Abstract
2-Aminonumconic 6-semialdehyde is an unstable intermediate in the biodegradation of nitrobenzene and 2-aminophenol by Pseudomonas pseudoalcaligenes JS45. Previous work has shown that enzymes in cell extracts convert 2-aminophenol to 2-aminomuconate in the presence of NAD+. In the present work, 2-aminomuconic semialdehyde dehydrogenase was purified and characterized. The purified enzyme migrates as a single band on sodium dodecyl sulfate-polyacrylamide gel electrophoresis with a molecular mass of 57 kDa. The molecular mass of the native enzyme was estimated to be 160 kDa by gel filtration chromatography. The optimal pH for the enzyme activity was 7.3. The enzyme is able to oxidize several aldehyde analogs, including 2-hydroxymuconic semialdehyde, hexaldehyde, and benzaldehyde. The gene encoding 2-aminomuconic semialdehyde dehydrogenase was identified by matching the deduced N-terminal amino acid sequence of the gene with the first 21 amino acids of the purified protein. Multiple sequence alignment of various semialdehyde dehydrogenase protein sequences indicates that 2-aminomuconic 6-semialdehyde dehydrogenase has a high degree of identity with 2-hydroxymuconic 6-semialdehyde dehydrogenases.
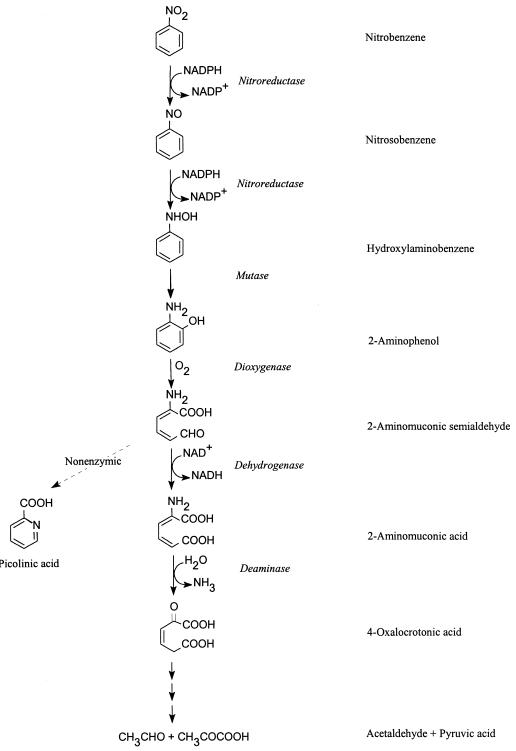
Pseudomonas pseudoalcaligenes JS45 (hereafter referred to as JS45) grows on nitrobenzene as the sole source of carbon, nitrogen, and energy (Fig. 1) (3, 10). 2-Aminophenol 1,6-dioxygenase catalyzes the cleavage of the aromatic ring of 2-aminophenol, yielding 2-aminomuconate 6-semialdehyde (2-AMS), which is unstable and spontaneously converts to picolinic acid (9, 10). Pseudomonas sp. strain AP-3 (16) degrades 2-aminophenol in an identical step to the ring fission of 2-aminophenol by JS45. The enzymes involved in metabolism after ring cleavage have been difficult to characterize biochemically, in part due to the instability of 2-AMS. Recently, we have demonstrated that in JS45, 2-aminophenol is degraded to pyruvate and acetaldehyde via a pathway similar to that of the meta cleavage of catechol (Fig. 1) (2, 3). In the pathway, 2-AMS is converted enzymatically to 2-aminomuconate in the presence of NAD+ by 2-AMS dehydrogenase (3).
FIG. 1.
Metabolic pathway for the degradation of nitrobenzene by P. pseudoalcaligenes JS45 (2, 3, 10).
2-AMS dehydrogenase was first investigated in the metabolism of tryptophan in mammalian tissues with 2-hydroxymuconic 6-semialdehyde (2-HMS) as a substrate (6, 11). Several genes of bacterial 2-HMS dehydrogenases have been sequenced (5, 8, 12, 18). However, only one 2-HMS dehydrogenase from P. putida has been purified and characterized, initially as a benzaldehyde dehydrogenase (7, 15). To investigate the properties of the bacterial 2-AMS dehydrogenase and to determine its relationship to 2-HMS as well as other semialdehyde dehydrogenases, purification, characterization, and sequence analysis of 2-AMS dehydrogenase from JS45 were performed in the present study.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
JS45 was grown with nitrobenzene as described previously (10). Escherichia coli DH5α was grown in Luria broth (Difco) with shaking or on Luria agar plates at 37°C. When necessary, ampicillin was added to a final concentration of 100 μg/ml.
Protein purification.
All purification procedures were carried out at 4°C. Crude extracts were prepared as described previously (2). The crude extract (25 ml) was loaded onto a DEAE-Sepharose column (Pharmacia; 2.6 by 10 cm). The column was washed with 200 ml of 50 mM potassium phosphate buffer (pH 7.0), and proteins were eluted with a linear NaCl gradient (0 to 0.4 M in buffer, 400 ml at 2 ml/min). Fractions containing 2-AMS dehydrogenase activity were pooled, and proteins were fractionated by ammonium sulfate precipitation. The material that precipitated between 1.2 and 2.5 M ammonium sulfate was dissolved in 3.2 ml of buffer, loaded onto a Sephacryl S-300 gel filtration column (Pharmacia; 1.6 by 100 cm), and eluted with 50 mM potassium phosphate–0.1 M NaCl (pH 7.0, 1 ml/min). Active fractions were pooled and diluted with an equal volume of potassium phosphate buffer (pH 7.0) and loaded onto a Mono Q HR10/10 column (Pharmacia; 8 ml). The column was washed with 30 ml of 0.05 M NaCl in buffer. The active fractions were eluted around 0.21 M NaCl in a NaCl gradient (0.05 to 0.30 M NaCl in buffer, 90 ml at 1 ml/min). The enzyme was stored at −70°C and used for characterization studies.
Enzyme assays.
2-AMS dehydrogenase activity was routinely assayed by measurement of the decrease in absorbance of the substrate analog, 2-HMS, at 375 nm in the presence of 0.1 mM NAD+ in 100 mM potassium phosphate buffer (pH 7.5; ε = 44,000 M−1) (11). The initial rate of dehydrogenation (in less than 1 min) was recorded. The physiological substrate, 2-AMS, was prepared in the reaction mixture by the addition of 2-aminophenol and excess partially purified 2-aminophenol 1,6-dioxygenase (3, 9). The activity on 2-AMS was determined by the increase in absorbance at 326 nm, concomitant with appearance of the product, 2-aminomuconate (2, 11). Activity on the other substrate analogs was measured by the increase in absorbance at 340 nm due to production of NADH. Protein concentrations were determined by the Coomassie Plus protein assay reagent from Pierce (Rockford, Ill.) by using bovine serum albumin as a standard.
Estimation of molecular mass.
The subunit molecular mass of 2-AMS dehydrogenase was determined by comparison with protein molecular mass standards (low-range SigmaMarkers) on a sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis gel. The native molecular mass was determined by gel filtration on a Superdex 200 HR 10/10 column (Pharmacia) with 50 mM potassium phosphate–0.1 M NaCl (pH 7.0, 1 ml/min). The molecular size standards for gel filtration were ferritin (440 kDa), β-amylase (200 kDa), alcohol dehydrogenase (150 kDa), and bovine serum albumin (66 kDa).
DNA and amino acid sequencing.
The N-terminal amino acid sequence was determined at the Protein Core Facility of the University of Florida, Gainesville. The gene (designated amnC) encoding the enzyme was identified by matching the amino acid sequence of the purified enzyme with that of a deduced open reading frame located 258 bp downstream of the amnBA genes of 2-aminophenol 1,6-dioxygenase in E. coli clones (1a). The N-terminal part of the DNA sequence was determined at the Genetic Engineering Facility at the University of Illinois. The remainder of the sequence was determined with an ALF sequencer (Pharmacia) according to the manufacturer’s instructions. Alignment of the sequence of the amnC product with those of other dehydrogenases was performed by using CLUSTALX (17) in the multiple-alignment mode with all parameters set to default. The GenBank accession number of the sequence is AF036343.
Chemicals.
2-HMS was enzymatically prepared from catechol by a method described previously (11) by using the resting cells of E. coli JM109/pDTG603 (20). All other chemicals were from Sigma (St. Louis, Mo.) or Aldrich (Milwaukee, Wis.), unless stated otherwise.
RESULTS AND DISCUSSION
Purification and molecular mass of 2-aminomuconic 6-semialdehyde dehydrogenase.
A typical purification procedure (Table 1) yielded a 12.5-fold purification with a recovery of 37.5% of the 2-AMS dehydrogenase activity. Analysis of the purified protein by sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis revealed a single band which corresponds to a molecular mass of 57 kDa. The amnC gene of 2-AMS dehydrogenase consists of 1,629 nucleotides, which would encode a protein of 542 amino acids with a molecular mass of 57.8 kDa. This subunit of 2-AMS is larger than that of 2-HMS dehydrogenase (51.5 to 51.7 kDa) (5, 8, 12, 18). Gel filtration chromatography of 2-AMS dehydrogenase revealed a native molecular mass of 160 kDa, suggesting that the enzyme is composed of three identical subunits. The active form of 2-HMS dehydrogenase from P. putida is a homodimer (15). Although there is no information on the native molecular mass and the subunit structure of 2-AMS dehydrogenase from cat liver, the quaternary structures of other eukaryotic aldehyde dehydrogenases are variable and the majority are homodimers or homotetramers (4). Because a homotrimeric structure is uncommon, more research is required to rigorously determine the native structure of the 2-AMS dehydrogenase.
TABLE 1.
Purification of 2-AMS dehydrogenase
| Step | Total protein (mg) | Total activity (μmol · min−1) | Sp act (μmol · min−1 · mg−1) | Purifi- cation (fold) | Yield (%) |
|---|---|---|---|---|---|
| Cell extract | 295 | 38 | 0.13 | 1 | 100 |
| DEAE-Sepharose | 53 | 36 | 0.49 | 3.8 | 68.7 |
| Sephacryl S-300 | 26 | 21 | 0.80 | 6.2 | 54.1 |
| Mono Q | 8.8 | 14 | 1.62 | 12.5 | 37.5 |
Catalytic properties of the enzyme.
The optimal pH for 2-AMS dehydrogenase activity was 7.3. Calculated from Lineweaver-Burk plots, at pH 7.5 and 25°C, the Km for 2-HMS was 26 ± 2 μM (mean ± standard deviation) in the presence of 500 μM NAD+. The Km for NAD+ was 74 ± 1 μM in the presence of 45 μM 2-HMS. The Km values for cat liver 2-AMS dehydrogenase were 16 (2-HMS) and 19 (NAD+) μM (6). The 2-HMS dehydrogenase from P. putida had Km values of 17 μM for 2-HMS and 330 μM for NAD+ (7). The high affinity of 2-AMS dehydrogenase for NAD+ might enhance the binding of NAD+ to the enzyme to facilitate the conversion of 2-AMS to 2-aminomuconate and to avoid unnecessary spontaneous conversion of 2-AMS to picolinate.
Substrate specificity.
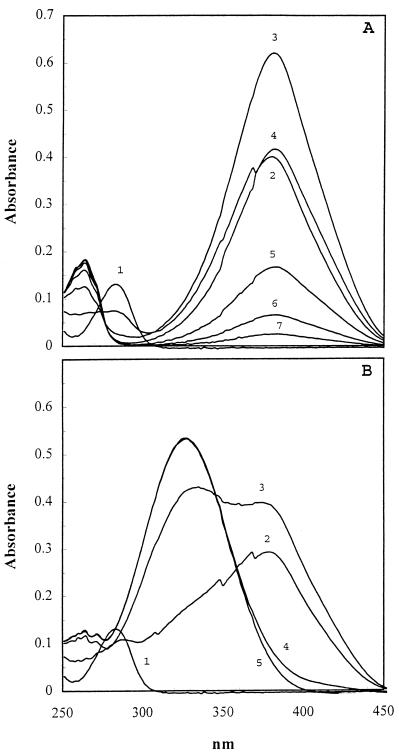
2-AMS dehydrogenase was purified by using 2-HMS as a substrate analog. As shown in Fig. 2A, 2-aminophenol 1,6-dioxygenase cleaved 2-aminophenol (A282) to 2-AMS (A380), which spontaneously cyclized to picolinate (A263). An absorbance maximum appeared at 326 nm rather than at 263 nm when 2-AMS dehydrogenase was included in the incubation mixture (Fig. 2B), indicating that 2-AMS was converted to 2-aminomuconate by 2-AMS dehydrogenase. The rapid spontaneous conversion of 2-AMS to picolinate made it impossible to measure the dehydrogenation reaction rate at a saturating (or stable) substrate concentration. By measuring the formation of 2-aminomuconate, the relative activity of 2-AMS dehydrogenase on 2-AMS was about 300% of the activity towards 2-HMS under the same conditions, indicating that 2-AMS is the physiological substrate for the enzyme.
FIG. 2.
Enzymatic formation of 2-aminomuconate from 2-aminophenol. (A) 2-Aminophenol (A282) was transformed to 2-AMS (A380) by 2-aminophenol 1,6-dioxygenase. 2-AMS was spontaneously converted to picolinic acid (A263). The reaction mixture contained 100 mM potassium phosphate (pH 7.5), 0.04 mM 2-aminophenol, 0.05 mg of partially purified dioxygenase per ml, 0.033 mM NAD+, and 0.133 mM pyruvate along with 4 U of lactate dehydrogenase/ml as an NAD+ regenerating system to eliminate the spectral interference of NADH with the absorbance of 2-aminomuconate during dehydrogenation. Recordings were taken at 0 time (curve 1), 10 s (curve 2), and later at 0.5-min intervals. (B) 2-Aminophenol was transformed to 2-aminomuconate (A326) in the presence of 2-aminophenol 1,6-dioxygenase and 2-AMS dehydrogenase. All the assay conditions were unchanged except for the presence of 2-AMS dehydrogenase (0.011 mg/ml).
2-AMS dehydrogenase was tested for its ability to oxidize several other aldehydes (Table 2). All the tested analogs served as substrates for the dehydrogenase, but the activity was much lower than that with 2-AMS and 2-HMS. 2-AMS dehydrogenase from JS45 showed a broader substrate range than either 2-AMS dehydrogenase from cat liver or 2-HMS dehydrogenase from Pseudomonas putida. Further investigations of the substrate specificity will be helpful in understanding the catalytic mechanism and in elucidating the structure of the active site of these dehydrogenases.
TABLE 2.
Comparison of substrate specificity of three similar semialdehyde dehydrogenases
| Substrate | Relative activity (%)
|
||
|---|---|---|---|
| 2-AMS dehydrogenase from JS45a | 2-AMS dehydrogenase from cat liverb | 2-HMS dehydrogenase from P. putidac | |
| 2-AMS | 300 | +d | NDe |
| 2-HMS | 100 | 100 | + |
| Formaldehyde | 0.009 | 50 | −f |
| Acetaldehyde | 0.02 | 7 | − |
| Propionaldehyde | 0.14 | − | − |
| Butyraldehyde | 0.81 | 32 | − |
| Crotonaldehyde | 0.086 | − | ND |
| Hexaldehyde | 7.0 | ND | − |
| Benzaldehyde | 5.6 | ND | + |
See text for details of the comparison of 2-AMS with 2-HMS. Other aldehyde substrate concentrations were 0.1 mM. The enzyme concentration was 0.034 mg/ml. One hundred percent activity corresponded to 8.7 μmol · min−1 · mg−1 of protein (Vmax of 2-HMS).
Data from reference 6.
+, able to serve as a substrate, but no quantitative data available.
ND, not determined.
−, not able to serve as a substrate.
Sequence analysis of 2-AMS dehydrogenase.
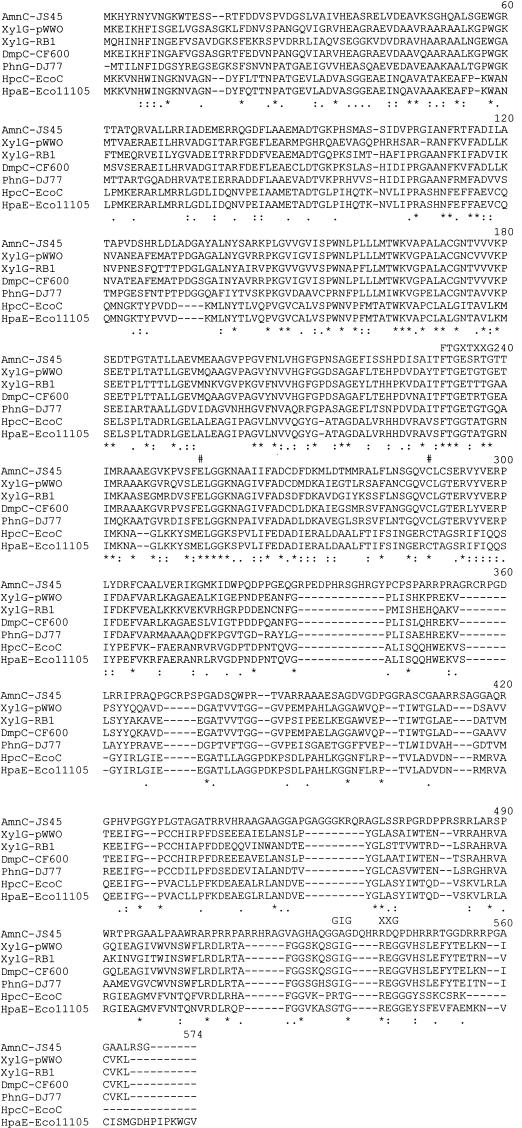
AmnC has high amino acid sequence identity to 2-HMS dehydrogenases (43 to 55% to the first 329 amino acid residues) and somewhat less identity (33%) to the same region of 5-carboxymethyl-2-hydroxymuconic semialdehyde dehydrogenases (Fig. 3). The high sequence identity of the JS45 enzyme with 2-HMS dehydrogenases explains the ability of 2-AMS dehydrogenase to act on 2-HMS. It is clear from the alignment that 2-AMS dehydrogenase from JS45 is homologous with the 2-HMS dehydrogenases. Conserved residues are present at positions 255 (Glu) and 289 (Cys), and a putative NAD+ binding site at residues 231 to 238 is conserved (1, 5, 19). A second putative NAD+ binding site (GIGXXG) does not exist in 2-AMS dehydrogenase even though it is conserved in other 2-HMS dehydrogenases. This finding supports the argument of Nordlund and Shingler (12) that GXGXXG is not likely to be an NAD+ binding site.
FIG. 3.
CLUSTALX alignment of AmnC from JS45 (AmnC-JS45) with 2-HMS dehydrogenase XylG from Pseudomonas putida (XylG-pWW0) (5), XylG from Cycloclasticus oligotrophus (XylG-RB1) (18), DmpC from Pseudomonas strain CF600 (DmpC-CF600) (12), and PhnG from Pseudomonas sp. strain DJ77 (PhnG-DJ77) (8) and of 5-carboxymethyl-2-hydroxymuconic acid semialdehyde dehydrogenases HpcC from E. coli C (HpcC-EcoC) (14) and HpaE from E. coli W (HpaE-Eco11105) (13). Numeral symbols above positions 255 and 289 indicate conserved residues shown to be essential for catalysis (1, 4). FTGXTXXG (FTGXSXXG in AmnC) and GIGXXG above the aligned sequences indicate the locations of proposed NAD binding sites (4, 19). Asterisks below the aligned sequences indicate amino acids present in all seven sequences. Positions with colons below contain a residue of the strongly conserved groups in all seven sequences, and periods indicate more weakly conserved groups in all seven sequences.
In JS45, 2-aminophenol was degraded to 4-oxalocrotonate in a pathway similar to the meta cleavage pathway of catechol (2, 3). Both biochemical and genetic analyses indicate that the 2-AMS dehydrogenase is homologous to the 2-HMS dehydrogenase in the meta cleavage of catechol. Since 2-HMS was a substrate for 2-AMS dehydrogenase, it would be of interest to determine whether 2-HMS dehydrogenase is able to act on 2-AMS. Exploration of the genetic origin and evolution of the novel pathway of degradation of nitrobenzene, via 2-aminophenol, in JS45 is currently under way.
ACKNOWLEDGMENTS
The work was supported in part by the U.S. Air Force Office of Scientific Research and the Strategic Environmental Defense Research Program. Z.H. acknowledges an NRC Postdoctoral Research Associateship awarded by the National Research Council.
We thank B. E. Haigler for providing E. coli JM109/pDTG603.
REFERENCES
- 1.Abriola D P, Fields R, Stein S, MacKerell A D, Jr, Pietruazko R. Active site of human liver aldehyde dehydrogenase. Biochemistry. 1987;26:5679–5684. doi: 10.1021/bi00392a015. [DOI] [PubMed] [Google Scholar]
- 1a.Davis, J., and J. Spain. Unpublished data.
- 2.He Z, Spain J C. A novel 2-aminomuconate deaminase in the nitrobenzene degradation pathway of Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1998;180:2502–2506. doi: 10.1128/jb.180.9.2502-2506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.He Z, Spain J C. Studies of the catabolic pathway of degradation of nitrobenzene by Pseudomonas pseudoalcaligenes JS45: removal of the amino group from 2-aminomuconic semialdehyde. Appl Environ Microbiol. 1997;63:4839–4843. doi: 10.1128/aem.63.12.4839-4843.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hempel J, Lindahl R. Class III aldehyde dehydrogenase from rat liver: superfamily relationship to class I and II and functional interpretations. In: Weiner H, Flynn T G, editors. Enzymology and molecular biology of carbonyl metabolism. 2. Alan R. New York, N.Y: Liss, Inc.; 1989. [Google Scholar]
- 5.Horn J M, Harayama S, Timmis K N. DNA sequence determination of the TOL plasmid (pWW0) xylGFJ genes of Pseudomonas putida: implications for the evolution of aromatic catabolism. Mol Microbiol. 1991;5:2459–2474. doi: 10.1111/j.1365-2958.1991.tb02091.x. [DOI] [PubMed] [Google Scholar]
- 6.Ichiyama A, Nakamura S, Kawai H, Honjo T, Nishizuka Y, Hayashi O, Senoh S. Studies on the metabolism of the benzene ring of tryptophan in mammalian tissues. II. Enzymic formation of α-aminomuconic acid from 3-hydroxyanthranilic acid. J Biol Chem. 1965;240:740–749. [PubMed] [Google Scholar]
- 7.Inoue J, Shaw J P, Rekik M, Harayama S. Overlapping substrate specificities of benzaldehyde dehydrogenase (the xylC gene product) and 2-hydroxymuconic semialdehyde dehydrogenase (the xylG gene product) encoded by TOL plasmid pWW0 of Pseudomonas putida. J Bacteriol. 1995;177:1196–1201. doi: 10.1128/jb.177.5.1196-1201.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim S, Shin H-J, Kim S J K Y, Kim Y-C. Nucleotide sequence of the Pseudomonas sp. DJ77 phnG gene encoding 2-hydroxymuconic semialdehyde dehydrogenase. Biochem Biophys Res Commun. 1997;240:41–45. doi: 10.1006/bbrc.1997.7595. [DOI] [PubMed] [Google Scholar]
- 9.Lendenmann U, Spain J C. 2-Aminophenol 1,6-dioxygenase: a novel aromatic ring-cleavage enzyme purified from Pseudomonas pseudoalcaligenes JS45. J Bacteriol. 1996;178:6227–6232. doi: 10.1128/jb.178.21.6227-6232.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nishino S F, Spain J C. Degradation of nitrobenzene by a Pseudomonas pseudoalcaligenes. Appl Environ Microbiol. 1993;59:2520–2525. doi: 10.1128/aem.59.8.2520-2525.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nishizuka Y, Ichiyama A, Hayaishi O. Metabolism of the benzene ring of tryptophan (mammals) Methods Enzymol. 1970;17A:463–491. [Google Scholar]
- 12.Nordlund I, Shingler V. Nucleotide sequence of the meta-cleavage pathway enzymes 2-hydroxymuconic semialdehyde dehydrogenase and 2-hydroxymuconic semialdehyde hydrolase from Pseudomonas CF600. Biochim Biophys Acta. 1990;1049:227–230. doi: 10.1016/0167-4781(90)90046-5. [DOI] [PubMed] [Google Scholar]
- 13.Prieto M A, Díaz E, García J L. Molecular characterization of the 4-hydroxyphenylacetate catabolic pathway of Escherichia coli W: engineering a mobile aromatic degradative cluster. J Bacteriol. 1996;178:111–120. doi: 10.1128/jb.178.1.111-120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roper D I, Stringfellow J M, Cooper R A. Sequence of the hpcC and hpcG genes of the meta-fission homoprotocatechuic acid pathway of Escherichia coli C: nearly 40% amino-acid identity with the analogous enzymes of the catechol pathway. Gene. 1995;156:47–51. doi: 10.1016/0378-1119(95)00082-h. [DOI] [PubMed] [Google Scholar]
- 15.Shaw J P, Harayama S. Purification and characterization of TOL plasmid-encoded benzyl alcohol dehydrogenase and benzaldehyde dehydrogenase of Pseudomonas putida. Eur J Biochem. 1990;191:705–714. doi: 10.1111/j.1432-1033.1990.tb19179.x. [DOI] [PubMed] [Google Scholar]
- 16.Takenaka S, Murakami S, Shinke R, Hatakeyama K, Yukawa H, Aoki K. Novel genes encoding 2-aminophenol 1,6-dioxygenase from Pseudomonas species AP-3 growing on 2-aminophenol and catalytic properties of the purified enzyme. J Biol Chem. 1997;272:14727–14732. doi: 10.1074/jbc.272.23.14727. [DOI] [PubMed] [Google Scholar]
- 17.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang Y, Lau P C K, Button D K. A marine oligobacterium harboring genes known to be part of aromatic hydrocarbon degradation pathways of soil pseudomonads. Appl Environ Microbiol. 1996;62:2169–2173. doi: 10.1128/aem.62.6.2169-2173.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wierenga R K, Terpstra P, Hol W G. Prediction of the occurrence of the ADP-binding beta-fold in proteins, using an amino acid sequence fingerprint. J Mol Biol. 1986;187:101–107. doi: 10.1016/0022-2836(86)90409-2. [DOI] [PubMed] [Google Scholar]
- 20.Zylstra G J, Gibson D T. Toluene degradation by Pseudomonas putida F1. J Biol Chem. 1989;264:14940–14946. [PubMed] [Google Scholar]