Abstract
Background
Recent studies describe an emerging role for percutaneous left ventricular assist devices such as Impella CP® as rescue therapy for refractory cardiac arrest. We hypothesized that the addition of mechanical chest compressions to percutaneous left ventricular assist device assisted CPR would improve hemodynamics by compressing the right ventricle and augmenting pulmonary blood flow and left ventricular filling. We performed a pilot study to test this hypothesis using a swine model of prolonged cardiac arrest.
Methods
Eight Yorkshire swine were anesthetized, intubated, and instrumented for hemodynamic monitoring. They were subjected to untreated ventricular fibrillation for 5.75 (SD 2.90) minutes followed by mechanical chest compressions for a mean of 20.0 (SD 5.0) minutes before initiation of percutaneous left ventricular assist device. After percutaneous left ventricular assist device initiation, mechanical chest compressions was stopped (n = 4) or continued (n = 4). Defibrillation was attempted 4, 8 and 12 minutes after initiating percutaneous left ventricular assist device circulatory support.
Results
The percutaneous left ventricular assist device + mechanical chest compressions group had significantly higher percutaneous left ventricular assist device flow prior to return of spontaneous heartbeat at four- and twelve-minutes after percutaneous left ventricular assist device initiation, and significantly higher end tidal CO2 at 4-minutes after percutaneous left ventricular assist device initiation, when compared with the percutaneous left ventricular assist device alone group. Carotid artery flow was not significantly different between the two groups.
Conclusion
The addition of mechanical chest compressions to percutaneous left ventricular assist device support during cardiac arrest may generate higher percutaneous left ventricular assist device and carotid artery flow prior to return of spontaneous heartbeat compared to percutaneous left ventricular assist device alone. Further studies are needed to determine if this approach improves other hemodynamic parameters or outcomes after prolonged cardiac arrest.
Keywords: Cardiac Arrest, OHCA, Chest Compressions, Percutaneous Left Ventricular Assist Device, Mechanical Support, Impella
Introduction
Out-of-hospital cardiac arrest (OHCA) and in-hospital cardiac arrest (IHCA) occur approximately 600,000 times annually in the United States with neurologically intact survival to hospital discharge of less than 10%.1, 2, 3 The majority of cardiac arrest patients do not achieve a return of spontaneous heartbeat (ROSHB) despite the current advances in cardiovascular life support and resuscitative efforts.2 Recently, percutaneous placement of a left ventricular assist device (pL-VAD) has been explored for use during refractory cardiac arrest and is emerging as a feasible resuscitation strategy in both animal models and in cardiac arrest patients who are unresponsive to conventional care.4, 5, 6, 7.
Panagides et al. demonstrated that patients undergoing pL-VAD Impella CP®, insertion during ongoing CPR achieved a 37.1% rate of 30-day survival without neurological impairment.4 Similarly, Derwall et al. demonstrated an improved coronary perfusion pressure and increased rates of ROSHB in a swine model.8 Lotun et al. also demonstrated improved survival with both a pL-VAD and pL-VAD + mCPR in an in-hospital cardiac arrest (IHCA) swine model.9 However, the optimal resuscitation strategy for patients in cardiac arrest following the initiation of pL-VAD therapy remains unclear. The pL-VAD continuously extracts blood from the left ventricle through an inlet cage, bypassing the aortic valve before ejecting it into the ascending aorta.10, 11 The hemodynamic support offered by the pL-VAD is dependent on left ventricular preload. In the case of cardiac arrest, the absence of right ventricular contraction combined with the decrease in venous return may result in decreased pulmonary blood flow and therefore a decrease in left ventricular preload. Disruption of pulmonary blood flow and inadequate preload could prevent optimal pL-VAD flow during cardiac arrest. In this study, we investigated the effect of adding mechanical CPR (mCPR) during pL-VAD assisted resuscitation on pL-VAD flow and intra-cardiac arrest hemodynamics in a swine model of prolonged cardiac arrest. We hypothesized that the addition of mCPR to pL-VAD assisted resuscitation would result in an improvement of pL-VAD flow, carotid artery blood flow, and end-tidal CO2.
Methods
All experimental procedures were conducted under an approved institutional animal care and use committee protocol (IACUC # PRO00010393. Approved on 7/20/2021 and expires on 7/20/2024) and in accordance with the Guide for the Care and Use of Laboratory Animals. This pilot study was completed as part of a larger study to develop a swine model of pL-VAD supported resuscitation during prolonged cardiac arrest limiting the sample size. In this pilot study, eight Yorkshire mix swine (62.5% male) were a priori assigned into one of two interventional groups (n = 4 per group) which received either pL-VAD alone or pL-VAD + mCPR to have similar animal characteristics in each group. There were no additional exclusion criteria. Due to the interventions, blinding was not feasible.
Experimental design
Animals were anesthetized using a combination of tiletamine/zolazepam and xylazine and subsequently intubated, mechanically ventilated, and maintained under 1–2% inhalant isoflurane for the duration of all experimental procedures. The carotid artery, femoral artery, internal jugular vein, and external jugular vein were surgically instrumented according to the Supplemental Material Section 1 and a pL-VAD (Impella CP®, Abiomed, Danvers, MA) was placed under fluoroscopic guidance. Animals were administered 200–300 U/kg of heparin to a target-activated clotting time of >300 seconds.
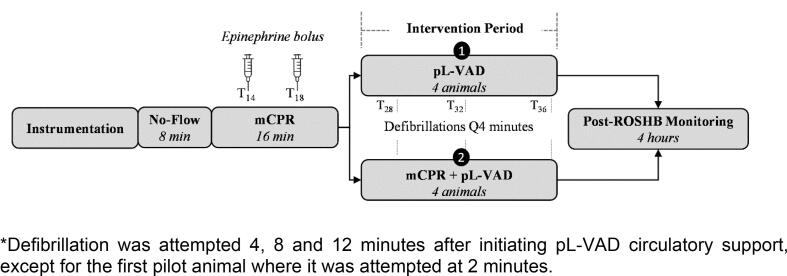
Experimental design is displayed in Fig. 1. Ventricular fibrillation was induced by applying 9 V to the right ventricle using a pacing wire (Arrow®, Teleflex, Wayne PA). Fibrillation was left untreated for a mean of 5.75 (2.90) minutes. Five animals remained in VF for eight minutes, while the remaining animals remained in VF for 2 minutes. Anesthesia and ventilation were paused during cardiac arrest. Mechanical chest compressions (LUCAS 2, Stryker Medical, Portage MI) and ventilation (10 breaths/min & 100% FiO2) were initiated following the untreated ventricular fibrillation period and continued throughout the CPR period until the intervention period, for a mean of 20.0 (5.0) minutes. Intravenous adrenaline boluses (0.02 mg/Kg) were administered 14 and 18-minutes after cardiac arrest. The pL-VAD was turned to P0 setting (off) until the intervention period.
Fig. 1.
*Defibrillation was attempted 4, 8 and 12 minutes after initiating pL-VAD circulatory support, except for the first pilot animal where it was attempted at 2 minutes.
The pL-VAD was initiated at the beginning of the intervention period in both groups and optimized for maximum flow. Mechanical compressions were continued in the pL-VAD + mCPR group. At 4 minutes after intervention, a 200 J biphasic defibrillation (Medtronic LIFEPAK 20e, Minneapolis MN) was attempted. If return of spontaneous circulation heartbeat (ROSHB) was achieved (indicated by organized electrical rhythm or pulse for 2 minutes), chest compressions were discontinued and Impella position was confirmed with transthoracic echocardiography. Otherwise, both interventions were continued for a total of 12 minutes with a single defibrillation attempt occurring every subsequent four minutes. After the last defibrillation attempt, the experiment was terminated if ROSHB was not achieved. Isoflurane was immediately resumed in animals which achieved ROSHB, and they were maintained on pL-VAD support for four additional hours. Following this four-hour period, animals were subsequently euthanized using intravenous potassium chloride injection while under general anesthesia.
Primary outcomes
The primary outcomes for the study were pL-VAD flow (L/min), end-tidal carbon dioxide (PetCO2), and carotid artery (CA) flow (mL/min) in animals receiving pL-VAD support with and without mCPR.
Statistical analysis
Continuous data are presented as mean (standard deviation). Inter-group comparisons were performed using a t-test. Pooled group comparisons were assessed by a mixed effect model. Type I error was set at 0.05. Prism 9 (GraphPad, San Diego, CA) was used for data analysis.
Results
There was no significant difference in baseline animal characteristics, cardiac arrest duration prior to intervention, or rate of ROSHB (Table 1). Mechanical CPR duration prior to intervention was 24.0 (4.24) minutes in the pL-VAD alone group and 16.0 (0) minutes in the pL-VAD + mCPR group (p = 0.047).
Table 1.
Pl-vad + mCPR vs. pL-VAD alone.
| pL-VAD + mCPR (n = 4) |
pL-VAD alone (n = 4) |
p-value | |
|---|---|---|---|
| Male (%) | 50% | 75% | 0.54 |
| Weight [kg (SD)] | 51.75 (2.86) | 49 (3.54) | 0.33 |
| ROSHB | 50% | 50% | 1 |
| Time intervals (min) mean (SD) | |||
| No-flow (VF) duration | 8.00 (0) | 3.50 (2.6) | 0.058 |
| mCPR duration | 16.00 (0) | 24.00 (4.24) | 0.04* |
| CA duration before pL-VAD initiation | 24.00 (0) | 27.50 (4.33) | 0.26 |
| pL-VAD Flow (L/Min) mean (SD) | |||
| 0 minutes | 1.38 (0.72) | 0.48 (0.09) | 0.11 |
| 4 minutes | 1.70 (0.41) | 0.50 (0.22) | 0.008 |
| 8 minutes | 1.45 (0.09) | 0.50 (0.20) | 0.11 |
| 12 minutes | 1.68 (0.50) | 0.40 (0.20) | 0.02 |
| Pooled 0–12 min. | 1.55 (0.52) | 0.47 (0.19) | <0.007 |
| Carotid Flow (mL/min) mean (SD) | |||
| 0 minutes | 113.30 (57.89) | 61.20 (15.75) | 0.22 |
| 4 minutes | 133.83 (61.87) | 41.45 (63.3) | 0.08 |
| 8 minutes | 120.68 (41.75) | 32.95 (18.65) | 0.05 |
| 12 minutes | 94.95 (70.58) | 39.70 (21.30) | 0.30 |
| Pooled 0–12 min. | 115.68 (62.59) | 46 (26.77) | 0.029 |
| PetCO2 (mmHg) mean (SD) | |||
| 0 minutes | 31.03 (4.18) | 26.5 (6.87) | 0.37 |
| 4 minutes | 33.99 (3.20) | 23.5 (3.20) | 0.007 |
| 8 minutes | 31.16 (5.22) | 22 (1) | 0.05 |
| 12 minutes | 28.08 (5.10) | 21.5 (1.5) | 0.12 |
| Pooled 0–12 min. | 31.06 (5.12) | 23.92 (5.07) | 0.144 |
CA = cardiac arrest; kg = kilograms; L = liter; mCPR = mechanical CPR; mL = milliliter; Min = minutes; PetCO2 = end-tidal CO2; pL-VAD = percutaneous ventricular assist device; ROSHB = Return of Spontaneous Heartbeat; SD = standard deviation; VF = ventricular fibrillation.
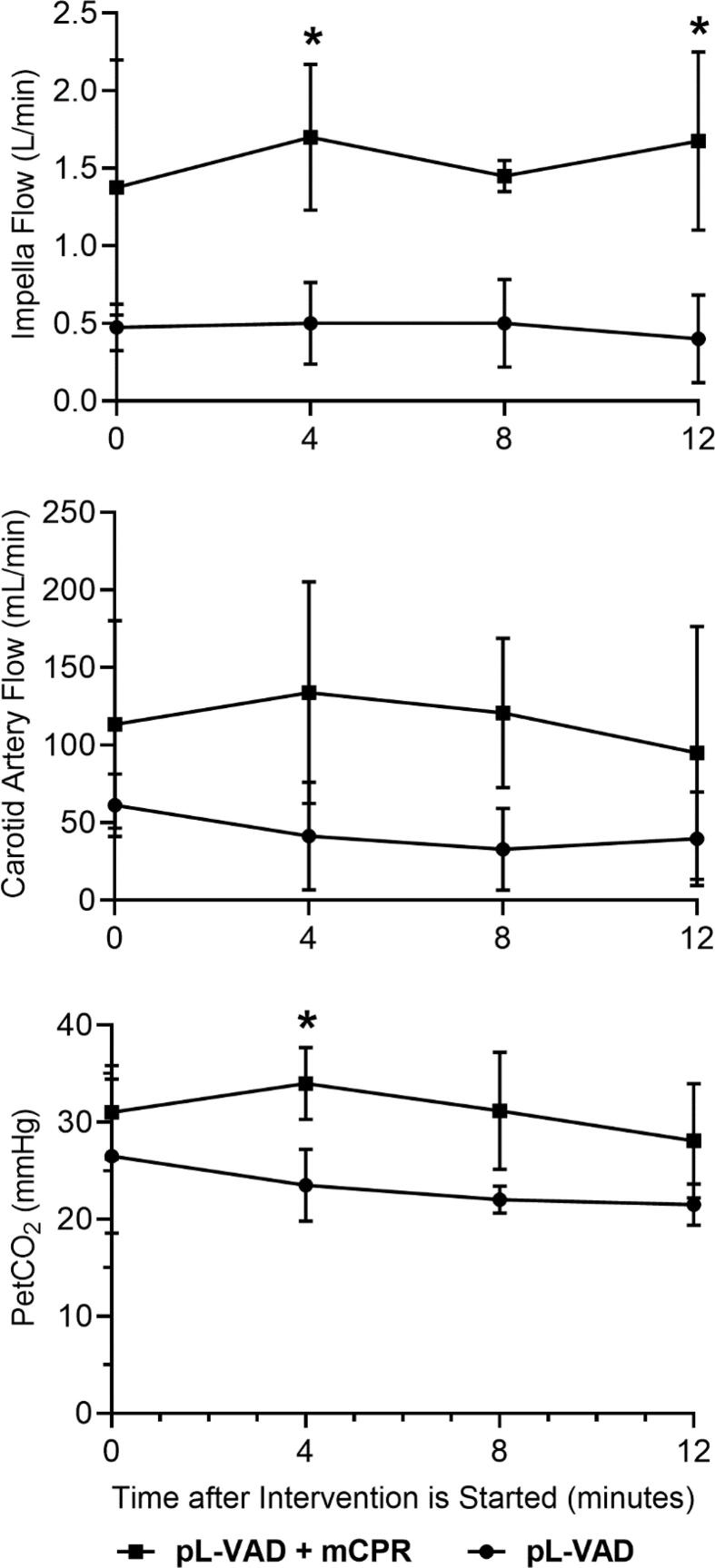
Detailed experimental data for pL-VAD flow, CA-flow, and end-tidal CO2 (PetCO2) are presented in Table 1. There was an overall significant increase in pL-VAD flow when pL-VAD was combined with mCPR. Pooled data analysis indicated a mean (SD) pL-VAD flow over the duration of intervention of 1.6 (0.52) L/min in the pL-VAD + mCPR group compared to a pL-VAD flow of 0.5 (0.19) L/min in the pL-VAD alone group (p = 0.007). Intergroup comparisons at each timepoint also indicated that the pL-VAD + mCPR group had significantly higher pL-VAD flow at individual timepoints of 4- and 12-minutes after intervention (Table 1, Fig. 2a). Similarly, there was an overall significant increase in CA-flow that averaged 115.7 (62.59) mL/min in the pL-VAD + mCPR group compared to 46 (26.77) mL/min in the pL-VAD alone group (p = 0.029). However, there were no statistically significant differences at any specific time point between groups (Table 1, Fig. 2b). PetCO2 values were higher following intervention in the pL-VAD + mCPR group compared to the pL-VAD alone group [31.1 (5.12) mmHg vs. 23.9 (5.07) mmHg]. However, the pooled analysis did not reveal a statistically significant difference between the groups.
Fig. 2.
Intra-arrest A) pL-VAD flow (liters/minute), B) carotid artery (CA) flow (milliliters/minute), and C) end-tidal carbon dioxide (PetCO2; mmHg) in swine support with pL-VAD + mCPR and pL-VAD alone.
Discussion
Although pL-VAD devices only provide left ventricular support, recent data suggests that the early implantation of a pL-VAD device during refractory cardiac arrest is feasible and associated with an increased survival.4, 7 However, in the study by Panagides et al., less than 50% of patients required extended mCPR following pL-VAD therapy initiation.4 Therefore, it is unclear if continued mCPR is still required in the resuscitation of refractory cardiac arrest patients following initiation of pL-VAD therapy.
In this study, we demonstrated that the addition of mCPR during pL-VAD use produced a significant increase in pL-VAD flow and carotid artery flow. The lower pL-VAD and carotid artery flow observed in the group without mCPR can most likely be attributed to the acute biventricular failure encountered during cardiac arrest. Without the forward flow from the right heart provided by mCPR, pulmonary blood flow and left ventricular filling are decreased, and independent pL-VAD flows are decreased when compared to pL-VAD with mCPR. Our study demonstrated findings consistent with previous studies that demonstrated favorable neurologic outcomes in swine treated with mCPR and pL-VAD.9 The study by Lotun et al. study modeled an IHCA with two minutes of no-flow time to simulate initiation of manual chest compressions in the cardiac catheterization laboratory. In contrast, our study simulated refractory OHCA with a more prolonged no-flow time that was similar to EMS arrival time and prehospital continuous high-quality CPR. Our study demonstrated that the addition of mCPR to pL-VAD augmented the pL-VAD flow and carotid artery flow compared to pL-VAD alone. Therefore, continuous mCPR might be beneficial following pL-VAD insertion for refractory cardiac arrest patients.
Our study has several limitations. First, this study used preliminary data from a larger study to develop a swine model of pL-VAD supported resuscitation during refractory cardiac arrest.12 Therefore, while equal numbers of animals were included in both intervention groups, the sample size of the study was not specifically designed to test our hypotheses. This study had a limited sample size and was not statistically powered to detect differences in hemodynamics. The small sample size might have contributed to the lack of significant difference at many independent time points, and similarly, any significant differences should be interpreted with caution. The duration of untreated ventricular fibrillation (no-flow) and mCPR prior to intervention was also variable between groups which may influence the hemodynamics of the pL-VAD, transpulmonary flow (PetCO2), and carotid artery flow. This variability in duration prior to intervention could contribute to variability in the data produced from the model. Additionally, the increase in intra-cardiac arrest pL-VAD and carotid artery flow was demonstrated in a swine model that may have limited applicability to humans, and long-term outcomes were not evaluated. Lastly, the translation of this technology to the clinical environment may be challenging, given the technical difficulty of placing a pL-VAD during ongoing CPR.
Conclusions
The addition of mCPR to pL-VAD support during refractory cardiac arrest may generate higher pL-VAD flow and carotid artery flow compared to pL-VAD alone. Further studies are needed to determine if this approach improves the hemodynamic parameters, probability of ROSHB, or survival and neurologic outcomes after refractory cardiac arrest.
Relevant regulations
This study was conducted in accordance with the Animal Research Reporting of In Vivo Experiments (ARRIVE) guidelines, National Research Council’s Guide for the Care and Use of Laboratory Animals, and in accordance with the Guide for the Care and Use of Laboratory Animals. All experimental procedures were conducted under an approved institutional animal care and use committee protocol (IACUC # PRO00010393. Approved on 7/20/2021 and expires on 7/20/2024).
Funding
This study was supported by funding from Abiomed.
CRediT authorship contribution statement
Adam L. Gottula: Conceptualization, Methodology, Software, Data curation, Writing – original draft, Writing – review & editing. Brendan M. McCracken: Data curation, Writing – original draft, Writing – review & editing. Takahiro Nakashima: Data curation, Writing – original draft, Visualization, Investigation, Writing – review & editing. Nicholas L. Greer: Data curation, Writing – original draft, Visualization, Investigation. Traci A. Cramer: Data curation, Writing – original draft, Visualization, Investigation, Writing – review & editing. Nadia R. Sutton: Supervision, Writing – review & editing. Kevin R. Ward: Supervision, Writing – review & editing. Mohamad Hakam Tiba: Conceptualization, Methodology, Software, Data curation, Writing – original draft, Visualization, Investigation, Supervision, Writing – review & editing. Cindy H. Hsu: Conceptualization, Methodology, Software, Data curation, Writing – original draft, Visualization, Investigation, Supervision, Writing – review & editing. Robert Neumar: Supervision, Writing - review & editing.
Declaration of competing interest
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests: ‘N.R.S. has received honoraria for speaking from Zoll, Cordis, and Shockwave and is a consultant for Abbott and Philips. The other authors declare no conflict of interest. C.H.H. is part of the Resuscitation Plus Editorial Board’.
Acknowledgements
We would like to thank the Max Harry Weil Institute for Critical Care Research and Innovation staff for their technical support.
Footnotes
Supplementary material to this article can be found online at https://doi.org/10.1016/j.resplu.2023.100488.
Contributor Information
Adam L. Gottula, Email: adgottul@umich.edu.
Brendan M. McCracken, Email: bmccrac@umich.edu.
Takahiro Nakashima, Email: takana@umich.edu.
Nicholas L. Greer, Email: nlgreer@umich.edu.
Traci A. Cramer, Email: crtraci@umich.edu.
Nadia R. Sutton, Email: nadia.sutton@vumc.org.
Kevin R. Ward, Email: keward@umich.edu.
Robert W. Neumar, Email: takana@umich.edu.
Mohamad Hakam Tiba, Email: tibam@umich.edu.
Cindy H. Hsu, Email: hcindy@umich.edu.
Appendix A. Supplementary material
The following are the Supplementary material to this article:
References
- 1.Virani S.S., Alonso A., Aparicio H.J., et al. Heart disease and stroke statistics-2021 update: A report from the American Heart Association. Circulation. 2021;143:e254–e743. doi: 10.1161/CIR.0000000000000950. [DOI] [PubMed] [Google Scholar]
- 2.CARES Summary Report – Demographic and Survival Characteristics of OHCA/Non-Traumatic Etiology (Date of Arrest: 01/01/20–12/31/20), https://mycares.net/sitepages/uploads/2021/2020Non-TraumaticNationalSummaryReport.pdf, accessed on June 10, 2021.
- 3.Andersen L.W., Holmberg M.J., Berg K.M., Donnino M.W., Granfeldt A. In-hospital cardiac arrest: A review. J Am Med Assoc. 2019;321:1200–1210. doi: 10.1001/jama.2019.1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Panagides V., Vase H., Shah S.P., et al. Impella CP Implantation during cardiopulmonary resuscitation for cardiac arrest: A multicenter experience. J Clin Med. 2021;10 doi: 10.3390/jcm10020339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Støttrup N.B., Jakobsen L., Krusell L.R., Terkelsen C.J. Utility of Impella® left ventricular assist device during cardiac arrest: A case report. Int J Cardiol. 2016;225:111–112. doi: 10.1016/j.ijcard.2016.09.074. [DOI] [PubMed] [Google Scholar]
- 6.Davidsen C., Packer E.J.S., Løland K.H., et al. Impella use in acute myocardial infarction complicated by cardiogenic shock and cardiac arrest: Analysis of 10 years registry data. Resuscitation. 2019;140:178–184. doi: 10.1016/j.resuscitation.2019.04.022. [DOI] [PubMed] [Google Scholar]
- 7.Vase H., Christensen S., Christiansen A., et al. The Impella CP device for acute mechanical circulatory support in refractory cardiac arrest. Resuscitation. 2017;03:70–74. doi: 10.1016/j.resuscitation.2016.10.003. [DOI] [PubMed] [Google Scholar]
- 8.Derwall M., Brücken A., Bleilevens C., et al. Doubling survival and improving clinical outcomes using a left ventricular assist device instead of chest compressions for resuscitation after prolonged cardiac arrest: a large animal study. Crit Care. 2015;19:123. doi: 10.1186/s13054-015-0864-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lotun K., Truong H.T., Cha K.C., et al. Cardiac arrest in the cardiac catheterization laboratory: Combining mechanical chest compressions and percutaneous LV assistance. J Am Coll Cardiol Intv. 2019;12:1840–1849. doi: 10.1016/j.jcin.2019.05.016. [DOI] [PubMed] [Google Scholar]
- 10.Abiomed I. Impella ventricular support systems for use during cardiogenic shock. Impella 2.5, 5.0, LD and Impella CP instructions for use and clinical reference manual.
- 11.Burzotta F., Trani C., Doshi S.N., et al. Impella ventricular support in clinical practice: Collaborative viewpoint from a European expert user group. Int J Cardiol. Dec 2015;201:684–691. doi: 10.1016/j.ijcard.2015.07.065. [DOI] [PubMed] [Google Scholar]
- 12.Tiba MH, Nakashima T, McCracken BM, Hsu CH, Gottula AL, Greer NL, Cramer TA, Sutton NR, Ward KR, Neumar RW. Haemodynamic impact of aortic balloon occlusion combined with percutaneous left ventricular assist device during cardiopulmonary resuscitation in a swine model of cardiac arrest. Resuscitation. 2023 Aug;189:109885. https://doi.org/10.1016/j.resuscitation.2023.109885. Epub 2023 Jun 28. PMID: 37385400. [DOI] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.