trend of triglycerides on LFD, MCT oil and PUFA prevented us from resorting to more aggressive therapeutic interventions. In the hindsight, earlier initiation of fibrates may have prevented the episode of pancreatitis in our patient. Initiation of fibrates in the early third trimester to prevent complications may be a prudent approach in the selected patient. Nevertheless, it cannot be denied that a multidisciplinary approach, close follow-up and proactive therapeutic interventions, improve the outcome for both the child and the mother.
Funding
Not applicable.
Declarations
Conflict of interest
None of the authors have anything to disclose with regard to this manuscript.
Ethics Approval
Institutional ethics committee approval was obtained for this manuscript.
Patient Consent
Informed consent has been obtained from the patient for publication of the case report and accompanying images.
Footnotes
Dr. Krishna Mori, M.D. (General Medicine), Senior Resident, Department of Endocrinology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India. Dr. Krishna Mori is currently pursuing D.M. Endocrinology in Sri Ramachandra Medical College. He has an avid interest in reproductive endocrinology and endocrine disorders in pregnancy. Dr. Priyadarshini Rajakumar, M.D., Senior Resident, Department of Endocrinology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India. Dr. Amulya Yalamanchi, M.D., Senior Resident, Department of Endocrinology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India. Dr. K. S. Rajeswari, M.S., DNB, Professor, Department of Obstretics and Gynecology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India. Dr. Karthik Balachandran, M.D. D.M., Assistant Professor, Department of Endocrinology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India. Dr. Adlyne Reena Asirvatham, M.D. D.M., Professor, Department of Endocrinology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India. Dr. Shriraam Mahadevan M.D. D.M., Professor, Department of Endocrinology, Sri Ramachandra Institute of Higher Education and Research, Chennai, India.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.YalcinBahat P, Turan G, Aslan CB. Abruptio placentae caused by hypertriglyceridemia-induced acute pancreatitis during pregnancy: case report and literature review. Case Rep Obstet Gynecol. 2018;2018:3869695. doi: 10.1155/2018/3869695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cruciat G, Nemeti G, Goidescu I, Anitan S, Florian A. Hypertriglyceridemia triggered acute pancreatitis in pregnancy—diagnostic approach, management and follow-up care. Lipids Health Dis. 2020;19(1):2. doi: 10.1186/s12944-019-1180-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kleess LE, Janicic N. Severe hypertriglyceridemia in pregnancy: a case report and review of the literature. AACE Clin Case Rep. 2018;5(2):e99–e103. doi: 10.4158/ACCR-2018-0168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tan SYT, Teh SP, Kaushik M, Yong TT, Durai S, Tien CJ, Gardner DS. Hypertriglyceridemia-induced pancreatitis in pregnancy: case review on the role of therapeutic plasma exchange. Endocrinol Diabetes Metab Case Rep. 2021;2021:21–0017. doi: 10.1530/EDM-21-0017. [DOI] [PMC free article] [PubMed] [Google Scholar]