Abstract
We examined the association between exposure to PM2.5, focused on individual exposure level, and metabolic dysfunction during pregnancy. APPO study (Air Pollution on Pregnancy Outcome) was a prospective, multicenter, observational cohort study conducted from January 2021 to March 2023. Individual PM2.5 concentrations were calculated using a time-weighted average model. Metabolic dysfunction during pregnancy was assessed based on a modified definition of metabolic syndrome and its components, accounting for pregnancy-specific criteria. Exposure to PM2.5 during pregnancy was associated with worsened metabolic parameters especially glucose metabolism. In comparison to participants exposed to the low PM2.5 group, those exposed to high PM2.5 levels exhibited increased odds of gestational diabetes mellitus (GDM) after adjusting for confounding variables in different adjusted models. Specifically, in model 1, the adjusted odds ratio (aOR) was 3.117 with a 95% confidence interval (CI) of 1.234–7.870; in model 2, the aOR was 3.855 with a 95% CI of 1.255–11.844; in model 3, the aOR was 3.404 with a 95% CI of 1.206–9.607; and in model 4, the aOR was 2.741 with a 95% CI of 0.712–10.547. Exposure to higher levels of PM2.5 during pregnancy was associated with a tendency to worsen metabolic dysfunction markers specifically in glucose homeostasis. Further research is needed to investigate the mechanisms underlying the effects of ambient PM2.5 on metabolic dysfunction during pregnancy.
Supplementary Information
The online version contains supplementary material available at 10.1007/s11356-023-30921-x.
Keywords: Particulate matter, Indoor air pollution, Pregnancy complications, Metabolic dysfunction, Glucose intolerance, Lipid metabolism
Background
Fine particulate matter 2.5 (PM2.5), consisting of particles with aerodynamic diameters ≤ 2.5 μm, has exhibited a global increase in concentration despite efforts to reduce air pollution resulting from industrial development (Wallwork et al. 2017). Exposure to PM2.5 can result in respiratory ailments, cardiovascular complications, and impaired pulmonary function. Individuals at heightened vulnerability, such as pediatric and geriatric populations, have increased susceptibilities (Liu et al. 2019). In fact, the World Health Organization (WHO) has recognized air pollution as one of the top 10 global health hazards in 2019 (Wojtyla et al. 2020). Given the amplification of urbanization and industrial undertakings, comprehending the health ramifications of PM2.5 holds crucial significance for the formulation of efficacious strategies in public health management.
Previous studies have established the association between particulate matter and adverse pregnancy and neonatal outcomes, as pregnant women and fetuses are vulnerable to the effects of air pollution (Bai et al. 2020; Eze et al. 2015; Jirtle and Skinner 2007; Klepac et al. 2018; Lee et al. 2019; Li et al. 2021). However, the specific window of harmful exposure to PM2.5 during pregnancy remains inconclusive (Zhang et al. 2022).
Metabolic syndrome, characterized by central obesity, glucose intolerance, hypertriglyceridemia, and low high-density lipoprotein cholesterol (HDL-c), poses a significant public health concern and is associated with various complications, including chronic diseases such as cerebrovascular disease, diabetes mellitus, and cancer. The prevalence of metabolic syndrome in pregnancy has been increasing in tandem with advanced maternal age (Li et al. 2013). Previous animal studies have suggested that particulate matter induces oxidative stress and systemic inflammation, leading to metabolic dysfunction such as elevated blood pressure and altered lipid and glucose metabolism (Li et al. 2013). Similar findings have been reported in general populations (Brook and Rajagopalan 2009; Cheng et al. 2022).
Accurate individual measurement of particulate matter is crucial to comprehensively explore its effects. However, most studies investigating PM2.5 have been retrospective due to limitations in measurement techniques. Notably, particulate matter emissions originate not only from outdoor sources but also from indoor sources. Previous studies examining the association between particulate matter and pregnancy have primarily focused on outdoor particulate matter density, with limited evaluation of indoor particulate matter (Cheng et al. 2022; Jedrychowski et al. 2009; Shezi et al. 2020). Given that modern individuals, including pregnant women who may have reduced physical activity, spend a significant amount of time indoors, indoor particulate matter density should be considered when analyzing the impact of particulate matter pollution. Thus, the objective of this study was to investigate the effect of PM2.5 exposure on metabolic dysfunction in pregnant women using a personalized measurement approach.
Methods
Study design and sample collection
APPO study (Air Pollution on Pregnancy Outcome) was a prospective, multicenter, and observational cohort study conducted from January 2021 to March 2023. A total of 456 pregnant individuals were enrolled from the outpatient clinics of all participating institutions (Ewha Womans University Mokdong Hospital, Yonsei University Severance Hospital, Ewha Womans University Seoul Hospital, Korea University Guro Hospital, Kangwon National University Hospital, Keimyung University Dongsan Medical Center, and Ulsan University Hospital). Inclusion criteria for the study were: mothers aged 19 years or older, less than 28 weeks of singleton gestation, and no history of chronic medical illness, other gynecologic disease, and cancer. The identification of preexisting medical conditions was ascertained via either self-report or clinical assessment.
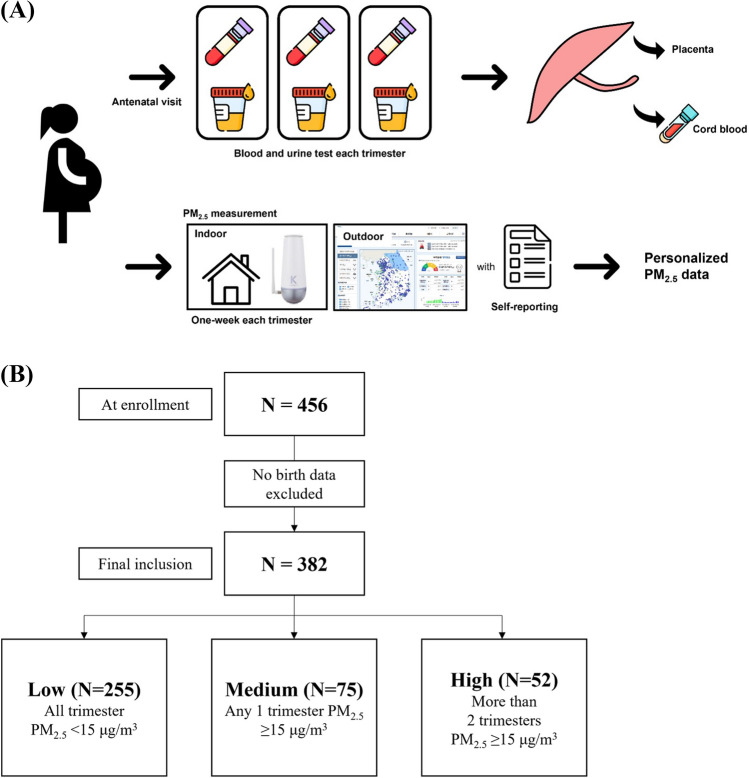
Sample collection involved obtaining blood and urine specimens from the pregnant women during routine antenatal visits at each trimester of pregnancy. Blood and urine specimens were collected in quantities of no less than 10 ml each. In addition, placenta and umbilical cord blood samples were collected at the time of delivery. The recruitment of participants and the sample collection processes are depicted in Fig. 1 for better understanding.
Fig. 1.
A Participant recruitment and sample collection processes. PM2.5, particulate matter 2.5. B Study flowchart
During the study period, self-reporting was conducted to collect data on various factors related to pregnant women, including age, pre-pregnancy body mass index (BMI), residential region, marriage status, education status, occupation, monthly household income level, gravidity (number of pregnancies), artificial reproductive technique, passive smoking exposure, drinking habits, exercise levels, physical activity levels, cooking device and technique, and air cleaner use (Hur et al. 2023).
Measurement of particulate matter exposure
AirguardK® (Kweather, Co, Korea) is a small electronic device equipped with a light scattering laser photometer sensor that is capable of detecting levels of air pollution. The device was placed inside the participants’ homes for a minimum of 1 week during each trimester of pregnancy to measure indoor air quality. The device collected data on various air pollutants such as PM2.5, PM10, and CO2, as well as temperature, humidity, and indoor integrated index, with a data collection interval of 1 min. The collected indoor air quality data was transmitted to an indoor air quality monitoring platform through Long-Term Evolution (LTE) communication network to prevent data loss, and data was collected and stored every minute. The measurement was carried out in real-time using Internet of Things (IoT) and Information and Communication Technology (ICT) for a week during each trimester of pregnancy to obtain concentration values of pollutants (Fig. 2).
Fig. 2.

AirguardK® (Kweather, Co, Korea); a device for measurement of air pollutants, placed inside the participants’ house at least 1 week per trimester
Outdoor air pollutant concentrations were collected from a nearby urban atmospheric measurement network based on the addresses of the recruited pregnant women. The Urban Air Monitoring Station data used in this study were obtained from AirKorea (https://www.airkorea.or.kr/web) by The Korean Ministry of Environment. AirKorea has 614 stations in 162 cities and counties for measurement of air pollutant including sulfur dioxide (SO2), nitrogen dioxide (NO2), ozone (O3), carbon monoxide (CO), PM10, and PM2.5 as of year in Korea.
To assess the potential harmful effects of particulate matter, we evaluated human exposure levels, time-activity patterns, and indoor pollution levels. Participants completed a time-activity questionnaire for one week to capture their daily activities and locations, and outdoor PM exposure data was obtained from Air Korea and a mapping/application tool. We used a time-weighted average model to estimate individual PM exposure, which takes into account the duration and location of various activities, as described in previous studies (Edwards et al. 2001; Moschandreas et al. 2002; Park et al. 2020). This approach allows us to better understand the relationship between PM exposure and metabolic dysfunction during pregnancy by obtaining more accurate measurements of individual PM exposure through a time-activity pattern analysis.
where
- Cper
predicted personal exposure
- Tindoor
indoor activity time rate (indoor activity time/24 h)
- Cindoor
indoor PM2.5 concentration
- Toutdoor
outdoor activity time rate (outdoor activity time/24 h)
- Coutdoor
outdoor PM2.5 concentration
According to the World Health Organization's (WHO) air quality guideline, a concentration of PM2.5 below 15 μg per cubic meter (μg/m3) is considered as the acceptable air quality level. In our study, we categorized the participants into three groups based on their PM2.5 exposure levels during pregnancy: low group: participants who were exposed to PM2.5 levels below 15 μg/m3 throughout the entire pregnancy; medium group: participants who were exposed to PM2.5 levels equal to or greater than 15 μg/m3 during any trimester of pregnancy; high group: participants who were exposed to PM2.5 levels above 15 μg/m3 more than two trimesters in pregnancy (Organization 2021). This categorization was done to assess the potential association between PM2.5 exposure levels and metabolic dysfunction during pregnancy in a more systematic and standardized manner.
Definition of metabolic dysfunction in pregnancy
Based on the criteria for metabolic syndrome outlined by the International Diabetes Federation, metabolic syndrome was defined as a waist circumference of ≥ 35 inches for women, along with abnormal metabolic components such as elevated blood pressure, altered lipid metabolism, and glucose intolerance. However, we found it inappropriate to apply the original diagnostic criteria for metabolic syndrome in our study due to the unique metabolic changes that occur during pregnancy. Additionally, there were inconsistencies in the trimester of pregnancy during which measurements were taken, and not all pregnant women underwent testing in a fasting state. Therefore, we modified the definition of metabolic parameters in our study by gestational diabetes mellitus (GDM) diagnosis, as a measure of fasting glucose levels. Additionally, we modified the lipid profile criteria to include triglyceride (TG) levels ≥ 175 mg/dl and high-density lipoprotein (HDL) levels < 40 mg/dl, based on previous research (Nordestgaard et al. 2016; Rammah et al. 2021). These modifications were made to account for the unique metabolic changes that occur during pregnancy and to ensure the appropriateness and accuracy of our analysis with regard to metabolic dysfunction. If a participant met the criteria for two or more metabolic parameters (elevated blood pressure over 130/85 mmHg, diagnosis of GDM, TG ≥ 175 mg/dl, and HDL < 40 mg/dl), it was considered indicative of metabolic dysfunction.
To diagnose GDM, following the rule of each institution, all pregnant women underwent the GDM screening tests between 24 and 28 weeks of gestation. GDM was diagnosed when thresholds were met or exceeded (Cunningham et al. 2022). If there is any abnormal value in OGTT, it is defined as glucose intolerance.
Lipid profiles including LDL cholesterol, triglycerides, and total cholesterol were measured through maternal blood samples in the third trimester. HDL cholesterol (HDL-c) was calculated using the traditional Friedewald equation (Friedewald et al. 1972). It has been known that elevated triglycerides (TG) are a risk factor for insulin resistance while an increase in HDL-c is a protective factor, especially since TG/HDL-c ratio ≥ 3.0 mg/dl has a higher association with insulin resistance than TG and HDL itself. Thus, we evaluated the TG/HDL-c ratio as a marker of metabolic dysfunction in this study (Barat et al. 2018; Gong et al. 2021; Marotta et al. 2010).
Statistical analysis
Categorical variables were expressed by frequencies out of available cases. Quantitative variables were expressed as mean (standard deviation), median and ranges. Differences in the continuous and categorical variables were analyzed using Student t-test, ANOVA, and chi-square test or Fisher’s exact test, as appropriate. The chi-square test for linear-by-linear association was used for analysis of trends. The logistic regression models were used to analyze the associations between exposure to PM2.5 and metabolic dysfunction using odds ratio (OR) and 95% confidence intervals (CI). Covariates, which are variables that may confound the relationship between the exposure and outcome, were selected based on previous research and included maternal age, monthly household income, education levels, pre-pregnancy BMI, exercise levels, physical activity levels, passive smoking exposure, season of conception (spring [March to May], summer [June to August], autumn [September to November], winter [December to February]), cooking device, cooking technique, and air cleaner use (Eze et al. 2015; Zhang et al. 2020). We examined the association by 4 steps: a crude (unadjusted) model 1; model 2, adjusted for demographical factors (age, education, income), pre-pregnancy BMI, and season of conception; model 3, adjusted for lifestyle factors such as smoking history, alcohol habit, physical activity levels, exercise, cooking method, and air cleaner use; and model 4, adjusted for environment factors (VOC, CO2, Temp, humidity, and PM10). The dose–response curves were drawn using Probit analysis. Receiver operating curves (ROC) and the area under the curve (AUC) were used to analysis. The exposure effects were regarded as statistically significant at the p < 0.05 level. Data was analyzed using SPSS (IBM) software version 26.0.
Results
Demographics
During the study period, we included 382 participants who had measured PM2.5 concentrations for more than two trimesters and had complete birth data out of a total of 456 participants (Fig. 1B). The baseline characteristics of the study population, such as maternal age, marital status, education level, smoking status, and alcohol consumption, are presented in Table 1. The mean maternal age was 33.59 ± 4.09 years, and most participants were married, well-educated (91.4% completed university education), non-smokers, and non-alcohol consumers.
Table 1.
Demographic characteristics and clinical data of the participants at baseline visit (N = 382)
| Characteristics | Total (n = 382) | PM2.5 exposure levels | |||
|---|---|---|---|---|---|
| Low (n = 255) | Medium (n = 75) | High (n = 52) | p-value | ||
| Age (year) | 33.49 ± 4.06 | 33.59 ± 3.71 | 33.36 ± 4.68 | 33.23 ± 4.75 | 0.834 |
| Pre-pregnancy BMI (kg/m2) | 21.58 ± 3.25 | 21.69 ± 3.46 | 21.26 ± 2.99 | 21.49 ± 2.47 | 0.590 |
| Pre-pregnancy BMI over 25 kg/m2 | 44 (11.5) | 30 (11.8) | 10 (13.3) | 4 (7.7) | 0.605 |
| Region (n, %) | 0.895 | ||||
| Urban | 358 (93.7) | 236 (92.5) | 72 (96.0) | 50 (96.2) | |
| Suburban | 16 (4.2) | 12 (4.7) | 3 (4.0) | 1 (1.9) | |
| Mountainous area | 2 (0.5) | 2 (0.8) | 0 (0.0) | 0 (0.0) | |
| Industrial area | 6 (1.6) | 5 (2.0) | 0 (0.0) | 1 (1.9) | |
| Marital status (n, %) | 0.131 | ||||
| Married | 377 (98.7) | 253 (99.2) | 74 (98.7) | 50 (96.2) | |
| Unmarried, divorced, diseased | 5 (1.3) | 2 (0.8) | 1 (1.3) | 2 (3.8) | |
| Education status (n, %) | 0.208 | ||||
| Lower than high school | 18 (4.8) | 15 (5.9) | 2 (2.7) | 1 (1.9) | |
| High school | 15 (3.9) | 6 (2.4) | 4 (5.3) | 5 (9.6) | |
| College or university | 272 (71.2) | 182 (71.4) | 57 (76.0) | 33 (63.5) | |
| Postgraduate | 77 (20.2) | 52 (20.4) | 12 (16.0) | 13 (25.0) | |
| Occupation status (n, %) | 0.887 | ||||
| No | 119 (31.2) | 83 (32.5) | 25 (33.3) | 11 (21.2) | |
| Yes | 263 (68.8) | 172 (67.5) | 50 (66.7) | 41 (78.8) | |
| Monthly income level (n, %) | 0.734 | ||||
| < KRW 400 (× 104) | 94 (24.6) | 54 (26.5) | 25 (36.8) | 15 (32.5) | |
| KRW 400 ~ 600 (× 104) | 67 (17.5) | 43 (21.1) | 13 (19.1) | 11 (23.9) | |
| ≥ KRW 600 (× 104) | 112 (16.0) | 75 (36.7) | 21 (30.9) | 16 (34.8) | |
| Gravidity (n, %) | 0.765 | ||||
| Primigravida | 188 (49.2) | 122 (47.8) | 37 (49.3) | 29 (55.8) | |
| Multigravida | 194 (50.8) | 133 (52.2) | 38 (50.7) | 23 (44.2) | |
| Pregnancy route (n, %) | 0.676 | ||||
| Natural | 325 (85.1) | 220 (86.3) | 61 (81.3) | 44 (84.6) | |
| ART (IUI, IVF-ET) | 57 (14.9) | 35 (13.7) | 14 (18.7) | 8 (15.4) | |
| Smoking status (n, %) | 0.736 | ||||
| Former | 348 (91.1) | 232 (91.0) | 70 (93.3) | 46 (88.5) | |
| Never | 34 (8.9) | 23 (9.0) | 5 (6.6) | 6 (11.3) | |
| Passive smoking in house (n, %) | 0.119 | ||||
| Yes | 21 (5.5) | 10 (3.9) | 6 (8.0) | 5 (9.6) | |
| No | 361 (94.5) | 245 (96.1) | 69 (92.0) | 47 (90.4) | |
| Passive smoking in work (n, %) | 0.918 | ||||
| Yes | 30 (7.9) | 21 (8.2) | 6 (8.0) | 3 (5.9) | |
| No | 351 (91.9) | 234 (91.8) | 69 (92.0) | 48 (94.1) | |
| Drinking status (days/week) (n, %) | 0.525 | ||||
| Former | 269 (70.4) | 169 (82.8) | 58 (85.3) | 42 (89.4) | |
| Never | 50 (13.1) | 35 (17.2) | 10 (14.7) | 5 (10.6) | |
| Exercise (n, %) | 0.114 | ||||
| Yes | 78 (20.4) | 198 (78.0) | 66 (88.0) | 39 (75.0) | |
| No | 303 (79.3) | 56 (22.0) | 9 (12.0) | 13 (25.0) | |
| Physical activity (n, %) | 0.445 | ||||
| Active | 9 (2.4) | 7 (2.8) | 1 (1.3) | 1 (2.0) | |
| Moderate | 86 (22.5) | 60 (23.6) | 16 (21.3) | 10 (19.6) | |
| Inactive | 285 (74.6) | 187 (73.6) | 58 (77.3) | 40 (78.4) | |
| Cooking device (n, %) | 0.738 | ||||
| Stove | 192 (50.3) | 119 (51.7) | 46 (63.0) | 27 (52.9) | |
| Induction | 134 (35.1) | 91 (39.6) | 23 (31.5) | 20 (39.2) | |
| Etc | 28 (7.4) | 20 (8.7) | 4 (5.5) | 4 (7.8) | |
| Cooking technique (n, %) | 0.533 | ||||
| Fry/grill/roast | 282 (73.8) | 186 (78.8) | 61 (85.9) | 35 (72.9) | |
| Steam/boil | 44 (11.5) | 33 (14.0) | 5 (7.0) | 6 (12.5) | |
| Raw | 6 (1.6) | 4 (1.7) | 1 (1.4) | 1 (2.1) | |
| Etc | 23 (6.0) | 13 (5.5) | 4 (5.6) | 6 (12.5) | |
| Season of conception (n, %) | NA | ||||
| Spring (March to May) | 114 (29.8) | 99 (39.0) | 10 (13.3) | 5 (9.6) | |
| Summer (June to August) | 115 (30.1) | 40 (15.7) | 42 (56.0) | 33 (63.5) | |
| Autumn (September to November) | 88 (23.0) | 59 (23.2) | 18 (24.0) | 11 (21.2) | |
| Winter (December to February) | 64 (16.8) | 56 (22.0) | 5 (6.7) | 3 (5.8) | |
Data are presented as N (%) or mean ± SD
Low group: participants who were exposed to PM2.5 levels below 15 μg/m3 throughout the entire pregnancy. Medium group: participants who were exposed to PM2.5 levels equal to or greater than 15 μg/m3 during any trimester of pregnancy. High group: participants who were exposed to PM2.5 levels above 15 μg/m3 more than two trimester in pregnancy
BMI body mass index, KRW Korean won, ART artificial reproductive technique, IUI intrauterine insemination, IVF-ET in vitro fertilization-embryo transfer
NA (not applicable) as statistically not interpretable
PM2.5 distribution and environment factors of study population
The distribution of PM2.5 concentrations and other air conditions are described in Table 2. The median concentration of PM2.5 weighted for time-activity log was 9.89 μg/m3 (IQR 6.35–16.02) over the entire follow-up period. The median indoor PM2.5 exposure level was 9.52 μg/m3 (IQR 5.85–15.75) and outdoor PM2.5 exposure was 17.51 μg/m3 (IQR 14.28–21.75). During the study period, median daily temperature was 25.10 °C (IQR 23.89–26.43) and relative humidity was 37.99% (IQR 31.96–43.55).
Table 2.
Descriptive statistics of air pollutants in study period
| Variables | Mean (SD) | Min | Q1 | Median | Q3 | Max |
|---|---|---|---|---|---|---|
| Air pollutant concentration | ||||||
| PM2.5 (μg/m3) | ||||||
| Indoor | 12.41 (10.60) | 0.90 | 5.85 | 9.52 | 15.75 | 99.05 |
| Outdoor | 18.14 (5.96) | 6.00 | 14.28 | 17.51 | 21.75 | 38.95 |
| Weighted PM2.5# | 12.71 (10.19) | 1.04 | 6.35 | 9.89 | 16.02 | 99.05 |
| PM10 (μg/m3) | 23.08 (19.81) | 2.49 | 11.96 | 17.48 | 26.46 | 199.73 |
| CO2 (ppm) | 873.64 (327.40) | 277.12 | 651.74 | 833.20 | 1019.61 | 2820.20 |
| VOC (μg/m3) | 5069.34 (5306.09) | 31.26 | 1332.73 | 2960.65 | 6681.92 | 28276.41 |
| Weather conditions | ||||||
| Daily temperature (℃) | 25.22 (2.25) | 17.45 | 23.89 | 25.10 | 26.428 | 34.75 |
| Relative humidity (%) | 38.61 (9.51) | 17.04 | 31.96 | 37.99 | 43.551 | 84.00 |
VOC volatile organic compounds, Min minimum, Q1 25 quartile, Q3 75 quartile, Max maximum
#Weighted PM2.5 Cper= ∑( Tindoor × Cindoor + Toutdoor × Coutdoor); where Cper: predicted personal exposure, Tindoor: indoor activity time rate (indoor activity time/24 h), Cindoor: indoor PM2.5 concentration, Toutdoor: outdoor activity time rate (outdoor activity time/24 h); Coutdoor: outdoor PM2.5 concentration
PM2.5 exposure and metabolic dysfunction in pregnancy
We observed significant correlations between PM2.5 exposure and metabolic components, such as elevated blood pressure, glucose intolerance (defined as any one abnormal oral glucose tolerance test (OGTT) results), gestational diabetes mellitus (GDM), and altered lipid metabolism as shown in Table 3. The longer the duration of exposure to high PM2.5 concentrations, the higher the risk of glucose intolerance and GDM (p = 0.023, 0.045, respectively). There was also a trend of increasing levels of triglycerides over 175 mg/dl with prolonged exposure to high PM2.5 concentrations (p for trend 0.055).
Table 3.
Association between each PM2.5 levels and metabolic components
| Variables | PM2.5 exposure levels | ||||
|---|---|---|---|---|---|
| Low (n = 255) | Medium (n = 75) | High (n = 52) | p-value | p for trend | |
| HBP (SBP ≥ 130 mmHg or DBP ≥ 85 mmHg) | 6 (2.4) | 7 (9.5) | 1 (2.0) | 0.020* | 0.345 |
| Glucose intolerance | 12 (4.7) | 6 (8.0) | 8 (15.4) | 0.023* | 0.006* |
| GDM | 14 (5.5) | 7 (9.3) | 8 (15.4) | 0.045* | 0.012* |
| HDL-c < 40 mg/dl | 27 (19.4) | 9 (21.4) | 1 (4.2) | 0.157 | 0.171 |
| TG ≥ 175 mg/dl | 211 (93.8) | 63 (96.9) | 45 (100.0) | 0.187 | 0.055 |
| TG/HDL ≥ 3.0 | 119 (85.6) | 31 (73.8) | 19 (79.2) | 0.178 | 0.170 |
| Metabolic dysfunction | 53 (20.8) | 19 (25.3) | 14 (26.9) | 0.507 | 0.147 |
Data are presented as N (%)
Analysis was conducted using the chi-square test, Fisher’s exact test, and the linear-by-linear association. Increased exposure to PM impacts glucose metabolism and may also influence elevated blood pressure and triglyceride levels, although the significance of these effects is limited or statistically insignificant
Low group: participants who were exposed to PM2.5 levels below 15 /m3 throughout the entire pregnancy. Medium group: participants who were exposed to PM2.5 levels equal to or greater than 15 μg/m3 during any trimester of pregnancy. High group: participants who were exposed to PM2.5 levels above 15 μg/m3 more than two trimesters in pregnancy
HBP high blood pressure, GDM gestational diabetes, HDL-c high density lipoprotein cholesterol, TG triglycerides
*p-value < 0.05
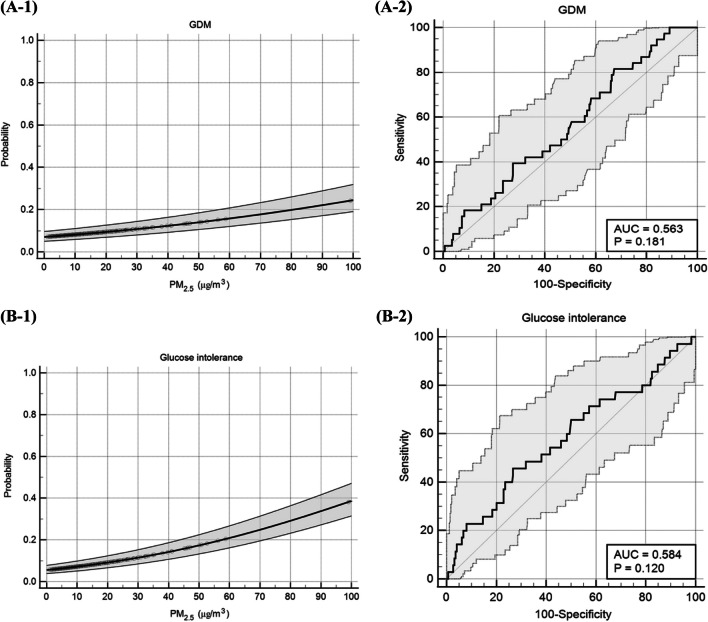
Table 4 presents the odds ratio (OR) of metabolic components by PM2.5 exposure period during pregnancy, after adjusting for four types of models; demographic, lifestyle, and environmental factors (as described in the “Methods” section). As the exposure to higher concentrations of PM2.5 for an extended period increased, changes in glucose homeostasis observed. Glucose intolerance showed an odds ratio (OR) of 3.682 (95% CI 1.423–9.525, p = 0.007) in the crude regression model for the high group, and similar results were obtained when considering other covariates. In the case of gestational diabetes mellitus (GDM), the crude model for the High group showed an OR of 3.117 (95% CI 1.234–7.870, p = 0.016), and even in models considering other variables, generally consistent results were observed. The highest exposure to PM2.5 in most trimesters was found to be associated with GDM, as evidenced by the concentration–response curves shown in Fig. 3A-1 and ROC curve in Fig. 3A-2 (AUC 0.563, p = 0.161). Exposure to high PM2.5 concentrations was also associated with glucose intolerance after adjustments, as shown by the concentration–response curve in Fig. 3B-1 and ROC curve in Fig. 3B-2 (AUC 0.584, p = 0.130).
Table 4.
Adjusted odds ratios with 95% CI of metabolic dysfunction and its components in overall population with increase PM2.5 exposure levels
| Low (ref) | Medium | High | ||||
|---|---|---|---|---|---|---|
| OR (95% CI) | p-value | OR (95% CI) | p-value | |||
| HBP | Model 1 | 1 | 4.318 (1.404–13.279) | 0.011* | 0.827 (0.097–7.017) | 0.827 |
| Model 2 | 4.038 (0.948–17.192) | 0.059 | 0.703 (0.065–7.592) | 0.703 | ||
| Model 3 | 4.536 (1.379–14.920) | 0.013* | 0.810 (0.092–7.128) | 0.810 | ||
| Model 4 | 6.484 (0.813–51.725) | 0.078 | NA | |||
| Glucose intolerance | Model 1 | 1 | 1.761 (0.638–4.863) | 0.275 | 3.682 (1.423–9.525) | 0.007* |
| Model 2 | 2.593 (0.722–9.321) | 0.144 | 5.792 (1.681–19.961) | 0.050* | ||
| Model 3 | 2.222 (0.681–7.248) | 0.186 | 5.026 (1.669–15.137) | 0.004* | ||
| Model 4 | 2.272 (0.477–10.829) | 0.303 | 5.001 (1.228–20.359) | 0.025* | ||
| GDM | Model 1 | 1 | 1.765 (0.685–4.547) | 0.239 | 3.117 (1.234–7.870) | 0.016* |
| Model 2 | 1.654 (0.544–5.025) | 0.375 | 3.855 (1.255–11.844) | 0.018* | ||
| Model 3 | 2.180 (0.742–6.402) | 0.156 | 3.404 (1.206–9.607) | 0.021* | ||
| Model 4 | 2.142 (0.542–8.467) | 0.277 | 2.741 (0.712–10.547) | 0.142 | ||
| HDL-c < 40 | Model 1 | 1 | 1.131 (0.484–2.643) | 0.776 | 0.180 (0.023–1.395) | 0.101 |
| Model 2 | 1.161 (0.430–3.136) | 0.769 | 0.185 (0.022–1.572) | 0.185 | ||
| Model 3 | 0.974 (0.387–2.454) | 0.956 | 0.195 (0.024–1.554) | 0.195 | ||
| Model 4 | 2.059 (0.634–6.692) | 0.230 | 0.316 (0.035–2.852) | 0.305 | ||
| TG ≥ 175 | Model 1 | 1 | 2.090 (0.463–9.443) | 0.338 | NA | |
| Model 2 | 1.635 (0.340–7.870) | 0.540 | NA | |||
| Model 3 | 1.329 (0.271–6.5110 | 0.726 | NA | |||
| Model 4 | 1.565 (0.156–15.685) | 0.703 | NA | |||
| Metabolic dysfunction | Model 1 | 1 | 1.293 (0.708–2.361) | 0.403 | 1.404 (0.709–2.781) | 0.330 |
| Model 2 | 1.305 (0.672–2.535) | 0.432 | 1.215 (0.556–2.656) | 0.625 | ||
| Model 3 | 1.247 (0.653–2.381) | 0.503 | 1.265 (0.600–2.667) | 0.537 | ||
| Model 4 | 1.294 (0.540–3.103) | 0.563 | 0.779 (0.256–2.376) | 0.661 | ||
Analysis was conducted using the logistic regression. Increased exposure to PM impacts glucose metabolism and may also influence elevated blood pressure and triglyceride levels, although the significance of these effects is limited or statistically insignificant
HBP high blood pressure, GDM gestational diabetes, HDL-c high density lipoprotein cholesterol, TG triglycerides, T-chol total cholesterol
Low group: participants who were exposed to PM2.5 levels below 15 μg/m3 throughout the entire pregnancy. Medium group: participants who were exposed to PM2.5 levels equal to or greater than 15 μg/m3 during any trimester of pregnancy. High group: participants who were exposed to PM2.5 levels above 15 μg/m3 more than two trimesters in pregnancy
Model 1, a crude (unadjusted) model; model 2, adjusted for demographical factors (age, education, income), pre-pregnancy BMI, and season of conception; model 3, adjusted for lifestyle factors such as smoking history, alcohol habit, physical activity levels, exercise, cooking method, and air cleaner use; and model 4, adjusted for environment factors (VOC, CO2, temperature, humidity, and PM10)
NA (not applicable): the effect size was judged to be clinically not interpretable
*p-value < 0.05
Fig. 3.
Concentration–response relationship and receiver operating curve (ROC) between exposure of PM2.5 and glucose metabolism. A GDM (1) concentration–response curve and (2) ROC curve; B Glucose intolerance (1) concentration–response curve and (2) ROC curve
Figure 4 illustrates the mean values of metabolic parameters corresponding to each cohort. Figure 4A reveals an elevation in diastolic blood pressure (DBP) and mean arterial pressure (MAP), albeit lacking a noteworthy alteration in systolic blood pressure (SBP). In Fig. 4B, a discernible trend towards increased triglyceride (TG) levels is evident. Furthermore, Fig. 4C demonstrates a gradual incline in fasting glucose levels, glucose levels at the 2-h mark following a 100-g oral glucose tolerance test (OGTT), and glucose levels at the 1-h mark following a 50-g OGTT.
Fig. 4.
Trend of metabolic components according to PM2.5 exposure level. A Blood pressure in 3rd trimester, B lipid profile, and (C) glucose metabolism. These graphs represent the average values of metabolic components for each group. In A, there was an increase in DBP and MAP, but no significant change in SBP. In B, a trend of increased TG was observed. Furthermore, C an increasing tendency in fasting glucose, post-2 h glucose after a 100-g OGTT, and post-1 h glucose after a 50-g OGTT. Abbreviations: SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; TG, triglycerides; T-chol, total cholesterol; LDL, low density lipoprotein; HDL, high density lipoprotein; Fast, fasting
Sensitivity analysis
When sensitivity analysis was performed on pregnant individuals with a pre-pregnancy BMI less than 27 kg/m3, sustained exposure to PM2.5 demonstrated an association with elevated blood pressure in the third trimester and impaired glucose metabolism (Table S1). Additionally, an observable trend of triglyceride increase suggests that PM2.5 exposure might impact lipid metabolism. The association between exposure to PM2.5 during pregnancy and metabolic components is presented in Table S2.
Discussion
This study aimed to investigate the association between particulate matter (PM) and metabolic dysfunction during pregnancy by utilizing personalized measurements of PM2.5 concentration. The findings of this study revealed a positive association between PM2.5 exposure during pregnancy and metabolic dysfunction. Specifically, it was observed that PM2.5 exposure during pregnancy was associated with alterations in blood pressure control, lipid metabolism, and glucose homeostasis. Pregnant women who were exposed to high concentrations of PM2.5 exhibited elevated blood pressure in their third trimester, increased levels of triglycerides (TGs), and higher odds of gestational diabetes mellitus (GDM) and glucose intolerance.
Particulate matter (PM) is derived from various sources, such as industrial emissions, vehicular emissions, and wildfires, with approximately 25% of PM attributed to combustion of fuel. However, during pregnancy, reduced mobility or increased prevalence of remote work may result in increased indoor activities. As a consequence, it is imperative to consider indoor sources of PM when analyzing its effects on pregnancy outcomes. Indoor sources of PM can have a significant impact on air quality and exposure during pregnancy. Indoor exposure to PM can be influenced by multiple factors such as ventilation, cleaning, cooking, heating, tobacco smoke, building materials, and individual behaviors, and may vary depending on geographical location, cultural practices, and other contextual factors. This approach can provide a comprehensive understanding of the potential risks and impacts of PM on maternal and fetal health during pregnancy. Therefore, incorporating the consideration of indoor sources of PM in the analysis and interpretation of research findings can enhance the accuracy of assessing the potential health effects of PM during pregnancy (Ambade et al. 2022; Kumar et al. 2020; Wojtyla et al. 2020; Zhang et al. 2022).
Several studies have provided evidence that is consistent with the findings of this study, indicating that exposure to PM2.5 is associated with metabolic dysfunction. Specifically, higher levels of PM2.5 have been associated with metabolic syndrome, hypertriglyceridemia, and elevated fasting blood glucose levels (Eze et al. 2015; Liu et al. 2022; Wallwork et al. 2017). Recent studies have also reported an association between PM2.5 exposure and gestational diabetes mellitus (GDM) (Bai et al. 2020; Zhang et al. 2020). Barat et al. demonstrated that long-term exposure to particulate matter is a risk factor for dyslipidemia and elevated TG/HDL ratio, and may even be associated with GDM (Barat et al. 2018; Mao et al. 2020).
In contrast, while the majority of previous studies have shown a positive association between particulate matter and metabolic dysfunction, some studies have reported negative or null associations. Honda et al. suggested that exposure to PM2.5 during the pre-conception period and first trimester did not increase the risk of gestational diabetes mellitus (GDM). Furthermore, some studies have reported no association between PM2.5 exposure and glucose intolerance, but have observed an increase in HbA1c levels with higher PM2.5 concentrations (Honda et al. 2017; Li et al. 2021; Lucht et al. 2018; Robledo et al. 2015). It is hypothesized that the lack of prospective studies may contribute to these discrepancies.
The mechanistic association between fine particulate matter exposure and metabolic disorders, in accordance with prior research, is summarized as follows. Exposure to PM2.5 triggers systemic inflammation and pulmonary oxidative stress. Elevated concentrations of inflammatory mediators such as tumor necrosis factor-α (TNF- α) and interleukin-6 (IL-6), pivotal cytokines in the inflammatory cascade, lead to an intensified inflammatory response. This heightened response could potentially induce endothelial cell impairment, precipitating insulin resistance. Furthermore, via fluid shifts to the third space, this detriment may also contribute to the initiation of preeclamptic conditions (Ambade et al. 2022; Baron et al. 1995; Herder et al. 2016; Kumar et al. 2020; Sun et al. 2009). Further research is needed to elucidate the mechanisms underlying the relationship between particulate matter exposure during pregnancy and adverse fetal outcomes.
The potential role of oxidative stress as a mechanistic pathway linking particulate matter exposure to metabolic dysfunction during pregnancy has been hypothesized and investigated in recent research. Oxidative stress refers to an imbalance between the production of reactive oxygen species (ROS) and the body’s capacity to counteract and repair ROS-induced damage. Various sources of oxidative stress, such as surrounding metals, tobacco, airborne particulate matter, and plastics, have been implicated in including systemic inflammation and disrupting metabolic pathways, ultimately leading to metabolic syndrome and insulin resistance (Ruano et al. 2022). The inhalation of particulate matter has been demonstrated to induce oxidative stress and trigger systemic inflammation, vascular dysfunction, atherosclerosis, and cardiovascular disease in non-pregnant populations (Dabass et al. 2016; Ruano et al. 2022). Moreover, ROS and oxygen-centered free radicals generated by particulate matter have been shown to impair the insulin signaling pathway, resulting in insulin resistance (Alderete et al. 2017; Araujo and Nel 2009; Brook et al. 2010; Houstis et al. 2006; Hutcheson and Rocic 2012; Münzel et al. 2017; Perticone et al. 2001; Roeckner et al. 2016). Furthermore, oxidative stress induced by particulate matter has been associated with various adverse obstetric outcomes, including recurrent pregnancy loss, prematurity, intrauterine growth restriction (IUGR), diabetes, and preeclampsia (Oke and Hardy 2021; Ornoy et al. 2021; van Westering-Kroon et al. 2021; Yu et al. 2020; Zejnullahu et al. 2021). To mitigate the detrimental effects of oxidative stress on inflammation and metabolic dysfunction, antioxidants have been investigated as a potential intervention (Casuso and Huertas 2018; Gregório et al. 2016; L.S et al. 2016). However, it should be noted that the use of antioxidants as a preventive or therapeutic strategy for particulate matter-induced metabolic dysfunction during pregnancy is still an area of ongoing research, and further studies are needed to comprehensively evaluate their efficacy, safety, and optimal dosages. Additionally, addressing the underlying causes of particulate matter pollution and implementing measures to reduce exposure to particulate matter remain critical in safeguarding maternal and fetal health during pregnancy.
Investigating the impact of particulate matter on adverse pregnancy outcomes in crucial in the context of fetal programming, as external stimuli experienced by the fetus in the uterus, such as oxidative stress, maternal physiological changes, and preeclampsia, can have long-term effects on fetal health (Hur et al. 2022; Kwon and Kim 2017; Öztürk and Türker 2021). Previous retrospective studies have suggested that particulate matter exposure during pregnancy may lead to fetal neurodevelopmental delay, possibly due to inflammation, although the underlying mechanisms are not fully understood (Lin et al. 2022; Su et al. 2022). These findings highlight that exposure to particulate matter during pregnancy may also influence maternal metabolic dysfunction. Consistent with the fetal programming theory, particulate matter exposure could alter the intrauterine environment, which plays a significant role in the fetal health beyond maternal well-being.
The principal strength of this study is its status as the first prospective investigation conducted in South Korea examining the impact of particulate matter on pregnant women. Additionally, this study concentrates on the individual concentration of particulate matter, which represents a unique contribution to the existing literature. Nevertheless, this study has limitations. Firstly, this study was limited by small sample size. We anticipate that more people will enroll in this study through ongoing investigations. Secondly, the study was conducted during the 2019 coronavirus pandemic, which may have resulted in reduced outdoor activities and may not fully represent outdoor PM2.5 concentrations. Thirdly, the lack of available data on PM2.5 exposure during the pre-conception period, which hinders the ability to assess the impact of PM2.5 prior to pregnancy. The omission of a measurement period is a potential limitation. Finally, it should be noted that the composition of PM2.5 can vary across different countries, and the present study did not assess the specific composition of PM2.5. This may have implications for the observed adverse health outcomes and represents a potential limitation of the study. However, this study was conducted during the period of the COVID-19 pandemic, thus indoor air quality measurements were exceedingly advantageous for the analysis. Even in the post-pandemic era, environmental pollution continues to intensify alongside global warming, and it is anticipated that indoor activities will correspondingly escalate. It is hoped that the findings of this study will be beneficial in the post-COVID era.
Conclusion
The findings of this study revealed a positive association between PM2.5 exposure during pregnancy and metabolic dysfunction especially in glucose metabolism. These results emphasize the significance of managing PM2.5 concentrations to mitigate potential alterations in metabolic components that may contribute to adverse pregnancy outcomes. Furthermore, further research is warranted to investigate the effects of particulate matter on metabolic dysfunction during pregnancy in more depth.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
We are grateful to the APPO participants and staff at Yonsei University Hospital, Ewha Womans University Mokdong Hospital, Ewha Womans University Seoul Hospital, Korea University Guro Hospital, Kangwon National University Hospital, Keimyung University Dongsan Medical Center, and Ulsan University Hospital.
APPO study group
The APPO study group also includes the following current members: Yeonseong Jeong, Sunwha Park, Eunjin Kwon, Young Min Hur, Young-Ah You, Soo Min Kim, Gain Lee, Kyung A Lee, Soo Jung Kim, Geum Joon Cho, Min-Jeong Oh, Sung Hun Na, Se jin Lee, Jin-Gon Bae, Yu-Hwan Kim, Soo-Jeong Lee, Young-Han Kim, Young Ju Kim.
Abbreviations
- PM2.5
Particulate matter 2.5
- sBP
Systolic blood pressure
- dBP
Diastolic blood pressure
- MAP
Mean arterial pressure
- TG
Triglycerides
- Tchol
Total cholesterol
- LDL-C
Low density lipoprotein cholesterol
- HDL-C
High density lipoprotein cholesterol
- SD
Standard deviation
- aOR
Adjusted odds ratio
- CI
Confidence intervals
- GDM
Gestational diabetes mellitus
- WHO
World Health Organization
- SO2
Sulfur dioxide
- CO2
Carbon dioxide
- NO2
Nitrogen dioxide
- CO
Carbon monoxide
- LTE
Long-Term Evolution
- BMI
Body mass index
- OGTT
Oral glucose tolerance test
- CRP
C-reactive protein
- IUGR
Intrauterine growth restriction
- TNF- α
Tumor necrosis factor-α
- IL-6
Interleukin-6
Author contribution
YSJ contributed to conceptualization, data curation, formal analysis, software, visualization, methodology, and writing—original draft. SP contributed to data curation, software, validation, and investigation. YMH contributed to investigation. EK contributed to data curation and investigation. YAY contributed to investigation. SMK contributed to investigation. GL contributed to investigation. KAL contributed to investigation. SJK contributed to investigation. GJC contributed to investigation. MO contributed to investigation. SHN contributed to investigation. SL contributed to investigation. JB contributed to investigation. YK contributed to investigation. SJL contributed to investigation. NKK contributed to investigation. The APPO study group contributed to clinical data curation. YHK contributed to conceptualization and validation. YJK contributed to conceptualization, funding acquisition, writing—review and editing, and supervision. All authors read and approved the final manuscript.
Funding
This study was supported by the National Institute of Health research project (project No. #2021-ER1208-01). This work was partly supported by Institute of Information & Communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (No.RS-2022–00155966, Artificial Intelligence Convergence Innovation Human Resources Development (Ewha Womans University)), and Korea Evaluation Institute of Industrial Technology (KEIT) grant funded by the Korea government (MOTIE).
Data availability
All data generated or analyzed during this study are included in this published article (and its additional information files).
Declarations
Ethics approval and consent to participate
IRB approval: the present study was approved by the ethics committees of all the participating hospitals from where the pregnant women were recruited. All participants provided written informed consent prior to enrolment.
Consent for publication
All authors declare their consent to publish this article.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Alderete TL, Habre R, Toledo-Corral CM, Berhane K, Chen Z, Lurmann FW, Weigensberg MJ, Goran MI, Gilliland FD. Longitudinal associations between ambient air pollution with insulin sensitivity, β-cell function, and adiposity in Los Angeles Latino children. Diabetes. 2017;66:1789–1796. doi: 10.2337/db16-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambade B, Sankar TK, Sahu LK, Dumka UC. Understanding sources and composition of black carbon and PM2.5 in urban environments in East India. Urb Sci. 2022;6:60. [Google Scholar]
- Araujo JA, Nel AE. Particulate matter and atherosclerosis: role of particle size, composition and oxidative stress. Part Fibre Toxicol. 2009;6:24. doi: 10.1186/1743-8977-6-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- B LS, M SA, S Y. The potentials of antioxidant micronutrients in the management of metabolic syndrome. J Antioxid Act. 2016;1:1–21. [Google Scholar]
- Bai W, Li Y, Niu Y, Ding Y, Yu X, Zhu B, Duan R, Duan H, Kou C, Li Y, Sun Z. Association between ambient air pollution and pregnancy complications: a systematic review and meta-analysis of cohort studies. Environ Res. 2020;185:109471. doi: 10.1016/j.envres.2020.109471. [DOI] [PubMed] [Google Scholar]
- Barat S, Ghanbarpour A, Bouzari Z, Batebi Z. Triglyceride to HDL cholesterol ratio and risk for gestational diabetes and birth of a large-for-gestational-age newborn. Caspian J Intern Med. 2018;9:368–375. doi: 10.22088/cjim.9.4.368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baron AD, Steinberg HO, Chaker H, Leaming R, Johnson A, Brechtel G. Insulin-mediated skeletal muscle vasodilation contributes to both insulin sensitivity and responsiveness in lean humans. J Clin Invest. 1995;96:786–792. doi: 10.1172/JCI118124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S. Particulate matter, air pollution, and blood pressure. J Am Soc Hypertens. 2009;3:332–350. doi: 10.1016/j.jash.2009.08.005. [DOI] [PubMed] [Google Scholar]
- Brook RD, Rajagopalan S, Pope CA, Brook JR, Bhatnagar A, Diez-Roux AV, Holguin F, Hong Y, Luepker RV, Mittleman MA, Peters A, Siscovick D, Smith SC, Whitsel L, Kaufman JD. Particulate matter air pollution and cardiovascular disease. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- Casuso RA, Huertas JR. Chapter 13 - antioxidant supplements in obesity and metabolic syndrome: angels or demons. In: del Moral AM, Aguilera García CM, editors. Obesity. Academic Press; 2018. pp. 263–275. [Google Scholar]
- Cheng X, Ji X, Yang D, Zhang C, Chen L, Liu C, Meng X, Wang W, Li H, Kan H, Huang H. Associations of PM2.5 exposure with blood glucose impairment in early pregnancy and gestational diabetes mellitus. Ecotoxicol Environ Saf. 2022;232:113278. doi: 10.1016/j.ecoenv.2022.113278. [DOI] [PubMed] [Google Scholar]
- Cunningham FG, Leveno KJ, Dashe JS, Hoffman BL, Spong CY, Casey BM (2022) Editors, Williams Obstetrics, 26e. McGraw Hill, New York
- Dabass A, Talbott EO, Venkat A, Rager J, Marsh GM, Sharma RK, Holguin F. Association of exposure to particulate matter (PM2.5) air pollution and biomarkers of cardiovascular disease risk in adult NHANES participants (2001–2008) Int J Hyg Environ Health. 2016;219:301–310. doi: 10.1016/j.ijheh.2015.12.002. [DOI] [PubMed] [Google Scholar]
- Edwards RD, Jurvelin J, Saarela K, Jantunen M. VOC concentrations measured in personal samples and residential indoor, outdoor and workplace microenvironments in EXPOLIS-Helsinki, Finland. Atmos Environ. 2001;35:4531–4543. [Google Scholar]
- Eze IC, Hemkens LG, Bucher HC, Hoffmann B, Schindler C, Künzli N, Schikowski T, Probst-Hensch NM. Association between ambient air pollution and diabetes mellitus in Europe and North America: systematic review and meta-analysis. Environ Health Perspect. 2015;123:381–389. doi: 10.1289/ehp.1307823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- Gong R, Luo G, Wang M, Ma L, Sun S, Wei X. Associations between TG/HDL ratio and insulin resistance in the US population: a cross-sectional study. Endocr Connect. 2021;10:1502–1512. doi: 10.1530/EC-21-0414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregório BM, De Souza DB, de Morais Nascimento FA, Pereira LM, Fernandes-Santos C. The potential role of antioxidants in metabolic syndrome. Curr Pharm Des. 2016;22:859–869. doi: 10.2174/1381612822666151209152352. [DOI] [PubMed] [Google Scholar]
- Herder C, Færch K, Carstensen-Kirberg M, Lowe GD, Haapakoski R, Witte DR, Brunner EJ, Roden M, Tabák AG, Kivimäki M, Vistisen D. Biomarkers of subclinical inflammation and increases in glycaemia, insulin resistance and beta-cell function in non-diabetic individuals: the Whitehall II study. Eur J Endocrinol. 2016;175:367–377. doi: 10.1530/EJE-16-0528. [DOI] [PubMed] [Google Scholar]
- Honda T, Pun VC, Manjourides J, Suh H. Associations between long-term exposure to air pollution, glycosylated hemoglobin and diabetes. Int J Hyg Environ Health. 2017;220:1124–1132. doi: 10.1016/j.ijheh.2017.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houstis N, Rosen ED, Lander ES. Reactive oxygen species have a causal role in multiple forms of insulin resistance. Nature. 2006;440:944–948. doi: 10.1038/nature04634. [DOI] [PubMed] [Google Scholar]
- Hur YM, Choi J, Park S, Oh SS, Kim YJ. Prenatal maternal alcohol exposure: diagnosis and prevention of fetal alcohol syndrome. Obstet Gynecol Sci. 2022;65:385–394. doi: 10.5468/ogs.22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hur YM, et al. The introduction to air pollution on pregnancy outcome (APPO) study: a multicenter cohort study. Obstet Gynecol Sci. 2023;66:169–180. doi: 10.5468/ogs.22278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutcheson R, Rocic P. The metabolic syndrome, oxidative stress, environment, and cardiovascular disease: the great exploration. Exp Diabetes Res. 2012;2012:271028. doi: 10.1155/2012/271028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedrychowski W, Perera F, Mrozek-Budzyn D, Mroz E, Flak E, Spengler JD, Edwards S, Jacek R, Kaim I, Skolicki Z. Gender differences in fetal growth of newborns exposed prenatally to airborne fine particulate matter. Environ Res. 2009;109:447–456. doi: 10.1016/j.envres.2009.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirtle RL, Skinner MK. Environmental epigenomics and disease susceptibility. Nat Rev Genet. 2007;8:253–262. doi: 10.1038/nrg2045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepac P, Locatelli I, Korošec S, Künzli N, Kukec A. Ambient air pollution and pregnancy outcomes: a comprehensive review and identification of environmental public health challenges. Environ Res. 2018;167:144–159. doi: 10.1016/j.envres.2018.07.008. [DOI] [PubMed] [Google Scholar]
- Kumar A, Ambade B, Sankar TK, Sethi SS, Kurwadkar S. Source identification and health risk assessment of atmospheric PM25-bound polycyclic aromatic hydrocarbons in Jamshedpur, India. Sustain Cities Soc. 2020;52:101801. [Google Scholar]
- Kwon EJ, Kim YJ. What is fetal programming?: a lifetime health is under the control of in utero health. Obstet Gynecol Sci. 2017;60:506–519. doi: 10.5468/ogs.2017.60.6.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Park H, Kim S, Lee EK, Lee J, Hong YS, Ha E. Fine particulate matter and incidence of metabolic syndrome in non-CVD patients: a nationwide population-based cohort study. Int J Hyg Environ Health. 2019;222:533–540. doi: 10.1016/j.ijheh.2019.01.010. [DOI] [PubMed] [Google Scholar]
- Li R, Navab M, Pakbin P, Ning Z, Navab K, Hough G, Morgan TE, Finch CE, Araujo JA, Fogelman AM, Sioutas C, Hsiai T. Ambient ultrafine particles alter lipid metabolism and HDL anti-oxidant capacity in LDLR-null mice. J Lipid Res. 2013;54:1608–1615. doi: 10.1194/jlr.M035014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Xiao X, Wang P, Meng X, Zhou Y, Shi H, Yin C, Zhang Y. PM2.5 exposure and maternal glucose metabolism in early pregnancy: associations and potential mediation of 25-hydroxyvitamin D. Ecotoxicol Environ Saf. 2021;224:112645. doi: 10.1016/j.ecoenv.2021.112645. [DOI] [PubMed] [Google Scholar]
- Lin L-Z, Zhan X-L, Jin C-Y, Liang J-H, Jing J, Dong G-H. The epidemiological evidence linking exposure to ambient particulate matter with neurodevelopmental disorders: a systematic review and meta-analysis. Environ Res. 2022;209:112876. doi: 10.1016/j.envres.2022.112876. [DOI] [PubMed] [Google Scholar]
- Liu C, et al. Ambient particulate air pollution and daily mortality in 652 cities. N Engl J Med. 2019;381:705–715. doi: 10.1056/NEJMoa1817364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu R, Zhang J, Chu L, Zhang J, Guo Y, Qiao L, Niu Z, Wang M, Farhat Z, Grippo A, Zhang Y, Ma C, Zhang Y, Zhu K, Mu L, Lei L. Association of ambient fine particulate matter exposure with gestational diabetes mellitus and blood glucose levels during pregnancy. Environ Res. 2022;214:114008. doi: 10.1016/j.envres.2022.114008. [DOI] [PubMed] [Google Scholar]
- Lucht SA, Hennig F, Matthiessen C, Ohlwein S, Icks A, Moebus S, Jöckel K-H, Jakobs H, Hoffmann B. Air pollution and glucose metabolism: an analysis in non-diabetic participants of the Heinz Nixdorf recall study. Environ Health Perspect. 2018;126:047001. doi: 10.1289/EHP2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao S, Chen G, Liu F, Li N, Wang C, Liu Y, Liu S, Lu Y, Xiang H, Guo Y, Li S. Long-term effects of ambient air pollutants to blood lipids and dyslipidemias in a Chinese rural population. Environ Pollut. 2020;256:113403. doi: 10.1016/j.envpol.2019.113403. [DOI] [PubMed] [Google Scholar]
- Marotta T, Russo BF, Ferrara LA. Triglyceride-to-HDL-cholesterol ratio and metabolic syndrome as contributors to cardiovascular risk in overweight patients. Obesity (Silver Spring) 2010;18:1608–1613. doi: 10.1038/oby.2009.446. [DOI] [PubMed] [Google Scholar]
- Moschandreas DJ, Watson J, D’Abreton P, Scire J, Zhu T, Klein W, Saksena S. Chapter three: methodology of exposure modeling. Chemosphere. 2002;49:923–946. doi: 10.1016/s0045-6535(02)00237-0. [DOI] [PubMed] [Google Scholar]
- Münzel T, Sørensen M, Gori T, Schmidt FP, Rao X, Brook J, Chen LC, Brook RD, Rajagopalan S. Environmental stressors and cardio-metabolic disease: part I-epidemiologic evidence supporting a role for noise and air pollution and effects of mitigation strategies. Eur Heart J. 2017;38:550–556. doi: 10.1093/eurheartj/ehw269. [DOI] [PubMed] [Google Scholar]
- Nordestgaard BG, et al. Fasting is not routinely required for determination of a lipid profile: clinical and laboratory implications including flagging at desirable concentration cutpoints—a joint consensus statement from the European Atherosclerosis Society and European Federation of Clinical Chemistry and Laboratory Medicine. Clin Chem. 2016;62:930–946. doi: 10.1373/clinchem.2016.258897. [DOI] [PubMed] [Google Scholar]
- Oke SL, Hardy DB (2021) The role of cellular stress in intrauterine growth restriction and postnatal dysmetabolism. Int J Mol Sci 22 (13):6986 [DOI] [PMC free article] [PubMed]
- Organization WHO (2021) WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide [PubMed]
- Ornoy A, Becker M, Weinstein-Fudim L, Ergaz Z (2021) Diabetes during pregnancy: a maternal disease complicating the course of pregnancy with long-term deleterious effects on the offspring. A clinical review. Int J Mol Sci 22 [DOI] [PMC free article] [PubMed]
- Öztürk HNO, Türker PF. Fetal programming: could intrauterin life affect health status in adulthood? Obstet Gynecol Sci. 2021;64:473–483. doi: 10.5468/ogs.21154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park J, Ryu H, Kim E, Choe Y, Heo J, Lee J, Cho S-H, Sung K, Cho M, Yang W. Assessment of PM2.5 population exposure of a community using sensor-based air monitoring instruments and similar time-activity groups. Atmos Pollut Res. 2020;11:1971–1981. [Google Scholar]
- Perticone F, Ceravolo R, Candigliota M, Ventura G, Iacopino S, Sinopoli F, Mattioli PL. Obesity and body fat distribution induce endothelial dysfunction by oxidative stress: protective effect of vitamin C. Diabetes. 2001;50:159–165. doi: 10.2337/diabetes.50.1.159. [DOI] [PubMed] [Google Scholar]
- Rammah A, Whitworth KW, Amos CI, Estarlich M, Guxens M, Ibarluzea J, Iñiguez C, Subiza-Pérez M, Vrijheid M, Symanski E (2021) Air pollution, residential greenness and metabolic dysfunction during early pregnancy in the INfancia y Medio Ambiente (INMA) cohort. Int J Environ Res Public Health 18(17):9354 [DOI] [PMC free article] [PubMed]
- Robledo CA, Mendola P, Yeung E, Männistö T, Sundaram R, Liu D, Ying Q, Sherman S, Grantz KL. Preconception and early pregnancy air pollution exposures and risk of gestational diabetes mellitus. Environ Res. 2015;137:316–322. doi: 10.1016/j.envres.2014.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roeckner JT, Sanchez-Ramos L, Jijon-Knupp R, Kaunitz AM. Single abnormal value on 3-hour oral glucose tolerance test during pregnancy is associated with adverse maternal and neonatal outcomes: a systematic review and metaanalysis. Am J Obstet Gynecol. 2016;215:287–297. doi: 10.1016/j.ajog.2016.04.040. [DOI] [PubMed] [Google Scholar]
- Ruano CSM, Miralles F, Méhats C, Vaiman D (2022) The impact of oxidative stress of environmental origin on the onset of placental diseases. Antioxidants (Basel) 11(1):106 [DOI] [PMC free article] [PubMed]
- Shezi B, Jafta N, Naidoo R (2020) Exposure assessment of indoor particulate matter during pregnancy: a narrative review of the literature. Rev Environ Health 35(4):427–442 [DOI] [PubMed]
- Su X, Zhang S, Lin Q, Wu Y, Yang Y, Yu H, Huang S, Luo W, Wang X, Lin H, Ma L, Zhang Z. Prenatal exposure to air pollution and neurodevelopmental delay in children: a birth cohort study in Foshan, China. Sci Total Environ. 2022;816:151658. doi: 10.1016/j.scitotenv.2021.151658. [DOI] [PubMed] [Google Scholar]
- Sun Q, Yue P, Deiuliis JA, Lumeng CN, Kampfrath T, Mikolaj MB, Cai Y, Ostrowski MC, Lu B, Parthasarathy S, Brook RD, Moffatt-Bruce SD, Chen LC, Rajagopalan S. Ambient air pollution exaggerates adipose inflammation and insulin resistance in a mouse model of diet-induced obesity. Circulation. 2009;119:538–546. doi: 10.1161/CIRCULATIONAHA.108.799015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Westering-Kroon E, Huizing MJ, Villamor-Martínez E, Villamor E. Male disadvantage in oxidative stress-associated complications of prematurity: a systematic review, meta-analysis and meta-regression. Antioxidants. 2021;10:1490. doi: 10.3390/antiox10091490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallwork RS, Colicino E, Zhong J, Kloog I, Coull BA, Vokonas P, Schwartz JD, Baccarelli AA. Ambient fine particulate matter, outdoor temperature, and risk of metabolic syndrome. Am J Epidemiol. 2017;185:30–39. doi: 10.1093/aje/kww157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtyla C, Zielinska K, Wojtyla-Buciora P, Panek G (2020) Prenatal fine particulate matter (PM(2.5)) exposure and pregnancy outcomes-analysis of term pregnancies in Poland. Int J Environ Res Public Health 17(16):5820 [DOI] [PMC free article] [PubMed]
- Yu H, Yin Y, Zhang J, Zhou R. The impact of particulate matter 2.5 on the risk of preeclampsia: an updated systematic review and meta-analysis. Environ Sci Pollut Res. 2020;27:37527–37539. doi: 10.1007/s11356-020-10112-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zejnullahu VA, Zejnullahu VA, Kosumi E. The role of oxidative stress in patients with recurrent pregnancy loss: a review. Reprod Health. 2021;18:207. doi: 10.1186/s12978-021-01257-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Wang Q, He S, Wu K, Ren M, Dong H, Di J, Yu Z, Huang C. Ambient air pollution and gestational diabetes mellitus: a review of evidence from biological mechanisms to population epidemiology. Sci Total Environ. 2020;719:137349. doi: 10.1016/j.scitotenv.2020.137349. [DOI] [PubMed] [Google Scholar]
- Zhang H, Zhang X, Zhang H, Luo H, Feng Y, Wang J, Huang C, Yu Z (2022) Assessing the effect of fine particulate matter on adverse birth outcomes in Huai River Basin, Henan, China, 2013–2018. Environ Pollut (Barking, Essex : 1987) 306:119357. 10.1016/j.envpol.2022.119357 [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article (and its additional information files).