Abstract
In the current investigation, we explored the benefits of aucubin against rodent ischemia/reperfusion (I/R) damages in brains and elucidated the role of 5'-AMP-activated protein kinase (AMPK) in its neuroprotective action. I/R model of brain was established in male three-month-old rats through 2 h of middle cerebral artery occlusion followed by two days of reperfusion. Aucubin boosted phosphorylation of AMPKα in ipsilateral cortex of injured rats. Then, rats were exposed to cerebral I/R damage and received treatment of aucubin and compound C (a well-known AMPK inhibitor). It was found that aucubin administration improved neurological symptom score, decreased infarct volume, and mitigated cerebral edema in injured rats. Aucubin administration upregulated Nrf2 expression and abated oxidative stress in ipsilateral cortex of injured rats. Aucubin administration reduced levels of multiple pro-inflammatory cytokines, suppressed microglial activation and neutrophil infiltration, and promoted M2 polarization in injured rats. More importantly, compound C abolished the neuroprotective, anti-oxidant and inflammation-modulating effects of aucubin in injured rats, at least in part. Therefore, we concluded that activation of AMPK by aucubin alleviated I/R injury in brain through abating oxidative stress and suppressing inflammation, identifying a potential candidate for those patients of ischemic stroke.
Keywords: Oxidative stress, 5'-AMP-activated protein kinase, M2 polarization, Neurological impairment, Inflammation
Introduction
Ischemic stroke results from thromboembolic occlusion of cerebral arteries and is responsible for physical disability and mortality globally (Katan and Luft 2018). The incidence of ischemic stroke is fast increasing with the growing population aged 65 and over (Donkor 2018). Up to now, options for treating ischemic stroke are still limited. Thus, there is of great clinical value for developing better neuroprotective agents for patients with ischemic stroke.
There is a growing interest in developing natural medicines for treatment of ischemic stroke in recent years (Tao et al. 2020). Aucubin is an iridoid glycoside with abundant potential sources and has multiple beneficial properties including antioxidant, anti-fibrogenic, anti-aging, and anti-inflammatory activities (Zeng et al. 2020). Aucubin treatment protected neurons against H2O2-induced damages in vitro (Li et al. 2021; Wang et al. 2020). Aucubin administration attenuated weight-drop-induced experimental murine traumatic brain injury (Wang et al. 2020). Additionally, aucubin provided neuroprotective effects in streptozotocin-induced diabetic encephalopathy (Xue et al. 2009), 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-related cerebral toxicity (Zhu et al. 2018), and lithium-pilocarpine-induced status epilepticus (Wang et al. 2017; Chen et al. 2019).
Mechanistically, ischemic stroke is composed of series of pathophysiological events: apoptosis, oxidative and nitrative stress, excitotoxicity, blood–brain barrier dysfunction, and inflammatory reaction (Maida et al. 2020). Numerous reports have demonstrated the beneficial role of 5'-AMP-activated protein kinase (AMPK) signaling in the cerebrum under ischemic stimuli (Jiang et al. 2018), such as activating downstream nuclear factor erythroid 2-related factor 2 (Nrf2) (Yu et al. 2020). The network of Nrf2 not only counteracted oxidative stress, but also regulated inflammatory reaction (Liu et al. 2019).
Our work aimed to explore the benefit of aucubin on the oxidative damage and inflammation in rats with cerebral I/R and simultaneously investigate the role of AMPK in its neuroprotective action.
Materials and methods
Ethical statement and animal
The animals’ studies were done under the supervision of the Animal Ethics Committee of the 305 Hospital of the People’s Liberation Army (NO. 2021–24), and reported in accordance with ARRIVE guidelines. Vital River (Beijing, China) provided the Sprague–Dawley (SD) rats (male, three-month-old) used in our study.
Rodent model of MCAO and study design
As described previously (Zhang et al. 2012), I/R model in rodent brains was established via MCAO by investigators blinded to experimental design. Following sterile preparation and anesthesia induction for animals with isoflurane (induction using 4% isoflurane and maintenance using 2% isoflurane), the neck skin incision was scissored, and the left common carotid artery (CCA), external carotid artery, and internal carotid artery were exposed and separated. Then, an incision was made in the proximal CCA to allow for inserting a nylon filament (long: 18 mm; diameter: 0.26 mm) to the end of internal carotid artery, for occluding middle cerebral artery. 120 min later, the filaments were withdrawn for reperfusion. The sham rats received similar procedures, but the nylon filament was not inserted. The physiological parameters were monitored during the whole operation.
Aucubin (dissolved in physiological saline, Winherb Medical Science, Shanghai, China) was administrated intraperitoneally (1 mg/kg and 10 mg/kg) (Yang et al. 2018) twice at 4 h and 24 h after reperfusion. Compound C (CC, 20 mg/kg body weight, P5499, Sigma, USA) (Wang et al. 2018), an AMPK inhibitor, was administrated intraperitoneally 30 min before aucubin administration.
Experiment 1: Animas were exposed to cerebral I/R damage and divided randomly into three groups as follows: (1) vehicle-treated group (n = 6); (2) low-dose (1 mg/kg) aucubin-treated group (n = 6); (3) high-dose (10 mg/kg) aucubin-treated group (n = 7).
Experiment 2: Animas were divided randomly into four groups as follows: (1) Sham-operated group (n = 3); (2) vehicle-treated MCAO group (n = 3); (3) low-dose (1 mg/kg) aucubin-treated MCAO group (n = 3); (4) high-dose (10 mg/kg) aucubin-treated MCAO group (n = 3).
Experiment 3: Animas were exposed to cerebral I/R damage and divided randomly into three groups as follows: (1) vehicle-treated group (n = 6); (2) group treated with aucubin (10 mg/kg) (n = 7); (3) group treated with aucubin (10 mg/kg) and Compound C (20 mg/kg) (n = 7).
Experiment 4: Animas were divided randomly into four groups as follows: (1) Sham-operated group (n = 25); (2) vehicle-treated MCAO group (n = 23); (3) MCAO group treated with aucubin (10 mg/kg) (n = 25); (4) MCAO group treated with aucubin (10 mg/kg) and Compound C (20 mg/kg) (n = 23).
Neurological function assessment
Rodent neurological function was measured at 2 days post-operation as the method previously described (Longa et al. 1989), on a five-point scale system by three operators blinded to experimental design. Grade 4: very severe (no spontaneous motor activity); grade 3: severe (falling to right); grade 2: moderate (circling to right); grade 1: mild (failure to extend right forepaw fully); grade 0: normal (no observable neurological deficit).
Assessment of infarct volume and cerebral edema
Two days after cerebral reperfusion, brains of animals were removed, cut into slices, and used for staining of 2,3,5-triphenyltetrazolium chloride (TTC, #17779, Sigma). Finally, all slices were photographed and analyzed using the software of Image J (NIH, Maryland, USA) by the operators blinded to experimental design.
Ipsilateral hemispheres were harvested and immediately weighed. The dry weight of ipsilateral hemispheres was obtained after being dried at 110 °C for 16 h. Water content of ipsilateral hemispheres was obtained using the formula of (1-dry weight/wet weight) × 100%.
Biochemical analysis
Two days after cerebral reperfusion, the sera were separated through centrifuging for 15 min at 2000 × g. The ipsilateral cortices were harvested. Homogenates were then extracted using the RIPA lysis buffer (HY-K1001, MedChemExpress). Concentrations of protein samples were detected using Bradford reagent (P0006, Beyotime). The heme oxygenase-1 (HO-1) and myeloperoxidase (MPO) activities were detected as the methods described previously (Choi et al. 2003; Hillegass et al. 1990). The levels of IL-1β (ab255730, Abcam), neuron-specific enolase (NSE, NBP2-76,684, Novus Biologicals, USA), TNF-α (RTA00, Novus Biologicals), S100 calcium-binding protein beta (S-100β, ab234573, Abcam). and IL-6 (R6000B, R&D) in sera were assayed using specific kits. The activities of glutathione peroxidase (GPx, S0058, Beyotime), superoxide dismutase (SOD, S0101S, Beyotime), and catalase (S0051, Beyotime) and levels of 8-hydroxy-2'-deoxyguanosine (8-OHdG, ab285254, Abcam) were assayed with kits. The levels of INF-γ (RIF00), IL-10 (R1000), IL-17A (DY8410-05), and ATP (NBP2-54,855) in ipsilateral cortex were assayed using kits from Novus Biologicals. The NAD+ levels in ipsilateral cortex were detected with an assay kit (K958, BioVision, USA).
Western blot
The nuclear proteins were separated and collected using a Kit (Beyotime, #P0027). Proteins of 25 μg were loaded on 8% SDS–polyacrylamide gel, electrotransferred to Immobilon-E PVDF Membrane (IEVH85R, Merck Millipore, Billerica, MA, USA), and incubated with the primary antibodies including Nrf2 (NBP1-32,822, Novus Biologicals), AMPKα (No.5831, Cell Signaling, Danvers, MA, USA), p-AMPKα (Thr172, No.50081, Cell Signaling), histone H3 (ab1791, Abcam, USA), or GAPDH (NB300-221, Novus Biologicals) at room temperature using slow rocking for 4 h. Protein bands of samples were quantified by software of ImageJ in densitometry measurements.
Real-time PCR measurements
Ipsilateral cortices were harvested for extracting RNA with TRIzol reagent (R0011, Beyotime). The RT-PCR procedure was done using ABI PRISM 7000 Sequence Detection System (Applied Biosystems) (Zhao et al. 2016). The primers of target genes (Wang et al. 2018; Chen et al. 2021) were presented in Table 1. The ΔΔCt method was applied for calculation of relative changes of mRNA levels.
Table 1.
Primers of target genes
| Target genes | Sequence (5’-3’) |
|---|---|
| Mrc1 | GGTTCCGGTTTGTGGAGCAG |
| TCCGTTTGCATTGCCCAGTA | |
| Arg1 | ATCGGAGCGCCTTTCTCTAA |
| AGACCGTGGGTTCTTCACAA | |
| Nos2 | GCAGAATGTGACCATCATGG |
| ACAACCTTGGTGTTGAAGGC | |
| Gapdh | CCCCCAATGTATCCGTTGTG |
| TAGCCCAGGATGCCCTTTAGT |
Immunofluorescence analysis
Animals were perfused transcardially with icecold physiological saline and then paraformaldehyde (4%). The brains were then sliced into Sects. (8 μm thick). H&E staining and ionized calcium binding adaptor molecule 1 (IBA-1, Abcam, ab5076) immunofluorescence staining was conducted according to the standard method (Xiao et al. 2016).
Statistical analysis
Statistical significance among groups was defined when P value < 0.05. Data analysis was done in current investigation using one-way ANOVA followed by Bonferroni post hoc test. Obtained results in our work were presented as the mean ± SD.
Results
Aucubin administration protected against the MCAO-induced damages in brains
Neurological impairment and infarct volume were assessed in rats 2 days following I/R surgery. Aucubin administration (1 mg/kg, 10 mg/kg) dose-dependently mitigated neurological deficits (Fig. 1B) and decreased infarct volume (Fig. 1C) in injured rats.
Fig. 1.
Neuroprotective effect of aucubin. A, structure of aucubin. Graphs showed the neurological score (B) and infarct volume (C, scale bar = 1 cm) when treated with aucubin (1 mg/kg, 10 mg/kg). Western blot of AMPKα and p-AMPKα (D) and quantification analysis were presented. * P < 0.05 vs. Sham; # P < 0.05 vs. MCAO + vehicle
Phosphorylation of AMPKα was enhanced in the ipsilateral cortex of injured rats than that in control rats. Administration of injured rats with aucubin aggravated the phosphorylation of AMPKα in ipsilateral cortex (Fig. 1D).
The neuroprotective effect of aucubin was dependent on AMPK
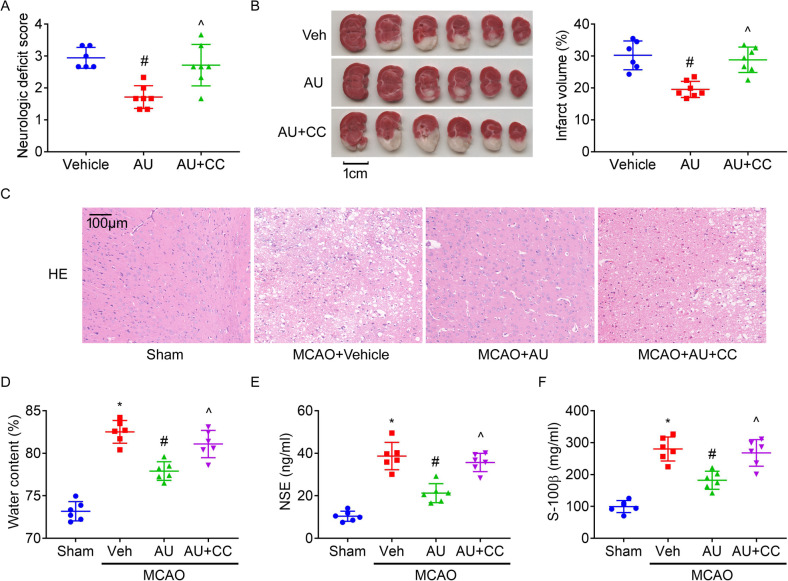
Rats were exposed to MCAO surgery and treated with aucubin and Compound C. It was found that inhibiting AMPK by co-treatment with Compound C abolished the benefits of aucubin on neurological impairment (Fig. 2A) and infarct size (Fig. 2B) in injured rats.
Fig. 2.
The neuroprotective effects of aucubin. Graphs showed the neurological score (A) and infarct volume (B, scale bar = 1 cm) when treated with aucubin (AU, 10 mg/kg) and Compound C (CC, 20 mg/kg) or not. H&E staining of cortical area (D). Graphs showed water content (D) of ipsilateral brains and levels of NSE (E) and S-100β (F) in sera. * P < 0.05 vs. Sham; # P < 0.05 vs. MCAO + vehicle; ^ P < 0.05 vs. MCAO + aucubin
In H&E staining results, no morphological change was found in cerebral cortex of Sham-operated rats. But, the MCAO rats presented neuronal loss, pyknotic nuclei and vacuoles around the nucleus (Fig. 2C) in cerebral cortex. Aucubin treatment attenuated the extent of the pathological damage, which was reversed by Compound C.
Furthermore, administration of injured rats with aucubin reduced water content (Fig. 2D, an indicator of cerebral edema) of ipsilateral brains and levels of NSE (Fig. 2E) and S-100β (Fig. 2F) (two biomarkers of cerebral damages) in serum, which were reversed by Compound C.
The anti-oxidative effect of aucubin in MCAO rats was dependent on AMPK
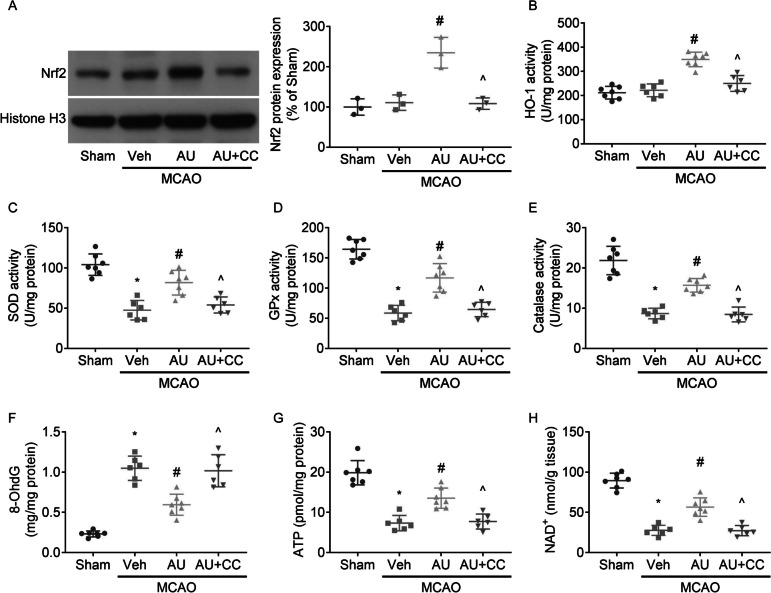
MCAO exposure in rats did not alter the nuclear protein levels of Nrf2 (Fig. 3A) or activities of HO-1 (Fig. 3B), but reduced SOD (Fig. 3C), GPx (Fig. 3D) and catalase (Fig. 3E) activities and ATP (Fig. 3F) and NAD + (Fig. 3H) levels, and enhanced 8-OhdG (Fig. 3G) production in ipsilateral cortex.
Fig. 3.
The anti-oxidative effects of aucubin. Western blot of Nrf2 protein expression (A) and quantification assessment were presented. Graphs showed the activities of HO-1 (B), SOD (C), GPx (D), and catalase (E) and levels of 8-OhdG (F), ATP (G), and NAD + (H) in ipsilateral cortex. * P < 0.05 vs. Sham; # P < 0.05 vs. MCAO + vehicle; ^ P < 0.05 vs. MCAO + aucubin
Aucubin administration upregulated nuclear Nrf2 expression, enhanced activities of HO-1, SOD, GPx, and catalase and levels of ATP and NAD + , and decreased 8-OhdG production in ipsilateral cortex, which were reversed by co-treatment with Compound C.
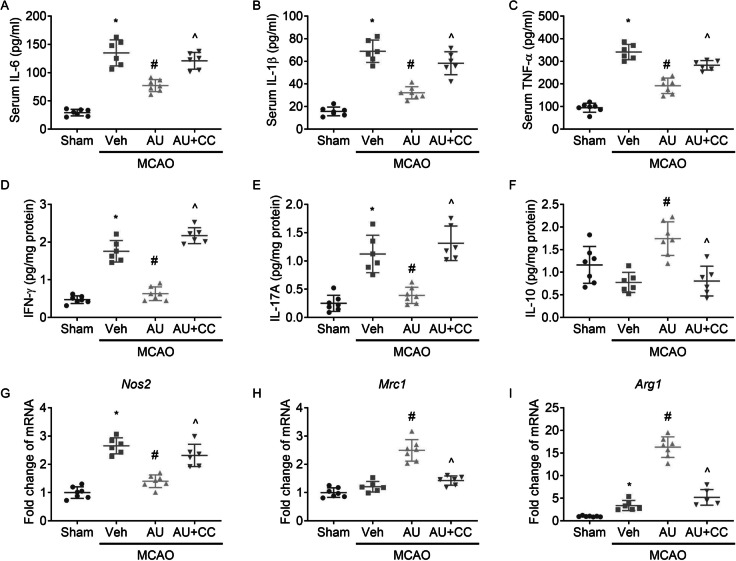
Aucubin-mediated inhibition on inflammation
MCAO exposure in rats led to enhanced content of IL-6, IL-1β and TNF-α (Fig. 4A, B, C) in sera and IFN-γ (Fig. 4D) and IL-17A (Fig. 4E) protein levels and Nos2 (Inos, Fig. 4G) and Arg1 (Arg-1, Fig. 4I) mRNA levels in ipsilateral cortex, as compared to sham-operated rats. MCAO exposure did not significantly alter IL-10 (Fig. 4F) protein expression or Mrc1 (CD206, Fig. 4H) mRNA expression in ipsilateral cortex.
Fig. 4.
Aucubin mitigated inflammation in an AMPK-dependent manner. Graphs showed the serum levels of IL-6 (A), IL-1 beta (IL-1β, B), and TNF alpha (TNF-α, C), and protein levels of interferon gamma (IFN-γ, D), IL-17A (E), and IL-10 (F) and mRNA levels of Nos2 (iNOS, G), Mrc1 (CD206, H) and Arg1 (Arg-1, I) in ipsilateral cortex. * P < 0.05 vs. Sham; # P < 0.05 vs. MCAO + vehicle; ^ P < 0.05 vs. MCAO + aucubin
Aucubin administration reduced IL-6, IL-1β and TNF-α levels in sera and protein levels of IFN-γ and IL-17A and mRNA level of Nos2 in ipsilateral cortex, and enhanced IL-10 protein level and Mrc1 and Arg1 mRNA levels in ipsilateral cortex. Our results suggested that aucubin administration promotes M2 polarization and inhibited inflammatory stress in injured brains.
Inhibiting AMPK by Compound C abolished the benefits of aucubin on inflammation and M2 polarization in injured brains.
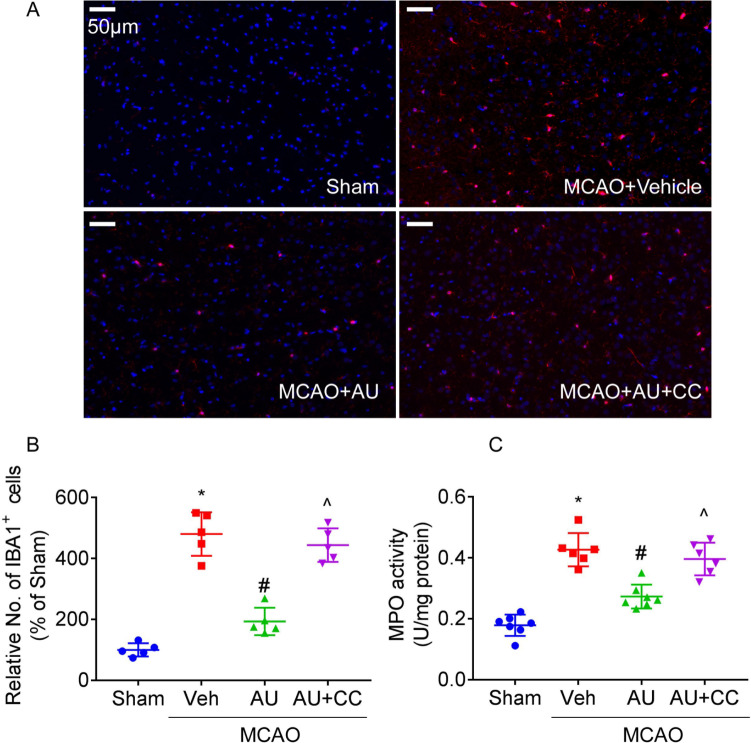
Aucubin-mediated inhibition on microglial activation and neutrophil infiltration
MCAO exposure in rats led to enhancement of number of IBA-positive cells (Fig. 5A, B) in ischemic penumbra region and activities of MPO (Fig. 5C, an indicator of neutrophil infiltration) in ipsilateral cortex. Aucubin administration reduced number of IBA-positive cells and activity of MPO in injured brains. The benefits of aucubin on numbers of IBA-positive cells and activities of MPO in injured brains were abolished by Compound C.
Fig. 5.
The inhibitory effects of aucubin on microglial activation and neutrophil infiltration. Representative pictures (A, scale bar = 50 μm) and responding quantification (B) of ischemic penumbra region for immunofluorescence using antibody recognizing IBA-1 were presented. Graph (C) showed the activities of MPO in ipsilateral cortex. * P < 0.05 vs. Sham; # P < 0.05 vs. MCAO + vehicle; ^ P < 0.05 vs. MCAO + aucubin
Discussion
In our study, we investigated the neuroprotective effects of aucubin against I/R damages in brain of rats, as well as the potential mechanisms. The results we have obtained demonstrated the benefits of aucubin against ischemic stroke and suggested that aucubin-induced neuroprotection was due to its inhibition of inflammation and oxidative stress through activating AMPK, at least in part.
The current treatment for acute ischemic stroke was to recanalize the blood flow, which had the disadvantage that reperfusion resulted in excessive formation of reactive oxygen species (ROS), generating subsequent oxidative damage (Orellana-Urzúa et al. 2020). Nrf2 targeted more than 200 ARE-driven genes and controlled several antioxidant and cytoprotective proteins (Bellezza et al. 2018). It was demonstrated that upregulation of Nrf2 contributed to the mitigation of acute injury and functional recovery from cerebral ischemic stroke (Farina et al. 2021; Wang et al. 2018). In our work, aucubin treatment upregulated Nrf2 expression in injured brains, which contributed to the mitigation of deleterious ROS during the ischemic cascade.
Apart from oxidative stress, inflammation from activated resident microglia and infiltration of circulating immune cells into the infarct and peri-infarct core is another important pathological event in acute phase of ischemic stroke (Jayaraj et al. 2019). The activated microglia and infiltrated monocytes/macrophages played a dual role in the ischemic stroke, because of distinct phenotypes including alternative protective M2 and pro-inflammatory M1 (Kanazawa et al. 2017). It was reported that aucubin treatment in rat articular chondrocytes prevented IL-1β-induced inflammation (Wang et al. 2015). In the parkinsonian mouse model, aucubin inhibited the activation of both astrocyte and microglia in substantia nigra (Wang et al. 2017). In our work, aucubin treatment upregulated M2-associated genes expression (CD206 and Arg1) and downregulated M1-associated gene expression (NOS2) in ischemic brains. It indicated that aucubin promoted M2 polarization, which contributed to the mitigation of ischemic brain injury and promoted post-stroke repair.
Mechanistically, treatment with aucubin promoted AMPK activation in ischemic brains and inhibition of AMPK could abolish the promoting effects of aucubin on Nrf2 pathway and M2 polarization. AMPK exerted protective properties in brain when exposed to ischemic stimuli (Jiang et al. 2018). AMPK activation directly enhanced Nrf2 abundance by promoting Nrf2 phosphorylation at Serine 550 (Joo et al. 2016), by p62-dependent activation of Nrf2 (Lee et al. 2016), or by impeding GSK3β-mediated degradation of Nrf2 (Lv et al. 2017). Additionally, transfection of bone marrow-derived and monocyte-derived macrophages with a constitutively active form of AMPKα led to reduced LPS-induced generation of proinflammatory cytokines and upregulated IL-10 production (Sag et al. 2008). AMPK activation enhanced microglia M2 polarization and suppressed inflammation in ischemic stroke (Wang et al. 2018). Thus, aucubin activated Nrf2 pathway and promoted anti-inflammatory M2 polarization through activating AMPK in ischemic stroke.
In conclusion, treatment with aucubin in rats mitigated ischemic stroke, abated oxidative stress and suppressed neuroinflammation through activating AMPK. In addition, the aucubin exhibited good safety and tolerability with a minimum lethal dose at 900 mg/kg body weight in intraperitoneal administration of mice (Li et al. 2021). Therefore, aucubin may become a potential candidate for treating ischemic stroke.
Author contributions
Conceptualization: Jin-jing Zhao, Bo Zhao, Rui Xu; Methodology: Jin-jing Zhao; Formal analysis and investigation: Jin-jing Zhao, Bo Zhao, Xiao Bai, Shuang Zhang, Rui Xu; Writing—original draft preparation: Jin-jing Zhao, Rui Xu; Writing—review and editing: Jin-jing Zhao, Bo Zhao, Rui Xu; Supervision: Rui Xu.
Data availability
The datasets analyzed or used in our investigation are available from the corresponding author without undue reservation.
Declarations
Ethics approval
All experiments involving animals were approved by the Animal Ethics Committee of the 305 Hospital of the People’s Liberation Army and were conducted with strict adherence to the American Association for the Accreditation of Laboratory Animal Care International and National Institutes of Health Guidelines embodied in the Guide for the Care and Use of Laboratory Animals.
Competing interests
The authors declare that there are no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Jin-jing Zhao and Bo Zhao equally contributed to the current investigation
References
- Bellezza I, Giambanco I, Minelli A, Donato R. Nrf2-Keap1 signaling in oxidative and reductive stress. Biochim Biophys Acta Mol Cell Res. 2018;1865:721–733. doi: 10.1016/j.bbamcr.2018.02.010. [DOI] [PubMed] [Google Scholar]
- Chen S, Zeng X, Zong W, Wang X, Chen L, Zhou L, Li C, Huang Q, Huang X, Zeng G, Hu K, Ouyang DS. Aucubin alleviates seizures activity in li-pilocarpine-induced epileptic mice: Involvement of inhibition of neuroinflammation and regulation of neurotransmission. Neurochem Res. 2019;44:472–484. doi: 10.1007/s11064-018-2700-y. [DOI] [PubMed] [Google Scholar]
- Chen F, Hu M, Shen Y, Zhu W, Cao A, Ni B, Qian J, Yang J. Isorhamnetin promotes functional recovery in rats with spinal cord injury by abating oxidative stress and modulating M2 macrophages/microglia polarization. Eur J Pharmacol. 2021;895:173878. doi: 10.1016/j.ejphar.2021.173878. [DOI] [PubMed] [Google Scholar]
- Choi BM, Pae HO, Chung HT. Nitric oxide priming protects nitric oxide-mediated apoptosis via heme oxygenase-1 induction. Free Radic Biol Med. 2003;34:1136–1145. doi: 10.1016/s0891-5849(03)00064-9. [DOI] [PubMed] [Google Scholar]
- Ding R, Wu W, Sun Z, Li Z. AMP-activated protein kinase: An attractive therapeutic target for ischemia-reperfusion injury. Eur J Pharmacol. 2020;888:173484. doi: 10.1016/j.ejphar.2020.173484. [DOI] [PubMed] [Google Scholar]
- Donkor ES. Stroke in the 21st century: A snapshot of the burden, epidemiology and quality of life. Stroke Res Treat. 2018;2018:3238165. doi: 10.1155/2018/3238165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina M, Vieira LE, Buttari B, Profumo E, Saso L. The Nrf2 Pathway in Ischemic Stroke: A Review. Molecules. 2021;26:5001. doi: 10.3390/molecules26165001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillegass LM, Griswold DE, Brickson B, Albrightson-Winslow C. Assessment of myeloperoxidase activity in whole rat kidney. J Pharmacol Methods. 1990;24:285–295. doi: 10.1016/0160-5402(90)90013-B. [DOI] [PubMed] [Google Scholar]
- Jayaraj RL, Azimullah S, Beiram R, Jalal FY, Rosenberg GA. Neuroinflammation: friend and foe for ischemic stroke. J Neuroinflammation. 2019;16:142. doi: 10.1186/s12974-019-1516-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang S, Li T, Ji T, Yi W, Yang Z, Wang S, Yang Y, Gu C. AMPK: Potential Therapeutic Target for Ischemic Stroke. Theranostics. 2018;8:4535–4551. doi: 10.7150/thno.25674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo MS, Kim WD, Lee KY, Kim JH, Koo JH, Kim SG. AMPK facilitates nuclear accumulation of Nrf2 by phosphorylating at serine 550. Mol Cell Biol. 2016;36:1931–1942. doi: 10.1128/MCB.00118-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa M, Ninomiya I, Hatakeyama M, Takahashi T, Shimohata T. Microglia and monocytes/macrophages polarization reveal novel therapeutic mechanism against stroke. Int J Mol Sci. 2017;18:2135. doi: 10.3390/ijms18102135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katan M, Luft A. Global burden of stroke. Semin Neurol. 2018;38:208–211. doi: 10.1055/s-0038-1649503. [DOI] [PubMed] [Google Scholar]
- Lee DH, Han DH, Nam KT, Park JS, Kim SH, Lee M, Kim G, Min BS, Cha BS, Lee YS, Sung SH, Jeong H, Ji HW, Lee MJ, Lee JS, Lee HY, Chun Y, Kim J, Komatsu M, Lee YH, Bae SH. Ezetimibe, an NPC1L1 inhibitor, is a potent Nrf2 activator that protects mice from diet-induced nonalcoholic steatohepatitis. Free Radic Biol Med. 2016;99:520–532. doi: 10.1016/j.freeradbiomed.2016.09.009. [DOI] [PubMed] [Google Scholar]
- Li YC, Hao JC, Shang B, Zhao C, Wang LJ, Yang KL, He XZ, Tian QQ, Wang ZL, Jing HL, Li Y, Cao YJ. Neuroprotective effects of aucubin on hydrogen peroxide-induced toxicity in human neuroblastoma SH-SY5Y cells via the Nrf2/HO-1 pathway. Phytomedicine. 2021;87:153577. doi: 10.1016/j.phymed.2021.153577. [DOI] [PubMed] [Google Scholar]
- Liu L, Locascio LM, Doré S. Critical Role of Nrf2 in Experimental Ischemic Stroke. Front Pharmacol. 2019;10:153. doi: 10.3389/fphar.2019.00153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y, Chu G, Shen W, Zhang Y, Xu W, Yu Y. XMU-MP-1 protects heart from ischemia/reperfusion injury in mice through modulating Mst1/AMPK pathway. Eur J Pharmacol. 2022;919:174801. doi: 10.1016/j.ejphar.2022.174801. [DOI] [PubMed] [Google Scholar]
- Longa EZ, Weinstein PR, Carlson S, Cummins R. Reversible middle cerebral artery occlusion without craniectomy in rats. Stroke. 1989;20:84–91. doi: 10.1161/01.str.20.1.84. [DOI] [PubMed] [Google Scholar]
- Lv H, Liu Q, Wen Z, Feng H, Deng X, Ci X. Xanthohumol ameliorates lipopolysaccharide (LPS)-induced acute lung injury via induction of AMPK/GSK3β-Nrf2 signal axis. Redox Biol. 2017;12:311–324. doi: 10.1016/j.redox.2017.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maida CD, Norrito RL, Daidone M, Tuttolomondo A, Pinto A. Neuroinflammatory Mechanisms in Ischemic Stroke: Focus on Cardioembolic Stroke, Background, and Therapeutic Approaches. Int J Mol Sci. 2020;21:6454. doi: 10.3390/ijms21186454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orellana-Urzúa S, Rojas I, Líbano L, Rodrigo R. Pathophysiology of ischemic stroke: Role of oxidative stress. Curr Pharm Des. 2020;26:4246–4260. doi: 10.2174/1381612826666200708133912. [DOI] [PubMed] [Google Scholar]
- Sag D, Carling D, Stout RD, Suttles J. Adenosine 5'-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J Immunol. 2008;181:8633–8641. doi: 10.4049/jimmunol.181.12.8633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao T, Liu M, Chen M, Luo Y, Wang C, Xu T, Jiang Y, Guo Y, Zhang JH. Natural medicine in neuroprotection for ischemic stroke: Challenges and prospective. Pharmacol Ther. 2020;216:107695. doi: 10.1016/j.pharmthera.2020.107695. [DOI] [PubMed] [Google Scholar]
- Wang SN, Xie GP, Qin CH, Chen YR, Zhang KR, Li X, Wu Q, Dong WQ, Yang J, Yu B. Aucubin prevents interleukin-1 beta induced inflammation and cartilage matrix degradation via inhibition of NF-κB signaling pathway in rat articular chondrocytes. Int Immunopharmacol. 2015;24:408–415. doi: 10.1016/j.intimp.2014.12.029. [DOI] [PubMed] [Google Scholar]
- Wang J, Li Y, Huang WH, Zeng XC, Li XH, Li J, Zhou J, Xiao J, Xiao B, Ouyang DS, Hu K. The protective effect of Aucubin from eucommia ulmoides against status epilepticus by inducing autophagy and inhibiting necroptosis. Am J Chin Med. 2017;45:557–573. doi: 10.1142/S0192415X17500331. [DOI] [PubMed] [Google Scholar]
- Wang Y, Huang Y, Xu Y, Ruan W, Wang H, Zhang Y, Saavedra JM, Zhang L, Huang Z, Pang T. A dual AMPK/Nrf2 activator reduces brain inflammation after stroke by enhancing microglia M2 polarization. Antioxid Redox Signal. 2018;28:141–163. doi: 10.1089/ars.2017.7003. [DOI] [PubMed] [Google Scholar]
- Wang H, Zhou XM, Wu LY, Liu GJ, Xu WD, Zhang XS, Gao YY, Tao T, Zhou Y, Lu Y, Wang J, Deng CL, Zhuang Z, Hang CH, Li W. Aucubin alleviates oxidative stress and inflammation via Nrf2-mediated signaling activity in experimental traumatic brain injury. J Neuroinflammation. 2020;17:188. doi: 10.1186/s12974-020-01863-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Zhou D, Tan RJ, Fu H, Zhou L, Hou FF, Liu Y. Sustained activation of Wnt/β-Catenin signaling drives AKI to CKD progression. J Am Soc Nephrol. 2016;27:1727–1740. doi: 10.1681/ASN.2015040449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue HY, Jin L, Jin LJ, Li XY, Zhang P, Ma YS, Lu YN, Xia YQ, Xu YP. Aucubin prevents loss of hippocampal neurons and regulates antioxidative activity in diabetic encephalopathy rats. Phytother Res. 2009;23:980–986. doi: 10.1002/ptr.2734. [DOI] [PubMed] [Google Scholar]
- Yang Z, Wu QQ, Xiao Y, Duan MX, Liu C, Yuan Y, Meng YY, Liao HH, Tang QZ. Aucubin protects against myocardial infarction-induced cardiac remodeling via nNOS/NO-regulated oxidative stress. Oxid Med Cell Longev. 2018;2018:4327901. doi: 10.1155/2018/4327901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J, Wang WN, Matei N, Li X, Pang JW, Mo J, Chen SP, Tang JP, Yan M, Zhang JH. Ezetimibe attenuates oxidative stress and neuroinflammation via the AMPK/Nrf2/TXNIP pathway after MCAO in rats. Oxid Med Cell Longev. 2020;2020:4717258. doi: 10.1155/2020/4717258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng X, Guo F, Ouyang D. A review of the pharmacology and toxicology of aucubin. Fitoterapia. 2020;140:104443. doi: 10.1016/j.fitote.2019.104443. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Chen J, Li F, Li D, Xiong Q, Lin Y, Zhang D, Wang XF, Yang P, Rui YC. A pentapeptide monocyte locomotion inhibitory factor protects brain ischemia injury by targeting the eEF1A1/endothelial nitric oxide synthase pathway. Stroke. 2012;43:2764–2773. doi: 10.1161/STROKEAHA.112.657908. [DOI] [PubMed] [Google Scholar]
- Zhao JJ, Song JQ, Pan SY, Wang K. Treatment with isorhamnetin protects the brain against ischemic injury in mice. Neurochem Res. 2016;41:1939–1948. doi: 10.1007/s11064-016-1904-2. [DOI] [PubMed] [Google Scholar]
- Zhu YL, Sun MF, Jia XB, Zhang PH, Xu YD, Zhou ZL, Xu ZH, Cui C, Chen X, Yang XS, Shen YQ. Aucubin alleviates glial cell activation and preserves dopaminergic neurons in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced parkinsonian mice. NeuroReport. 2018;29:1075–1083. doi: 10.1097/WNR.0000000000001075. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets analyzed or used in our investigation are available from the corresponding author without undue reservation.