Abstract
Escherichia coli has two primary pathways for glutamate synthesis. The glutamine synthetase-glutamate synthase (GOGAT) pathway is essential for synthesis at low ammonium concentration and for regulation of the glutamine pool. The glutamate dehydrogenase (GDH) pathway is important during glucose-limited growth. It has been hypothesized that GDH is favored when the organism is stressed for energy, because the enzyme does not use ATP as does the GOGAT pathway. The results of competition experiments between the wild-type and a GDH-deficient mutant during glucose-limited growth in the presence of the nonmetabolizable glucose analog α-methylglucoside were consistent with the hypothesis. Enzyme measurements showed that levels of the enzymes of the glutamate pathways dropped as the organism passed from unrestricted to glucose-restricted growth. However, other conditions influencing pathway choice had no substantial effect on enzyme levels. Therefore, substrate availability and/or modulation of enzyme activity are likely to be major determinants of pathway choice in glutamate synthesis.
Most biochemical conversions are mediated by a single pathway (enzyme or sequence of enzymes). When seemingly redundant pathways exist, as for some steps in the citric acid cycle (5), detailed examination usually reveals that the parallel paths operate under different conditions and are subject to different controls. Thus, the ability to choose from parallel pathways is a mechanism for control of biological function.
Most cellular nitrogen enters metabolism through glutamate. In enteric bacteria such as Escherichia coli and in many other organisms, glutamate is synthesized by either of two primary pathways, each beginning with 2-oxoglutarate (2OG). In one, glutamate is formed directly through reductive amination of 2OG by glutamate dehydrogenase (GDH). Alternatively, synthesis can proceed indirectly through amidation of glutamate to form glutamine by glutamine synthetase (GS) followed by reductive transfer of the amide group to oxoglutarate by glutamate synthetase (GOGAT) to give two glutamate molecules (a net gain of one) (17). Mutants of E. coli lacking either pathway show no glutamate requirement under usual laboratory conditions; mutants lacking both require external glutamate for growth. Thus, one or the other pathway is necessary for glutamate synthesis, but not both.
In E. coli the two-step GOGAT pathway is known to be important for synthesizing glutamate when the concentration of ammonium is low and for controlling the glutamine pool size (17). Accordingly, gltBD mutants lacking GOGAT grow normally in usual laboratory media but have a glutamate requirement when grown in low ammonium, even though they retain GDH.
Understanding the role of GDH has been a major problem for those studying nitrogen metabolism. Mutants lacking GDH have no obvious growth alteration under usual laboratory conditions. However, it has been shown recently that such gdhA mutants are impaired in growth relative to the wild type when limited for energy (and carbon) but ammonium and phosphate are present in excess (6). It was hypothesized that the primary role of GDH is to form glutamate during energy limitation because, in contrast to the GOGAT pathway, the GDH pathway does not use ATP; the requirement for ATP in biosynthesis would increase by nearly 20% if the GOGAT pathway were used instead of GDH (6). Thus, the parallel pathways for glutamate synthesis may play roles analogous to those of the sets of parallel pathways in the respiratory chain (NADH dehydrogenases and ubiquinol oxidases) in balancing the speed and efficiency of growth (6, 16).
At low ammonium concentration, glutamate is made primarily through GS and GOGAT, apparently because the affinity (Km) for ammonium of GDH is poor relative to that of GS (13, 15, 17–19). Under other conditions, the mechanisms for determining the pathway used in glutamate synthesis remain largely unknown.
As Reitzer has pointed out (17), GS is the first step in the GOGAT pathway, and so the relative contributions of the two pathways to glutamate synthesis may reflect the relative activities of the two ammonia-assimilating enzymes, GS and GDH. No specific regulation of GDH activity is known in enteric bacteria, although it has been reported to be subject to phosphorylation in vivo (12). Control of transcription of its structural gene, gdhA, is not well understood (17, 19).
GS is a highly controlled enzyme, subject to multiple forms of regulation (13, 17). A cascade of regulatory proteins and small molecule effectors, primarily glutamine and 2-oxoglutarate, control synthesis of GS. Each GS monomer in the 12-member holoenzyme is subject to rapid adenylylation and deadenylylation in response to the same effectors. The adenylylated form is much less active. Partial adenylylation of the holoenzyme sensitizes it to feedback inhibition by a variety of small molecules that may be construed as end products of GS activity.
The relative importance of each of the two glutamate pathways during glucose-limited growth varies (in opposite directions) as a function of phosphate and ammonium concentrations (6). However, the levels of two of the three relevant enzymes, GDH and GOGAT, remain low during glucose-limited growth in continuous culture and do not change markedly with change in ammonium or phosphate level under conditions in which pathway choice appears to be controlled by these compounds (6). In the work described in this report, I looked at the level and form (adenylylated or unadenylylated) of the third enzyme, glutamine synthetase, to see if either varied with conditions controlling choice of pathway for glutamate synthesis. The results show that regulation of the form or amount of GS is also insufficient to control the choice of pathway. Thus, concentration of substrates and/or modulation of enzyme activity is likely to be a major determinant of pathway choice in glutamate synthesis.
MATERIALS AND METHODS
Strains, media, and culture conditions.
The E. coli K-12 strains used were RH828 and RH830, isogenic except that RH828 is gdhA1 and RH830 is gdhA+ (6). The gdhA1 mutant is devoid of GDH activity as a result of replacement of an essential lysine (Lys-92) by glutamate at the active site (10). RH842 is isogenic with RH830 except that it is Arar and so can be distinguished from RH828 in samples from competition experiments (6). Media and culture conditions have been described previously (6, 8). The standard medium contained 7.6 mM (NH4)2SO4, 22 mM KH2PO4, 40.2 mM K2HPO4, and 0.8 mM MgSO4 (6). In addition, glucose was present at 0.0125% (0.694 mM) for continuous culture and 0.05% for unlimited growth, and thiamine-HCl was present at 50 μg/liter. A trace metals-iron-vitamin B12 mixture (14) was added when the effect of these additives on growth in continuous culture was tested. Continuous cultures were grown at a dilution rate of approximately 0.2 h−1 unless otherwise stated. In all experiments, growth was at 30°C.
Competition experiments.
Detailed procedures have been described previously (6, 7). In brief, the strains were grown separately in glucose-limited continuous culture and mixed, and during continuous growth over 30 to 40 generations, samples were removed and plated, and frequencies of the competing genotypes were determined by testing individual colonies. The growth rate of the gdhA1 strain (RH828) relative to that of the gdhA+ strain (RH842) is expressed as relative fitness, or 1 − s (selection coefficient). Each datum point (Fig. 2 and 5) represents the average of at least two independent experiments.
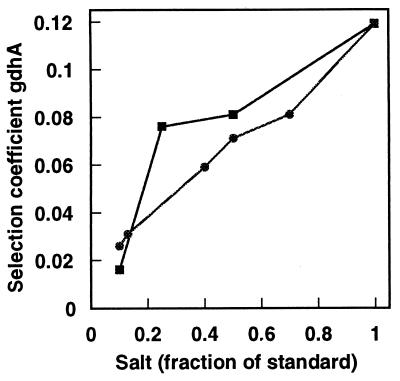
FIG. 2.
Growth disadvantage of a gdhA mutant during glucose-limited growth as a function of ammonium or phosphate concentration. Ammonium (■) and phosphate (•) concentrations are represented as fractions of those in 1× medium (see Materials and Methods). Data are taken from reference 4.
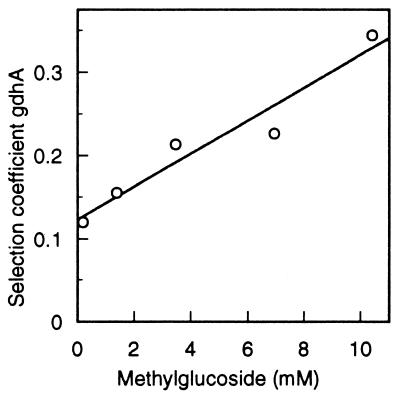
FIG. 5.
The growth disadvantage of glucose-limited cells lacking GDH as a function of the concentration of the nonmetabolizable glucose analog α-methylglucoside.
GS measurements.
Continuous cultures (190 ml running volume) were initiated with 100 ml of an overnight standing culture in the same medium and grown for 12 to 25 generations. Unlimited cultures were initiated in the continuous culture apparatus at an A550 of 0.02 and harvested at an A550 of 0.18 to 0.24 after exponential growth at the maximum specific growth rate (μmax = 0.44 h−1). It was shown previously that the relative fitness of a gdhA strain did not change over (at least) the pH range 6.8 to 7.5 (6). During steady-state glucose-limited culture in standard medium, the pH was 7.14; in 0.1× phosphate medium, the pH was 6.84 (6). Unlimited cultures in 0.1× phosphate medium had a pH at harvest (A550 of 0.18) of approximately 6.7.
Harvest and assay at the isoactivity point (which was determined to be 7.02) were essentially as described previously (3). In brief, hexadecyltrimethylammonium bromide (CTAB) was added to the growing culture to permeabilize cells and inactivate GS-adenylyltransferase (3), and after brief mixing 100 ml was collected by centrifugation for GS determination by the γ-glutamyl transferase assay. Both unadenylylated and total activities were determined, and the ratio was used as an approximation of the average proportion of active subunits (out of the total of 12 monomers per holoenzyme molecule). Specific activity is expressed as nanomoles of product formed at 37°C per min per milligram of protein. Each datum point corresponds to the average of at least two independent experiments except for those taken at dilution rates between 0.3 and 0.42 h−1, which represent single experiments (Fig. 1).
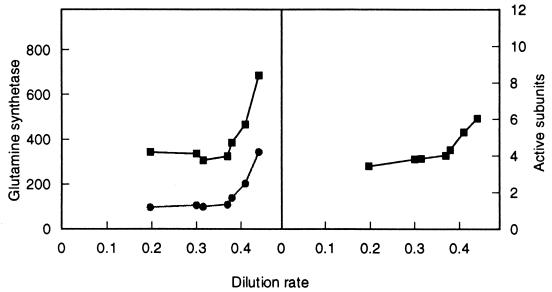
FIG. 1.
Comparison of GS levels during unlimited and glucose-limited growth. (Left) Total (upper) and unadenylylated (lower) enzyme activity. (Right) Average number of active monomers in the holoenzyme, as a function of dilution rate. The maximum specific growth rate is 0.44 h−1.
RESULTS AND DISCUSSION
Preliminary studies.
Previous work had shown that the GDH pathway became more important to the organism as the phosphate concentration increased (6). This was not the result of changes in ionic strength or pH. In order to see if the effect of phosphate might be due to trace contaminants rather than to phosphate itself, I carried out competition experiments under standard conditions, using medium supplemented with trace metals, iron, vitamin B12, and additional B1. These additives did not change the relative fitness of the gdhA strain (results not shown). I conclude that phosphate itself is likely to control pathway choice in glutamate synthesis. Although the rationale for control by phosphate is unclear, it may reflect linkage between energy and nitrogen metabolism (see reference 6).
The GDH pathway is less energy demanding than the GOGAT pathway because it does not require ATP, so it seemed possible that gdhA mutants forced to make glutamate via the more expensive pathway would not survive starvation as well as the wild type (2, 4, 21, 22). In order to see if gdhA mutants were impaired in recovery from starvation, I compared survival at 30°C of gdhA (RH828) and gdhA+ (RH842) strains mixed after growth in equal amounts or at a 100-fold excess of the wild type. There was no difference in survival over at least 150 days after growth in minimal glucose (0.1%) medium. Survival after growth in tryptone broth-glucose over 3 weeks gave inconsistent results. In several experiments the gdhA mutant showed lower survival, but in others it showed no significant difference from that of the gdhA+ strain. I conclude that if GDH plays a role in survival of starvation, it is a minor one under the conditions of the present study.
Glutamine synthetase during unlimited and glucose-limited growth.
Earlier it was found that GDH played no essential role in glutamate synthesis during unlimited growth but that it was important during glucose-restricted growth (6). Thus, glutamate might be made largely or entirely via the GOGAT pathway in unlimited growth, but it is made at least in part by GDH during restricted growth. The transition from unlimited to limited growth might be reflected in a decrease in the amount and/or increase in adenylylation state of GS if pathway choice were determined by the relative activities of GS and GDH.
I determined the levels of glutamine synthetase in RH830 (gdhA+) cells harvested during unrestricted growth and in cells harvested from glucose-limited growth at different dilution rates to see if the enzyme level might change accordingly. As shown in Fig. 1 (also compare Fig. 3 and 4), total enzyme was relatively high during unrestricted growth (μmax = 0.44) (8). (Note that the ammonium concentration is high, so the organism should be under ammonium repression.) As growth became restricted by a reduction in the dilution rate, total GS dropped sharply and then stabilized at about half the specific activity of cells in unrestricted growth. The average number of active subunits (unadenylylated) also dropped (Fig. 1).
FIG. 3.
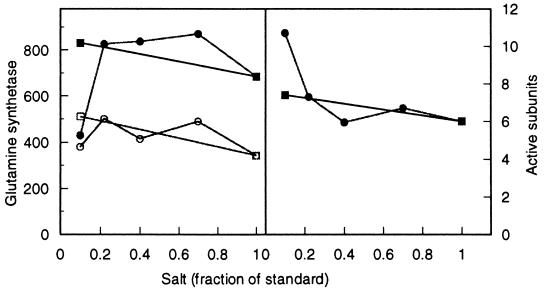
GS during glucose-limited growth as a function of ammonium or phosphate concentration. (Left panel) The uppermost curves (•, ■) show total enzyme activity from RH830 cells, and the lowermost curves (○, □) represent unadenylylated GS activity. (Right panel) Average number of active monomers in the 12-monomer holoenzyme. Ammonium (■, □) and phosphate (•, ○) concentrations are represented as fractions of those in 1× medium (see Materials and Methods).
FIG. 4.
GS during unrestricted growth on glucose as a function of ammonium or phosphate concentration. (Left panel) The uppermost curves (•, ■) show total enzyme activity from RH830 and the lowermost curves (○, □) represent unadenylylated GS activity. (Right panel) Average number of active monomers in the holoenzyme. Ammonium (■, □) and phosphate (•, ○) concentrations are represented as fractions of those in 1× medium.
Together with previous results (6), this shows that under the growth conditions employed, the levels of the three enzymes in the pathways for glutamate synthesis drop about twofold when cells pass from unrestricted growth to glucose-restricted growth. The drop in levels of active enzyme presumably results in reduced synthesis of glutamine, glutamate, and organic nitrogen. The number of active subunits (unadenylylated) of glutamine synthetase simultaneously drops, showing that an increase in the ratio of glutamine to 2OG follows the growth transition (13, 17, 20). (The roles of glutamine and 2OG are separate and complex [11, 13, 17].) At the same time, 2OG becomes channeled through both the GDH and GOGAT pathways to form glutamate instead of substantially or solely through the GOGAT pathway as it appears to be during steady-state unrestricted growth. Although differences in the medium and other growth conditions preclude a detailed comparison, Senior has shown that the change in glutamine-2OG results from a modest drop in the glutamine pool and a large drop in the 2OG pool as cells progress from unrestricted to glucose-limited growth with ammonium excess (20; see also reference 9).
The drop in the level of all three enzymes during the transition to restricted growth presumably reflects a change in the transcription frequencies of the structural genes (glnA, gdhA, and gltBD) in response to increasing nitrogen repression as the cell becomes progressively stressed for a carbon and energy source (13, 17).
GS level and adenylylation state as a function of phosphate and ammonium concentration.
Previous work had also shown that during glucose-limited growth, the selection coefficient (disadvantage) of a gdhA mutant increased as the ammonium or phosphate concentration increased (6). These correlations are shown in Fig. 2.
As noted above, the activity of GS could in principle control pathway choice in glutamate synthesis. By this rationale the amount of unadenylylated enzyme might be high relative to GDH at low concentrations of ammonium or phosphate when the role of GDH is minimized (Fig. 2) and lower as those concentrations increase. Direct measurement showed little change in total GS with ammonium concentration, and the proportion of active subunits (unadenylylated) did not change substantially (Fig. 3). Similarly, total enzyme and number of active subunits were little affected by changing the phosphate concentration (Fig. 3). GS activity in a gdhA mutant showed a similar pattern, but the total enzyme and proportion of active subunits were slightly higher (data not shown).
For comparison, GS measurements were also made on cells from unrestricted growth. It was already known that GDH played no essential role under these conditions (6). Total enzyme and number of active subunits were found to change little as the ammonium concentration changed (Fig. 4). They also changed little with phosphate at higher phosphate concentrations, but there was a drop in total enzyme (but not unadenylylated enzyme) as phosphate went from 0.2 to 0.1 that of the standard medium (Fig. 4). As a result, the proportion of active subunits increased to nearly 100% (Fig. 4). This change in the amount of adenylylated enzyme during unrestricted growth on glucose in low-phosphate medium was not studied further.
Confirming the importance of GDH during energy limitation.
The hypothesis that GDH is important during energy-restricted growth was tested by determining the relative fitness of a gdhA mutant under conditions of increasing energy stress. The mutant organism (RH828) was grown in competition with the wild type (RH842) on limiting glucose as described previously (6) except with the addition of the nonmetabolizable glucose analog α-methylglucoside (αMG) (1). αMG competes for uptake with glucose and is taken into the cell by the glucose phosphotransferase system, with an ATP equivalent expended for every αMG, but no ATP is generated from the analog. Uptake of the glucose analog should increase the energy stress and therefore decrease the fitness of a gdhA mutant relative to that of the wild type, if the operating hypothesis were correct.
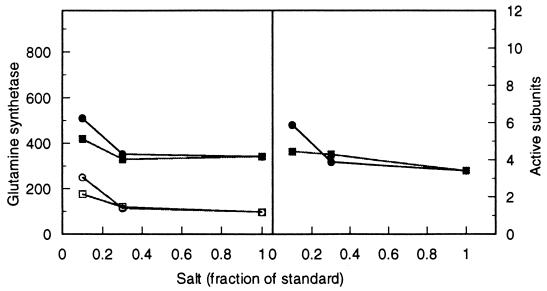
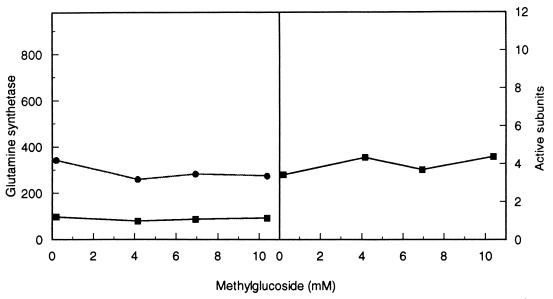
The results conform to expectation (Fig. 5). The data show clearly that as the concentration of αMG and, hence, energy stress increased, the selection coefficient (1 − relative fitness) of the mutant increased. Over the range of approximately 0 to 10 mM αMG (molar ratio αMG:glucose, 0 to 15), the growth rate of the mutant dropped from 88% of that of the competing wild type to about 66% (Fig. 5). In order to see if the amount of GS changed correspondingly, I measured the enzyme level under the same growth conditions. The results show that neither total GS nor the proportion of active subunits changed significantly over the range of αMG concentration in which the selection coefficient of the gdhA mutant changed markedly (Fig. 6).
FIG. 6.
GS during glucose-restricted growth in the presence of the nonmetabolizable glucose analog αMG. (Left panel) Total (upper) and unadenylylated (lower) enzyme activity from strain RH830. (Right panel) average number of active monomers in the holoenzyme.
The molecular bases for pathway determination must result from regulation of enzyme level, enzyme activity, or availability of substrates. As reported above, the levels of the three enzymes drop as the organism passes from unlimited to limited growth. In contrast, no substantial change in the amount of any of the three enzymes or in the proportion of active subunits of GS accompanied a change in phosphate or ammonium level, and the presence of αMG did not affect the amount or form of GS during glucose-limited growth, even though these variables affect the relative importance of the pathways for glutamate synthesis. The changing flux through the separate pathways for glutamate synthesis during the latter transitions must reflect either modulation of enzyme activity (other than by covalent modification) or a change in substrate availability or both. These kinds of control are expected to be exerted rapidly relative to change in amount of enzymes and to be readily reversible. This suggests that the ability to control flux through the separate glutamate pathways is most important for dealing with rapid fluctuation in variables such as levels of specific metabolites rather than for long-term adaptations.
ACKNOWLEDGMENTS
I thank J. R. Guest, M. M. Attwood, and their colleagues at The University of Sheffield for their generous hospitality while part of this work was done and the NIH for support as a John E. Fogarty Senior International Fellow. I thank R. Bender and T. Goss for their criticisms.
REFERENCES
- 1.Anderson K B, von Meyenburg K. Are growth rates of Escherichia coli limited by respiration? J Bacteriol. 1980;144:114–123. doi: 10.1128/jb.144.1.114-123.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Archer C D, Wang X, Elliott T. Mutants defective in the energy-conserving NADH dehydrogenase of Salmonella typhimurium identified by a decrease in energy-dependent proteolysis after carbon starvation. Proc Natl Acad Sci USA. 1993;90:9877–9881. doi: 10.1073/pnas.90.21.9877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bender R A, Janssen K A, Resnick A D, Blumenberg M, Foor F, Magasanik B. Biochemical parameters of glutamine synthetase from Klebsiella aerogenes. J Bacteriol. 1977;129:1001–1009. doi: 10.1128/jb.129.2.1001-1009.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Goldman B S, Gabbert K K, Kranz R G. The temperature-sensitive growth and survival phenotypes of Escherichia coli cydDC and cydAB are due to deficiencies in cytochrome bd and are corrected by exogenous catalase and reducing agents. J Bacteriol. 1996;178:6348–6351. doi: 10.1128/jb.178.21.6348-6351.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Guest J R. The Leeuwenhoek lecture, 1995. Adaptation to life without oxygen. Philos Trans R Soc Lond B. 1995;350:189–202. doi: 10.1098/rstb.1995.0152. [DOI] [PubMed] [Google Scholar]
- 6.Helling R B. Why does Escherichia coli have two primary pathways for synthesis of glutamate? J Bacteriol. 1994;176:4664–4668. doi: 10.1128/jb.176.15.4664-4668.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helling R B, Kinney T, Adams J. The maintenance of plasmid-containing organisms in populations of Escherichia coli. J Gen Microbiol. 1981;123:129–141. doi: 10.1099/00221287-123-1-129. [DOI] [PubMed] [Google Scholar]
- 8.Helling R B, Vargas C N, Adams J. Evolution of Escherichia coli during growth in a constant environment. Genetics. 1987;116:349–358. doi: 10.1093/genetics/116.3.349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ikeda T P, Shauger A E, Kustu S. Salmonella typhimurium apparently perceives external nitrogen limitation as internal glutamine limitation. J Mol Biol. 1996;259:589–607. doi: 10.1006/jmbi.1996.0342. [DOI] [PubMed] [Google Scholar]
- 10.Jones K M, McPherson M J, Baron A J, Mattaj I W, Riodan C L, Wooton J C. The gdhA1 point mutation in Escherichia coli CLR207 alters a key lysine residue of glutamate dehydrogenase. Mol Gen Genet. 1993;240:286–289. doi: 10.1007/BF00277068. [DOI] [PubMed] [Google Scholar]
- 11.Kamberov E S, Atkinson M R, Ninfa A J. The Escherichia coli PII signal transduction protein is activated upon binding 2-ketoglutarate and ATP. J Biol Chem. 1995;270:17797–17807. doi: 10.1074/jbc.270.30.17797. [DOI] [PubMed] [Google Scholar]
- 12.Lin H-P P, Reeves H C. In vivo phosphorylation of NADP+ glutamate dehydrogenase in Escherichia coli. Curr Microbiol. 1994;28:63–65. [Google Scholar]
- 13.Magasanik B. Regulation of nitrogen utilization. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: ASM Press; 1996. pp. 1344–1356. [Google Scholar]
- 14.McGarvey P, Helling R B, Lee J-Y, Engelke D R, El-Gewely M R. Initiation of rrn transcription in chloroplasts of Euglena gracilis bacillaris. Curr Genet. 1988;14:493–500. doi: 10.1007/BF00521275. [DOI] [PubMed] [Google Scholar]
- 15.Miller R E, Stadtman E R. Glutamate synthase from Escherichia coli. An iron-sulfide flavoprotein. J Biol Chem. 1972;247:7407–7419. [PubMed] [Google Scholar]
- 16.Neijssel O M, Teixeira de Mattos M J. The energetics of bacterial growth: a reassessment. Mol Microbiol. 1994;13:179–182. doi: 10.1111/j.1365-2958.1994.tb00413.x. [DOI] [PubMed] [Google Scholar]
- 17.Reitzer L J. Ammonia assimilation and the biosynthesis of glutamine, glutamate, aspartate, asparagine, l-alanine, and d-alanine. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C: ASM Press; 1996. pp. 391–407. [Google Scholar]
- 18.Sakamoto N, Kotre A M, Savageau M A. Glutamate dehydrogenase from Escherichia coli: purification and properties. J Bacteriol. 1975;124:775–783. doi: 10.1128/jb.124.2.775-783.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Saroja G N, Gowrishankar J. Roles of SpoT and FNR in NH4+ assimilation and osmoregulation in GOGAT (glutamate synthase)-deficient mutants of Escherichia coli. J Bacteriol. 1996;178:4105–4114. doi: 10.1128/jb.178.14.4105-4114.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Senior P J. Regulation of nitrogen metabolism in Escherichia coli and Klebsiella aerogenes: studies with the continuous-culture technique. J Bacteriol. 1975;123:407–418. doi: 10.1128/jb.123.2.407-418.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Siegele D A, Imlay K R C, Imlay J A. The stationary-phase-exit defect of cydC (surB) mutants is due to lack of a functional cytochrome oxidase. J Bacteriol. 1996;178:6091–6096. doi: 10.1128/jb.178.21.6091-6096.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zambrano M M, Kolter R. Escherichia coli mutants lacking NADH dehydrogenase I have a competitive disadvantage in stationary phase. J Bacteriol. 1993;175:5642–5647. doi: 10.1128/jb.175.17.5642-5647.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]