Abstract
Adriamycin (ADR) is an important chemotherapeutic drug, but it has serious side effects such as hepatotoxicity. This study aimed to evaluate whether N-acetylcysteine (NAC) has hepatoprotective effects against ADR-induced hepatotoxicity in rats. In addition, it was aimed to determine how Meteorin-Like (MtrnL), which has pleiotropic effects on immunology, inflammation, and metabolism, is affected by ADR and/or NAC applications in liver tissue. 28 rats were randomly assigned to one of four equal groups in the study: control (no treatment), NAC (150 mg/kg/day of NAC intraperitoneally (i.p), ADR (15 mg/kg only on the first day of the experiment), and ADR + NAC (ADR 15 mg/kg on the first day of the experiment + 150 mg/kg/day NAC i.p). After 15 days, liver enzyme levels in serum, oxidant/antioxidant parameters in liver tissue, histopathological changes, caspase 3 (Casp3) and heat shock protein 70 (HSP-70) immunoreactivities, and MtrnL levels were examined. Histopathological changes, liver enzyme levels, as well as HSP-70, and Casp3 immunoreactivities increased due to ADR application. Additionally, MtrnL levels in liver tissue were significantly increased as a result of ADR application. However, it was detected that the NAC application significantly regulated the ADR-induced changes. Furthermore, it was determined that NAC administration regulated the changes in ADR-induced oxidative stress parameters. We propose that NAC may exert a hepatoprotective effect by regulating ADR-induced altered oxidative stress parameters, MtrnL levels, Casp3, and HSP-70 immunoreactivities in the liver.
Keywords: Adriamycin, N-Acetylcysteine, Meteorin-like, Heat shock protein 70, Caspase3, Hepatotoxicity
Introduction
Adriamycin (ADR) is a frequently used antineoplastic agent in the clinic. However, it is known to have serious side effects such as nephrotoxicity, hepatotoxicity, and cardiotoxicity (El Sayed et al. 2017; Omobowale et al. 2018; Kaya et al. 2023b). Two theories attempting to explain the toxicity of ADR have been proposed. The first theory is that ADR blocks the biosynthesis of macromolecules by interfering with the nitrogenous bases of DNA and inhibiting topoisomerase II enzyme activity. Disruption of the replication process terminates the division of cancerous cells. The other is oxidative stress due to overproduction of ADR-derived free oxygen radicals (ROS) (Prasanna et al. 2020). Both procedures cause lipid peroxidation, DNA damage, and overproduction of ROS, which ultimately leads to apoptosis in both malignant and non-malignant cells (Xiao et al. 2019; Renu et al. 2022). Oxidative stress is the main and most significant factor to be considered when examining the toxic effects of ADR on different organs (Renu et al 2018; Renu et al 2022; Akın et al. 2021b). However, there are many factors that increase ADR-related oxidative stress. These factors are excessive ROS production that accelerates lipid peroxidation, dysregulation of the electron transport chain that impairs energy metabolism, and irregularities in the expression of antioxidant regulatory genes. Therefore, ADR drives cells to apoptosis in non-target tissues (Renu et al. 2018, 2022). In addition, according to reports, ADR has been linked to significant hepatotoxicity (Omobowale et al. 2018; Bilgic and Ozgocmen 2019).
N-Acetylcysteine (NAC) is an antioxidant, and the thiol groups it contains may react rapidly with ROS (Hashim et al. 2021). NAC increases cellular capacity in order to reduce ROS production and terminate apoptosis (Kahraman et al. 2013). It is also beneficial in managing various conditions such as liver dysfunction, malignancies, and cardiac problems (Kaya Tektemur et al. 2021; Osman et al. 2021). Studies have shown that NAC has a wide range of protective effects against liver damage caused by the organophosphate pesticide Monocrotophos (Singh et al. 2022), the environmental pollutant Arsenic (Zhang et al.2022), high-dose Acetaminophen (Fisher and Curry 2019), and Irinotecan (Gençosman et al. 2022) which is one of the most important antitumor drugs developed in recent years. In addition, research indicate that NAC reduces cellular damage by suppressing apoptosis, oxidative stress, and heat shock response (Kahraman et al. 2013; Al Hajm and Ozgun 2022). The positive effect of NAC on heat shock response and proteasome inhibition has been associated with its antioxidant and cytoprotective effect (Al Hajm and Ozgun 2022). Heat shock response is one of the few survival pathways that protect cells from harsh conditions. In addition, heat shock proteins (HSPs) are also expressed under physiological conditions and play a key role in the normal functions of cells (Ozaydin et al. 2016). Heat shock protein 70 (HSP-70) belongs to a 70 kDa chaperone family that plays significant roles in protein degradation, separation, and folding. HSP-70 has been reported to play a key role in processes including protein degradation (Fernandez-Fernandez and Valpuesta 2018). HSP-70 is also an important molecular marker for the HSP response, in which chaperone synthesis is increased due to cellular stress factors such as heavy metals, pathological conditions, increased temperature or oxidative stress (Evans et al. 2010). Protein homeostasis is essential in maintaining the secretory and metabolic functions of the liver, and liver diseases are associated with protein degradation disorders (Allaire et al. 2019). In addition, studies have shown that NAC reduces cellular damage by suppressing apoptosis, oxidative stress, and heat shock response (Kahraman et al. 2013; Al Hajm and Ozgun 2022).
Meteorin-Like (MtrnL), a recently discovered cytokine, regulates energy expenditure, macrophage activation, and inflammation (Wang et al. 2022). MtrnL is expressed at high levels in tissues such as the lung, intestine, subcutaneous adipose tissue, and skin (Li et al. 2014, 2016). It has been reported that MtrnL is found in large amounts particularly in organs related to metabolism as well as barrier tissues (Li et al. 2014). MtrnL is known to be secreted by skeletal muscle following physical activity and to attenuate inflammation in white adipose tissue (Rao et al. 2014). MtrnL is also strongly induced by alternatively activated macrophages (Ushach et al. 2015). It triggers the expression of MtrnL, interleukin 4 (IL4) and IL13 in adipose tissues, supports the activation of macrophages (M2 type), and inhibits inflammation (Ushach et al. 2018). It is known to increase beige adipose thermogenesis, insulin sensitivity, and glucose tolerance (Rao et al. 2014; Ushach et al. 2015, 2018; Jung et al. 2018; Yalcın and Kaya 2023). A study reported that patients with psoriasis, an autoimmune disease had increased MtrnL levels (Bridgewood et al. 2019). Another study reported that MtrnL overexpression in cardiomyocytes with ischemia/reperfusion injury attenuated apoptosis by activating the AMPK-PAK2 signaling pathway (Xu et al 2020). In another research, it was demonstrated that MtrnL overexpression may play a role in reducing ADR-mediated cardiac dysfunction, oxidative stress, and apoptosis (Hu et al. 2020). In a recent study, serum and hepatic MtrnL expression were significantly increased in mice with Concanavalin A-induced fulminant hepatitis. The same study showed that MtrnL overexpression ameliorated fulminant hepatitis by inhibiting chemokine-dependent immune cell infiltration (Du et al. 2023). However, the signaling pathways or molecular processes through which MtrnL operates are not yet fully understood (Wang et al. 2022).
The purpose of this study was to investigate the biochemical, histological, and immunohistochemical effects of NAC on ADR-induced liver damage in rats. In addition, these treatments aimed to determine the expression of MtrnL, whose secretion and regulation depend on the physiological and pathological context, in liver tissue, which is categorized as a barrier tissue and actively participates in metabolic processes.
Materials and methods
Experimental design
The study was initiated following the approval of the Fırat University Animal Experiments Ethics Committee (decision dated 05.07.2021 and numbered 2831). The rats employed in the study were housed and fed under ideal circumstances (22 ± 2 °C temperature, 12 h day/night cycle, add-libitum water and food). A total of 28 male Sprague–Dawley rats (8–10 week-old, 220 ± 20 g weight) were used, and they were split into 4 groups at random: control, NAC, ADR, and ADR + NAC (n = 7). The rats in the control group received no treatments. The NAC group received intraperitoneally (i.p.) administered NAC (Hüsnü Arsan Farma, Turkey) at a dose of 150 mg/kg every day for 14 days. For the ADR group, 15 mg/kg ADR was applied i.p. only once at the beginning of the experimental process. As for the ADR + NAC group, at the beginning of the experimental process, 15 mg/kg ADR was applied i.p only once, followed by 150 mg/kg NAC i.p. application on a daily basis. ADR was purchased from Saba Farma Turkey. The lyophilized ADR was reconstituted with sterile water to 2 mg/ml. The ADR (Karabulut et al. 2021) and NAC (Yalçın et al. 2023) doses used in the experiment were referenced from previous studies. The rats were sacrificed under anesthesia (using xylazine 10 mg/kg and ketamine 75 mg/kg i.p) on the fifteenth day of the experiment. Blood samples were taken to determine liver enzyme levels. The remainder of the liver tissues were preserved at -80 °C for use in qRT-PCR and biochemical investigations, while others were used for histological and immunohistochemical exams.
Power Analysis: The number of animals to be used in the experiments was determined by power analysis with a power of 0.90 and a type 1 error (α) of 0.05. The sample size (n) was calculated to be 7 animals in each group (Faul et al. 2009).
Histopathological evaluation
Liver tissue was quickly excised and placed in a 10% formalin solution for fixation. The Hematoxylin–Eosin (HE) staining procedure was applied to 5 µm thick sections taken for use in general histopathological evaluations. Prepared preparations were examined under a light microscope and photographed (DM-2500 – MC-170, Leica, Germany). Histopathological changes were evaluated and scored in liver tissue preparations prepared separately for each rat. The histopathological evaluation histoscore was established by taking into account the presence of score criteria (0, absent; 1, less; 2, moderate; 3, more) in 10 non-overlapping random areas at X10 magnification (Yalçın et al. 2023). The histopathological score criteria were; sinusoid dilatation, hepatocyte vacuolization, foci of inflammation, degenerated hepatocyte cords, and hemorrhagic areas.
Immunohistochemical examination
The Avidin Biotin Peroxidase Complex Method was utilized to detect the HSP-70 (sc-32239, Santa Cruz Biotechnology), Casp3 (bs-0081R, Bioss, China), and MtrnL (Q641Q3, Cusabio Diagnostics, China) immunoreactivities in the liver tissues (Kaya et al. 2023a). Sections that underwent immunohistochemical staining procedures separately for each rat were examined with a light microscope and photographed (DM-2500 – MC-170, Leica, Germany). Immunoreactivity was calculated according to the formula of prevalence (0.1 = < %25, 0.4 = %26–50, 0.6 = %51–75, 0.9 = %76–100) X severity (0 = none, 0.5 = very little, 1 = little, 2 = medium, 3 = high) (Yalcın and Kaya 2023) by examining 20 random non-overlapping areas at X20 magnification.
Biochemical analyses
Liver enzyme levels
The levels of the serum liver enzymes alkaline phosphatase (ALP), alanine transaminase (ALT), aspartate transaminase (AST), and gamma glutamyl transferase (GGT) were measured using an automatic biochemical analyzer (Siemens ADVIA 2400).
Detection of MtrnL, TOS, and TAS levels
Total oxidant/antioxidant (TOS/TAS) and MtrnL levels in liver tissue samples were determined using the enzyme-linked immunosorbent assay (ELISA) method. MtrnL (201–11-6038, SunRed, China), TOS (AD3282Ra, AndyGene, China) and TAS (AD3283Ra, AndyGene, China) levels in rat liver tissue homogenates were determined according to ELISA kits protocols. The results of the analyses were presented as ng/ml, pg/ml, and U/ml, respectively. MtrnL sensitivity was 0.092 ng/ml and the test range was 0.1–30 ng/ml; TOS sensitivity was 0.7 pg/ml and the test range was 3.75–120 pg/ml; TAS sensitivity was 0.1 U/ml and the test range was 0.2–8 U/ml. Utilizing the TOS/TAS methodology, the Oxidative Stress Index (OSI) was detected (Günes-Bayir et al. 2018).
qRT-PCR analysis
In the study, qRT-PCR was used to detect differences in MtrnL (Sente Bio Lab, Turkey) mRNA expression. Total RNA was isolated from materials made of rat liver tissue that had been kept at -80 °C using an RNA extraction solution (RiboExTM, GeneAll, Korea). RNA isolation was performed following the procedure instructions recommended by the manufacturer. RNA concentrations were measured using a microspectrophotometer device (Nano-400a, Allsheng, China). RNA samples containing equal amounts of total RNA were collected from each group in their own pools. 10 μl of pooled RNA samples of each group were used for the synthesis of complementary DNA (cDNA), which is used to detect gene expression differences at the mRNA level. cDNA synthesis was performed using the cDNA synthesis kit (High capacity cDNA Reverse Transcription kit, Applied Bios., Foster, CA), following the procedure recommended by the manufacturer. The cDNA samples obtained were stored at -20 °C. In the presence of primers specific to the desired sequence, obtained cDNAs were amplified by qRT-PCR. Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) was used as a reference gene for the determination of mRNA levels in rat liver samples (Table 1). qRT-PCR analysis was performed in a total volume of 10 µl in 96-well plates in triplicate (1 µl cDNA—9 µl mix). This analysis was performed using BrightGreen 2X qPCR Master Mix (MasterMix-S, abm, Canada) in Applied Biosystems 7500 Real-Time PCR in accordance with the procedure specified by the manufacturer.
Table 1.
Primers used in qRT-PCR
| PRIMER | SEQUENCE (5’-3’) | |
|---|---|---|
| GAPDH | FORWARD | AGGTCGGTGTGAACGGATTTG |
| REVERSE | TGTAGACCATGTAGTTGAGGTCA | |
| MtrnL | FORWARD | ACTTTGGGGAGGCACAACTT |
| REVERSE | TGTCACCCTGCAAGTCACTC | |
Statistical analysis
The Statistical Package for Social Sciences (SPSS) 22.0 application was used to statistically analyze the data. The conformity of the data to the normal distribution was determined with the Shapiro–Wilk test. Normally distributed parameters were statistically analyzed using the one-way ANOVA and post hoc Tukey tests. The data were presented as the mean ± standard error. Parameters that did not show a normal distribution were analyzed by Kruskal–Wallis and then Mann–Whitney U tests. In addition, differences between mRNA expressions in qRT-PCR analysis were determined using the 2-ΔΔCT method. Statistical significance was evaluated as p < 0.05. GraphPad Prism 9.3 was used to create graph drawings.
Results
Effect of ADR and/or NAC applications on liver histopathology
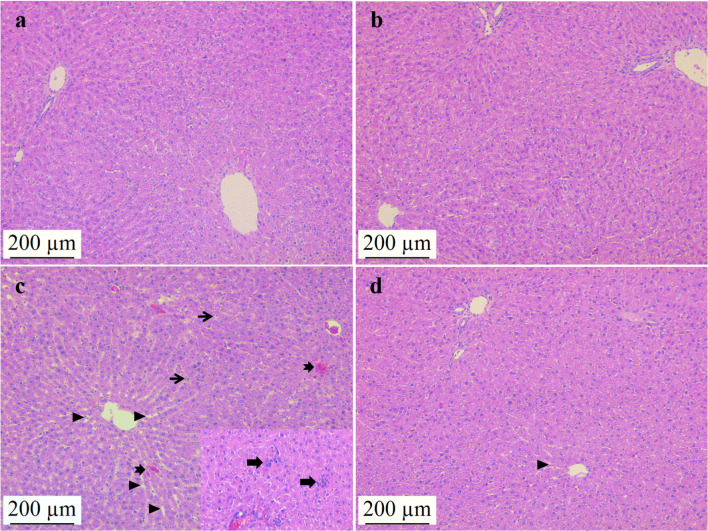
In the control and NAC groups, the histological liver structure, hepatocyte cords, close-located hepatocytes, and sinusoidal structure were all observed to be regular (Fig. 1; a, b). Histopathological examination of liver tissues revealed that ADR application caused sinusoidal dilatation, hepatocyte vacuolization, inflammation foci, degenerated hepatocyte cords, and hemorrhagic areas. ADR application significantly increased histopathological changes in liver tissue as compared to the control group (p < 0.05). However, when compared to the ADR group, it was observed that the histopathological alterations were lessened in the ADR + NAC group. (p < 0.05) (Table 2, Fig. 1; c, d).
Fig. 1.
Effects of NAC administration on histopathological changes in ADR-induced liver tissue. Control (a) and NAC group (b) liver tissues had normal histological appearance. In the ADR group (c); sinusoidal dilatation (triangle), hemorrhagic areas (notched arrow), hepatocyte vacuolızation (thin arrow), foci of inflammation (thick arrow) and hemorrhagic areas (notched arrow) were observed. Histopathological changes were significantly reduced in the ADR + NAC group (d) compared to the ADR group (p < 0.05). Hematoxylin Eosin, scale bar: 200µm, × 100. ADR: Adriamycin; NAC: N-Acetylcysteine
Table 2.
Histopathological evaluation results of ADR and/or NAC applications on liver tissue
|
Control (n = 7) Median(Min–Max) |
NAC (n = 7) Median(Min–Max) |
ADR (n = 7) Median(Min–Max) |
ADR + NAC (n = 7) Median(Min–Max) |
P* | |
|---|---|---|---|---|---|
| Sinusoid dilatation | 0.20(0.00–0.30)b | 0.20(0.00–0.40)b | 2.10(1.40–2.60)a | 0.90(0.50–1.50)a b | < 0.001 |
| Hepatocyte vacuolization | 0.20(0.00–0.50)b | 0.20(0.10–0.40)b | 2.20(1.70–2.60)a | 0.90(0.60–1.30)a b | < 0.001 |
| Foci of inflammation | 0.00(0.00–0.10)b | 0.10(0.00–0.20)b | 2.40(1.90–2.70)a | 0.60(0.50–1.60)a b | < 0.001 |
| Degenerated hepatocyte cords | 0.20(0.00–0.30)b | 0.20(0.00–0.40)b | 2.40(1.70–2.60)a | 0.70(0.50–1.80)a b | < 0.001 |
| Hemorrhagic areas | 0.00(0.00–0.10)b | 0.00(0.00–0.20)b | 1.90(0.80–2.30)a | 0.50(0.30–1.30)a b | < 0.001 |
Data are presented as median (min–max)
a: Compared with the control group (p < 0.05),
b: Compared with the ADR group (p < 0.05)
p* Kruskal Wallis
Effect of NAC and/or ADR applications on liver HSP-70 and Casp3 immunoreactivities
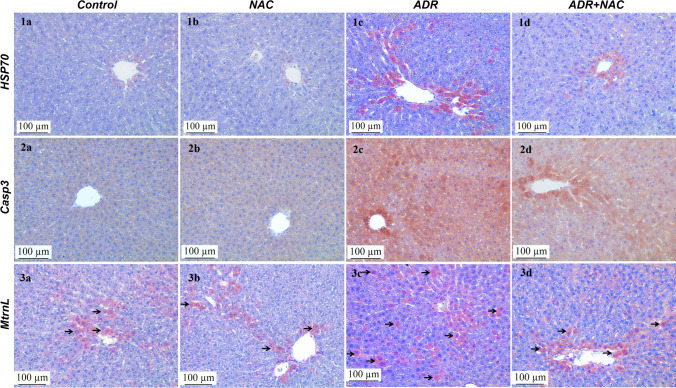
The immunoreactivities of HSP-70 and Casp3 in liver tissue were comparable in the NAC and control groups (p > 0.05) (Fig. 2; 1a, 1b; 2a, 2b). ADR application significantly increased (p < 0.001) HSP-70 immunoreactivity in liver tissue compared to the control group (Fig. 2; 1c). NAC application significantly decreased the ADR induced HSP-70 immunoreactivity compared to the ADR group (p < 0.001) (Table 3, Fig. 2; 1d). Similarly, ADR application significantly increased Casp3 immunoreactivity in liver tissue compared to the control group (p < 0.001) (Fig. 2; 2c). NAC application significantly decreased ADR-induced Casp3 immunoreactivity compared to the ADR group (p < 0.001) (Table 3, Fig. 2; 2d,).
Fig. 2.
Effects of ADR and/or NAC application on the HSP70, Casp3 and MtrnL immunoreactivity of the liver tissue. Liver tissues in the control and NAC groups were observed to have similar HSP70 immunoreactivity (p > 0.05) (1a, 1b). ADR group (1c) HSP70 immunoreactivity was observed to be significantly increased (p < 0.05) compared to the control group. The HSP70 immunoreactivity of the ADR + NAC group (1d) was observed to decrease at a statistically significant level (p < 0.05) compared to the ADR group. Liver tissues in the control and NAC groups were observed to have similar Casp3 immunoreactivity (p > 0.05) (2a, 2b). The Casp3 immunoreactivity of the ADR group (2c) was observed to increase at a statistically significant level (p < 0.05) compared to the control group. Casp3 immunoreactivity of the ADR + NAC group (2d) was observed to decrease at a statistically significant level (p < 0.05) compared to the ADR group. Liver tissues in the control and NAC groups were observed to have similar MtrnL immunoreactivity (p > 0.05) (3a, 3b). MtrnL immunoreactivity was increased in hepatocytes in the ADR group compared to the control group (arrow) (3c). MtrnL immunoreactivity was decreased in the ADR + NAC group compared to the ADR group (3d). HSP70, Casp3 and MtrnL immunohistochemical images, scale bar: 100 μm, × 200. HSP70: The 70-kDa heat shock protein; Casp3: Caspase 3; MtrnL: Meteorin-Like, ADR: Adriamycin; NAC: N-Acetylcysteine
Table 3.
Effect of ADR and/or NAC treatments on HSP-70 and Casp3 immunoreactivities in liver
| Control | NAC | ADR | ADR + NAC | p | |
|---|---|---|---|---|---|
| HSP-70 | 0.23 ± 0.06b | 0.27 ± 0.10b | 1.27 ± 0.14a | 0.57 ± 0.06b | < 0.001 |
| Casp3 | 0.16 ± 0.04b | 0.23 ± 0.05b | 1.80 ± 0.25a | 0.62 ± 0.06b | < 0.001 |
Values are presented as mean ± standard error. a: Compared with the control group (p < 0.05), b: Compared with the ADR group (p < 0.05). ADR: Adriamycin; Casp3: Caspase 3, HSP70: The 70-kDa heat shock protein, NAC: N-Acetylcysteine
Effect of ADR and/or NAC treatment on liver tissue MtrnL immunoreactivity, ELISA and mRNA levels
In the NAC and control groups, MtrnL immunoreactivities in liver tissues were similar (p > 0.05) (Fig. 2; 3a, 3b). ADR application increased MtrnL immunoreactivity in liver (p = 0.024) (Fig. 2; 3c). In the ADR + NAC group, MtrnL immunoreactivity was decreased (Fig. 2; 3d, Table 4).
Table 4.
Effect of ADR and/or NAC treatment on liver MtrnL immunoreactivities and ELISA levels
| Control | NAC | ADR | ADR + NAC | p | |
|---|---|---|---|---|---|
| MtrnL (IHC) | 1.20 ± 0.11b | 1.32 ± 0.12 | 1.97 ± 0.20a | 1.54 ± 0.23 | = 0.026 |
|
MtrnL (ng/ml) |
3.48 ± 0.12b | 3.19 ± 0.10b | 4.59 ± 0.11a | 3.66 ± 0.09b | < 0.001 |
Values are presented as mean ± standard error. a: Compared with the control group (p < 0.05), b: Compared with the ADR group (p < 0.05). ADR: Adriamycin; NAC: N-Acetylcysteine, MtrnL: Meteorin-Like, IHC; Immunohistochemical staining
MtrnL ELISA levels in liver tissues were similar in the NAC and control groups (p > 0.05). When compared to the control group, the MtrnL ELISA level in the ADR group considerably rose (p < 0.001). However, as a result of NAC treatment, it was significantly reduced in the ADR + NAC group compared to the ADR group (p < 0.001) (Table 4).
A substantial rise in the MtrnL mRNA level was observed in the ADR group as compared to the control group (p = 0.008). In comparison to the control group, MtrnL mRNA levels were lower in the NAC group (p = 0.042). MtrnL mRNA levels in the ADR + NAC group did not differ significantly from those in the control group (p > 0.05). There was a statistically significant decrease in the MtrnL mRNA level in the NAC group compared to the ADR group (p = 0.005). When compared to the ADR group, MtrnL mRNA levels significantly decreased in the ADR + NAC group (p = 0.007). However, there was no significant change in the MtrnL mRNA level in the ADR + NAC group compared to the NAC group (p > 0.05) (Fig. 3).
Fig. 3.
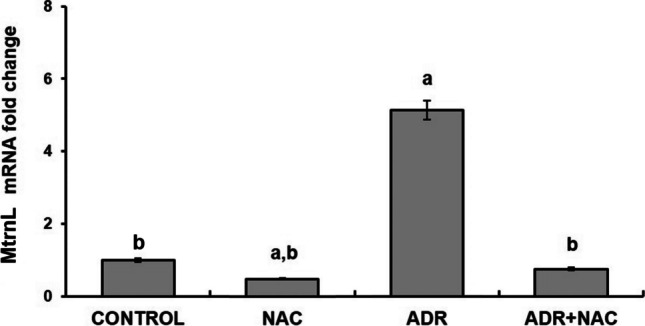
Effect of ADR and/or NAC treatment on MtrnL mRNA fold change. MtrnL mRNA expression was significantly increased in the ADR group compared to the control group. In the ADR + NAC group, when compared to the ADR group, there was a significant decrease in MtrnL mRNA expression. a: Compared with control group (p < 0.05), b: Compared to the ADR group (p < 0.05). ADR; Adriamycin, NAC; N-Acetylcysteine, MtrnL; Meteorin-Like
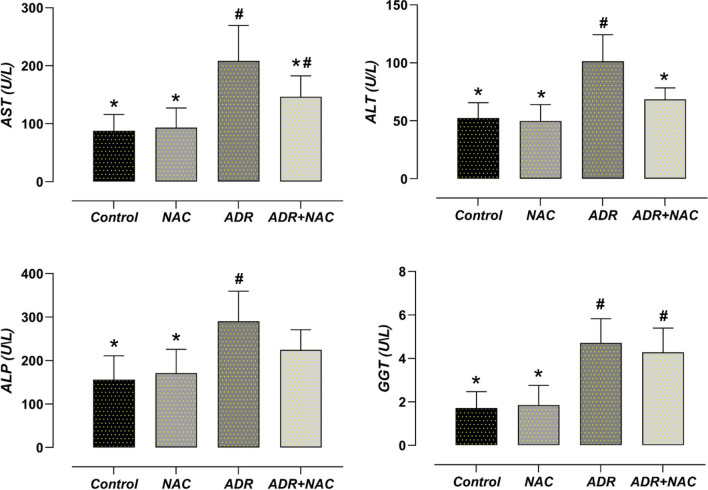
Effect of NAC treatment on liver enzyme levels after ADR treatment
Serum liver enzyme levels in the NAC and control groups were found to be similar (p > 0.05). The ADR group's AST, ALT, ALP, and GGT levels were considerably higher than those of the control group (p < 0.001). The ADR + NAC group's AST and ALT levels were lower than those of the ADR group (p = 0.049, p = 0.004, respectively) (Fig. 4).
Fig. 4.
Effect of ADR and/or NAC treatment on liver enzyme levels. AST, ALT, ALP and GGT were significantly increased in the ADR group compared to the control group (p < 0.05). In the ADR + NAC group, when compared to the ADR group, there was a significant decrease in AST and ALT levels (p < 0.05). #: Compared to the control group (p < 0.05), *: Compared with ADR group (p < 0.05). ADR: Adriamycin; ALT: Alanine Transaminase; ALP: Alkaline phosphatase; AST: Aspartate transaminase; GGT: Gamma glutamyl transferase; NAC: N-Acetylcysteine
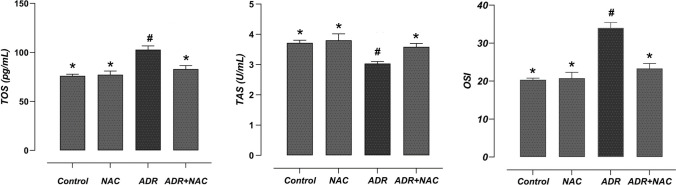
Effect of NAC treatment on ADR-induced oxidative stress parameters
The NAC and control groups' liver tissue TOS and TAS values were determined to be similar (p > 0.05). When compared to the control group, ADR treatment significantly elevated TOS levels (p < 0.001), whereas the TAS level decreased significantly in comparison to the control group (p = 0.007). On the other hand, NAC treatment significantly increased the TAS level in the ADR + NAC group compared to the ADR group (p = 0.034), whereas it significantly reduced the TOS level (p = 0.002). A statistically significant rise in OSI was found in the ADR group when compared to the control group (p < 0.001). When compared to the ADR group, OSI considerably decreased in the ADR + NAC group (p < 0.001) (Fig. 5).
Fig. 5.
Effect of ADR and/or NAC treatment on oxidative stress parameters. OSI was significantly increased in the ADR group compared to the control group. In the ADR + NAC group, when compared to the ADR group, there was a significant decrease in OSI. #: Compared to the control group (p < 0.05), *: Compared with ADR group (p < 0.05). ADR; Adriamycin, NAC; N-Acetylcysteine, TAS; Total antioxidant status, TOS; Total oxidant status, OSI; Oxidative Stress Index
Discussion
ADR is an anthracycline cytotoxic chemotherapeutic drug extensively used in cancer treatment (Adiyaman et al. 2022). However, hepatotoxicity is a significant adverse effect that limits its clinical use (Omobowale et al. 2018). Studies have associated ADR toxicity with oxidative stress (Akin et al. 2021b). ADR-induced ROS production can activate apoptotic pathways in cells through DNA damage, membrane damage, and lipid peroxidation (Prasanna et al. 2020). It is known that NAC, which has potent antioxidant properties, has a protective effect on cells by inhibiting ROS production and reducing apoptosis (Kahraman et al. 2013).
Previous studies reported that in liver tissue, ADR leads to enlargement of the sinusoidal, bile duct hyperplasia, vacuolization of hepatocytes, sinusoid dilatation, degeneration of hepatocyte cords, cellular edema, focal necrosis, irregular hepatic trabeculae, biliary duct proliferation, parenchymal necrosis, intercellular space enlargement, vacuolization of mitochondria, and lymphocyte infiltration (Prasanna et al. 2020; Akin et al. 2021a). Consistent with these data, in the present study, sinusoid dilatation, vacuolization of hepatocytes, foci of inflammation, degeneration of hepatocyte cords, and hemorrhagic areas were all observed in the liver tissue following ADR treatment. Likewise, many studies showed that ADR significantly increases ALT, AST, ALP, and GGT levels, which indicate liver damage (Djabir et al. 2017; Mohebbati et al. 2018; Gotama et al. 2019; Rafiee et al. 2021; Owumi et al. 2021). In the current study, it was detected that ADR-induced histopathological changes and liver enzyme levels were significantly decreased as a result of NAC treatment.
In oxidative stress, the balance between oxidants and antioxidants that cause damage to cells, tissues, and organs is in favor of the former (Erdem Guzel et al. 2023). One study reported that excessive ROS production and/or oxidative stress associated with reduced antioxidant defense are the main mechanisms of ADR toxicity (Akin et al. 2021b). In this current study, it was determined that TOS levels increased to a greater extent in the ADR group compared to the control group, but TAS levels decreased. As a result, OSI increased significantly in the ADR group compared to the control group. These data support the idea that ADR causes oxidative stress, in line with previous studies (Nagai et al. 2015; Rafiee et al. 2021; Owumi et al. 2021). However, it was observed that oxidative stress parameters were significantly regulated in the ADR + NAC group compared to the ADR group. HSPs are critical for cell survival against harmful stimuli such as heat shock, ischemia, and inflammation. The most fundamental and prevalent HSP that is activated in practically all cell types is HSP-70 (Qiao et al. 2019). HSP-70 overexpression via acetylation has been shown to increase cellular resistance to liver ischemia/reperfusion injury (Sun et al. 2014). The current study showed that ADR significantly increased HSP-70 immunoreactivity in liver tissue. Similarly, it was reported in a study that exposure to oxidative stress may induce HSP-70 expression (Pockley 2003). Additionally, in this current study, regulation of oxidative parameters with NAC treatment resulted in a decrease in oxidative stress and HSP-70 immunoreactivity in the ADR + NAC group. One study suggests that the role of HSP is to protect the cell against oxiradical-induced changes (Choudhary and Devi 2016). According to another study, HSP-70 is a protein with an antiapoptotic role that protects tissue from oxidative stress-induced cytotoxicity (Zorzi and Bonvini 2011). However, it was reported that ADR induced Casp3 expression by significantly increasing the expression of intracellular and extracellular apoptotic molecules such as Bax and Fas (Nagai et al. 2015). This study demonstrated that ADR significantly increases Casp3 immunoreactivity in liver tissue. Likewise, other studies reported that ADR exposure increases Casp3 expression, supporting these data (Nagai et al. 2016; Owumi et al. 2021). This study demonstrated that NAC treatment significantly reduces ADR-induced Casp3 immunoreactivity.
MtrnL is a recently identified cytokine (Wang et al. 2022). A previous study defined MtrnL as a hormone that can be selectively induced in various tissues depending on physiological stimuli (Rao et al. 2014). Recent studies have reported that MtrnL plays a role in pathophysiological as well as various physiological events (Alizadeh 2021; Kaya et al. 2023b). A study has stated that the increase in MtrnL after local muscle injury contributes to muscle regeneration (Baht et al. 2020). Previous studies reported increased MtrnL excretion due to downhill running (Rao et al. 2014) and other exercises (Bae et al. 2018) that cause damage to skeletal muscle. The idea that MtrnL plays a significant role in muscle tissue regeneration is supported by the fact that it is stimulated by tissue damage and contributes to immune modulation (Baht et al. 2020). Numerous studies have reported that MtrnL, which is expressed in cardiac tissue, plays a crucial role in the pathogenesis of cardiovascular diseases (Miao et al. 2020; Rupérez et al. 2021). In a study, it was observed that the decrease in endogenous MtrnL increased the negative effects of ADR-induced cardiotoxicity, while exogenous MtrnL administration significantly reduced oxidative stress and apoptosis in the ADR-induced cardiotoxicity model used (Hu et al. 2020). Similarly, in a different study, MtrnL was shown to have a reducing effect on ADR-induced cardiomyocyte apoptosis (Xu et al. 2020). Consistent with these data, in the current study, it was determined that ADR administration significantly increased MtrnL levels in liver tissue. Similarly, a recent study reported that MtrnL levels were higher in patients with nonalcoholic fatty liver disease activity scores and that the newest definition of MtrnL as a hepatokine could pave the way for new targeting strategies in liver-related disorders (Grander et al. 2021). The data obtained in this study showed that NAC treatment may have a hepatoprotective effect on ADR-induced liver injury by regulating oxidative stress, Casp3, HSP-70, and MtrnL levels.
Conclusion
It was observed that NAC treatment showed an antioxidant effect and alleviated ADR-induced oxidative stress in liver tissue. It was determined that Casp3 and HSP-70 immunoreactivities, which increased due to ADR application, were alleviated by NAC treatment. In addition, it was observed that MtrnL immunoreactivity, ELISA, and gene expression levels increased following ADR application. However, NAC treatment exhibited a hepatoprotective effect by regulating the ADR-induced altered MtrnL levels. These findings are significant given that NAC administration may represent a therapeutic strategy against ADR-induced hepatotoxicity.
Authors Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Sercan Kaya, Tuba Yalcın, Ahmet Tektemur and Tuncay Kuloglu. The first draft of the manuscript was written by Sercan Kaya and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Funding
This study was supported by Fırat University Scientific Research Projects Unit, Elazığ, TURKEY with project number TF.21.11.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
Declarations
Ethical Approval
This study has been approved by the decision of Fırat University Animal Experiments Ethics Committee with the number 2831 and dated 05.07.2021.
Competing Interests
There is no conflict of interest between the authors. All authors have read and approved this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Adıyaman MS, Adıyaman ÖA, Dağlı AF, Karahan MZ, Dağlı MN. Prevention of doxorubicin-induced experimental cardiotoxicity by Nigella sativa in rats. Revista Portuguesa de Cardiologia. 2022;41(2):99–105. doi: 10.1016/j.repc.2020.12.015. [DOI] [PubMed] [Google Scholar]
- Akin AT, Kaymak E, Öztürk E, Karabulut D, Kuloğlu N, Ceylan T, Toluk A. Investigation of the therapeutic effects of chloroquine in adriamycin-ınduced hepatotoxicity. The EuroBiotech Journal. 2021;5(1):8–14. doi: 10.2478/ebtj-2021-0003. [DOI] [Google Scholar]
- Akin AT, Öztürk E, Kaymak E, Karabulut D, Yakan B. Therapeutic effects of thymoquinone in doxorubicin-induced hepatotoxicity via oxidative stress, inflammation and apoptosis. Anat Histol Embryol. 2021;50(6):908–917. doi: 10.1111/ahe.12735. [DOI] [PubMed] [Google Scholar]
- Al Hajm AYS, Ozgun E. Effects of acrylamide on protein degradation pathways in human liver-derived cells and the efficacy of N-acetylcysteine and curcumin. Drug Chem Toxicol. 2022;45(4):1536–1543. doi: 10.1080/01480545.2020.1846548. [DOI] [PubMed] [Google Scholar]
- Alizadeh H. Myokine-mediated exercise effects: the role of myokine meteorin-like hormone (Metrnl) Growth Factors. 2021;39(1–6):71–78. doi: 10.1080/08977194.2022.2032689. [DOI] [PubMed] [Google Scholar]
- Allaire M, et al. Autophagy in liver diseases: time for translation? J Hepatol. 2019;70(5):985–998. doi: 10.1016/j.jhep.2019.01.026. [DOI] [PubMed] [Google Scholar]
- Bae JY, Woo J, Kang S, Shin KO. Effects of detraining and retraining on muscle energy-sensing network and meteorin-like levels in obese mice. Lipids Health Dis. 2018;17(1):1–9. doi: 10.1186/s12944-018-0751-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baht GS, Bareja A, Lee DE, Rao RR, Huang R, Huebner JL, White JP. Meteorin-like facilitates skeletal muscle repair through a Stat3/IGF-1 mechanism. Nat Metab. 2020;2(3):278–289. doi: 10.1038/s42255-020-0184-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilgic S, Ozgocmen M. The protective effect of misoprostol against doxorubicin induced liver injury. Biotech Histochemical. 2019;94(8):583–591. doi: 10.1080/10520295.2019.1605457. [DOI] [PubMed] [Google Scholar]
- Bridgewood C, Russell T, Weedon H, Baboolal T, et al. The novel cytokine Metrnl/IL-41 is elevated in psoriatic arthritis synovium and inducible from both entheseal and synovial fibroblasts. Clin Immunol. 2019;208:108253. doi: 10.1016/j.clim.2019.108253. [DOI] [PubMed] [Google Scholar]
- Choudhary AK, Devi RS. Effects of aspartame on hsp70, bcl-2 and bax expression in immune organs of Wistar albino rats. J Biomed Res. 2016;30(5):427–435. doi: 10.7555/JBR.30.20140097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djabir YY, Arsyad MA, Sartini S, Lallo S. Potential roles of kleinhovia hospita L. leaf extract in reducing doxorubicin acute hepatic, cardiac and renal toxicities in rats. Pharmacognosy Res. 2017;9(2):168–173. doi: 10.4103/pr.pr_129_16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du YN, Teng JM, Zhou TH, Du BY, Cai W. Meteorin-like protein overexpression ameliorates fulminant hepatitis in mice by inhibiting chemokine-dependent immune cell infiltration. Acta Pharmacol Sin. 2023;44:1404–1415. doi: 10.1038/s41401-022-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Sayed EM, Mansour AM, El-Sawy WS (2017) Protective effect of proanthocyanidins against doxorubicin-induced nephrotoxicity in rats. J Biochemical and Molecular Toxicology 31(11). 10.1002/jbt.21965. [DOI] [PubMed]
- Erdem Guzel E, Kaya Tektemur N, Tektemur A, Etem Önalan E. Carbamazepine-induced renal toxicity may be associated with oxidative stress and apoptosis in male rat. Drug Chem Toxicol. 2023;46(1):136–143. doi: 10.1080/01480545.2021.2014859. [DOI] [PubMed] [Google Scholar]
- Evans CG, Chang L, Gestwicki JE. Heat shock protein 70 (hsp70) as an emerging drug target. J Med Chem. 2010;53(12):4585–4602. doi: 10.1021/jm100054f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Buchner A, Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior Res Methods. 2009;41(4):1149–1160. doi: 10.3758/BRM.41.4.1149. [DOI] [PubMed] [Google Scholar]
- Fernandez-Fernandez MR, Valpuesta JM (2018) Hsp70 chaperone: a master player in protein homeostasis. F1000Res 19:7. 10.12688/f1000research.15528.1 [DOI] [PMC free article] [PubMed]
- Fisher ES, Curry SC. Evaluation and treatment of acetaminophen toxicity. Adv Pharmacol. 2019;85:263–272. doi: 10.1016/bs.apha.2018.12.004. [DOI] [PubMed] [Google Scholar]
- Gençosman S, Ceylanlı D, Şehirli AÖ, Teralı K, Bölükbaşı F, Çetinel Ş, et al. Investigation of the Possible Protective Effect of N-Acetylcysteine (NAC) against Irinotecan (CPT-11)-Induced Toxicity in Rats. Antioxidants. 2022;11(11):2219. doi: 10.3390/antiox11112219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotama KT, Soetikno V, Louisa M, Arozal W. Hepatoprotective effects of l-citrulline against doxorubicin-induced liver damage in rats: an analysis of serum biomarkers. Int J Appl Pharmaceutics. 2019;11:230–233. doi: 10.22159/ijap.2019.v11s1.19099. [DOI] [Google Scholar]
- Grander C, Grabherr F, Enrich B, et al. Hepatic Meteorin-like and krüppel-like factor 3 are associated with weight loss and liver ınjury. Exp Clin Endocrinol Diabetes. 2021 doi: 10.1055/a-1537-8950. [DOI] [PubMed] [Google Scholar]
- Günes-Bayir A, Kocyigit A, Güler EM, Bilgin MG, Ergün İS, Dadak A. Effects of carvacrol on human fibroblast (WS-1) and gastric adenocarcinoma (AGS) cells in vitro and on Wistar rats in vivo. Mol Cell Biochem. 2018;448(1):237–249. doi: 10.1007/s11010-018-3329-5. [DOI] [PubMed] [Google Scholar]
- Hashim AR, Bashir DW, Yasin NAE, et al. Ameliorative effect of N-acetylcysteine against glyphosate-induced hepatotoxicity in adult male albino rats: histopathological, biochemical, and molecular studies. Environ Sci Pollut Res. 2021;28(31):42275–42289. doi: 10.1007/s11356-021-13659-2. [DOI] [PubMed] [Google Scholar]
- Hu C, Zhang X, et al. Meteorin-like protein attenuates doxorubicin-induced cardiotoxicity via activating cAMP/PKA/SIRT1 pathway. Redox Biol. 2020;37:101747. doi: 10.1016/j.redox.2020.101747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung TW, Lee SH, et al. METRNL attenuates lipid-induced inflammation and insulin resistance via AMPK or PPARdelta-dependent pathways in skeletal muscle of mice. Experimental Molecul Med. 2018;50(9):1–11. doi: 10.1038/s12276-018-0147-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahraman H, Kurutas E, et al. Protective effects of erythropoietin and N-acetylcysteine on methotrexate-induced lung injury in rats. Balkan Med J. 2013;30(1):99–104. doi: 10.5152/balkanmedj.2012.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karabulut D, Ozturk E, Kaymak E, Akin AT, Yakan B. Thymoquinone attenuates doxorubicin-cardiotoxicity in rats. J Biochem Mol Toxicol. 2021;35(1):e22618. doi: 10.1002/jbt.22618. [DOI] [PubMed] [Google Scholar]
- Kaya Tektemur N, Erdem Güzel E, Gül M, et al. The combination of N-acetylcysteine and cyclosporin A reduces acetaminophen-induced hepatotoxicity in mice. Ultrastruct Pathol. 2021;45(1):19–27. doi: 10.1080/01913123.2020.1850964. [DOI] [PubMed] [Google Scholar]
- Kaya S, Yalçın T, Boydak M, Dönmez HH (2023a) Protective Effect of N-Acetylcysteine Against Aluminum-Induced Kidney Tissue Damage in Rats. Biol Trace Elem Res 201:1806–1815. 10.1007/s12011-022-03276-6 [DOI] [PubMed]
- Kaya S, Yalçın T, Kuloğlu T (2023b) Resveratrol may reduce oxidative stress and apoptosis in doxorubicin-induced cardiotoxicity by regulating meteorin-like and TRPM2 levels. Comp Clin Pathol 32:393–404. 10.1007/s00580-023-03449-2
- Li ZY, Zheng SL, et al. Subfatin is a novel adipokine and unlike Meteorin in adipose and brain expression. CNS Neurosci Therapeutics. 2014;20(4):344–354. doi: 10.1111/cns.12219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li ZY, Fan MB, et al. Intestinal Metrnl released into the gut lumen acts as a local regulator for gut antimicrobial peptides. Acta Pharmacol Sin. 2016;37:1458–1466. doi: 10.1038/aps.2016.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao ZW, Hu WJ, Li ZY, et al. Involvement of the secreted protein Metrnl in human diseases. Acta Pharmacol Sin. 2020;41(12):1525–1530. doi: 10.1038/s41401-020-00529-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohebbati R, Maryam Paseban M, Soukhtanloo M, et al. Effects of standardized Zataria multiflora extract and its major ingredient, Carvacrol, on Adriamycin-induced hepatotoxicity in rat. Biomedical Journal. 2018;41(6):340–347. doi: 10.1016/j.bj.2018.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K, Oda A, Konishi H. Theanine prevents doxorubicin-induced acute hepatotoxicity by reducing intrinsic apoptotic response. Food Chem Toxicol. 2015;78:147–152. doi: 10.1016/j.fct.2015.02.009. [DOI] [PubMed] [Google Scholar]
- Nagai K, Fukuno S, Oda A, Konishi H. Protective effects of taurine on doxorubicin-induced acute hepatotoxicity through suppression of oxidative stress and apoptotic responses. Anticancer Drugs. 2016;27:17–23. doi: 10.1097/CAD.0000000000000299. [DOI] [PubMed] [Google Scholar]
- Omobowale TO, Oyagbemi AA, et al. Ameliorative effect of gallic acid in doxorubicin-ınduced hepatotoxicity in wistar rats through antioxidant defense system. Journal of Dietary Supplements. 2018;15(2):183–196. doi: 10.1080/19390211.2017.1335822. [DOI] [PubMed] [Google Scholar]
- Osman AT, Sharkawi SMZ, Hassan MIA, Abo-Youssef AM, Hemeida RAM. Empagliflozin and neohesperidin mitigate methotrexate hepatotoxicity via Nrf2/PPARγ/HO-1 signalling initiation and suppression of NF-κB/Keap1/HSP70/caspase-3 axis in rats. Life Sci. 2021;278:119638. doi: 10.1016/j.lfs.2021.119638. [DOI] [PubMed] [Google Scholar]
- Owumi S, Lewu D, Arunsi U, Oyelere A. Luteolin attenuates doxorubicin-induced derangements of liver and kidney by reducing oxidative and inflammatory stress to suppress apoptosis. Hum Exp Toxicol. 2021;40(10):1656–1672. doi: 10.1177/09603271211006171. [DOI] [PubMed] [Google Scholar]
- Ozaydin T, Sur E, Oznurlu Y, Celik I, Uluisik D. Immunohistochemical distribution of heat shock protein 70 and proliferating cell nuclear antigen in mouse placenta at different gestational stages. Microsc Res Tech. 2016;79(4):251–257. doi: 10.1002/jemt.22624. [DOI] [PubMed] [Google Scholar]
- Pockley AG. Heat shock proteins as regulators of the immune response. Lancet. 2003;362(9382):469–476. doi: 10.1016/S0140-6736(03)14075-5. [DOI] [PubMed] [Google Scholar]
- Prasanna PL, Renu K, Gopalakrishnan AV. New molecular and biochemical insights of doxorubicin-induced hepatotoxicity. Life Sci. 2020;250:117599. doi: 10.1016/j.lfs.2020.117599. [DOI] [PubMed] [Google Scholar]
- Qiao Y, Zhang X, Zhao G, Liu Z, Yu M, Fang Z, Li X. Hepatocellular iNOS protects liver from ischemia/reperfusion injury through HSF1-dependent activation of HSP70. Biochem Biophys Res Commun. 2019;512(4):882–888. doi: 10.1016/j.bbrc.2019.03.133. [DOI] [PubMed] [Google Scholar]
- Rafiee Z, Moaiedi MZ, et al. p-Coumaric acid alleviates adriamycin-induced hepatotoxicity in rats. Asian Pac J Trop Biomed. 2021;11:115–121. doi: 10.4103/2221-1691.306691. [DOI] [Google Scholar]
- Rao RR, Long JZ, et al. Meteorin-like is a hormone that regulates immune-adipose interactions to increase beige fat thermogenesis. Cell. 2014;157:1279–1291. doi: 10.1016/j.cell.2014.03.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renu K, et al. Molecular mechanism of doxorubicin-induced cardiomyopathy–an update. Eur J Pharmacol. 2018;818:241–253. doi: 10.1016/j.ejphar.2017.10.043. [DOI] [PubMed] [Google Scholar]
- Renu K, Pureti LP, Vellingiri B, Valsala Gopalakrishnan A. Toxic effects and molecular mechanism of doxorubicin on different organs–an update. Toxin Reviews. 2022;41(2):650–674. doi: 10.1080/15569543.2021.1912099. [DOI] [Google Scholar]
- Rupérez C, Ferrer-Curriu G, et al. Meteorin-like/Meteorin-beta protects heart against cardiac dysfunction. J Experiment Med. 2021;218(5):e20201206. doi: 10.1084/jem.20201206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh J, Phogat A, Kumar V, Malik V. N-acetylcysteine ameliorates monocrotophos exposure-induced mitochondrial dysfunctions in rat liver. Toxicol Mech Methods. 2022;32(9):686–694. doi: 10.1080/15376516.2022.2064258. [DOI] [PubMed] [Google Scholar]
- Sun J, Wu Q, Sun H, Qiao Y. Inhibition of histone deacetylase by butyrate protects rat liver from ischemic reperfusion injury. Int J Mol Sci. 2014;15:21069–21079. doi: 10.3390/ijms151121069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushach I, Burkhardt AM, et al. METEORIN-LIKE is a cytokine associated with barrier tissues and alternatively activated macrophages. Clinical Immunolog. 2015;156(2):119–127. doi: 10.1016/j.clim.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ushach I, Arrevillaga-Boni G, et al. Meteorin-like/Meteorin-beta is a novel immunoregulatory cytokine associated with inflammation. J Immunol. 2018;201(12):3669–3676. doi: 10.4049/jimmunol.1800435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jia Z, Dang H, Zou J. Meteorin-like/Meteorin-β upregulates proinflammatory cytokines via nf-κb pathway in grass carp ctenopharyngodon idella. Develop Comparative Immunol. 2022;127:104289. doi: 10.1016/j.dci.2021.104289. [DOI] [PubMed] [Google Scholar]
- Xiao B, et al. The true colors of autophagy in doxorubicin-induced cardiotoxicity. Oncol Lett. 2019;18(3):2165–2172. doi: 10.3892/ol.2019.10576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Cai Y, Wang Y, Xu C. Meteorin-Like (METRNL) attenuates myocardial ischemia/reperfusion injury-induced cardiomyocytes apoptosis by alleviating endoplasmic reticulum stress via activation of AMPK-PAK2 signaling in H9C2 cells. Med Sci Monit. 2020;26:e924564. doi: 10.12659/MSM.924564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yalcın T, Kaya S. Thymoquinone may alleviate cisplatin-induced muscle atrophy in rats by regulating mitofusin 2 and meteorin-like levels. Comp Clin Pathol. 2023;32:339–345. doi: 10.1007/s00580-023-03442-9. [DOI] [Google Scholar]
- Yalçın T, Kaya S, Kuloğlu T, Yiğin A (2023) N-Acetylcysteine May Regulate Altered Meteorin-Like Levels in Testicular Tissue due to Aluminum Exposure. Biological Trace Element Research 1–11. 10.1007/s12011-023-03656-6 [DOI] [PubMed]
- Zhang H, He Z, Deng P, et al. PIN1-mediated ROS production is involved in antagonism of N-acetyl-L-cysteine against arsenic-induced hepatotoxicity. Toxicol Res. 2022;11(4):628–643. doi: 10.1093/toxres/tfac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zorzi E, Bonvini P. Inducible hsp70 in the regulation of cancer cell survival: analysis of chaperone induction, expression and activity. Cancers. 2011;3(4):3921–3956. doi: 10.3390/cancers3043921. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.