Abstract
Background
Erythrodermic psoriasis is an acute inflammatory condition presenting as erythema and scaling involving more than 90% of body surface area in patients with a history of psoriasis vulgaris. If not treated promptly, metabolic complications and infections due to acute skin failure can cause significant morbidity and mortality in this condition. Interleukin-17 (IL-17) is considered to be the key player in initiating the inflammatory cascade in psoriasis. IL-17 blockers have been successfully used in the management of psoriasis vulgaris. However, its use in unstable erythrodermic psoriasis is limited to isolated case reports.
Methods
We hereby report an observational study of nine patients of unstable psoriatic erythroderma successfully managed with injection secukinumab and followed up over the next 24 months.
Results
Nine patients were managed during the study period, and a successful outcome was noted in all the patients. The Psoriasis Area and Severity Index response rate improved by at least 75% from baseline in 33.3% (3⁄9) at week 4 and improved to 88.9% (8⁄9) at week 12. None of the patients had a recurrence of erythroderma till 24 months of followup.
Conclusion
The study concluded that secukinumab is quick, safe, and efficient in psoriatic erythroderma, and there was no relapse of erythroderma in any of the patients in the 24 months of followup.
Keywords: Psoriasis, Erythroderma, Secukinumab
Introduction
Erythroderma has pronounced erythema and extensive scaling involving more than 90% of body surface area. It is caused due to various dermatological and systemic disorders, including erythrodermic psoriasis, which constitutes about 25–30% of all cases of erythroderma.1, 2, 3 There are two very distinct forms of psoriatic erythroderma. In one stable form, the long standing chronic plaques of psoriasis gradually spread to involve a majority of body surface area. It can be considered an extension of chronic plaque psoriasis vulgaris extending to involve a majority of the cutaneous surface.4,5 There are usually areas of normal skin between the affected plaques. The skin characteristics of psoriasis are retained, and the prognosis is better as patients respond to topical agents as well.
The second form of psoriatic erythroderma exists, which is termed as “unstable psoriasis.” It can occur at any time during the chronic course of psoriasis, either presenting unexpectedly and suddenly or be precipitated by a period of increasing intolerance to UV phototherapy, local applications, and loss of control over the disease. Generally, arthropathy is frequently associated with this spectrum of psoriatic erythroderma.6 Generalized pustular psoriasis can also lead to erythrodermic psoriasis due to infection, tar application, hypocalcemia, and most commonly due to withdrawal of oral corticosteroids. In this spectrum of unstable erythroderma, the whole skin is involved, and the basic characteristics of the disease are often lost. The patient may be ill and febrile, relapses are frequent, the disease course is often prolonged, and there is significant mortality. In contrast to the stable form, pruritus is often severe. The metabolic consequences of erythroderma are marked, which include altered thermoregulation, hemodynamic dysregulation, intestinal malabsorption, hypoproteinemia and dyselectrolytemia.
Psoriatic erythroderma if not treated promptly can lead to high rates of mortality and morbidity due to acute skin failure. Loss of protective barrier of skin predisposes to secondary infection, and loss of nutrients and proteins in the form of scales leads to anemia and hypoproteinemia. Poikilothermic is a known complication, and the patient needs good nursing care in a barrier-controlled environment. The immunosuppressive agents used in the management of psoriasis also predispose the patients to secondary infection and have altered pharmacokinetics due to altered renal and cardiac functions in an erythrodermic patient. The definitive management of underlying psoriasis has to be prompt, effective, and with minimal immune suppression. Along with targeting the key molecules with limited collateral damage, a detailed understanding of the pathogenesis of psoriatic erythroderma can significantly alter the outcome of this dermatological emergency.
T lymphocytes are the key inflammatory cells in the pathogenesis of psoriasis, which release several cytokines, which finally lead to angiogenesis and keratinocyte proliferation. T helper 17 lymphocytes produce Interleukin-17 (IL-17) that is a proinflammatory cytokine.7 It is mainly involved in inflammatory responses, promoting angiogenesis and neutrophilic chemotaxis. IL-17 induces keratinocytes to express various chemokines that promote the recruitment of Th17 cells, myeloid dendritic cells, and neutrophils at the site of the lesion.8, 9, 10, 11 Several studies have demonstrated that IL-17 is the key cytokine in the pathogenesis of psoriasis and psoriatic erythroderma and thus represents a crucial therapeutic target.12
Secukinumab is a recombinant, fully humanized, monoclonal anti-IL-17A G1 kappa antibody.13 Selective targeting of IL-17A and blocking its interaction with the IL-17 receptor leads to inhibition of the downstream effects of this proinflammatory cytokine, thereby interfering with the key psoriasis disease pathways and finally promoting normalization of immune function and skin histology.13 With therapeutic doses, which are used in psoriasis it fully neutralizes IL-17A without neutralizing IL-17F. It does not affect other Th17 functions or the Th1 pathway. This target specificity offers the potential for minimal adverse effects compared with other currently available systemic therapies. Secukinumab was approved by the US Food and Drug Administration (FDA) for the treatment of moderate to severe plaque psoriasis (January 2015), ankylosing spondylitis (January 2016), and Psoriatic arthritis (January 2016). The recommended dosage of secukinumab for plaque psoriasis is two 150 mg (300 mg) subcutaneous injections at weeks 0, 1, 2, 3, and 4, followed by 300 mg every 4 weeks.13 Infliximab has been used in cases with extensive psoriasis, and so far, it has been the most reported drug in literature in cases of erythroderma due to psoriasis.14
Here we report the results from prospective observational study from a tertiary care center of patients with erythrodermic psoriasis treated with Inj Secukinumab and followed up for a period of two years thereafter.
Materials and methods
This was an observational study in a tertiary care center, which had dedicated facilities to manage acute skin failure, including a dermatological Intensive Care Unit (ICU). Although this was an observational study using an approved drug, ethical clearance was not mandated, but still, it was obtained and attached. Over a period of two years nine patients with an unstable variant of erythrodermic psoriasis were followed up with Injection Secukinumab and subsidiary management of erythroderma. Patients with stable chronic plaque psoriasis progressing to erythroderma were not included as they could be managed with conventional therapy for psoriasis and did not necessitate a biologic for their management. Data of these nine patients with erythrodermic psoriasis (defined as a generalized erythematous inflammatory dermatosis, with associated exfoliation or scaling involving at least 90% of the body surface area, with the characteristic clinical and/or histological features of psoriasis and the exclusion of the other main differential diagnoses for erythroderma), treated with standard doses of secukinumab between January 2016 and December 2018 were included and followed up. Systemic therapies (Disease-modifying antirheumatic drugs (DMARDs), including methotrexate and leflunomide, immunosuppressants, including oral and injectable corticosteroids, cyclosporine, acitretin, and phototherapy) were prohibited concurrent with Secukinamab throughout the treatment period. Each patient was evaluated with the Psoriasis Area and Severity Index (PASI) at baseline, every week till 4 weeks, and then every four weeks till 24 weeks of initiation of treatment. Thereafter, patients were followed up every 12 weeks after stopping therapy for a total duration of 24 months. All patients were managed with the standard regimen for psoriasis, which included Inj secukinumab 300 mg subcutaneous at 0, 1, 2, 3, and 4 weeks, and then 300 mg once a month for the next 5 months. The patients were followed up to two years post-therapy for recurrence of erythroderma or deterioration of PASI. For no improvement or worsening of unstable psoriasis during the treatment period or recurrence of unstable erythroderma during the follow-up period, it was planned to shift the patient to Inj Infliximab or Cap Cyclosporine for rapid onset of action. For the development of plaque psoriasis or pustular psoriasis during the follow-up period, patients were planned for conventional topical therapy ± conventional systemic therapy (methotrexate/Cyclosporine/Acitretin). All patients were investigated prior to the institution of therapy, which included a battery of tests to diagnose the skin condition, including a skin biopsy, test prior to Inj secukinumab, including haemogram, liver, and renal function tests and infection profile, including HIV, Hepatitis B, Hepatitis C and a Chest radiograph for Tuberculosis. As most patients had extensive skin involvement and there was difficulty in interpreting Mantoux intradermal test hence Interferon-Gamma Release Assay (IGRA) was performed on the blood samples of all the patients before initiating therapy. Investigations for underlying erythroderma and its possible complications were performed, including blood sugar, electrolytes, sepsis screening, and protein levels. The patients were continuously monitored and managed in a thermo-regulated setting with nursing care as per the protocol for acute skin failure. Vital parameters, including temperature and weight, were regularly charted. The patients were evaluated on the basis of clinical response and the time for resolution of erythroderma. Complications of drug therapy, disease progression, and erythroderma were noted. All patients were followed for a period of two years for recurrence of psoriasis or erythroderma. Concerning drug safety evaluation, the presence or absence of any abnormal or unfavorable symptoms, signs, or diseases, including general laboratory values, throughout the study period was monitored. On the injection administration day, a general physical examination was performed. Adverse events that occurred during the injection from the start to 2 h after administration of secukinumab were defined as injection reactions. The advent of adverse reactions along with reactivation of any latent infection was noted at each follow-up visit. A written informed consent was taken from all patients.
Results
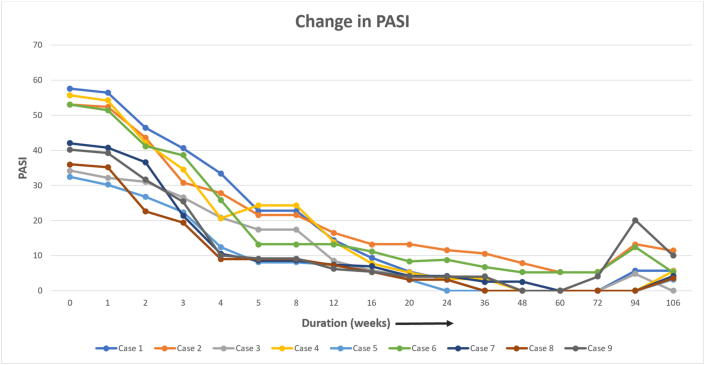
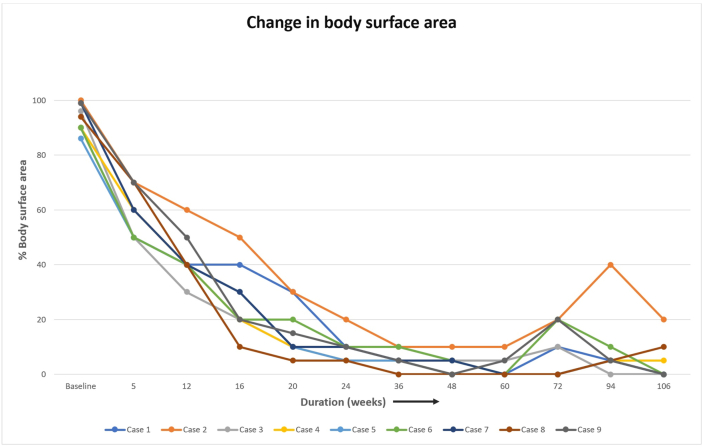
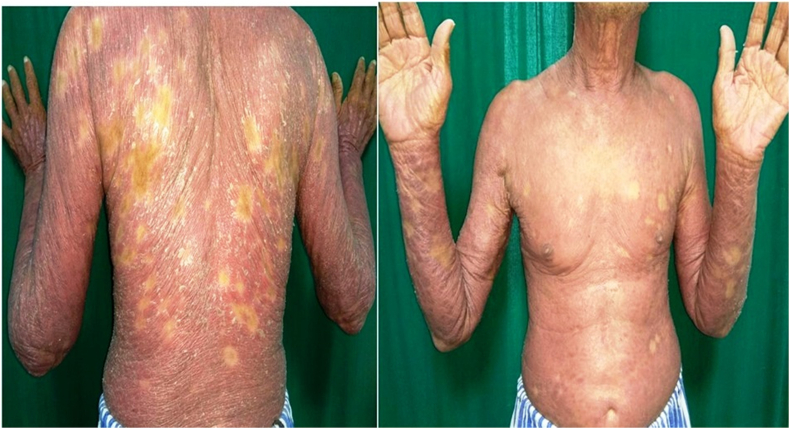
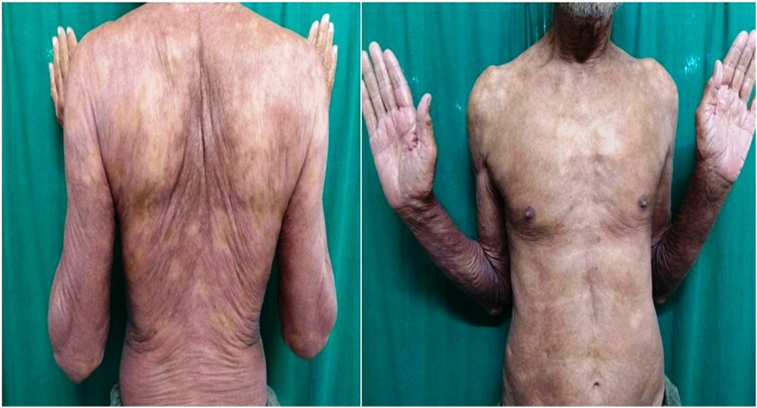
Nine patients were managed during the study period, and a successful outcome was noted in all the patients. Table 1 lists the baseline characteristics of the patients and the likely duration of chronic plaque psoriasis, the duration of present erythroderma, and the past episodes of erythroderma. The average age of the patients was 40.5 (±13.4) years. Regarding the sex distribution of all nine patients, 77.8% (7 / 9) were male and 22.2% (2 / 9) female. The average disease was 15.4 years. Regarding the treatment history, two patients were treatment naïve patients, i.e. they had not received any systemic medicine for psoriasis. Five patients had received biologics in the form of Tumour Necrosis Factor alpha (TNF alpha) inhibitors, and one patient among them had received secukinumab in the past. PASI and body surface area improvement was assessed in each visit, and this is shown in Fig. 1, Fig. 2. All patients had a significant reduction in body surface area involvement. Fig. 3, Fig. 4 show a 78 years old male patient with dramatic response in erythroderma over a period of 7 days.
Table 1.
Baseline characteristics of the nine studied patients.
| Patient | Sex | Age of onset of psoriasis | Age at present | Previous treatment for psoriasis (Topical therapies not included) | Last episode of erythroderma | Duration of present erythroderma | Baseline PASI | PASI75 achieved |
|---|---|---|---|---|---|---|---|---|
| 1 | M | 52 | 78 | Cyclosporine, NBUVB, Methotrexate, Etanercept | 18 months back | 3 weeks | 57.6 | 12 weeks |
| 2 | M | 49 | 65 | Acitretin, Etanercept, Methotrexate, Secukinumab | – | 6 weeks | 53 | 16 weeks |
| 3 | M | 18 | 31 | – | – | 2 months | 34.2 | 12 weeks |
| 4 | F | 14 | 35 | Cyclosporine, Infliximab, NBUVB | – | 3 weeks | 55.7 | 12 weeks |
| 5 | F | 59 | 59 | – | – | 2 weeks | 32.4 | 5 weeks |
| 6 | M | 46 | 48 | Methotrexate, Etanercept | 6 months back | 2 weeks | 53 | 5 weeks |
| 7 | M | 49 | 66 | NBUVB, Methotrexate | – | 2 weeks | 42 | 4 weeks |
| 8 | M | 57 | 67 | Methotrexate, NBUB | – | 3 weeks | 36 | 4 weeks |
| 9 | M | 21 | 55 | Systemic corticosteroids, Etanercept, Adalimumab, Acitretin | 8yrs, 5 yrs and 3 yrs back | 4 months | 40.2 | 4 weeks |
Fig. 1.
Psoriasis area and severity index change over 24 months.
Fig. 2.
Percentage of body surface area involvement.
Fig. 3.
78 Years old male patient with generalized erythema and scaling.
Fig. 4.
7 days after first dose of Injection Secukinumab.
The drug efficacy was assessed by the PASI score. The PASI 75 response rate, indicating the proportion of patients whose PASI score improved by at least 75% from baseline, was 33.3% (3 / 9) at week 4, improved to 55.6% (5 / 9) at week 5, 88.9% (8 / 9) at week 12 and 100% (9/9) at week 16. Thereafter, the PASI 75 response rate remained between 55.6% and 88.8% during the period from weeks 20–48, and it was 88.8% (8/9) at the end of 48 weeks. The drug efficacy was also assessed based on the reduction of erythema and scaling over the body surface area involved, which is shown in Fig. 2. 77.8% (7/9) had more than 30% body surface area improvement after the third dose of Secukinumab, and by eight weeks all patients had improvement in erythroderma. Thereafter, the response rate of improvement varied between 87.5% and 100.0%. The response rate at week 48 was 87.5%, and four patients were rated as “resolved,” five patients as “improved,” 0 patients as “unchanged,” and no patients as “worsened.” Itching and burning sensation of skin was the first clinical response noted with Inj Secukinumab within the first 24–48 h in all the patients and were a clinical sign of patient response. Erythema and hypoproteinemia resolved parallelly, and the patient was discharged after an average stay of about 2–3 weeks. The changes in the symptoms and laboratory parameters are shown in Table 2.
Table 2.
Clinical response of all the patients (resolved in number of days).
| Itching and skin burning | Skin redness and scaling | Fever | Hypoproteinemia | Hospital stay | |
|---|---|---|---|---|---|
| Patient 1 | 1 | 7 | 3 | 10 | 18 |
| Patient 2 | 2 | 5 | 2 | 8 | 14 |
| Patient 3 | 1 | 6 | 1 | 11 | 20 |
| Patient 4 | 1 | 10 | 2 | 12 | 20 |
| Patient 5 | 2 | 10 | 4 | 15 | 20 |
| Patient 6 | 5 | 12 | 7 | 21 | 25 |
| Patient 7 | 4 | 5 | 2 | 12 | 18 |
| Patient 8 | 3 | 7 | 2 | 6 | 18 |
| Patient 9 | 4 | 8 | 6 | 20 | 24 |
| Average | 2.5 days | 7.8 days | 3.2 days | 12.8 days | 19.7 days |
None of the patients had a relapse of erythroderma during the follow-up period. Two patients at the end of 60 weeks, and the remaining seven patients by 72 weeks, required topical therapy (corticosteroid/coaltar) for recurrence of chronic plaque psoriasis lesions involving less than <10% BSA or PASI <10. Two patients required initiation of methotrexate by 94 weeks for failure to control of lesions (>10% BSA or >10 PASI) with topical therapy alone and improved by the end of the follow-up period by 106 weeks. No severe adverse effect or life-threatening emergency was noted. 22.2% (2 / 9) patients complained of sore throat and nasal congestion, which lasted for 2–3 days after the first four injections and the symptoms were self-limiting and did not require any added intervention. No malignancy or reactivation of tuberculosis or any latent infection was noted in any patient during the follow-up period.
Discussion
Erythrodermic psoriasis is a multifactorial inflammatory disease, which may transform from pustular psoriasis or psoriasis vulgaris due to serious complications, infection or irregular treatments. Management of this condition is not standardized, difficult, and often inefficient. Traditionally employed systemic therapies for erythrodermic psoriasis include cyclosporine, methotrexate, oral retinoids, and systemic steroids have been used in many cases. Both safety and efficacy of conventional therapeutic options are unsatisfactory and unpredictable. Although biologics have been studied in psoriasis vulgaris, their use in erythrodermic psoriasis has been reported in the literature, including a few pilot studies.15, 16, 17 One clinical trial has evaluated the safety and efficacy of an anti-IL17 biologic for the treatment of erythrodermic psoriasis; Brodalumab (anti-IL17-receptor antibody) was used in a cohort of Japanese patients. Ixekizumab, a new humanized anti-interleukin-17A monoclonal antibody, has recently been evaluated for erythrodermic psoriasis in a phase 3 study (UNCOVER-J) on a small cohort of Japanese subjects. Results of these studies suggest that newer/developmental antipsoriatic biological agents targeting the IL-23/Th17 pathway, including anti-IL23p19 antibodies (Tildrakizumab and Guselkumab), anti-IL17 agents (Secukinumab, Ixekizumab, and Brodalumab), might play a positive role in the therapy of erythrodermic psoriasis.16, 17, 18 IL-17A is considered a key “driver” of proinflammatory cytokines in psoriasis pathogenesis since it can activate keratinocytes and promote hyper-proliferation, production of cytokines, chemokines, and antimicrobial peptides and ensues further promoting the inflammation cascade.19,20 Off late, more studies are emerging from clinical experiences and trials suggesting that secukinumab could become a treatment choice for erythrodermic psoriasis, especially in patients that have had previous unsuccessful therapies, even with biologics.15,21, 22, 23, 24, 25, 26, 27
Our study had the following key highlights.
-
1.
The rapid onset of action of Secukinumab in erythrodermic psoriasis.
-
2.
Clinical response in the form of reduced burning and itching sensation was observed as early as 24 h.
-
3.
Secukinumab was a successful, safe, quick-acting alternative and reduced the duration of hospital stay.
-
4.
It is efficient in the geriatric population (four of our patients were aged more than 65 years: multisystem failure and poor prognosis is expected in erythroderma of elderly)
-
5.
All nine patients did not have a recurrence of erythroderma in the 24 months observation period.
-
6.
Psoriatic erythroderma responds to the same standard dose of secukinumab that is advocated for psoriasis vulgaris.
-
7.
Secukinumab also reduced the metabolic complications of acute skin failure due to treatment of the underlying disease and arresting the inflammatory cascade.
Conclusion
Although the use of secukinumab in erythrodermic psoriasis has been limited to case reports, this study highlights the successful outcome in nine patients with unstable erythroderma, mostly in the geriatric age group. The key observation of this study is the rapid reversal of symptoms of erythroderma in all the patients with the clinical response as early as 24 h of the institution of therapy. It can be safely concluded that Secukinumab can be considered as safe option in erythrodermic psoriasis for quick and safe results.
Limitations
-
1.
Further studies with large sample sizes and well-designed Randomized trials are required to formulate treatment guidelines for erythrodermic psoriasis.
-
2.
Patients need to be followed up further for long-term complications of secukinumab and relapse rates.
-
3.
Cost of secukinumab is a major limiting factor in a resource-limited setup.
Disclosure of competing interest
The authors have none to declare.
References
- 1.Rosenbach M., Hsu S., Korman N.J., et al. Treatment of erythrodermic psoriasis: from the medical board of the National Psoriasis Foundation. J Am Acad Dermatol. 2010;62(4):655–662. doi: 10.1016/j.jaad.2009.05.048. [DOI] [PubMed] [Google Scholar]
- 2.Mistry N., Gupta A., Alavi A., et al. A review of the diagnosis and management of erythroderma (generalized red skin) Adv Skin Wound Care. 2015;28(5):228–236. doi: 10.1097/01.ASW.0000463573.40637.73. [DOI] [PubMed] [Google Scholar]
- 3.Zhang P., Chen H.X., Xing J.J., et al. Clinical analysis of 84 cases of erythrodermic psoriasis and 121 cases of other types of erythroderma from 2010-2015. J Huazhong Univ Sci Technolog Med Sci. 2017 Aug;37(4):563–567. doi: 10.1007/s11596-017-1773-1. [DOI] [PubMed] [Google Scholar]
- 4.Goeckerman W.H., O'Leary P.A. Erythroderma psoriaticum. J Am Med Assoc. 1932;99:2102–2105. [Google Scholar]
- 5.Cornbleet T. Action of synthetic antimalarial drugs on psoriasis. J Invest Dermatol. 1956;26:435–436. doi: 10.1038/jid.1956.58. [DOI] [PubMed] [Google Scholar]
- 6.Wright V., Reed W.B. The link between Reiter's syndrome and psoriatic arthritis. Ann Rheum Dis. 1964;23:12–21. doi: 10.1136/ard.23.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fargnoli M.C. Secukinumab: the anti-IL-17A biologic for the treatment of psoriasis. Case Rep Dermatol. Sep. 2019;11(suppl 1):1–3. doi: 10.1159/000501991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zaba L.C., Fuentes-Duculan J., Eungdamrong N.J., et al. Psoriasis is characterized by accumulation of immunostimulatory and Th1/Th17 cell-polarizing myeloid dendritic cells. J Invest Dermatol. 2009 Jan;129(1):79–88. doi: 10.1038/jid.2008.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghoreschi K., Laurence A., Yang X.P., Hirahara K., O'Shea J.J. T helper 17 cell heterogeneity and pathogenicity in autoimmune disease. Trends Immunol. 2011 Sep;32(9):395–401. doi: 10.1016/j.it.2011.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nograles K.E., Zaba L.C., Guttman-Yassky E., et al. Th17 cytokines interleukin (IL)-17 and IL-22 modulate distinct inflammatory and keratinocyte-response pathways. Br J Dermatol. 2008 Nov;159(5):1092–1102. doi: 10.1111/j.1365-2133.2008.08769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiricozzi A. Pathogenic role of IL-17 in psoriasis and psoriatic arthritis. Actas Dermosifiliogr. 2014 Oct;105(suppl 1):9–20. doi: 10.1016/S0001-7310(14)70014-6. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y., Qian T., Zhang D., Yan H., Hao F. Clinical efficacy and safety of anti-IL-17 agents for the treatment of patients with psoriasis. Immunotherapy. 2015;7(9):1023–1037. doi: 10.2217/imt.15.50. [DOI] [PubMed] [Google Scholar]
- 13.Frieder J., Kivelevitch D., Menter A. Secukinumab: a review of the anti-IL-17A biologic for the treatment of psoriasis. Ther Adv Chronic Dis. 2018;9(1):5–21. doi: 10.1177/2040622317738910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Leman J., Burden A. Treatment of severe psoriasis with infliximab. Therapeut Clin Risk Manag. 2008;4(6):1165–1176. doi: 10.2147/tcrm.s3094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Damiani G., Pacifico A., Russo F., et al. Use of secukinumab in a cohort of erythrodermic psoriatic patients: a pilot study. J Clin Med. 2019;8(6):770. doi: 10.3390/jcm8060770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mugheddu C., Atzori L., Lappi A., et al. Successful Secukinumab treatment of generalized pustular psoriasis and erythrodermic psoriasis. J Eur Acad Dermatol Venereol. 2017 doi: 10.1111/jdv.14234. [DOI] [PubMed] [Google Scholar]
- 17.Galluzzo M., D'Adamio S., Bianchi L., et al. Brodalumab for the treatment of psoriasis. Expet Rev Clin Immunol. 2016;12(12):1255–1271. doi: 10.1080/1744666X.2016.1246957. [DOI] [PubMed] [Google Scholar]
- 18.Saeki H., Nakagawa H., Nakajo K. Efficacy and safety of ixekizumab treatment for Japanese patients with moderate to severe plaque psoriasis, erythrodermic psoriasis and generalized pustular psoriasis: results from a 52-week, open-label, phase 3 study (UNCOVER-J) J Dermatol. 2017;44(4):355–362. doi: 10.1111/1346-8138.13622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galluzzo M., D'Adamio S., Campione E., Mazzilli S., Bianchi L., Talamonti M. A clinical case of severe disease burden: an erythrodermic psoriatic patient treated with secukinumab. J Dermatol Treat. 2018 Sep 26:1–11. doi: 10.1080/09546634.2018.1524818. [DOI] [PubMed] [Google Scholar]
- 20.Stinco G., Errichetti E. Erythrodermic psoriasis: current and future role of biologicals. Biopharmaceuticals and gene therapy. 2015;29(2):91–101. doi: 10.1007/s40259-015-0119-4. [DOI] [PubMed] [Google Scholar]
- 21.Dogra S., Bishnoi A., Narang T., Handa S. Long-term remission induced by secukinumab in a 13-year-old boy having recalcitrant chronic erythrodermic psoriasis. Dermatol Ther. 2018 Apr 23 doi: 10.1111/dth.12611. [DOI] [PubMed] [Google Scholar]
- 22.Mateu-Puchades A., Santos-Alarcón S., Martorell-Calatayud A., Pujol-Marco C., SánchezCarazo J.L. Erythrodermic psoriasis and secukinumab: our clinical experience. Dermatol Ther. 2018 Apr 16 doi: 10.1111/dth.12607. [DOI] [PubMed] [Google Scholar]
- 23.Weng H.J., Wang T.S., Tsai T.F. Clinical experience of secukinumab in the treatment of erythrodermic psoriasis: a case series. Br J Dermatol. 2018 Jun;178(6):1439–1440. doi: 10.1111/bjd.16252. [DOI] [PubMed] [Google Scholar]
- 24.Mugheddu C., Atzori L., Lappi A., Pau M., Murgia S., Rongioletti F. Successful Secukinumab treatment of generalized pustular psoriasis and erythrodermic psoriasis. J Eur Acad Dermatol Venereol. 2017 Sep;31(9):e420–e421. doi: 10.1111/jdv.14234. [DOI] [PubMed] [Google Scholar]
- 25.Fujita H., Ohtsuki M., Morita A., et al. Safety and effectiveness of secukinumab in psoriasis vulgaris and psoriatic arthritis: real-world evidence in Japan. J Dermatol. 2021 Feb;48(2):175–183. doi: 10.1111/1346-8138.15655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin L., Boggs J., Ramsay B., Hackett C., Ahmad K., Lynch M. Practical experience of secukinumab in the treatment of psoriasis: experience from a single centre. Ir J Med Sci. 2021 May;190(2):639–641. doi: 10.1007/s11845-020-02336-x. [DOI] [PubMed] [Google Scholar]
- 27.Bagel J., Blauvelt A., Nia J., et al. Secukinumab maintains superiority over ustekinumab in clearing skin and improving quality of life in patients with moderate to severe plaque psoriasis: 52-week results from a double-blind phase 3b trial (CLARITY) J Eur Acad Dermatol Venereol. 2021 Jan;35(1):135–142. doi: 10.1111/jdv.16558. [DOI] [PMC free article] [PubMed] [Google Scholar]