Abstract
Background: Powassan virus is an emerging neurotropic arbovirus transmitted by the tick Ixodes scapularis. This systematic review was conducted to aggregate data on its clinical manifestations, diagnostic findings, and complications. Methods: PubMed was searched until August 2023 using the term “Powassan”, to identify all published cases of Powassan virus infections, as per PRISMA guidelines. Results: Among the 380 abstracts identified, 45 studies describing 84 cases (70 adult, 14 pediatric) were included. Cases were reported from the USA and Canada. Complications included paralysis in 44.1% of adult and 42.6% of pediatric cases, cognitive deficits in 33.3% of adult and 25% of pediatric cases, while the mortality rate was 19.1% and 7.1% in the adult and pediatric populations, respectively. Correlation analysis revealed an association between mortality and age (r = 0.264, p = 0.029), development of paralysis (r = 0.252, p = 0.041), or respiratory distress or failure (r = 0.328, p = 0.006). Factors associated with persistent neurological deficits were development of ataxia (r = 0.383, p = 0.006), paralysis (r = 0.278, p = 0.048), speech disorder (r = 0.319, p = 0.022), and cranial nerve involvement (r = 0.322, p = 0.017). Other significant correlations included those between speech disorders and ataxia (r = 0.526, p < 0.001), and between paralysis and respiratory distress or failure (r = 0.349, p = 0.003). Conclusion: Powassan virus infections have significant morbidity and mortality and should be suspected in cases of encephalitis and possible tick exposure. PROSPERO registration number: CRD42023395991.
Keywords: tick-borne infection, climate change, expanding habitat, emerging infectious disease
1. Introduction
Powassan virus is an arbovirus and a member of the Flaviviridae family. It is a single-stranded, positive-sense RNA virus, and has significant genetic similarities with another arbovirus, the deer tick virus, with a 84% nucleotide sequence identity [1]. It is also closely related to the tick-borne encephalitis virus, which is found in Asia and Europe [2]. It was originally isolated in 1958 from the brain of a child with fulminant encephalitis of unknown origin, from the town of Powassan in Ontario, Canada [3].
Powassan virus infection mainly manifests as encephalitis. However, the illness severity can vary significantly, from asymptomatic infection to the development of meningitis and encephalitis [4]. Lymphocytic infiltration of perivascular neuronal tissue can be seen on pathology, with a predilection for gray matter, including the thalamus, midbrain, and cerebellum [5]. In patients that develop meningoencephalitis, complications can include seizures, aphasia, cranial nerve palsies, and paresis [6], with approximately 10% of cases resulting in death [7].
The virus is transmitted by tick bites and has been isolated in various species, such as Ixodes cookei (groundhog tick), I. scapularis (black-legged or deer tick), I. marxi (squirrel tick), I. spinipalpus, and Dermacentor andersoni [8]. Cases of Powassan virus reflect the ecology of its hosts and their interaction with humans. In general, cases begin increasing in April, peaking during the summer and continuing into the fall, before decreasing during the winter [9,10]. Unlike Borrelia burgdorferi or Babesia microti (pathogens carried by I. scapularis), which require 48 h and 24 h of tick attachment for transmission to occur, respectively [11], Powassan virus transmission can occur 15–30 min after tick attachment, with symptoms appearing 1–4 weeks later [12].
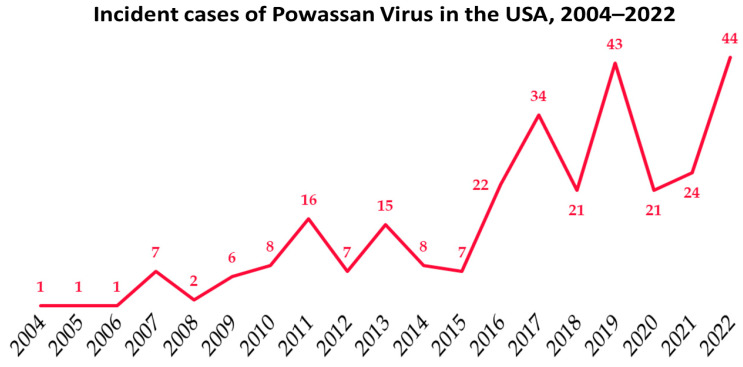
Between 2004 and 2022, a total of 288 cases have been reported to the CDC, of which 267 (92.7%) pertained to neuroinvasive disease, 264 (91.7%) required hospitalization, and 36 (12.5%) resulted in death. The reported incidence of Powassan virus infection is increasing (Figure 1), with 44 incident cases noted in the USA in 2022 [9].
Figure 1.
Incident cases of Powassan virus infection in the USA, as reported by the CDC [9].
Given the increasing reported incidence of this devastating disease, we conducted a systematic review with the objective of identifying all published cases of Powassan virus infection, compiling aggregate data on its clinical manifestations, diagnostic findings and complications.
2. Materials and Methods
This systematic review was conducted according to the international Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines [13]. The study protocol has been published in the international prospective register of systematic reviews PROSPERO, (https://www.crd.york.ac.uk/prospero/display_record.php?RecordID=395991, accessed on 31 August 2023), registration number CRD42023395991.
2.1. Data Sources and Searches
A systematic literature review was conducted through PubMed, using the search term “Powassan [title/abstract]”. No limitations were imposed on publication date or language, with the search concluding on August 31st, 2023. Screening of article titles and abstracts was based on selection criteria as presented in Table 1. To identify further relevant publications, references from the extracted articles were also reviewed.
Table 1.
Inclusion and exclusion criteria for this study.
| Inclusion Criteria | Exclusion Criteria |
|---|---|
| Studies providing detailed description of cases of Powassan virus infection | In vitro, in vivo, epidemiological studies, or reviews on Powassan virus |
| Cohort studies, case reports, and series were eligible for inclusion | Studies providing aggregate data on Powassan virus infections, without detailed descriptions of individual cases |
2.2. Study Selection
The selection of studies for inclusion in this review was carried out by a team of three reviewers (L.K., V.R.V., D.K.), who applied the selection criteria outlined in Table 1 to determine each study’s eligibility. Subsequently, the reviewers further assessed the relevance and quality of the studies that met the inclusion criteria.
Study design: case reports, case series, and cohort studies describing cases of Powassan virus infections.
Type of participants: adult and pediatric patients that are diagnosed with Powassan virus infection through immunoassays via the state or CDC.
Type of exposure: Powassan virus infection
Type of outcome: The primary outcome was identifying and describing the clinical manifestations, results of laboratory and imaging studies, and outcomes of patients with Powassan virus infections. Secondary outcomes were evaluating whether the presence of specific clinical manifestations and laboratory and imaging findings was associated with specific outcomes.
2.3. Data Extraction and Quality Assessment
The following data were extracted from full-text articles included in this review: eligibility criteria, study design, patient demographic parameters, symptoms and signs on presentation, findings of cerebrospinal fluid (CSF) and imaging studies, treatment with steroids or intravenous immunoglobulin (IVIG), development of complications, and final outcome.
The risk of bias in the included studies was independently evaluated by three authors (L.K., V.R.V., D.K.) using the Murad scale, which is a modified version of the Newcastle–Ottawa Scale (NOS) [14] used for non-randomized trials. The assessment involved the evaluation of six evidence-based criteria, which assessed for selection, representativeness of cases, ascertainment of outcomes and exposure, and adequate reporting [15]. The questions used for evaluating each study are presented in Supplementary Table S1. Discrepancies between the reviewers were resolved through group discussions until a consensus was reached. Based on the number of criteria met, a study was categorized as having low risk of bias (all 6 criteria met), moderate risk of bias (5 or 4 criteria met), or high risk of bias (3 or less criteria met) [16]. The scores assigned to each study are presented in Supplementary Table S2.
2.4. Data Synthesis and Analysis
Data relating to the primary and secondary endpoints are extracted and presented descriptively. Statistical analysis was performed using IBM SPSS for Windows version 28. For continuous variables, means are reported for parametric variables and medians for non-parametric variables. Statistical analysis was also conducted in order to evaluate the effect of the presence of specific manifestations, laboratory and imaging findings on mortality or long-term neurological deficits. The data were analyzed by converting them into binary variables, and correlations between these variables were identified using Pearson correlation. p-values were calculated using Fisher’s Exact Test (2-sided), and only p-values less than 0.05 were considered statistically significant.
2.5. Role of the Funding Source
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
3. Results
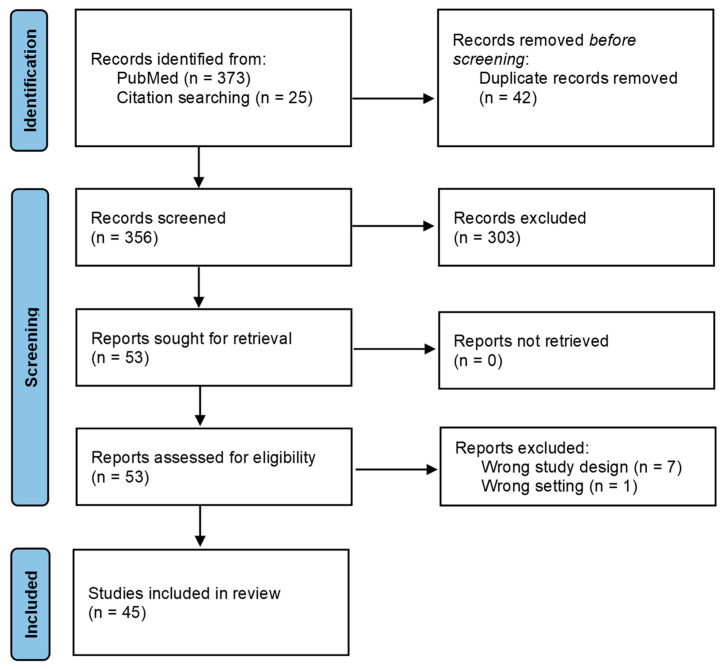
The original PubMed search retrieved 373 articles, and citation searching led to the identification of an additional 25 articles. A total of 45 studies met the inclusion criteria and were included in the review (Figure 2). The extracted data are presented in Table 2, while the quality assessment of each study is provided in Supplementary Table S2.
Figure 2.
Flow chart of evaluated studies, as per PRISMA guidelines [13].
3.1. Demographics
A total of 84 cases were identified; 70 were adults and 14 were pediatric patients. Among the adult patients, 23 individuals (32.9%) were female. The mean age of the adult patients was 60.1 years. In the pediatric population, three patients (21.4%) were female, with a mean age of 4.8 years. The diagnosis was confirmed in all cases through testing of the serum samples or CSF for Powassan virus-neutralizing antibodies or genetic material. Specifically, immunoassays for Powassan virus IgM were used to identify 80 cases (95.2%)—either in serum for 61 cases (72.6%), in CSF for 38 cases (45.2%), or in both for 17 cases (20.2%). The virus was identified in the CSF through either PCR for 6 cases (7.1%) or metagenomic next-generation sequencing in 2 cases (2.4%). In total, genetic testing was the sole diagnostic method used in four cases (4.8%).
3.2. Geographic Distribution
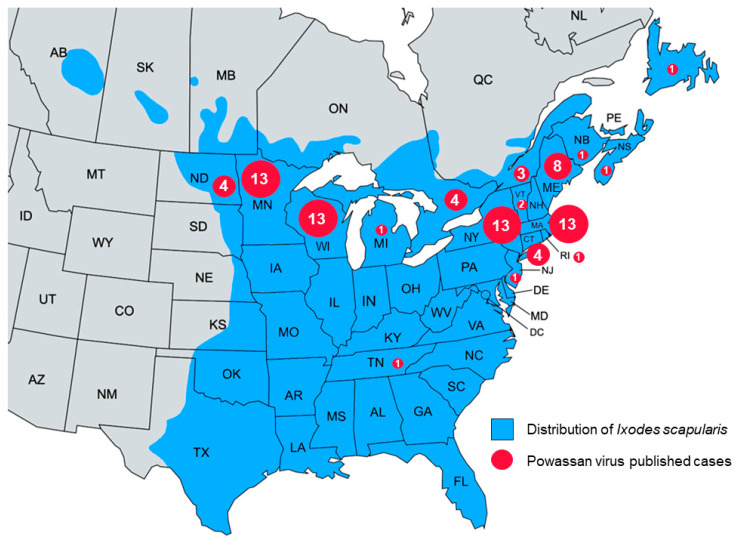
All identified cases were recorded in North America, with 75 cases (89.3%) reported from the USA and 9 cases (10.7%) from Canada. The cases were mostly distributed between northeastern United States, midwestern states bordering Canada, and Canadian states on the border with the USA. Most cases were recorded in New York, Massachusetts, Minnesota, and Wisconsin with 13 cases each. Maine followed with 8 cases; Ontario, Connecticut, and North Dakota had 4 cases each; and Quebec had 3 cases. Vermont had 2 cases, while Michigan, Tennessee, New Jersey, Rhode Island, New Brunswick, Nova Scotia, and Newfoundland each had 1 recorded case (Figure 3).
Figure 3.
Map depicting the distribution of Powassan virus cases reported in this review along with the distribution of Ixodes scapularis in the USA [17] and Canada [18].
3.3. Data on Individual Cases
Data on individual cases, including patient presentation, CSF, and imaging findings, as well as final outcome are presented in Table 1.
Table 2.
Data on each individual published case.
| 1st Author, Year | Patient Presentation | CSF Studies | Imaging Findings | Outcome |
|---|---|---|---|---|
| McLean, 1959 [3] | 5M, right-sided headache, drowsiness, fever, twitching, tremors, nystagmus, followed by worsening mentation, neck stiffness, left-sided hemiplegia, ataxia | WBC: 150 c/mL, PMN: 60 c/mL, LY: 90 c/mL, MONO: NA, Glu: NA, Prot: NA, sPVAb + | NA | Death |
| Goldfield, 1973 [19] | 57F, severe retro-orbital headache, hearing loss, dizziness, lethargy, delirium | WBC: 44 c/mL, PMN: 10 c/mL, LY: 35 c/mL, MO: NA, Glu: 41 mg/dL, Prot: NA, sPVAb + | NA | Persistent neurological deficit |
| Smith, 1974 [20] | 7M, sudden-onset fever 39–40 °C, headache, three generalized tonic–clonic seizures lasting 5 min each | WBC: 460 c/mL, PMN: 438 c/mL, LY: 22 c/mL, MONO: NA, Glu: 77 mg/dL, Prot: 36 mg/dL, sPVAb 1:80 | NA | Improved |
| 14-month M, sudden onset of URI, sore throat, fever 39 °C, a single convulsion followed by status epilepticus | WBC: 152 c/mL, PMN: 52 c/mL, LY: 100 c/mL, MONO: NA, Glu: 60 mg/dL, Prot: 138 mg/dL, sPVAb 1:1280 | NA | Persistent neurological deficit, movement | |
| 12M, fever, convulsions, somnolence, disorientation, severe headache | WBC: 77 c/mL, PMN: 16 c/mL, LY: 61 c/mL, MONO: NA, Glu: 72 mg/dL, Prot: 31 mg/dL, sPVAb 1:1280 | NA | Improved | |
| Rossier, 1974 [21] | 8M, headache, malaise, anorexia, vomiting, fever, somnolence, neck stiffness, followed by stupor, seizures. Progressed to coma, right gaze deviation, left facial palsy, and bilateral pyramidal tract signs | WBC: 495 c/mL, PMN: 406 c/mL, LY: 89 c/mL, MONO: NA, Glu: 74 mg/dL, Prot: 28 mg/dL, sPVAb + | Normal reading | Improvement with physical and speech therapy |
| Wilson, 1979 [22] | 13-month F, 4-day history of anorexia, lethargy, fever 38–39 °C, rash |
WBC: NA, PMN: 175 c/mL, LY: 36 c/mL, MONO: NA, Glu: 30 mg/dL, Prot: NA, sPVAb 1:160 | NA | Persistent neurological deficit, movement and cognitive |
| Partington, 1980 [23] | 7M, 3-day history of fever, vomiting, increasing lethargy, headache |
WBC: 53 c/mL, PMN: NA, LY: NA, MONO: NA, Glu: 63 mg/dL, Prot: 28 mg/dL, sPVAb 1:80 | NA | Persistent neurological deficit, movement and cognitive |
| Embil, 1983 [24] | 8M, malaise, olfactory hallucination, reduced appetite, seizures, fever | WBC: 91 c/mL, PMN: NA, LY: NA, MONO: 91 c/mL, Glu: NA, Prot: NA, sPVAb 1:320 | NA | Complete recovery |
| Fitch, 1990 [25] | 76M, 4-day history of malaise, fever, intermittent headache, vomiting | WBC: NA, PMN: NA, LY: NA, MO: NA, Glu: 107 mg/dL, Prot: NA, sPVAb 1:80 | NA | Persistent neurological deficit, cognitive |
| Gholam, 1999 [26] | 64M, headache, fever, expressive and nominal dysphasia, mild right facial weakness | WBC: 106 c/mL, PMN: 69 c/mL, LY: 34 c/mL, MO: 3 c/mL, Glu: 55 mg/dL, Prot: 175 mg/dL, sPVAb 1:160 |
Normal reading | Improved neurologically, died during hospitalization from a massive pulmonary embolism |
| Courtney, 2001 [27] | 70M, fever, generalized muscle weakness, somnolence, diarrhea, anorexia | WBC: 40 c/mL, PMN: NA, LY: 35 c/mL, MO: NA, Glu: 96 mg/dL, Prot: 640 mg/dL, sPVAb 1:1640 | Parietal changes consistent with microvascular ischemia or demyelinating disease | Persistent neurological deficit, movement |
| 53F, agitation, ataxia, bilateral lateral gaze palsy, dysarthria, loss of balance, visual disturbance, fever | WBC: 148 c/mL, PMN: 68 c/mL, LY: 59 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:640 | Bilateral temporal lobe microvascular ischemia or demyelinating disease | Residual deficits, persistent neurological deficit | |
| 25M, fever, headache, vomiting, somnolence, confusion, bilateral hand twitching, muscle weakness, pronounced lip smacking | WBC: 920 c/mL, PMN: NA, LY: 681 c/mL, MO: NA, Glu: 77 mg/dL, Prot: 80 mg/dL, cPVAb 1:80 | NA | Improved | |
| 66M, somnolence, severe headache, increasing confusion, progressive bilateral leg weakness, slow speech, memory loss, wide-based gait | WBC: 54 c/mL, PMN: NA, LY: 51 c/mL, MO: NA, Glu: 67 mg/dL, Prot: 640 mg/dL, sPVAb 1:640 | NA | Persistent neurological deficit, cognitive | |
| Lessell, 2003 [28] | 53F, nausea, vomiting, diarrhea, dizziness, diplopia, ataxia, followed by arm weakness, urinary retention, fever, delirium, ophthalmoplegia | WBC: 148 c/mL, PMN: 68 c/mL, LY: 59 c/mL, MO: 21 c/mL, Glu: NA, Prot: 640 mg/dL, sPVAb 1:640 | Hyperintensity in the white matter of each temporal lobe | Persistent neurological deficit |
| Hinten, 2008 [7] | 70M, Somnolence, AMS, generalized muscle weakness progressing to left-sided hemiplegia, encephalopathy, renal insufficiency, anemia. | WBC: 40 c/mL, PMN: 5 c/mL, LY: 35 c/mL, MO: 0 c/mL, Glu: NA, Prot: 96 mg/dL, cPVAb 1:640. | Old infarct in right parietal lobe, bilateral parietal lobe abnormalities suggestive of microvascular ischemia or demyelinating disease | Dense left-sided hemiplegia at the time of discharge |
| 53F, ataxia, bilateral lateral gaze palsy, dysarthria, AMS, generalized muscle weakness, complete ophthalmoplegia | WBC: 148 c/mL, PMN: 68 c/mL, LY: 59 c/mL, MO: 0 c/mL, Glu: NA, Prot: NA, sPVAb 1:640. | Bilateral temporal lobe abnormalities consistent with microvascular ischemia or demyelinating disease | Ophthalmoplegia | |
| 25M, fever 38.5 °C, headache, vomiting, somnolence, confusion, inability to walk, bilateral hand twitching, bilateral upper extremity weakness, pronounced lip-smacking | WBC: 920 c/mL, PMN: 239 c/mL, LY: 681 c/mL, MO: 0 c/mL, Glu: 77 mg/dL, Prot: 80 mg/dL, cPVAb 1:80 | NA | Persistent neurological deficit, movement | |
| 74F, headache, fever 40.5 °C, myalgias, confusion, progressive weakness with inability to speak or walk, combative, tremors | WBC: 28 c/mL, PMN: 5 c/mL, LY: 23 c/mL, MO: 0 c/mL, Glu: 34 mg/dL, Prot: 8 mg/dL, cPVAb 1:8 | NA | Persistent neurological deficit, movement | |
| 66M, somnolence, severe headache, increasing confusion, slow speech, short-term memory loss, bilateral leg weakness, wide-based gait | WBC: 54 c/mL, PMN: 3 c/mL, LY: 51 c/mL, MO: 0 c/mL, Glu: 67 mg/dL, Prot: 640 mg/dL, sPVAb 1:640 | NA | Persistent neurological deficit, cognitive | |
| 69M, abdominal pain, vomiting, fever 38.7 °C, chills, lethargy | WBC: 60 c/mL, PMN: NA, LY: NA, MO: NA, Glu: NA, Prot: 60 mg/dL, cPVAb 1:640 | NA | NA | |
| 60F, fever 39.2 °C, diplopia, acute onset of proximal muscle weakness in all extremities (upper > lower), paralysis, respiratory failure requiring mechanical ventilation. | WBC: 198 c/mL, PMN: NA, LY: NA, MO: NA, Glu: 61 mg/dL, Prot: 16 mg/dL, cPVAb 1:16 | NA | Persistent B/L upper extremity weakness requiring total assistance with feeding and dressing | |
| 83M, fever, headache, AMS, stiff neck, generalized muscle weakness | WBC: 80 c/mL, PMN: 42 c/mL, LY: 38 c/mL, MO: 0 c/mL, Glu: 61 mg/dL, Prot: 320 mg/dL, sPVAb 1:320 | NA | Improvement in symptoms | |
| 91F, fever, headache, AMS, stiff neck, muscle pain, generalized muscle weakness | WBC: 28 c/mL, PMN: 3 c/mL, LY: 25 c/mL, MO: 0 c/mL, Glu: 64 mg/dL, Prot: 960 mg/dL, sPVAb 1:960 | Cerebral atrophy | Improvement in symptoms | |
| Tavakoli, 2009 [29] | 62M, fatigue, fever, bilateral maculopapular palmar rash, diplopia, dysarthria, weakness of right arm and leg | WBC: 891 c/mL, PMN: 9 c/mL, LY: 829 c/mL, MO: NA, Glu: 47 mg/dL, Prot: 192 mg/dL, cPVAb +, cPCR + | Hyperintensities in superior cerebellum, left pons, and bilateral basal ganglia, restricted diffusion in the superior cerebellum | Death |
| Trépanier, 2010 [30] | 61M, 3 days of fever, AMS, disorientated with incoherent speech, dizziness, headache | WBC: 65 c/mL, PMN: 9 c/mL, LY: NA, MO: NA, Glu: NA, Prot: NA, sPVAb 1:40. | FLAIR/T2 hyperintensity at the junction between the left internal capsule and the caudate nucleus and of the junction between putamen and external capsule | Improved |
| Hicar, 2011 [8] | 9F, 3 days of headache, abdominal pain, emesis, fever to 39.4 °C, nuchal rigidity | WBC: 68 c/mL, PMN: 44 c/mL, LY: 17 c/mL, MONO: 10 c/mL, Glu: 63 mg/dL, Prot: 52 mg/dL, sPVAb + | T2 hyperintensities in the white matter, left putamen, and caudate nucleus | Persistent neurological deficit, movement, and cognitive |
| Raval, 2012 [31] | 18M, severe headache for 2 days, 1 episode of seizure | WBC: 237 c/mL, PMN: 218 c/mL, LY: 19 c/mL, MO: NA, Glu: 66 mg/dL, Prot: 53 mg/dL, sPVAb + | Normal reading | Improved |
| 60M, fevers, headaches, dizziness | WBC: 12 c/mL, PMN: NA, LY: 12 c/mL, MO: NA, Glu: 166 mg/dL, Prot: 80 mg/dL, cPVAb 1:640 | NA | Improved | |
| 61M, progressive headaches, body aches, high-grade fever, AMS | WBC: 3 c/mL, PMN: NA, LY: NA, MO: NA, Glu: 57 mg/dL, Prot: 46 mg/dL, cPCR + | Normal reading | Improved | |
| 69M, progressive weakness, headaches, fevers | NA. Diagnosis through cPCR + | NA | Persistent neurological deficit, movement | |
| Choi, 2012 [32] | 43M, fever, chills, arthralgia, myalgia, rash, diarrhea, sudden-onset left-sided weakness, progressive AMS | WBC: 60 c/mL, PMN: NA, LY: NA, MO: NA, Glu: NA, Prot: NA, sPVAb + | Right thalamic lesion with restricted diffusion | Persistent neurological deficit, movement |
| Birge, 2012 [5] | 67F, 3-day history of dizziness, fever 39.4 °C, chills, malaise, nausea, confusion, dysarthria, mild neck tenderness | WBC: 80 c/mL, PMN: 71 c/mL, LY: 9 c/mL, MO: NA, Glu: NA, Prot: 64 mg/dL, sPVAb +, cPVAb + | Nonspecific inflammatory changes within the thalamus, midbrain, and cerebellum. Mass effect of structures at the foramen magnum, acute hydrocephalus | Death |
| Sung, 2013 [33] | 22M, influenza-like illness, recurred after few weeks with fever, eye pain, lateral gaze palsy, ataxia, dysarthria, stomach pain, neck stiffness | WBC: 212 c/mL, PMN: 11 c/mL, LY: 201 c/mL, MO: NA, Glu: 60 mg/dL, Prot: 55 mg/dL, cPVAb 1:320 | Bilateral caudate and basal ganglia hyperintensities consistent with encephalitis | Persistent dysarthria, mild tremors |
| 34M, rash on trunk followed by headache, fever, chills, bilateral ankle pain, progressed to bilateral proximal leg weakness, confusion, diplopia | WBC: 145 c/mL, PMN: NA, LY: NA, MO: NA, Glu: 39 mg/dL, Prot: 142 mg/dL, cPVAb 1:320 | Hyperintensities in bilateral temporal lobes | Residual leg weakness | |
| Piantadosi, 2016 [34] | 82M, sudden onset of dizziness followed by fever, nausea, vomiting. | WBC: 169 c/mL, PMN: NA, LY: 140 c/mL, MO: 0 c/mL, Glu: 45 mg/dL, Prot: 230 mg/dL, cPVAb 1:8 | Diffuse T2 signal intensity in cerebellum and vermis | Death |
| 74M, 2 weeks of upper respiratory symptoms, followed by fever, right eye pain, visual blurring | WBC: 108 c/mL, PMN: 33 c/mL, LY: 55 c/mL, MO: NA, Glu: 76 mg/dL, Prot: 201 mg/dL, cPVAb 1:512 | T2 and FLAIR hyperintensities throughout the brainstem and basal ganglia bilaterally, extending to the right anterior frontal subcortical white matter | Persistent neurological deficit, movement, and cognitive | |
| 21M, 3 days of vomiting, confusion, fever, rash | WBC: 156 c/mL, PMN: 47 c/mL, LY: 80 c/mL, MO: NA, Glu: 85 mg/dL, Prot: 320 mg/dL, sPVAb 1:320 | T2/FLAIR hyperintensities in basal ganglia (insula, caudate heads, putamen) and thalami bilaterally and diffusion restriction throughout the cortex. | Improved | |
| 67M, 4 days of confusion, encephalopathy, vomiting, diarrhea, fever, faint maculopapular rash over the chest, upper back. | WBC: 557 c/mL, PMN: 11 c/mL, LY: 418 c/mL, MO: NA, Glu: 49 mg/dL, Prot: 89 mg/dL, sPVAb 1:160 | T2/FLAIR hyperintensities in the caudate heads, putamina, and thalami | Improved | |
| 65F, 7 days of fever, confusion, slow speech, headache, vomiting | WBC: 220 c/mL, PMN: 31 c/mL, LY: 161 c/mL, MO: NA, Glu: 44 mg/dL, Prot: 84 mg/dL, sPVAb 1:160 | T2/FLAIR hyperintensity was visible involving the basal ganglia bilaterally | Improved | |
| 52M, 2 days of fever, myalgias | WBC: 420 c/mL, PMN: 8 c/mL, LY: 336 c/mL, MO: NA, Glu: 53 mg/dL, Prot: 113 mg/dL, sPVAb 1:160 | T2/ FLAIR hyperintensity in bilateral basal ganglia and thalami, diffusion restriction in dorsal mid-brain | Persistent neurological deficit, movement, and cognitive | |
| 49M, 4 days of fever, headache | WBC: 146 c/mL, PMN: NA, LY: 136 c/mL, MO: NA, Glu: 54 mg/dL, Prot: 107 mg/dL, sPVAb + | Asymmetric T2/FLAIR hyperintensities in the basal ganglia and thalami | Death | |
| 44M, 3 days of headache, fatigue, diplopia, diffuse rash over trunk and extremities | WBC: 720 c/mL, PMN: 288 c/mL, LY: 338 c/mL, MO: NA, Glu: 91 mg/dL, Prot: 58 mg/dL, sPVAb 1:10240 (reported concern for cross reactivity with other arboviruses) | Normal reading | Improved | |
| Cavanaugh, 2017 [35] | 72F, erythema migrans on left scapula, myalgias, chills, fever, headache, AMS, hypotension, oliguria | WBC: 119 c/mL, PMN: 115 c/mL, LY: NA, MO: NA, Glu: 79 mg/dL, Prot: 42 mg/dL, sPVAb 1:2560 | T2 hyperintensities in cerebellar cortex and vermis, pons, midbrain, ventrolateral thalami, and dentate nuclei | Death |
| Tutolo, 2017 [36] | 5-month M, fever, vomiting, right-sided facial twitching that progressed to seizures | WBC: 125 c/mL, PMN: NA, LY: 101 c/mL, MONO: NA, Glu: NA, Prot: 32 mg/dL, cPVAb 1:32 | Restricted diffusion involving the basal ganglia, rostral thalami, and left pulvinar, consistent with encephalitis | Persistent neurological deficit |
| Mittal, 2017 [37] | 35F, headache, vomiting, confusion, fever 38.4 °C, severe hypertension (240/140 mm Hg) | WBC: 343 c/mL, PMN: 7 c/mL, LY: NA, MO: 336 c/mL, Glu: 47 mg/dL, Prot: 76 mg/dL, cPVAb 1:32. | T2 white matter hyperintensities in the cerebral hemispheres and posterior fossa bilaterally | Persistent neurological deficit, movement, and cognitive |
| Sanderson, 2018 [38] | 68F, right abducens nerve palsy, mild left-sided pyramidal weakness, erythematous mark on right shoulder | WBC: 31 c/mL, PMN: NA, LY: 28 c/mL, MO: NA, Glu: 54 mg/dL, Prot: 105 mg/dL, sPVAb 1:5120. | T2 and FLAIR hyperintensities in supratentorial white matter | Persistent neurological deficit, movement |
| Solomon, 2018 [39] | 60M, 1 week of testicular pain, fever, testicular ultrasonography demonstrated orchiepididymitis. Later developed meningismus, bilateral upper extremity dysmetria, dysarthria, gait instability | WBC: 10 c/mL, PMN: 4 c/mL, LY: 6 c/mL, MO: NA, Glu: 62 mg/dL, Prot: 83 mg/dL, cPCR +, sPCR + | Diffuse cerebellar edema, obstructive hydrocephalus, diffuse leptomeningeal enhancement, and periventricular, thalamo-mesencephalic, and basal ganglia T2 hyperintensities | Death |
| Patel, 2018 [40] | 81F, fever 41.1 C, somnolence, neck stiffness, diffuse motor weakness, increased tone and cogwheel rigidity, nonconvulsive status epilepticus. | WBC: 197 c/mL, PMN: 2 c/mL, LY: 160 c/mL, MO: 18 c/mL, Glu: 60 mg/dL, Prot: 121 mg/dL, sPVAb 1:2660 | T2/FLAIR hyperintensities in superficial and deep white matter and corpus callosum | Improved |
| Picheca, 2019 [41] | 62M, nausea, vomiting, abdominal pain, diplopia, ataxia, dysarthria, respiratory distress, weakness progressing to flaccid paralysis (upper > lower) with preserved sensation | WBC: 159 c/mL, PMN: 67 c/mL, LY: 68 c/mL, MO: NA, Glu: 79 mg/dL, Prot: 160 mg/dL, sPVAb 1:160 | Infratentorial and supratentorial leptomeningeal enhancement. T2 hyperintensity signal involving the anterior horns from C3 to C6 | NA |
| Khan, 2019 [42] | 87M, 2 weeks of worsening fatigue, malaise, intermittent lightheadedness, mild abdominal pain followed by fever 39.4 °C, rigors, vomiting, drowsiness. Co-infected with Babesia microti and Borrelia burgdorferi. | WBC: 20000 c/mL, PMN: NA, LY: 19200 c/mL, MO: 800 c/mL, Glu: 60 mg/dL, Prot: 48 mg/dL, sPVAb + | Normal reading | Persistent neurological deficit, movement, and cognitive |
| Allgaier, 2019 [43] | 55M, headache, nausea, vomiting followed by confusion, memory loss | WBC: 88 c/mL, PMN: 4 c/mL, LY: 74 c/mL, MO: 11 c/mL, Glu: 64 mg/dL, Prot: NA, sPVAb +, cPVAb + | T2 hyperintensities in bilateral caudate, putamen, and hippocampus, findings of inflammatory encephalitis. Enhancement of hippocampus in DWI | Short-term memory deficit, resolved within 5 months |
| Colman, 2020 [44] | 56M, worsening headache, decreased appetite, vomiting, trouble dressing, acute confusion, new-onset blurred vision. | WBC: 218 c/mL, PMN: NA, LY: 201 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb + | FLAIR hyperintensity about the cerebral convexities and cerebellar folia, leptomeningeal enhancement | Improved |
| Koester, 2020 [45] | 8F, 1-day history of headache, photophobia, fever to 38.3 °C, lethargy, poor oral intake, diffuse abdominal pain, vomiting | WBC: 88 c/mL, PMN: 20 c/mL, LY: 58 c/mL, MONO: 10 c/mL, Glu: 66 mg/dL, Prot: 35 mg/dL, cPVAb 1:40. | Normal reading | Improved |
| Yu, 2020 [46] | 88M, fever, AMS, dysarthria, falls, left arm weakness, hypoxic respiratory failure due to aspiration | WBC: 33 c/mL, PMN: NA, LY: 30 c/mL, MO: NA, Glu: NA, Prot: NA, cPCR + | Normal reading | Death |
| Feder, 2021 [47] | 5-month M, 2 days of fever (39.4 °C), vomiting, right-sided facial twitching progressing to seizures. Symptom onset 2 weeks after tick was removed from infant’s head. | WBC: 125 c/mL, PMN: 24 c/mL, LY: 101 c/mL, MONO: NA, Glu: 57 mg/dL, Prot: 55 mg/dL, cPVAb 1:32. | Edema of the basal ganglia, rostral thalami, and left pulvinar, consistent with encephalitis | Speech delay |
| 2-month M, Fever 38.9° C, listlessness for 1 day, followed by left-sided focal seizures | WBC: 215 c/mL, PMN: 22 c/mL, LY: 112 c/mL, MONO: 82 c/mL, Glu: 56 mg/dL, Prot: 75 mg/dL, sPVAb +, cPVAb + | Patchy edema of the thalami, right parietal lobe, and right mid brain | Paresis of the left upper extremity | |
| Pach, 2021 [48] | 62M, fevers, night sweats, headaches, fatigue, AMS, progressed to profound expressive aphasia, ataxia, unable to protect airway. | WBC: 59 c/mL, PMN: 2 c/mL, LY: 52 c/mL, MO: 5 c/mL, Glu: 80 mg/dL, Prot: 99 mg/dL, cPVAb 1:320 | Mild generalized cerebral and cerebellar atrophy | Persistent ataxia and expressive aphasia with spasticity in upper limbs requiring baclofen pump, wheelchair-bound |
| Dumic, 2021 [49] | 42M, 2 days of diplopia due to left abducens nerve palsy, dysarthria, headache, fever 38.5 °C, maculopapular rash torso | WBC: 193 c/mL, PMN: NA, LY: 154 c/mL, MO: NA, Glu: NA, Prot: NA, cPVAb 1:320 | Normal reading | Complete recovery |
| Dumic, 2021 [50] | 76M, confusion followed by abrupt onset of fever the next day. Co-infected with Borrelia burgdorferi. | WBC: 68 c/mL, PMN: NA, LY: 37 c/mL, MO: NA, Glu: NA, Prot: NA, cPVAb 1:8 | Normal reading | Persistent neurological deficit, movement, and cognitive |
| Taylor, 2021 [51] | 30F, severe headache, fever, weakness, myalgias, chills, confusion, photophobia, nausea, diarrhea, 24 days after undergoing kidney transplantation | WBC: 5 c/mL, PMN: NA, LY: NA, MO: NA, Glu: 55 mg/dL, Prot: 320 mg/dL, sPVAb 1:320 | Abnormal T2-FLAIR signal in the cerebellum | Persistent neurological deficit, cognitive |
| Kroopnick, 2021 [52] | 82F, 2 days of worsening back pain, headache, vomiting, followed by acute agitation and confusion. Co-infected with Anaplasma phagocytophilum. | NA, cPVAb + | T2/FLAIR hyperintensities dorsal midbrain, pons, and superior cerebellar peduncles | Death |
| Nord, 2021 [53] | 51M, acute AMS, fever 40 °C | NA, cPVAb + | NA | Improved |
| Bazer, 2022 [54] | 62M, acute onset AMS, dysarthria, left-sided facial droop, followed by recurrent strokes | WBC: 370 c/mL, PMN: 296 c/mL, LY: 74 c/mL, MO: NA, Glu: 59 mg/dL, Prot: 152 mg/dL, cPVAb + | Acute infarct involving cerebellum, left basal ganglia, and splenium of corpus callosum, left putamen infarct | Global aphasia at the time of discharge |
| Johnson, 2022 [55] | 68M, headache, recurrent fever, rapidly progressive weakness | WBC: 274 c/mL, PMN: 38 c/mL, LY: 236 c/mL, MO: NA, Glu: 107 mg/dL, Prot: 84 mg/dL, cPVAb + | Cerebellar cerebritis without hydrocephalus | Found to have diffuse large B-cell lymphoma, transitioned to comfort care |
| 75M, headache, encephalopathy, inability to follow commands, rigidity with saccades, vertical upgaze restriction | WBC: 76 c/mL, PMN: 8 c/mL, LY: 68 c/mL, MO: NA, Glu: 64 mg/dL, Prot: 34 mg/dL, CSF mNGS + | Cerebral cerebritis and obstructive hydrocephalus | No neurological improvement, comfort care | |
| Kakoullis, 2022 [56] | 75F, hypersomnia, apraxia, AMS, short-term memory loss, encephalitis | WBC: 69 c/mL, PMN: 2 c/mL, LY: 61 c/mL, MO: 6 c/mL, Glu: 49 mg/dL, Prot: 60 mg/dL, cPVAb + | Bilaterally increased T2 signal in posterior parietal lobes, cerebellum, and basal ganglia | Persistent neurological deficit, cognitive |
| Mendoza, 2023 [57] | 75M, encephalopathy, anarthria, quadriparesis, vertical gaze restriction | WBC: 6 c/mL, PMN: 3 c/mL, LY: 2 c/mL, MO: NA, Glu: NA, Prot: NA, CSF mNGS + | Cerebellitis with obstructive hydrocephalus | Death |
| 74F, left-sided weakness, dysarthria, tremor, truncal ataxia | WBC: 121 c/mL, PMN: 17 c/mL, LY: 87 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb +, sPCR + | Symmetric T2 hyperintensities in bilateral thalami | NA | |
| 34F, headache, fever, nausea, AMS | WBC: 257 c/mL, PMN: 8 c/mL, LY: 242 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:640 | NA | Cognitive impairment and headaches | |
| 2-month M, seizures | WBC: 480 c/mL, PMN: 230 c/mL, LY: 62 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:640 | Normal | Recovery | |
| 68M, rapidly progressive quadriparesis, encephalopathy, multiple cranial neuropathies (left 3rd and 4th, bilateral 6th, and bilateral optic perineuritis) | WBC: 274 c/mL, PMN: 0 c/mL, LY: 236 c/mL, MO: NA, Glu: NA, Prot: NA, cPVAb + | Cerebellitis and ventral cervical cord enhancement | Death | |
| 50F, headache, fever, neck pain | WBC: 40 c/mL, PMN: 16 c/mL, LY: 16 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:640 | Normal | NA | |
| 76M, headache, fever, neck pain | WBC: 55 c/mL, PMN: 32 c/mL, LY: 9 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:2560 | Normal | Cognitive impairment | |
| 76M, encephalopathy, multifocal myoclonus | WBC: 85 c/mL, PMN: 18 c/mL, LY: 43 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:640 | Normal | Cognitive impairment and gait disturbance | |
| 49M, left 6th nerve palsy, dysarthria, upper-extremity dysmetria | WBC: 193 c/mL, PMN: 15 c/mL, LY: 176 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:40 | Cerebellar and occipital lobe leptomeningeal enhancement | Gait disturbance | |
| 58M, headaches, dysarthria, appendicular and truncal ataxia | WBC: 66 c/mL, PMN: 5 c/mL, LY: 57 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:160 | Leptomeningeal enhancement at cerebellar folia and cerebral hemispheres | Gait disturbance | |
| 74M, truncal > appendicular ataxia, encephalopathy, anarthria | WBC: 5 c/mL, PMN: 0 c/mL, LY: 3 c/mL, MO: NA, Glu: NA, Prot: NA, cPVAb <1:10 | Diffuse sulcal hyperintensities on post-gadolinium FLAIR sequence | Persistent ataxia and dysarthria | |
| 33F, headache, encephalopathy, ataxia and left 6th nerve palsy | WBC: 585 c/mL, PMN: 6 c/mL, LY: 579 c/mL, MO: NA, Glu: NA, Prot: NA, cPVAb 1:32 | Midbrain, pons, and cerebellar FLAIR hyperintensities | Parkinsonism | |
| 71F, headache, fever, meningismus, encephalopathy | WBC: 43 c/mL, PMN: 1 c/mL, LY: 39 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:1280 | Normal | Gait disturbance, headaches | |
| 78F, fever, headache, encephalopathy | WBC: NA c/mL, PMN: NA c/mL, LY: NA c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:640 | Normal | Recovery | |
| 44M, fever, headache, meningismus, truncal ataxia, tremulousness, opsoclonus | WBC: 860 c/mL, PMN: NA c/mL, LY: 705 c/mL, MO: NA, Glu: NA, Prot: NA, sPVAb 1:640 | Normal | Recovery | |
| 56F, encephalopathy, truncal ataxia, quadriparesis, dysarthria, left 6th nerve palsy | WBC: 153 c/mL, PMN: 53 c/mL, LY: 58 c/mL, MO: NA, Glu: NA, Prot: NA, cPVAb + | Leptomeningeal enhancement and left basal ganglia FLAIR hyperintensities | Death |
Abbreviations: M, male; F, female; WBC, white blood cell count; PMN, polymorphonuclear; LY, lymphocytes; MO, monocytes; Glu, glucose; Prot, protein; cPVAb, cerebrospinal fluid Powassan virus-neutralizing antibody titers; sPVAb, serum Powassan virus-neutralizing antibody titers; +, positive immunoassay, titer not reported; sPCR, serum polymerase chain reaction; cPCR, CSF polymerase chain reaction; NA, not available; DWI, diffusion-weighted imaging; mNGS, metagenomic next-generation sequencing.
3.4. Presentation
Findings on clinical manifestations are outlined in Table 3. In the adult patients, fever was the most common symptom, reported by 77.1% of patients, followed by headache, affecting 54.3% of patients. Other common symptoms included confusion (42.9%), speech disorders (32.9%), weakness and fatigue (30%), nausea and vomiting (24.3%), and visual symptoms (27.1%). Less frequently reported were hypersomnia, somnolence, and lethargy (11.4%); myalgias and arthralgias (8.6%), memory loss (5.7%), and seizures (1.4%).
Table 3.
Prevalence of clinical manifestations of Powassan virus infections.
| Finding | Adults (N = 70) | Children (N = 14) |
|---|---|---|
| Symptoms | ||
| Fever | 77.1% | 100.00% |
| Headache | 54.3% | 50% |
| Confusion | 42.9% | - |
| Fatigue | 30% | 15.40% |
| Nausea and Vomiting | 24.3% | 42.9% |
| Speech Disorders | 32.9% | - |
| Visual Symptoms | 27.1% | - |
| Hypersomnia and Lethargy | 11.4% | 42.9% |
| Myalgias and Arthralgias | 8.6% | - |
| Memory Loss | 5.7% | - |
| Seizures | 1.4% | 64.3% |
| Signs | ||
| Altered Mental Status | 50% | 28.6% |
| Paresis | 25.7% | 14.3% |
| Cranial Nerve Involvement | 25.7% | 7.1% |
| Ataxia | 24.3% | 7.1% |
| Rash | 14.3% | 14.3% |
| Meningeal Signs | 12.9% | 23.10% |
| Tremors | 10% | 21.4% |
Signs in the adult population included altered mental status (50% of cases), paralysis or paresis (25.7%), cranial nerve involvement (25.7%), and ataxia (24.3%). Other signs included rash (14.3%), meningeal signs (12.9%), and tremors (10%).
The final diagnosis was encephalitis in 63 patients and meningoencephalitis in 16 patients; 5 patients had no final diagnosis reported. Furthermore, four patients had a concurrent tickborne infection.
In the pediatric population, fever was also the most commonly reported symptom, found in all 14 patients (100%). Seizures were the second-most frequently observed symptom (64.3%), followed by headache (50%). Hypersomnia, somnolence, lethargy, nausea, and vomiting were each reported in 42.9%. Weakness and fatigue were noted in 14.34% of patients.
Altered mental status was observed in 28.6% of pediatric cases, whereas meningeal signs, tremors, and twitching each occurred in 21.4%. Paralysis or paresis was noted in 14.3% of patients. A rash was present in 14.3% of patients, while ataxia and cranial nerve involvement were each present in 7.1% of cases. No cases of confusion, memory loss, myalgias, arthralgias, respiratory distress or failure, speech disorders, or visual symptoms were observed in the pediatric population.
The final diagnoses were encephalitis (10) and meningoencephalitis (4). No concurrent tickborne infections were noted in the pediatric population.
3.5. CSF Studies
Lumbar puncture was performed in 79 (94%) of patients, with findings summarized in Table 4. Among adult patients, 4 did not undergo lumbar puncture, while data were not available for 1 case. The median white blood cell (WBC) count was 119/mL, predominantly lymphocytic, with a median lymphocyte count of 59.2/mL, a median monocyte count of 1.6, and a median neutrophil count of 10.9/mL. The mean glucose level was 64.9 mg/dL, and the mean protein level was 88.2 mg/dL. Opening pressures were reported in three subjects, with a median value of 180 mm H2O. Powassan virus-neutralizing antibody titers were reported in 45 cases, with a median titer of 1:320.
Table 4.
Findings of cerebrospinal fluid analysis.
| Finding | Adults (N = 65) | Children (N = 14) |
|---|---|---|
| Median WBC (per mL) | 119 | 137.5 |
| Median Lymphocytes (per mL) | 59.2 | 75.5 |
| Median Neutrophils (per mL) | 10.9 | 46 |
| Mean Glucose (mg/dL) | 64.9 | 65.3 |
| Mean Protein (mg/dL) | 88.2 | 35.5 |
| Median Powassan Virus Antibody Titers | 1:320 | 1:120 |
All 14 pediatric patients underwent lumbar puncture. The median WBC count was 137.5/mL, also with a lymphocytic predominance, consisting of a median lymphocyte count of 75.5/mL, a median monocyte count of 46, and a median neutrophil count of 48.1/mL. The mean glucose level was 65.3 mg/dL and the median protein level was 35.5 mg/dL. Data on opening pressures were available for four pediatric cases, with a median value of 140 mm of H2O. Powassan virus-neutralizing antibody titers were measured in 10 pediatric cases, with a median titer of 1:120.
3.6. Imaging Studies
Brain MRI results were available for 62 cases, specifically for 56 adults and 6 pediatric patients. Findings are summarized in Table 5.
Table 5.
Summary of MRI Findings.
| Lesion Site | Adults (N = 56) | Children (N = 6) |
|---|---|---|
| Basal Ganglia | 32.1% | 50% |
| Cerebrum | 26.8% | 16.6% |
| Cerebellum | 21.4% | - |
| Thalamus | 14.3% | 50% |
| Midbrain | 8.9% | 16.6% |
| Normal MRI | 21.4% | 33.3% |
Among the adults, the most commonly affected location was the basal ganglia, observed in 32.1% of cases. This was followed by cerebral involvement (26.8%) and cerebellar involvement (21.4%). The thalamus was affected in 14.3% of cases, while the midbrain showed involvement in 8.9% of cases. Additionally, 8.9% of cases presented with leptomeningeal enhancement on MRI while 21.4% of cases showed a normal MRI result.
Among the pediatric patients, two had both basal ganglia lesions and thalamic lesions, one had basal ganglia lesions and cerebral lesions, and one had thalamic lesions and midbrain lesions, while two patients had a normal MRI result.
3.7. Treatment
The identified treatment modalities were the administration of corticosteroids and/or IVIG. Six adult patients received only corticosteroids, four received only IVIG, while another four received both treatments. An improvement was noted in 3 of the patients receiving only corticosteroids and in 3 of the patients receiving both steroids and IVIG.
Among the pediatric patients, two received corticosteroids, and both improved after treatment. No pediatric patients received IVIG.
3.8. Outcomes
Outcome data are summarized in Table 6. Among adult patients, data on mortality were available for all but two patients, with 13/68 (19.1%) patients expiring due to the disease. Regarding complications, 30/68 (44.1%) patients developed paralysis, 10/67 (14.9%) developed tremors, and 17/51 (33.3%) developed cognitive defects. The neurological deficits persisted in 32/51 (62.7%) patients for whom follow-up data were available, including paralysis in 14/18 (77.8%), tremors in 5/8 (62.5%), and cognitive defects in 14/17 (82.3%).
Table 6.
Summary of outcomes.
| Finding | Adults (N = 68) | Children (N = 14) |
|---|---|---|
| Mortality Rate | 19.1% | 7.1% |
| Paralysis | 44.1% | 42.6% |
| Cognitive Defects | 33.3% | 25% |
| Persistent Neurological Deficits | 62.7% | 50% |
Among the pediatric patients, death occurred in 1/14 (7.1%) patients, paralysis was noted in 6/14 (42.6%), tremors were noted in 2/14 (14.3%), and cognitive defects were noted in 3/12 (25%). Persistent neurological deficits were noted for 6/12 (50%) of patients for whom follow-up data were available: paralysis in 3/4 (75%) and cognitive defects in 3/3 (100%). Follow-up data were available for only one of the patients who developed tremor, in whom the tremors were not noted on follow-up.
3.9. Correlation Analysis
Pearson correlation analysis was conducted only in the adult population due to the small sample size in the pediatric population, which limited the feasibility of such an analysis. Factors significantly correlated with mortality were age (r = 0.264, p = 0.029), development of paralysis (r = 0.252, p = 0.041), or respiratory distress or failure (r = 0.328, p = 0.006). Factors associated with persistent neurological deficits were development of ataxia (r = 0.383, p = 0.006), paralysis (r = 0.278, p = 0.048), speech disorder (r = 0.319, p = 0.022), and cranial nerve involvement (r = 0.322, p = 0.017). Other significant correlations included those between speech disorders and ataxia (r = 0.526, p < 0.001), and between paralysis and respiratory distress or failure (r = 0.349, p = 0.003).
4. Discussion
This systematic review examines Powassan virus infections, an emerging arbovirus increasingly identified as a causative agent of encephalitis in the USA [9]. The review provides a comprehensive description of reported cases, including the prevalence of symptoms, signs, CSF, and imaging findings in these patients, as well as data on outcomes and prognostic factors.
Adult patients appeared to present with typical findings of viral encephalitis, with fever, headache, fatigue, and encephalopathy being among the most common findings. Some notable findings were the presence of speech disorders, visual symptoms, paresis, ataxia, and cranial nerve involvement. These findings may serve as clues for suspecting this pathogen, distinguishing it from other causes of encephalitis, such as HSV [58]. Furthermore, imaging studies suggest a predilection for the basal ganglia, which has been associated with Flaviviridae infections [59].
Powassan virus infections were associated with significant morbidity and mortality. Among 288 cases reported to the CDC between 2004 and 2022, 36 (12.5%) had a fatal outcome [9]. That mortality rate is lower than the rate calculated from the cases included in this review (14/78, 17.9%), suggesting the presence of publication bias. Furthermore, neurological deficits occurred at a significant rate, both cognitive and movement-related, which became persistent and long-term in the majority of adult cases. Correlation analysis identified age and complications such as paralysis and respiratory distress as associated with mortality, while the development of ataxia, paralysis or paresis, cranial nerve involvement, or speech disorders was associated with the development of long-term neurological deficits.
Notably, the pediatric patients frequently experienced seizures, which were the second-most commonly reported symptom. It is notable that the prevalence of this symptom was only 1.4% in the adult population, compared to 64.3% in the pediatric population. Imaging studies were conducted less often, but when performed, they also identified the basal ganglia as a commonly affected site. The prognosis was also unfavorable in this group, as long-term neurological deficits were common and persistent.
Identification of exposure to ticks can play a crucial role in making the diagnosis. However, this task may be unreliable, as even in cases of Lyme disease, which requires prolonged tick attachment for transmission to occur, patients rarely recall tick bites [60]. Consequently, clinicians should consider other indicators, such as the presence in or travel to an endemic area, as well as the engagement in activities that increase the likelihood of exposure to ticks, such as hiking, camping, gardening, or hunting [61]. Such exposures should raise the clinician’s suspicion regarding the possibility of Powassan virus as the underlying cause of the patient’s condition.
Furthermore, the geographic distribution of cases of Powassan virus infections appears to be expanding. From the original case in Ontario [3], cases have been reported as far west as North Dakota [31] and as far south as Tennessee [8]. This may be due to increased awareness and increased testing and diagnosis. It may also be due to the expanding habitat of its host. Indeed, studies evaluating the impact of climate change on I. scapularis have shown that there is a progressive expansion of its range, estimated to be approximately 46 km/year. This process is expected to continue, especially northward, as more areas become warmer and more suitable habitats for the tick [62]. Furthermore, climate change is leading to warmer winters, which may translate to increased tick survival and possibly more infections [63].
There are limitations to this study. First and foremost, the information included in each case was dependent on what was reported by each study, and in several cases, the data were incomplete. Furthermore, the mortality rate calculated in this systematic review exceeds that reported by the CDC, suggesting that there is publication bias favoring the more severe cases. Information on co-morbidities, which may affect mortality and morbidity, was not extracted. Therefore, there is a significant selection bias that may increase the prevalence of more severe symptoms, signs, complications, and long-term sequalae including mortality, which may have been overestimated. In addition, although this study did not encompass reports of deer tick virus, it is noteworthy that the term “Powassan virus” is occasionally used as an umbrella term encompassing both Powassan virus and deer tick virus; consequently, the authors lack the means to ascertain whether the publication might have inadvertently documented a case of deer tick virus as a Powassan virus infection. Lastly, only articles published in the English language were evaluated; therefore, publications on Powassan virus outside of the US and Canada may have not been included.
5. Conclusions
In conclusion, Powassan virus encephalitis is associated with considerable mortality and poses a risk of long-term neurological complications. Clinicians should maintain a high index of suspicion for this disease in patients presenting with encephalitis, particularly those with a known or suspected tick exposure. Additionally, individuals who exhibit a combination of speech disorders, paralysis, ataxia, or cranial nerve dysfunction, along with basal ganglia lesions on imaging, should raise suspicion for Powassan virus. Given the increasing incidence and expanding geographic range of the virus, it is expected that the disease will become more prevalent in the coming years. Comprehensive clinical reporting, along with surveillance studies, and further research are warranted to better understand and respond to this emerging arbovirus and public health threat. Development of a vaccine against Powassan virus may became crucial in mitigating its impact on public health.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/tropicalmed8120508/s1, Supplementary Table S1: Murad scale for the evaluation on non-randomized trials; Supplementary Table S2: Evaluation of the quality of studies fulfilling the inclusion criteria by the Murad scale [13].
Author Contributions
Conceptualization, L.K. and I.B.; methodology, L.K., V.R.V. and D.K.; software, L.K., V.R.V. and D.K.; formal analysis, L.K.; investigation, L.K., V.R.V. and D.K.; data curation, L.K., V.R.V. and D.K.; writing—original draft preparation, L.K., V.R.V. and D.K.; writing—review and editing, L.K., V.R.V., D.K., S.K., G.P., L.H.C. and I.B.; visualization, S.K. and L.K.; supervision, L.K. and I.B.; project administration, L.K. and I.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data pertaining to this study are presented in the current manuscript and its Supplementary Materials.
Conflicts of Interest
The authors declare no relevant conflicts of interest.
Declarations
Part of the data presented in this manuscript were presented in the American College of Physicians Massachusetts Chapter Annual Scientific Meeting in September of 2023.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Hermance M.E., Thangamani S. Powassan Virus: An Emerging Arbovirus of Public Health Concern in North America. Vector Borne Zoonotic Dis. 2017;17:453–462. doi: 10.1089/vbz.2017.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hills S.L., Poehling K.A., Chen W.H., Staples J.E. Tick-Borne Encephalitis Vaccine: Recommendations of the Advisory Committee on Immunization Practices, United States, 2023. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2023;72:1–29. doi: 10.15585/mmwr.rr7205a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.McLean D.M., Donohue W.L. Powassan Virus: Isolation of Virus from a Fatal Case of Encephalitis. Can. Med. Assoc. J. 1959;80:708–711. [PMC free article] [PubMed] [Google Scholar]
- 4.Khan M., Beckham J.D., Piquet A.L., Tyler K.L., Pastula D.M. An Overview of Powassan Virus Disease. Neurohospitalist. 2019;9:181–182. doi: 10.1177/1941874419844888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Birge J., Sonnesyn S. Powassan Virus Encephalitis, Minnesota, USA. Emerg. Infect. Dis. 2012;18:1669–1671. doi: 10.3201/eid1810.120621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kemenesi G., Bányai K. Tick-Borne Flaviviruses, with a Focus on Powassan Virus. Clin. Microbiol. Rev. 2019;32:10–1128. doi: 10.1128/CMR.00106-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hinten S.R., Beckett G.A., Gensheimer K.F., Pritchard E., Courtney T.M., Sears S.D., Woytowicz J.M., Preston D.G., Smith R.P.J., Rand P.W., et al. Increased Recognition of Powassan Encephalitis in the United States, 1999–2005. Vector Borne Zoonotic Dis. 2008;8:733–740. doi: 10.1089/vbz.2008.0022. [DOI] [PubMed] [Google Scholar]
- 8.Hicar M.D., Edwards K., Bloch K. Powassan Virus Infection Presenting as Acute Disseminated Encephalomyelitis in Tennessee. Pediatr. Infect. Dis. J. 2011;30:86–88. doi: 10.1097/INF.0b013e3181f2f492. [DOI] [PubMed] [Google Scholar]
- 9.CDC Historic Data (2004–2022)|Powassan Virus. [(accessed on 20 July 2023)]; Available online: https://www.cdc.gov/powassan/statistics-data/historic-data.html.
- 10.CDC Lifecycle of Blacklegged Ticks. [(accessed on 20 July 2023)]; Available online: https://www.cdc.gov/lyme/transmission/blacklegged.html.
- 11.Eisen L. Pathogen Transmission in Relation to Duration of Attachment by Ixodes scapularis Ticks. Ticks Tick. Borne. Dis. 2018;9:535–542. doi: 10.1016/j.ttbdis.2018.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ebel G.D., Kramer L.D. Short Report: Duration of Tick Attachment Required for Transmission of Powassan Virus by Deer Ticks. Am. J. Trop. Med. Hyg. 2004;71:268–271. doi: 10.4269/ajtmh.2004.71.3.0700268. [DOI] [PubMed] [Google Scholar]
- 13.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;372:105906. doi: 10.1136/BMJ.N71. [DOI] [PubMed] [Google Scholar]
- 14.Wells G., Shea B., O’Connell D., Peterson J., Welch V., Losos M., Tugwell P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomised Studies in Meta-Analyses. [(accessed on 7 December 2018)]. Available online: http://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 15.Murad M.H., Sultan S., Haffar S., Bazerbachi F. Methodological Quality and Synthesis of Case Series and Case Reports. Evid. Based. Med. 2018;23:60–63. doi: 10.1136/bmjebm-2017-110853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salah E. Primary Cutaneous CD4+ Small/Medium Pleomorphic T-Cell Lymphoproliferative Disorder: Where Do We Stand? A Systematic Review. JDDG-J. Ger. Soc. Dermatol. 2019;17:123–136. doi: 10.1111/ddg.13691. [DOI] [PubMed] [Google Scholar]
- 17.CDC Regions Where Ticks Live. [(accessed on 15 July 2023)]; Available online: https://www.cdc.gov/ticks/geographic_distribution.html.
- 18.Wilson C., Gasmi S., Bourgeois A.-C., Badcock J., Chahil N., Kulkarni M., Lee M.-K., Lindsay R., Leighton P., Morshed M., et al. Surveillance for Ixodes scapularis and Ixodes pacificus Ticks and Their Associated Pathogens in Canada, 2019. Can. Commun. Dis. Rep. 2022;48:208–218. doi: 10.14745/ccdr.v48i05a04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goldfield M., Austin S.M., Black H.C., Taylor B.F., Altman R. A Non-Fatal Human Case of Powassan Virus Encephalitis. Am. J. Trop. Med. Hyg. 1973;22:78–81. doi: 10.4269/ajtmh.1973.22.78. [DOI] [PubMed] [Google Scholar]
- 20.Smith R., Woodall J.P., Whitney E., Deibel R., Gross M.A., Smith V., Bast T.F. Powassan Virus Infection. A Report of Three Human Cases of Encephalitis. Am. J. Dis. Child. 1974;127:691–693. doi: 10.1001/archpedi.1974.02110240077010. [DOI] [PubMed] [Google Scholar]
- 21.Rossier E., Harrison R.J., Lemieux B. A Case of Powassan Virus Encephalitis. Can. Med. Assoc. J. 1974;110:1173–1174, 1179–1180. [PMC free article] [PubMed] [Google Scholar]
- 22.Wilson M.S., Wherrett B.A., Mahdy M.S. Powassan Virus Meningoencephalitis: A Case Report. Can. Med. Assoc. J. 1979;121:320–323. [PMC free article] [PubMed] [Google Scholar]
- 23.Partington M.W., Thomson V., O’Shaughnessy M. V Powassan Virus Encephalitis in Southeastern Ontario. Can. Med. Assoc. J. 1980;123:603–606. [PMC free article] [PubMed] [Google Scholar]
- 24.Embil J.A., Camfield P., Artsob H., Chase D.P. Powassan Virus Encephalitis Resembling Herpes Simplex Encephalitis. Arch. Intern. Med. 1983;143:341–343. doi: 10.1001/archinte.1983.00350020167030. [DOI] [PubMed] [Google Scholar]
- 25.Fitch W.M., Artsob H. Powassan Encephalitis in New Brunswick. Can. Fam. Physician. 1990;36:1289–1290. [PMC free article] [PubMed] [Google Scholar]
- 26.Gholam B.I., Puksa S., Provias J.P. Powassan Encephalitis: A Case Report with Neuropathology and Literature Review. CMAJ Can. Med. Assoc. J. 1999;161:1419–1422. [PMC free article] [PubMed] [Google Scholar]
- 27.Courtney T., Sears S., Woytowicz J., Preston D., Smith R. Outbreak of Powassan Encephalitis–Maine and Vermont, 1999–2001. Morb. Mortal. Wkly. Rep. 2001;50:761–764. [PubMed] [Google Scholar]
- 28.Lessell S., Collins T.E. Ophthalmoplegia in Powassan Encephalitis. Neurology. 2003;60:1726–1727. doi: 10.1212/01.WNL.0000064167.16083.02. [DOI] [PubMed] [Google Scholar]
- 29.Tavakoli N.P., Wang H., Dupuis M., Hull R., Ebel G.D., Gilmore E.J., Faust P.L. Fatal Case of Deer Tick Virus Encephalitis. N. Engl. J. Med. 2009;360:2099–2107. doi: 10.1056/NEJMoa0806326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Trépanier P., Loungnarath V., Gourdeau A., Claessens C., Savard M. Supranuclear Ophthalmoplegia in Powassan Encephalitis. Can. J. Neurol. Sci. 2010;37:890–892. doi: 10.1017/S0317167100051672. [DOI] [PubMed] [Google Scholar]
- 31.Raval M., Singhal M., Guerrero D., Alonto A. Powassan Virus Infection: Case Series and Literature Review from a Single Institution. BMC Res. Notes. 2012;5:594. doi: 10.1186/1756-0500-5-594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi E.E.J., Taylor R.A. A Case of Powassan Viral Hemorrhagic Encephalitis Involving Bilateral Thalami. Clin. Neurol. Neurosurg. 2012;114:172–175. doi: 10.1016/j.clineuro.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 33.Sung S., Wurcel A.G., Whittier S., Kulas K., Kramer L.D., Flam R., Roberts J.K., Tsiouris S. Powassan Meningoencephalitis, New York, New York, USA. Emerg. Infect. Dis. 2013;19:1549–1551. doi: 10.3201/eid1909.121846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Piantadosi A., Rubin D.B., McQuillen D.P., Hsu L., Lederer P.A., Ashbaugh C.D., Duffalo C., Duncan R., Thon J., Bhattacharyya S., et al. Emerging Cases of Powassan Virus Encephalitis in New England: Clinical Presentation, Imaging, and Review of the Literature. Clin. Infect. Dis. 2016;62:707–713. doi: 10.1093/cid/civ1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cavanaugh C.E., Muscat P.L., Telford S.R., 3rd, Goethert H., Pendlebury W., Elias S.P., Robich R., Welch M., Lubelczyk C.B., Smith R.P. Fatal Deer Tick Virus Infection in Maine. Clin. Infect. Dis. 2017;65:1043–1046. doi: 10.1093/cid/cix435. [DOI] [PubMed] [Google Scholar]
- 36.Tutolo J.W., Staples J.E., Sosa L., Bennett N. Notes from the Field: Powassan Virus Disease in an Infant—Connecticut, 2016. Morb. Mortal. Wkly. Rep. 2017;66:408–409. doi: 10.15585/mmwr.mm6615a3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Mittal S.O., Hassan A., Sanchez J., Robertson C. Powassan Virus Postencephalitic Parkinsonism. Neurol. Clin. Pract. 2017;7:527–530. doi: 10.1212/CPJ.0000000000000391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanderson M., Lindsay L.R., Campbell T.M., Morshed M. A Case of Powassan Encephalitis Acquired in Southern Quebec. CMAJ Can. Med. Assoc. J. 2018;190:E1478–E1480. doi: 10.1503/cmaj.180905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Solomon I.H., Spera K.M., Ryan S.L., Helgager J., Andrici J., Zaki S.R., Vaitkevicius H., Leon K.E., Wilson M.R., DeRisi J.L., et al. Fatal Powassan Encephalitis (Deer Tick Virus, Lineage II) in a Patient with Fever and Orchitis Receiving Rituximab. JAMA Neurol. 2018;75:746–750. doi: 10.1001/jamaneurol.2018.0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Patel K.M., Johnson J., Zacharioudakis I.M., Boxerman J.L., Flanigan T.P., Reece R.M. First Confirmed Case of Powassan Neuroinvasive Disease in Rhode Island. IDCases. 2018;12:84–87. doi: 10.1016/j.idcr.2018.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Picheca C., Yogendrakumar V., Brooks J.I., Torres C., Pringle E., Zwicker J. Polio-Like Manifestation of Powassan Virus Infection with Anterior Horn Cell Involvement, Canada. Emerg. Infect. Dis. 2019;25:1609–1611. doi: 10.3201/eid2508.190399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khan A.M., Shahzad S.R., Ashraf M.F., Naseer U. Powassan Virus Encephalitis, Severe Babesiosis and Lyme Carditis in a Single Patient. BMJ Case Rep. 2019;12:e231645. doi: 10.1136/bcr-2019-231645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Allgaier J., Quarles R., Skiest D. Possible Prognostic Value of Serial Brain MRIs in Powassan Virus Encephalitis. Emerg. Infect. Dis. 2019;25:1956–1958. doi: 10.3201/eid2510.181262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colman D., Peaslee S. Powassan Virus in a Hunter Returning from a Trip in the Adirondack Park. Wilderness Environ. Med. 2020;31:87–90. doi: 10.1016/j.wem.2019.09.002. [DOI] [PubMed] [Google Scholar]
- 45.Koester T.M., Timothy P., Meece J.K., Osborn R.A., Frost H.M. Suspected Neuro-Invasive Powassan Virus Infection in a Pediatric Patient. Clin. Med. Res. 2020;18:95–98. doi: 10.3121/cmr.2020.1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yu Q., Matkovic E., Reagan-Steiner S., Denison A.M., Osborn R., Salamat S.M. A Fatal Case of Powassan Virus Encephalitis. J. Neuropathol. Exp. Neurol. 2020;79:1239–1243. doi: 10.1093/jnen/nlaa094. [DOI] [PubMed] [Google Scholar]
- 47.Feder H.M., Telford S., Goethert H.K., Wormser G.P. Powassan Virus Encephalitis Following Brief Attachment of Connecticut Deer Ticks. Clin. Infect. Dis. 2021;73:e2350–e2354. doi: 10.1093/cid/ciaa1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pach J.J., Zubair A.S., Traner C., Falcone G.J., Dewey J.J. Powassan Meningoencephalitis: A Case Report Highlighting Diagnosis and Management. Cureus. 2021;13:e16592. doi: 10.7759/cureus.16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Dumic I., Madrid C., Vitorovic D. Unusual Cause at an Unusual Time-Powassan Virus Rhombencephalitis. Int. J. Infect. Dis. 2021;103:88–90. doi: 10.1016/j.ijid.2020.11.159. [DOI] [PubMed] [Google Scholar]
- 50.Dumic I., Glomski B., Patel J., Nordin T., Nordstrom C.W., Sprecher L.J., Niendorf E., Singh A., Simeunovic K., Subramanian A., et al. “Double Trouble”: Severe Meningoencephalitis Due to Borrelia burgdorferi and Powassan Virus Co-Infection Successfully Treated with Intravenous Immunoglobulin. Am. J. Case Rep. 2021;22:e929952. doi: 10.12659/AJCR.929952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Taylor L., Condon T., Destrampe E.M., Brown J.A., McGavic J., Gould C.V., Chambers T.V., Kosoy O.I., Burkhalter K.L., Annambhotla P., et al. Powassan Virus Infection Likely Acquired through Blood Transfusion Presenting as Encephalitis in a Kidney Transplant Recipient. Clin. Infect. Dis. 2021;72:1051–1054. doi: 10.1093/cid/ciaa738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kroopnick A., Jia D.T., Rimmer K., Namale V.S., Kim C., Ofoezie U., Thakur K.T. Clinical Use of Steroids in Viral Central Nervous System (CNS) Infections: Three Challenging Cases. J. Neurovirol. 2021;27:727–734. doi: 10.1007/s13365-021-01008-5. [DOI] [PubMed] [Google Scholar]
- 53.Nord J.M., Goldberg N.R. Novel Case of Multifocal Choroiditis Following Powassan Virus Infection. Ocul. Immunol. Inflamm. 2021;30:1651–1653. doi: 10.1080/09273948.2021.1929335. [DOI] [PubMed] [Google Scholar]
- 54.Bazer D.A., Orwitz M., Koroneos N., Syritsyna O., Wirkowski E. Powassan Encephalitis: A Case Report from New York, USA. Case Rep. Neurol. Med. 2022;2022:8630349. doi: 10.1155/2022/8630349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johnson I.M., Scheckel C., Parikh S.A., Enzler M., Fugate J., Call T.G. Fatal Powassan Virus Encephalitis in Patients with Chronic Lymphocytic Leukemia. Blood Cancer J. 2022;12:143. doi: 10.1038/s41408-022-00737-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kakoullis L., Yalcin A., AbdelRazek M.A., Sasson J.P., Behlau I. Powassan Virus Infection Presenting as Acute Encephalitis in a Patient from the New England Area: A Case Report. Neurol. Sci. 2022;43:5119–5123. doi: 10.1007/s10072-022-06058-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Mendoza M.A., Hass R.M., Vaillant J., Johnson D.R., Theel E.S., Toledano M., Abu Saleh O. Powassan Virus Encephalitis: A Tertiary Center Experience. Clin. Infect. Dis. 2023:ciad454. doi: 10.1093/cid/ciad454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tyler K.L. Acute Viral Encephalitis. N. Engl. J. Med. 2018;379:557–566. doi: 10.1056/NEJMra1708714. [DOI] [PubMed] [Google Scholar]
- 59.Solomon T. Flavivirus Encephalitis. N. Engl. J. Med. 2004;351:370–378. doi: 10.1056/NEJMra030476. [DOI] [PubMed] [Google Scholar]
- 60.Oksi J., Marttila H., Soini H., Heikki A.H.O., Uksila J., Viljanen M.K. Early Dissemination of Borrelia burgdorferi without Generalized Symptoms in Patients with Erythema MigransNote. APMIS. 2001;109:581–588. doi: 10.1034/j.1600-0463.2001.d01-178.x. [DOI] [PubMed] [Google Scholar]
- 61.CDC Preventing Tick Bites. [(accessed on 25 July 2023)]; Available online: https://www.cdc.gov/ticks/avoid/on_people.html.
- 62.Clow K.M., Leighton P.A., Ogden N.H., Lindsay L.R., Michel P., Pearl D.L., Jardine C.M. Northward Range Expansion of Ixodes scapularis Evident over a Short Timescale in Ontario, Canada. PLoS ONE. 2017;12:e0189393. doi: 10.1371/journal.pone.0189393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dumic I., Severnini E. “Ticking Bomb”: The Impact of Climate Change on the Incidence of Lyme Disease. Can. J. Infect. Dis. Med. Microbiol. 2018;2018 doi: 10.1155/2018/5719081. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data pertaining to this study are presented in the current manuscript and its Supplementary Materials.