Abstract
Background
Primary ovarian insufficiency (POI) is characterized by the development of hypergonadotropic hypogonadism before 40 years of age and leads to intractable infertility. Although in vitro fertilization and embryo transfer with donated eggs enables pregnancy, not a few patients desire pregnancy using their oocytes. However, follicular development is rare and unpredictable in patients with POI. Thus, there is a need for treatments that promote the development of residual follicles and methods to accurately predict infrequent ovulation.
Methods
This review discusses the effects of various treatments for obtaining eggs from POI patients. Furthermore, this study focused a potential marker for predicting follicular growth in patients with POI.
Main Findings
Different treatments such as hormone‐replacement therapy, dehydroepiandrosterone supplementation, platelet‐rich plasma injection, and in vitro activation have shown varying degrees of effectiveness in retrieving oocytes from patients with POI. To predict follicle development in the cycle, elevated serum estradiol and reduced follicle‐stimulating hormone (FSH) levels are important. However, these markers are not always reliable under continuous estradiol‐replacement therapy. As a novel marker for predicting follicle growth, serum anti‐Müllerian hormone (AMH) levels, measured using the picoAMH enzyme‐linked immunosorbent assay, were found to predict follicle growth in patients and the cycle.
Conclusion
This review highlights the challenges and available interventions for achieving pregnancy using a patient's oocytes in cases of POI. We believe that a combination of currently available treatments and prediction methods is the best strategy to enable patients with POI to conceive using their own eggs. Although AMH levels may predict follicle growth, further research is necessary to improve the chances of successful follicular development and conception in patients with POI.
Keywords: anti‐Müllerian hormone, enzyme‐linked immunosorbent assay, hypogonadism, premature ovarian failure, primary ovarian insufficiency
This review highlights the challenges and available interventions for achieving pregnancy using the patient's own oocytes in POI cases. Serum AMH levels, measured using picoAMH enzyme‐linked immunosorbent assay, may be useful to predict follicle growth of the patients with POI.
1. INTRODUCTION
Primary ovarian insufficiency (POI) is characterized by the development of hypergonadotropic hypogonadism before the age of 40 years. 1 The European Society of Human Reproduction and Embryology guidelines define POI as the presence of oligo‐/amenorrhea for at least 4 months and serum follicle‐stimulating hormone (FSH) levels >25 IU/L on two occasions >4 weeks apart, with onset before the age of 40 years. 2 Over the past few decades, POI has become more common and has drawn more attention. It occurs in approximately 1% of the population. 2 , 3 A recent meta‐analysis showed that the prevalence of POI was as high as 3.7% (95% confidence interval: 3.1–4.3). 4 The factors contributing to its onset are diverse, since the follicle pool cannot be recovered, POI is one of the causes of intractable infertility. Genetic defects, including chromosomal abnormalities, metabolic or enzymatic dysfunction, infection, environmental factors, iatrogenic causes, and autoimmunity, are potential etiologies of POI. 5 , 6 Chemotherapy and radiotherapy are the most common causes of iatrogenic toxin‐induced ovarian failure. Recently, the use of oocyte and ovarian cryopreservation prior to radiotherapy or chemotherapy has become widespread. However, depending on their clinical conditions, such as the time left before treatment for the primary disease, not all patients can preserve their oocytes. 7 , 8 , 9 , 10 , 11 , 12 , 13 , 14 , 15 Certain situations may require use of the remaining ovarian follicles to attempt pregnancy, not only in cases of unpredictable POIs but also in iatrogenic cases.
Patients with POI occasionally present follicular growth. 16 , 17 In addition, various treatments have been attempted to maximize the use of the remaining follicles. 18 Traditionally, hormone‐replacement therapy (HRT) has been considered important and applied to patients. More recently, treatments such as intraovarian injection of platelet‐rich plasma (PRP) 19 , 20 , 21 and dehydroepiandrosterone (DHEA), 22 , 23 , 24 and an innovative method, in vitro activation (IVA), 25 have been developed. In addition to the development of treatments, accurate prediction of cycles with follicle growth is important; however, it is challenging. Serum FSH or estradiol (E2) levels are commonly measured in clinical settings to predict follicle growth in patients with POI. Serum E2 levels have been reported to be a useful predictive factor of ovarian function in patients with POI. 26 However, a high serum E2 level is a result of follicle growth, which means that the follicles are already growing. Likewise, HRT affects serum FSH levels; consequently, a few cycles of low serum FSH levels during HRT did not show follicle growth, even with ovarian stimulation by human gonadotropin therapy. 27 , 28 Therefore, a marker that can predict follicle development with a higher predictive accuracy is desired. Serum anti‐Müllerian hormone (AMH) levels are commonly used as markers of ovarian reserve. AMH is produced from small antral follicles; therefore, it is substituted as the number of remaining primordial follicles. 29 In patients with POI, serum AMH levels are very low or below the lower limit of quantification when measured using conventional methods. 30 However, in cycles with small antral follicles, very low levels of serum AMH may appear in POI cases, which can help predict cycles with follicular growth to promote more effective use of precious follicle development.
Although in vitro fertilization and embryo transfer (IVF‐ET) using donated eggs is an option for patients with POI, patients desire pregnancy using their eggs because of limited access to egg donation, religious beliefs, etc. Furthermore, oocyte‐donated pregnancies are reported to have association with increased incidence of perinatal complications, particularly in patients with POI. 31 , 32 Additionally, studies have reported the induction of oocyte differentiation from pluripotent stem cells of animals. 33 , 34 However, various concerns such as technical and ethical issues must be addressed before its application in humans. 35 , 36 , 37
In this review, we aimed to consolidate knowledge on maximizing the utilization of the remaining follicles of POI patients, the current treatment of POI for obtaining own eggs ovulation prediction in patients with POI to enhance the possibility of conception using patients' oocytes.
2. CURRENT TREATMENT OF POI FOR OBTAINING OWN EGGS
Several methods have been reported for the treatment of POI that support the utilization of residual follicles. HRT is a widely used conventional method. There are new methods, some of which are effective in obtaining eggs from patients with POI. 18 A growing body of evidence is emerging for each of the new treatments, with improvements in pregnancy rates. However, in the absence of RCTs that directly compare treatments to the standard, further evidence on their usefulness is required.
2.1. HRT and gonadotropin hormone‐releasing hormone (GnRH) agonist
HRT for POI is necessary regardless of the absence of presence of the desire to raise a child or maintain health. 38 In addition, recent animal studies have indicated that E2 has important roles in follicle development. 39 , 40 Follicle development is observed when gonadotropins are decreased during HRT. Theoretically, elevated serum luteinizing hormone levels induce premature luteinization of the antral follicles, 41 and elevated FSH levels downregulate the expression of granulosa cell FSH receptors in patients with POI. Decreasing serum levels of gonadotropin using HRT is expected to improve these conditions and positively affect follicular development in patients with POI. However, these benefits have not yet been clearly demonstrated. 41 A comprehensive discussion of this matter is provided in the later section. Lower serum gonadotropin levels are necessary but not sufficient conditions for follicle development. Whether lowering gonadotropins by GnRH agonist therapy or other means contributes to follicle development has not been proven. 42 , 43
2.2. Dehydroepiandrosterone (DHEA)
Some randomized controlled trials (RCTs) have been conducted to compare DHEA with placebos in patients with POI. One study indicated that the DHEA group had higher antral follicle counts (AFC) and ovarian volumes at weeks 12 and 20, respectively. 23 Wong et al. 22 evaluated 12‐month DHEA supplementation in women with POI. However, FSH and AFC levels did not change significantly. There have been some meta‐analyses on the efficacy of DEHA supplementation in patients with diminished ovarian reserve (DOR) and/or poor ovarian response (POR). 44 , 45 , 46 , 47 In 2019, a meta‐analysis of RCTs was reported by Xu et al. 24 This analysis revealed that compared to the controls, patients with DOR or POR treated with DHEA exhibited tendency of increases in the number of retrieved oocytes (mean difference, 0.91; 95% confidence interval [CI], 0.23–1.59; p = 0.009), significant increase of clinical pregnancy rate (relative risk [RR] = 1.27; 95% CI, 1.01–1.61; p = 0.04), and live birth rate (RR, 1.76; 95% CI, 1.17–2.63; p = 0.006). However, a recent systematic review advocated that multiple observational and RCTs are conducted with varying DOR and POR definitions, and with different DHEA doses. To date, no pharmacological studies have been performed to determine the optimal dose, duration, or timing of DHEA supplementation in patients with DOR; hence, the true effect of this treatment should be carefully evaluated through additional studies that standardize patient definitions and administration methods. 48
2.3. Intra‐ovarian injection of PRP
PRP therapy is a treatment that involves the use of a patient's own blood, contains high concentration of platelets, to promote tissue regeneration. 49 In the field of assisted reproductive medicine, there have been a number of reports of intrauterine administration in patients with repeated implantation failure and thin endometrium. 50 , 51 , 52 Recent studies have shown that intraovarian injections of autologous PRP can enhance ovarian folliculogenesis. 53 , 54
Cakiroglu et al. initiated transvaginal PRP therapy in 311 patients with POI. Following PRP therapy, AFC and AMH levels significantly increased, whereas the FSH levels remained unchanged. Moreover, 186 (59.8%) patients showed zero AFC before PRP therapy and patients with no antral follicles decreased to 87 (30.0%) after treatment. In addition, 23 (7.4%) patients experienced spontaneous pregnancy after PRP therapy. 20 Another study reported that in 60% of patients with POI who received transvaginal PRP therapy, their normal menstrual cycles returned, FSH levels decreased, and AMH levels and AFC increased. 21 Previous studies have mainly been non‐randomized interventional studies, and well‐designed RCTs and meta‐analyses of RCTs are expected to be conducted in the future to evaluate the efficacy of PRP more accurately.
2.4. The IVA method
In 2013, Kawamura et al. reported innovative methods to promote follicular activation, i.e., an IVA method for infertility treatment for patients with POI. This method is based on the activation of dormant follicles using in vitro cultures of ovarian fragments treated with PI3K stimulators and PTEN inhibitors. 25 They first removed the ovaries of patients with POI, followed by fragmentation to disrupt Hippo signaling, and drug therapy was initiated to stimulate Akt signaling. After grafting ovarian tissues back to the patients, rapid follicle growth was observed in some patients, and mature eggs were retrieved successfully. After IVF‐ET, two successful full‐term births were reported following IVA in patients with POI. 25 , 55
3. FOLLICLE GROWTH AND ITS PREDICTION IN PATIENTS WITH POI
In patients with POI, cycles with the opportunity to achieve follicle development are precious. This period must be captured without missing any chance to obtain eggs successfully.
When follicle development is expected, after ovarian stimulation through gonadotropin injections, the following options can be considered for patients with POI. If there is a current desire for pregnancy, options include timely intercourse, artificial insemination, or IVF‐ET. In patients without a partner, oocyte cryopreservation is a considerable option for future pregnancy.
Some patients with POI have occasional follicular growth 56 ; however, predicting which cycles have positive follicular growth is difficult because ovulation is infrequent, with an approximate 4% chance per month. 56 , 57 Predicting the cycle of follicle development in patients with POI is challenging but important. Lower serum FSH levels after HRT, higher serum E2 levels, and the presence of small follicles were the most common predictors of follicle growth during the cycle. After discussing the advantages and disadvantages of using these factors for prediction, we propose measuring the serum picoAMH levels as a novel approach for predicting the follicle growth.
3.1. Lower serum FSH levels is a necessary condition, but does not always promise the follicle development of the cycle
Empirically, follicular development has been observed in patients with amenorrhea who undergo HRT. Follicle development was observed in patients with POI following a decrease in gonadotropin levels after HRT. As mentioned above, higher gonadotropin levels negatively affect follicular development in patients with POI, theoretically.
One RCT showed importance of lower FSH under HRT for follicle development in patients with POI. 28 This RCT comparing patients with POI who received ethinylestradiol and placebo before the initiation of ovarian stimulation with FSH, 32% of the patients in the ethinylestradiol group ovulated, whereas the placebo group had not ovulated. In this study, the FSH levels at the start of stimulation in the ovulated group were all <15 mIU/mL.
However, it should be noted that the lower FSH levels under HRT does not promise the follicular development in that cycle. Sato et al. 58 evaluated 20 patients with POI receiving HRT, and 11 (55%) did not have any follicle development, and 9 had follicle development. They were monitored weekly, and if their E2 levels were ≥80–100 pg/mL, transvaginal ultrasonography was performed. After confirming follicle development, ovarian stimulation was initiated using FSH agents, and timed intercourse, AIH, and IVF were performed. They compared the FSH levels during menstruation between a cycle with and without follicle development. The FSH levels during menstruation with follicle (27 cycles) and non‐follicle (110 cycles) development were 12.7 ± 12.5 and 13.5 ± 11.4 mIU/mL, respectively (p = 0.739), and no significant difference was observed. 58 In this protocol, estradiol is continuously administered during withdrawal bleeding. While it is a commonly used method to prevent FSH elevation, under such conditions, the decrease in FSH is not helpful in predicting subsequent follicular development. It can be said that low FSH levels in patients with POI are a necessary condition for follicle development of the cycle, however it is not a sufficient one.
3.2. Higher serum E2 levels in the withdrawal period is one of the important prediction factors for follicle growth
An observational study of 25 patients with POI receiving HRT revealed that the serum E2 levels on days 1–5 of withdrawal bleeding (Day 1–5 E2) were significantly higher in cycles with successful follicle growth and ovulation than in unsuccessful cycles (p < 0.05). Receiver operator characteristic curve analysis revealed that the cutoff value of the Day 1–5 E2 was 15.5 pg/mL, with areas under the curve of 0.674 for follicle growth and 0.752 for ovulation. Serum E2 levels in the withdrawal period of HRT may be useful for predicting follicle development and ovulation. 26 However, it is necessary for exogenous E2 to have been completely washed out. Therefore, under estradiol administration, serum E2 levels cannot be used to predict the follicle growth.
3.3. Small follicles detected by ultrasound can promise the growth in the cycle
AFC refers to the number of antral follicles visualized by ultrasound examination and is used as a marker for assessing ovarian reserve and follicle development in oocyte retrieval cycles. 59 , 60 , 61 , 62 , 63 However, in patients with POI, there are no small follicles and AFC cannot be counted. 64 In POI, small follicles observed during a cycle have the potential to grow, and gonadotropin injections are often administered. The presence of small follicles is a promising finding for follicular development in the cycle; however, in situations where it is not easily feasible to visit a hospital or undergo ultrasound examinations, the presence of a marker that supports the necessity of ultrasound testing is desired.
3.4. AMH is not only an indicator of the ovarian reserve but also useful in predicting the follicle development of patients with POI
Among several markers for ovarian reserve, the serum level of AMH, which is produced by the granulosa cells of early‐stage follicles, is considered one of the most informative markers of ovarian reserve. 29 , 65 , 66 The measurement of AMH levels in the clinical setting has been used extensively in the prediction of ovarian response to control ovarian stimulation by gonadotropins, optimization of protocols in assisted reproductive technology, assessment of ovarian toxicity in medical and surgical conditions, etc. 67 , 68 , 69 Low serum AMH levels are accompanied by the reduction of residual follicle pools (Figure 1). Therefore, women with POI who have diminished residual follicle pool show lower and undetectable AMH levels than normal women of the same age. Because AMH is produced by the granulosa cells of early stage follicles, if there are cycles in which small follicles develop, trace amounts of AMH can be detected in the serum. Serum AMH levels in patients with POI may predict the existence of residual follicles. Therefore, a method to measure small amounts of AMH is necessary. Hence, it is necessary to apply a more sensitive measurement method than the traditional AMH measurement methods. Here, we outline the conventional method and describe a new kit for the measurement of small amounts of serum AMH, highlighting their differences.
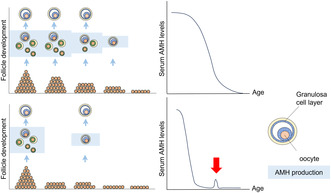
FIGURE 1.
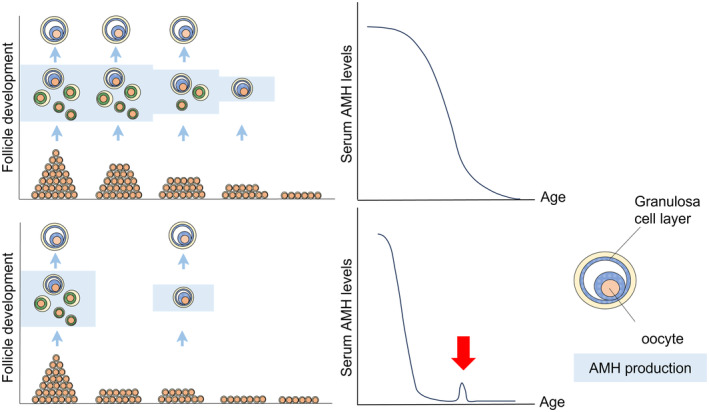
Schematic model of the residual follicle pool and serum AMH levels; comparison of age‐dependent decline in women with normal cycles and with women POI. The upper panel shows the model for women with normal cycles: residual follicles decrease with age, accompanied by a decrease in serum AMH levels. The lower panel shows the model for women with POI: the residual follicle pool is smaller than that in age‐matched women with normal cycles. Consequently, serum AMH levels were low from an early stage of reproductive life. If there are cycles where small follicles develop, it is supposed that trace amounts of AMH can be detected in the serum (red arrow).
3.5. Conventional methods for AMH measurement and its limitations
The concentration of AMH is measured using enzyme‐linked immunosorbent assay (ELISA) systems. The first and second commercially available kits for AMH were developed in 1999 by Immunotech (IOT, EIA AMH/MIS; Marseille, France) and 2003 by Diagnostics Systems Laboratories (Active AMH, Webster, TX, USA), respectively. Afterward, the second‐generation ELISA kit for AMH (AMH Gen II) was released when both companies became affiliated with Beckman Coulter (Brea, CA, USA). Thus far, most studies published have used EIA AMH/MIS, active AMH, and/or Gen II kits. Newer assays use automated formats with improved analytical sensitivity. However, none of these can detect very low serum AMH levels. 30 , 70
Evidence from a systematic review (41 studies, 28 858 women) supports the use of serum AMH to examine the age at menopause. The increased sensitivity of current AMH assays provides improved accuracy for the prediction of imminent menopause; however, the diagnostic use in individual patients is still incredulous. 71 In addition, very low AMH levels in young women indicate an increased risk of developing POI and may facilitate earlier diagnosis. A systematic review (75 studies, 9183 patients) evaluated AMH as a biomarker of ovarian reserve and POI before and after anticancer treatment and showed that AMH can be used to identify the damaging effect of cancer treatments on ovarian function. However, the clinical use of AMH is supported by very limited data relating post‐treatment AMH levels to the fertility, reproductive lifespan, or time to POI. 72
3.6. picoAMH ELISA have advantage to detect the lower levels of AMH
In recent years, Ansh Labs released two brand‐new kits. These kits, i.e., ultrasensitive AMH and picoAMH ELISA, can detect very low blood AMH levels. Although these kits use the same antibodies and calibrators, picoAMH ELISA can cover the lower range of the standard curve. 73 , 74 , 75 , 76 , 77 , 78 Good correlations of assay values have been found between these new kits and conventional kits 79 , 80 , 81 , 82 (Table 1). Compared with AMH Gen II, the new kits showed lower detection limits of serum AMH levels in women during the recovery phase after chemotherapy. 83 , 84
TABLE 1.
Comparison of current immunoassays for measuring the AMH.
| Description | MenoCheck® picoAMH ELISA | Ultrasensitive AMH/MIS ELISA | Gen II AMH ELISA | Elecsys® AMH immunoassay | Access AMH |
|---|---|---|---|---|---|
| Manufacturer | Ansh Laboratories (TX, USA) | Ansh Laboratories (TX, USA) | Beckman Coulter Diagnostics (TX, USA) | Roche Diagnostics International Ltd. (IN, USA) | Beckman Coulter Diagnostics (TX, USA) |
| Test format | Sandwich‐type immunoassay | Sandwich‐type ELISA | ELISA (2 site manual immunoassay) | Automated immunoassay | Automated immunoassay |
| Limit of detection | 1.3 pg/mL | 0.023 ng/mL | 0.05 ng/mL | 0.010 ng/mL | ≤0.02 ng/mL |
| Limit of quantification (LoQ) with <20% CV | 3.2 pg/mL | 0.06 ng/mL | 0.13 ng/mL | 0.030 ng/mL | ≤0.08 ng/mL |
Abbreviation: CV, coefficient of variation.
Su et al. compared the AMH levels among three commercially available AMH immunoassays, namely, AMH Gen II (Beckman Coulter), ultrasensitive AMH (Ansh Labs), and picoAMH ELISA (Ansh Labs), in 90 patients with breast cancer before cancer treatment. Compared with both Gen II (84%) and ultrasensitive (92%) assays, picoAMH ELISA (97%) revealed significantly higher proportions of detectable AMH levels. Furthermore, the Gen II AMH values were consistently lower than those measured by both Ultrasensitive and picoAMH ELISA. As AMH levels increased, the magnitude of the difference grew larger between Gen II and each of the other two assays. 79
During the same time, another study compared and well considered these three testing methods. The authors revealed that 15 of the 22 undetectable samples by the Ultrasensitive assay and the Gen‐assay yielded a measurable concentration result on the picoAMH ELISA. 80 Thus, serum AMH levels of patients with POI measured by picoAMH ELISA may predict the existence of residual follicles in patients with POI.
3.7. Measurement of serum AMH by picoAMH ELISA and prediction of follicle development in patients with POI
Previously, we reported that picoAMH ELISA is useful for the assessment of women with a low ovarian reserve. The AMH levels of 68 women with serum AMH levels undetectable using AMH Gen II ELISA was analyzed by picoAMH ELISA. The AMH concentrations of the 36 samples were detectable (≥1.3 pg/mL) using picoAMH ELISA, 32 were within the standard range, and 4 were out of the standard range but still detectable. Moreover, this study revealed that the 32 women whose AMH levels were undetectable in picoAMH ELISA all had amenorrhea, and five women with amenorrhea and detectable AMH levels eventually achieved follicle growth (Figure 2). 30
FIGURE 2.
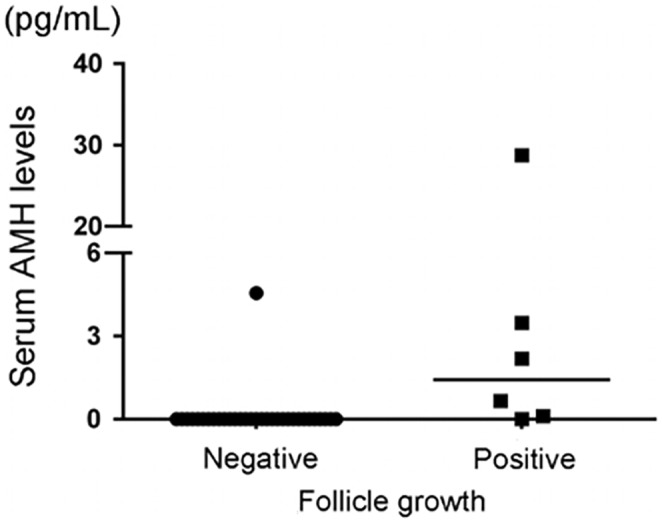
Serum AMH levels in patients with amenorrhea with or without follicle growth cycles. Patients with follicle growth showed significantly higher levels of serum AMH. Bars represent the medians. This figure has been reproduced with permission from “Reproductive Sciences volume 23, pages 756–760 (2016).”
In addition, Decanter et al. measured the AMH levels by a conventional AMH ELISA (EIA AMH/MIS) and picoAMH ELISA on the same sample of young patients with cancer (breast cancer, n = 13; hematologic malignancies, n = 17) during the ovarian recovery phase of their chemotherapy. The results indicated that serum AMH was detectable (≥3 pmol/L) in 6.7% and 10.7% of the samples corresponding to patients with amenorrhea and patients with menstrual cycles, respectively, using a conventional AMH ELISA (EIA AMH/MIS), and the rate of measurable AMH levels with the EIA AMH/MIS assay was not significantly higher in samples from patients with normal menstrual cycles than from patients with amenorrhea. With picoAMH, serum AMH was detectable (≧0.07 pmol/L) in 71.4% of the samples from women with menstrual cycles versus 16.7% of the samples from patients with amenorrhea. In addition, in the serum samples from patients who regained normal menstrual cycles, AMH was much more frequently detectable with the latter assay, and the difference was not highly significant. Accordingly, the AMH levels of patients with menstrual cycles were significantly higher than in those with amenorrhea using picoAMH ELISA. 84
To determine the usefulness of measuring serum AMH levels measured by picoAMH ELISA as a predictor of follicle growth in patients with POI, we further evaluated follicle growth in each menstruation during each cycle. We evaluated 91 cycles of 19 patients with POI retrospectively. The serum AMH levels of each patient were measured during the withdrawal period of each cycle. If the serum AMH level was higher than the limit of detection (1.3 pg/mL), the cycle was defined as AMH‐positive. In contrast, if the AMH levels of the cycle were below the limit of detection of the picoAMH ELISA, the cycle was defined as AMH‐negative. Five patients presented with AMH‐positive cycles, and they exhibited cycles with follicle development. In 14 patients with AMH‐negative cycles, only 2 (14.2%) experienced follicle growth during the observed periods. Then, to evaluate AMH as a predictor of follicle growth during a cycle, the serum AMH and FSH levels and amount of follicle growth in each cycle were compared. Of the total 91 cycles, 14 were positive for AMH and 14 showed follicle growth. Of the 14 AMH‐positive cycles, nine presented with follicle growth, and five did not show follicle growth. Of the 14 follicle growth cycles, five were AMH‐negative; however, two of these five cycles were AMH‐positive in the previous cycle. The serum FSH and AMH levels were significantly lower and significantly higher, respectively, in cycles with follicle growth than in those without. The median serum FSH and AMH levels in cycles with follicle growth were 15.44 (25th and 75th percentile 5.03, 26.85) and 2.77 pg/mL (25th and 75th percentile 0.0, 9.64), respectively. More cycles are showing follicle growth in AMH‐positive cycles than in AMH‐negative cycles (64.3% vs. 6.5%, p = 0.0001). Serum FSH levels in AMH‐positive cycles were significantly lower than those in AMH‐negative cycles. The positive‐predictive value (PPV) and negative‐predictive value (NPV) of the AMH‐positive serum for follicle growth in the cycle were 0.643 and 0.935, respectively. Moreover, the usefulness of serum FSH levels in the prediction of follicle growth was assessed. As a predictor of follicle growth FSH levels were set to <10 mIU/mL, and the PPV and NPV of FSH were 0.250 and 0.873, respectively. These results indicate that the AMH‐positive serum during the withdrawal period had superior to low serum FSH levels when used to predict follicle growth in the cycle (Table 2). 85 Based on these results, it is suggested that to avoid missing the follicular development cycle, picoAMH ELISA measurements should be performed in each cycle during the HRT in patients with POI who desire to conceive using their own eggs. Consequently, we propose performing ultrasound observations and ovarian stimulation with gonadotropin injections in cycles with positive picoAMH, as follicular development may be achieved in these cycles. Additionally, if follicular development is not observed in a picoAMH‐positive cycle, the next cycle as well as the positive cycle should be monitored because follicular development may be observed in the cycle following the picoAMH‐positive cycle. In our study, there are five AMH‐negative cycles showed follicle growth, however two of these five cycles were AMH‐positive in the previous cycle. It is possible that AMH secretion from small follicles that were not visible on ultrasound was detected in these cycles, and that the small follicles developed in the next cycle. As mentioned above, high E2 levels during the withdrawal period is another reliable predictive factor of follicle growth during the cycle. 26 However, when using E2 for prediction, it is necessary to perform blood sampling at a timing strictly without the effects of hormone replacement. In addition, when applying a protocol that involves the continuous administration of estrogen medications, serum E2 levels cannot be used as an indicator. 58 In such situations, we believe that predictions made using picoAMH ELISA would be useful.
TABLE 2.
Comparison of the predictive accuracy of AMH and FSH for follicle development in patients with POI.
| PPV | NPV | Sensitivity | Specificity | |
|---|---|---|---|---|
| AMH positive | 0.643 | 0.935 | 0.643 | 0.949 |
| FSH <15 mIU/mL | 0.269 | 0.892 | 0.500 | 0.753 |
| FSH <10 mIU/mL | 0.250 | 0.873 | 0.357 | 0.805 |
Note: AMH positive, serum AMH levels are over the limit of detection of picoAMH ELISA.
Abbreviations: AMH, anti‐Müllerian hormone; FSH, follicle‐stimulating hormone; NPV, negative‐predictive value; PPV, positive‐predictive value.
One of the problems with the clinical use of picoAMH ELISA is the long waiting time for the ELISA results. As potential contributions toward resolving such issues, a study reported the validation and characterization of the MenoCheck picoAMH ELISA using the Dynex‐DS2 automated immunoassay system. In this report, picoAMH ELISA was validated on the Dynex‐DS2 according to CLSI guidelines. The intra‐ and interassay coefficient of variations of picoAMH ELISA on DS2 was ≤4%, and the assay was linear between AMH concentrations of 0.0067 and 16.24 ng/mL (0.048–116.0 pmol/L). Methods were compared in the manufacturer's laboratory and indicated a good correlation. 86 The availability of the automated measurement of picoAMH ELISA in routine clinical practice may allow patients with POI to determine on the visit day whether or not follicle development is achieved during their current cycle.
In summary, the measurement of serum AMH levels in patients with POI using the picoAMH ELISA may be useful for predicting follicle development in patients with POI. Combined with serum E2 levels during withdrawal periods and follicular findings on ultrasonography, this may provide a more accurate prediction.
4. CONCLUSION
This review provides an overview of interventions in POI to maximize the utilization of a patient's remaining oocytes to achieve pregnancy by using currently available therapies and methods to predict follicular development. These interventions may be beneficial for patients with POI who are not considering or are unable to obtain donor oocytes for achieving pregnancy. However, retrieving one's eggs remains challenging in patients with POI, and high‐risk cases must be identified before POI onset. Currently, AMH is considered a potential marker for identifying high‐risk cases. Even if cases with decreased AMH levels are identified, regaining lost follicles is not possible. In cases of idiopathic POI, markers that can identify high‐risk cases before the decline in AMH levels and the follicles are intact must be developed.
CONFLICT OF INTEREST STATEMENT
Akira Iwase is an Editorial Board member of Reproductive Medicine and Biology and a co‐author of this article. To minimize the bias, he was excluded from all editorial decision‐making related to the acceptance of this article for publication. Akira Iwase and Satoko Osuka have received a grant from Grant in Aid for the Scientific Research (20H0381, 23K08865) related to POI. Akira Iwase is a chairperson of the Reproductive and Endocrine Committee, Japan Society of Obstetrics and Gynecology. There are no conflicts of interest with other authors.
ACKNOWLEDGMENTS
We would like to express our sincere gratitude to all lab members who have supported us throughout the completion of all the cited research. In particular, we would like to extend my appreciation to Bayasula and Nobuyoshi Takasaki for their invaluable technical support and insightful advice. This work was partially supported by a Grant in Aid for the Scientific Research (no. 23K08865 to S.O.).
Osuka S, Kasahara Y, Iyoshi S, Sonehara R, Myake N, Muraoka A, et al. Follicle development and its prediction in patients with primary ovarian insufficiency: Possible treatments and markers to maximize the ability to conceive with residual follicles. Reprod Med Biol. 2023;22:e12556. 10.1002/rmb2.12556
REFERENCES
- 1. Nelson LM. Primary ovarian insufficiency. N Engl J Med. 2009;360(6):606–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Webber L, Davies M, Anderson R, Bartlett J, Braat D, Cartwright B, et al. ESHRE guideline: management of women with premature ovarian insufficiency. Hum Reprod. 2016;31(5):926–937. [DOI] [PubMed] [Google Scholar]
- 3. Jiao X, Zhang H, Ke H, Zhang J, Cheng L, Liu Y, et al. Premature ovarian insufficiency: phenotypic characterization within different etiologies. J Clin Endocrinol Metab. 2017;102(7):2281–2290. [DOI] [PubMed] [Google Scholar]
- 4. Golezar S, Ramezani Tehrani F, Khazaei S, Ebadi A, Keshavarz Z. The global prevalence of primary ovarian insufficiency and early menopause: a meta‐analysis. Climacteric. 2019;22(4):403–411. [DOI] [PubMed] [Google Scholar]
- 5. Cox L, Liu JH. Primary ovarian insufficiency: an update. Int J Womens Health. 2014;6(1):235–243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Osuka S, Iwase A, Goto M, Takikawa S, Nakamura T, Murase T, et al. Thyroid autoantibodies do not impair the ovarian reserve in euthyroid infertile women: a cross‐sectional study. Horm Metab Res. 2018;50(7):537–542. [DOI] [PubMed] [Google Scholar]
- 7. Savage A. Screening and management of the hyperandrogenic adolescent. Obstet Gynecol. 2019;134(4):888–889. [DOI] [PubMed] [Google Scholar]
- 8. Rodgers RJ, Reid GD, Koch J, Deans R, Ledger WL, Friedlander M, et al. The safety and efficacy of controlled ovarian hyperstimulation for fertility preservation in women with early breast cancer: a systematic review. Hum Reprod. 2017;32(5):1033–1045. [DOI] [PubMed] [Google Scholar]
- 9. Loren AW, Mangu PB, Beck LN, Brennan L, Magdalinski AJ, Partridge AH, et al. Fertility preservation for patients with cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2013;31(19):2500–2510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24(18):2917–2931. [DOI] [PubMed] [Google Scholar]
- 11. Von Wolff M, Montag M, Dittrich R, Denschlag D, Nawroth F, Lawrenz B. Fertility preservation in women – a practical guide to preservation techniques and therapeutic strategies in breast cancer, Hodgkin's lymphoma and borderline ovarian tumours by the fertility preservation network FertiPROTEKT. Arch Gynecol Obstet. 2011;284(2):427–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kim SS, Donnez J, Barri P, Pellicer A, Patrizio P, Rosenwaks Z, et al. Recommendations for fertility preservation in patients with lymphoma, leukemia, and breast cancer. J Assist Reprod Genet. 2012;29(6):465–468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Kunitomi C, Harada M, Sanada Y, Kusamoto A, Takai Y, Furui T, et al. The possible effects of the Japan Society of Clinical Oncology Clinical Practice Guidelines 2017 on the practice of fertility preservation in female cancer patients in Japan. Reprod Med Biol. 2022;21(1):1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Sanada Y, Harada M, Kunitomi C, Kanatani M, Izumi G, Hirata T, et al. A Japanese nationwide survey on the cryopreservation of embryos, oocytes and ovarian tissue for cancer patients. J Obstet Gynaecol Res. 2019;45(10):2021–2028. [DOI] [PubMed] [Google Scholar]
- 15. Alvarez RM, Ramanathan P. Fertility preservation in female oncology patients: the influence of the type of cancer on ovarian stimulation response. Hum Reprod. 2018;33(11):2051–2059. [DOI] [PubMed] [Google Scholar]
- 16. Chen X, Chen S‐L, Ye D‐S, Liu Y‐D, He Y‐X, Tian X‐L, et al. Retrospective analysis of reproductive outcomes in women with primary ovarian insufficiency showing intermittent follicular development. Reprod Biomed Online. 2016;32(4):427–433. [DOI] [PubMed] [Google Scholar]
- 17. Welt CK, Hall JE, Adams JM, Taylor AE. Relationship of estradiol and inhibin to the follicle‐stimulating hormone variability in hypergonadotropic hypogonadism or premature ovarian failure. J Clin Endocrinol Metab. 2005;90(2):826–830. [DOI] [PubMed] [Google Scholar]
- 18. Kuang X, Tang Y, Xu H, Ji M, Lai D. The evaluation of ovarian function recovery following treatment of primary ovarian insufficiency: a systematic review. Front Endocrinol (Lausanne). 2022;13:855992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Aflatoonian A, Lotfi M, Saeed L, Tabibnejad N. Effects of intraovarian injection of autologous platelet‐rich plasma on ovarian rejuvenation in poor responders and women with primary ovarian insufficiency. Reprod Sci. 2021;28(7):2050–2059. [DOI] [PubMed] [Google Scholar]
- 20. Cakiroglu Y, Saltik A, Yuceturk A, Karaosmanoglu O, Kopuk SY Jr, Scott RT, et al. Effects of intraovarian injection of autologous platelet rich plasma on ovarian reserve and IVF outcome parameters in women with primary ovarian insufficiency. Aging (Albany NY). 2020;12(11):10211–10222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sfakianoudis K, Simopoulou M, Grigoriadis S, Pantou A, Tsioulou P, Maziotis E, et al. Reactivating ovarian function through autologous platelet‐rich plasma Intraovarian infusion: pilot data on premature ovarian insufficiency, perimenopausal, menopausal, and poor responder women. J Clin Med. 2020;9(6):1–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wong QHY, Yeung TWY, Yung SSF, Ko JKY, Li HWR, Ng EHY. The effect of 12‐month dehydroepiandrosterone supplementation on the menstrual pattern, ovarian reserve markers, and safety profile in women with premature ovarian insufficiency. J Assist Reprod Genet. 2018;35(5):857–862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yeung TWY, Li RHW, Lee VCY, Ho PC, Ng EHY. A randomized double‐blinded placebo‐controlled trial on the effect of dehydroepiandrosterone for 16 weeks on ovarian response markers in women with primary ovarian insufficiency. J Clin Endocrinol Metab. 2013;98(1):380–388. [DOI] [PubMed] [Google Scholar]
- 24. Xu L, Hu C, Liu Q, Li Y. The effect of Dehydroepiandrosterone (DHEA) supplementation on IVF or ICSI: a meta‐analysis of randomized controlled trials. Geburtshilfe Frauenheilkd. 2019;79(7):705–712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kawamura K, Cheng Y, Suzuki N, Deguchi M, Sato Y, Takae S, et al. Hippo signaling disruption and Akt stimulation of ovarian follicles for infertility treatment. Proc Natl Acad Sci USA. 2013;110(43):17474–17479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miyazaki K, Miki F, Uchida S, Masuda H, Uchida H, Maruyama T. Serum estradiol level during withdrawal bleeding as a predictive factor for intermittent ovarian function in women with primary ovarian insufficiency. Endocr J. 2015;62(1):93–99. [DOI] [PubMed] [Google Scholar]
- 27. Surrey ES, Cedars MI. The effect of gonadotropin suppression on the induction of ovulation in premature ovarian failure patients. Fertil Steril. 1989;52(1):36–41. [DOI] [PubMed] [Google Scholar]
- 28. Tartagni M, Cicinelli E, De Pergola G, De Salvia MA, Lavopa C, Loverro G. Effects of pretreatment with estrogens on ovarian stimulation with gonadotropins in women with premature ovarian failure: a randomized, placebo‐controlled trial. Fertil Steril. 2007;87(4):858–861. [DOI] [PubMed] [Google Scholar]
- 29. Iwase A, Osuka S, Goto M, Murase T, Nakamura T, Takikawa S, et al. Clinical application of serum anti‐Müllerian hormone as an ovarian reserve marker: a review of recent studies. J Obstet Gynaecol Res. 2018;44(6):998–1006. [DOI] [PubMed] [Google Scholar]
- 30. Iwase A, Osuka S, Nakamura T, Kato N, Takikawa S, Goto M, et al. Usefulness of the ultrasensitive anti‐Müllerian hormone assay for predicting true ovarian reserve. Reprod Sci. 2016;23(6):756–760. [DOI] [PubMed] [Google Scholar]
- 31. Söderström‐Anttila V, Tiitinen A, Foudila T, Hovatta O. Obstetric and perinatal outcome after oocyte donation: comparison with in‐vitro fertilization pregnancies. Hum Reprod. 1998;13(2):483–490. [DOI] [PubMed] [Google Scholar]
- 32. Salha O, Sharma V, Dada T, Nugent D, Rutherford AJ, Tomlinson AJ, et al. The influence of donated gametes on the incidence of hypertensive disorders of pregnancyNo title. Hum Reprod. 1999;14(9):2268–2273. [DOI] [PubMed] [Google Scholar]
- 33. Hikabe O, Hamazaki N, Nagamatsu G, Obata Y, Hirao Y, Hamada N, et al. Reconstitution in vitro of the entire cycle of the mouse female germ line. Nature. 2016;539(7628):299–303. [DOI] [PubMed] [Google Scholar]
- 34. Murakami K, Hamazaki N, Hamada N, Nagamatsu G, Okamoto I, Ohta H, et al. Generation of functional oocytes from male mice in vitro. Nature. 2023;615(7954):900–906. [DOI] [PubMed] [Google Scholar]
- 35. Yamashiro C, Sasaki K, Yokobayashi S, Kojima Y, Saitou M. Generation of human oogonia from induced pluripotent stem cells in culture. Nat Protoc. 2020;15(4):1560–1583. [DOI] [PubMed] [Google Scholar]
- 36. Saitou M, Hayashi K. Mammalian in vitro gametogenesis. Science. 2021;374(6563):eaaz6830. [DOI] [PubMed] [Google Scholar]
- 37. Ishii T, Saitou M. Promoting in vitro gametogenesis research with a social understanding. Trends Mol Med. 2017;23(11):985–988. [DOI] [PubMed] [Google Scholar]
- 38. Webber L, Anderson RA, Davies M, Janse F, Vermeulen N. HRT for women with premature ovarian insufficiency: a comprehensive review. Hum Reprod Open. 2017;2017(2):hox007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Wei W, Komatsu K, Osuka S, Murase T, Bayasula B, Nakanishi N, et al. Tamoxifen activates dormant primordial follicles in mouse ovaries. Reprod Sci. 2022;29(12):3404–3412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Komatsu K, Wei W, Murase T, Masubuchi S. 17β‐estradiol and cathepsins control primordial follicle growth in mouse ovaries. Reproduction. 2021;162(4):277–287. [DOI] [PubMed] [Google Scholar]
- 41. Popat VB, Vanderhoof VH, Calis KA, Troendle JF, Nelson LM. Normalization of serum luteinizing hormone levels in women with 46,XX spontaneous primary ovarian insufficiency. Fertil Steril. 2008;89(2):429–433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. van Kasteren YM, Schoemaker J. Premature ovarian failure: a systematic review on therapeutic interventions to restore ovarian function and achieve pregnancy. Hum Reprod Update. 1999;5(5):483–492. [DOI] [PubMed] [Google Scholar]
- 43. van Kasteren YM, Hoek A, Schoemaker J. Ovulation induction in premature ovarian failure: a placebo‐controlled randomized trial combining pituitary suppression with gonadotropin stimulation. Fertil Steril. 1995;64(2):273–278. [DOI] [PubMed] [Google Scholar]
- 44. Li J, Yuan H, Chen Y, Wu H, Wu H, Li L. A meta‐analysis of dehydroepiandrosterone supplementation among women with diminished ovarian reserve undergoing in vitro fertilization or intracytoplasmic sperm injection. Int J Gynaecol Obstet. 2015;131(3):240–245. [DOI] [PubMed] [Google Scholar]
- 45. Zhang M, Niu W, Wang Y, Xu J, Bao X, Wang L, et al. Dehydroepiandrosterone treatment in women with poor ovarian response undergoing IVF or ICSI: a systematic review and meta‐analysis. J Assist Reprod Genet. 2016;33(8):981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Qin JC, Fan L, Qin AP. The effect of dehydroepiandrosterone (DHEA) supplementation on women with diminished ovarian reserve (DOR) in IVF cycle: evidence from a meta‐analysis. J Gynecol Obstet Hum Reprod. 2017;46(1):1–7. [DOI] [PubMed] [Google Scholar]
- 47. Wang J, Liu B, Wen J, Qu B. The role of dehydroepiandrosterone in improving in vitro fertilization outcome in patients with DOR/POR: a systematic review and meta‐ analysis. Comb Chem High Throughput Screen. 2023;26(5):916–927. [DOI] [PubMed] [Google Scholar]
- 48. Neves AR, Montoya‐Botero P, Polyzos NP. The role of androgen supplementation in women with diminished ovarian reserve: time to randomize, not meta‐analyze. Front Endocrinol (Lausanne). 2021;12:653857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Redler LH, Thompson SA, Hsu SH, Ahmad CS, Levine WN. Platelet‐rich plasma therapy: a systematic literature review and evidence for clinical use. Phys Sportsmed. 2011;39(1):42–51. [DOI] [PubMed] [Google Scholar]
- 50. Anitua E, Allende M, de la Fuente M, Del Fabbro M, Alkhraisat MH. Efficacy of platelet‐rich plasma in women with a history of embryo transfer failure: a systematic review and meta‐analysis with trial sequential analysis. Bioengineering (Basel). 2023;10(3):303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Deng H, Wang S, Li Z, Xiao L, Ma L. Effect of intrauterine infusion of platelet‐rich plasma for women with recurrent implantation failure: a systematic review and meta‐analysis. J Obstet Gynaecol. 2023;43(1):2144177. [DOI] [PubMed] [Google Scholar]
- 52. Bos‐Mikich A, de Oliveira R, Frantz N. Platelet‐rich plasma therapy and reproductive medicine. J Assist Reprod Genet. 2018;35(5):753–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Seckin S, Ramadan H, Mouanness M, Kohansieh M, Merhi Z. Ovarian response to intraovarian platelet‐rich plasma (PRP) administration: hypotheses and potential mechanisms of action. J Assist Reprod Genet. 2022;39(1):37–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Panda SR, Sachan S, Hota S. A systematic review evaluating the efficacy of intra‐ovarian infusion of autologous platelet‐rich plasma in patients with poor ovarian reserve or ovarian insufficiency. Cureus. 2020;12(12):e12037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Suzuki N, Yoshioka N, Takae S, Sugishita Y, Tamura M, Hashimoto S, et al. Successful fertility preservation following ovarian tissue vitrification in patients with primary ovarian insufficiency. Hum Reprod. 2015;30(3):608–615. [DOI] [PubMed] [Google Scholar]
- 56. Nelson LM, Anasti JN, Kimzey LM, Defensor RA, Lipetz KJ, White BJ, et al. Development of luteinized graafian follicles in patients with karyotypically normal spontaneous premature ovarian failure. J Clin Endocrinol Metab. 1994;79(5):1470–1475. [DOI] [PubMed] [Google Scholar]
- 57. Taylor AE, Adams JM, Mulder JE, Martin KA, Sluss PM, Crowley WF. A randomized, controlled trial of estradiol replacement therapy in women with hypergonadotropic amenorrhea. J Clin Endocrinol Metab. 1996;81(10):3615–3621. [DOI] [PubMed] [Google Scholar]
- 58. Sato T, Kusuhara A, Kasahara Y, Haino T, Kishi H, Okamoto A. Follicular development during hormone replacement therapy in patients with premature ovarian insufficiency. Reprod Med Biol. 2021;20(2):234–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Nardo LG, Gelbaya TA, Wilkinson H, Roberts SA, Yates A, Pemberton P, et al. Circulating basal anti‐Müllerian hormone levels as predictor of ovarian response in women undergoing ovarian stimulation for in vitro fertilization. Fertil Steril. 2009;92(5):1586–1593. [DOI] [PubMed] [Google Scholar]
- 60. Barreto Melo MA, Garrido N, Alvarez C, Bellver J, Meseguer M, Pellicer A, et al. Antral follicle count (AFC) can be used in the prediction of ovarian response but cannot predict the oocyte/embryo quality or the in vitro fertilization outcome in an egg donation program. Fertil Steril. 2009;91(1):148–156. [DOI] [PubMed] [Google Scholar]
- 61. Hsu A, Arny M, Knee AB, Bell C, Cook E, Novak AL, et al. Antral follicle count in clinical practice: analyzing clinical relevance. Fertil Steril. 2011;95(2):474–479. [DOI] [PubMed] [Google Scholar]
- 62. Jayaprakasan K, Chan Y, Islam R, Haoula Z, Hopkisson J, Coomarasamy A, et al. Prediction of in vitro fertilization outcome at different antral follicle count thresholds in a prospective cohort of 1,012 women. Fertil Steril. 2012;98(3):657–663. [DOI] [PubMed] [Google Scholar]
- 63. Verhagen TEM, Hendriks DJ, Bancsi LFJMM, Mol BWJ, Broekmans FJM. The accuracy of multivariate models predicting ovarian reserve and pregnancy after in vitro fertilization: a meta‐analysis. Hum Reprod Update. 2008;14(2):95–100. [DOI] [PubMed] [Google Scholar]
- 64. Jiao X, Meng T, Zhai Y, Zhao L, Luo W, Liu P, et al. Ovarian reserve markers in premature ovarian insufficiency: within different clinical stages and different etiologies. Front Endocrinol (Lausanne). 2021;12:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Broer SL, Broekmans FJM, Laven JSE, Fauser BCJM. Anti‐Müllerian hormone: ovarian reserve testing and its potential clinical implications. Hum Reprod Update. 2014;20(5):688–701. [DOI] [PubMed] [Google Scholar]
- 66. Visser JA, Schipper I, Laven JSE, Themmen APN. Anti‐Müllerian hormone: an ovarian reserve marker in primary ovarian insufficiency. Nat Rev Endocrinol. 2012;8(6):331–341. [DOI] [PubMed] [Google Scholar]
- 67. Iwase A, Nakamura T, Kato N, Goto M, Takikawa S, Kondo M, et al. Anti‐Müllerian hormone levels after laparoscopic cystectomy for endometriomas as a possible predictor for pregnancy in infertility treatments. Gynecol Endocrinol. 2016;32(4):293–297. [DOI] [PubMed] [Google Scholar]
- 68. Iwase A, Nakamura T, Osuka S, Takikawa S, Goto M, Kikkawa F. Anti‐Müllerian hormone as a marker of ovarian reserve: what have we learned, and what should we know? Reprod Med Biol. 2016;15(3):127–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Saito A, Iwase A, Nakamura T, Osuka S, Bayasula, Murase T, et al. Involvement of mesosalpinx in endometrioma is a possible risk factor for decrease of ovarian reserve after cystectomy: a retrospective cohort study. Reprod Biol Endocrinol. 2016;14(1):72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Li HWR, Ng EHY, Wong BPC, Anderson RA, Ho PC, Yeung WSB. Correlation between three assay systems for anti‐Müllerian hormone (AMH) determination. J Assist Reprod Genet. 2012;29(12):1443–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Nelson SM, Davis SR, Kalantaridou S, Lumsden MA, Panay N, Anderson RA. Anti‐Müllerian hormone for the diagnosis and prediction of menopause: a systematic review. Hum Reprod Update. 2023;29(3):327–346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Anderson RA, Cameron D, Clatot F, Demeestere I, Lambertini M, Nelson SM, et al. Anti‐Müllerian hormone as a marker of ovarian reserve and premature ovarian insufficiency in children and women with cancer: a systematic review. Hum Reprod Update. 2022;28(3):417–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Bell RJ, Skiba MA, Sikaris K, Liu A, Islam RM, Davis SR. Differing performance of two assays for the measurement of anti‐Mullerian hormone in premenopausal women: a cross‐sectional study. Clin Endocrinol (Oxf). 2021;95(1):169–175. [DOI] [PubMed] [Google Scholar]
- 74. de Kat AC, Van Der Schouw YT, Eijkemans MJC, Broer SL, Verschuren WMM, Broekmans FJM. Can menopause prediction be improved with multiple AMH measurements? Results from the prospective Doetinchem cohort study. J Clin Endocrinol Metab. 2019;104(11):5024–5031. [DOI] [PubMed] [Google Scholar]
- 75. de Kat AC, Broekmans FJM, van Westing AC, Lentjes E, Verschuren WMM, van der Schouw YT. A quantitative comparison of anti‐Müllerian hormone measurement and its shifting boundaries between two assays. Maturitas. 2017;101:12–16. [DOI] [PubMed] [Google Scholar]
- 76. Jung S, Allen N, Arslan AA, Baglietto L, Brinton LA, Egleston BL, et al. Demographic, lifestyle, and other factors in relation to antimüllerian hormone levels in mostly late premenopausal women. Fertil Steril. 2017;107(4):1012–1022.e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. de Kat AC, van der Schouw YT, Eijkemans MJC, Herber‐Gast GC, Visser JA, Verschuren WMM, et al. Back to the basics of ovarian aging: a population‐based study on longitudinal anti‐Müllerian hormone decline. BMC Med. 2016;14(1):1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Burks HR, Ross L, Opper N, Paulson E, Stanczyk FZ, Chung K. Can highly sensitive antimüllerian hormone testing predict failed response to ovarian stimulation? Fertil Steril. 2015;104(3):643–648. [DOI] [PubMed] [Google Scholar]
- 79. Su HI, Sammel MD, Homer MV, Bui K, Haunschild C, Stanczyk FZ. Comparability of antimüllerian hormone levels among commercially available immunoassays. Fertil Steril. 2014;101(6):1766–1772.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Welsh P, Smith K, Nelson SM. A single‐centre evaluation of two new anti‐Müllerian hormone assays and comparison with the current clinical standard assay. Hum Reprod. 2014;29(5):1035–1041. [DOI] [PubMed] [Google Scholar]
- 81. Punchoo R, Bhoora S. Variation in the measurement of anti‐Müllerian hormone – what are the laboratory issues? Front Endocrinol (Lausanne). 2021;12:719029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Bungum L, Tagevi J, Jokubkiene L, Bungum M, Giwercman A, Macklon N, et al. The impact of the biological variability or assay performance on AMH measurements: a prospective cohort study with AMH tested on three analytical assay‐platforms. Front Endocrinol (Lausanne). 2018;9:603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Chai J, Howie AF, Cameron DA, Anderson RA. A highly‐sensitive anti‐Müllerian hormone assay improves analysis of ovarian function following chemotherapy for early breast cancer. Eur J Cancer. 2014;50(14):2367–2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Decanter C, Peigne M, Mailliez A, Morschhauser F, Dassonneville A, Dewailly D, et al. Toward a better follow‐up of ovarian recovery in young women after chemotherapy with a hypersensitive antimüllerian hormone assay. Fertil Steril. 2014;102(2):483–487. [DOI] [PubMed] [Google Scholar]
- 85. Kasahara Y, Osuka S, Bayasula, Nakanishi N, Murase T, Nakamura T, et al. Very low levels of serum anti‐Müllerian hormone as a possible marker for follicle growth in patients with primary ovarian insufficiency under hormone replacement therapy. Reprod Sci. 2021;28(1):31–36. [DOI] [PubMed] [Google Scholar]
- 86. Garnett ER, Jariwala P, Rector K, Gibbons WE, Zarutskie PW, Devaraj S. Validation of the picoAMH assay on the Dynex DS2 platform. Pract Lab Med. 2019;17:e00140. [DOI] [PMC free article] [PubMed] [Google Scholar]


