Abstract
The circular dichroism, CD, spectra of the telomere repeats of vertebrates, d(TTAGGG), indicate that parallel type quadruplex structures or disordered single-stranded structures are formed in low salt. Anti-parallel quadruplex structures are favored in the presence of high concentrations, 140 mM, of sodium. External loop, also known as propeller, parallel type structures are favored in the presence of high concentrations, 100 mM, of potassium in the presence of either 5 or 140 mM sodium. The cation dependence of the CD spectra of the vertebrate telomere repeat DNAs is distinctly different from that of the telomere repeats of Tetrahymena and Oxytricha as well as that of the thrombin binding aptamer. These results indicate that the external loop structures may be present in vertebrate telomeres under the conditions of high potassium and low sodium concentration found in nuclei.
INTRODUCTION
Telomeres are involved in the protection of the ends of linear chromosomes as well as in the maintenance of the length of chromosomes that are shortened during each round of replication (1–7). The telomere repeat of vertebrates is d(TTAGGG), of plants is d(TTTAGGG), of Tetrahymena is d(TTGGGG) and of Oxytricha is d(TTTTGGGG). The presence of runs of dG residues has lead to investigations of the conditions under which telomere repeat DNAs form quadruplex structures.
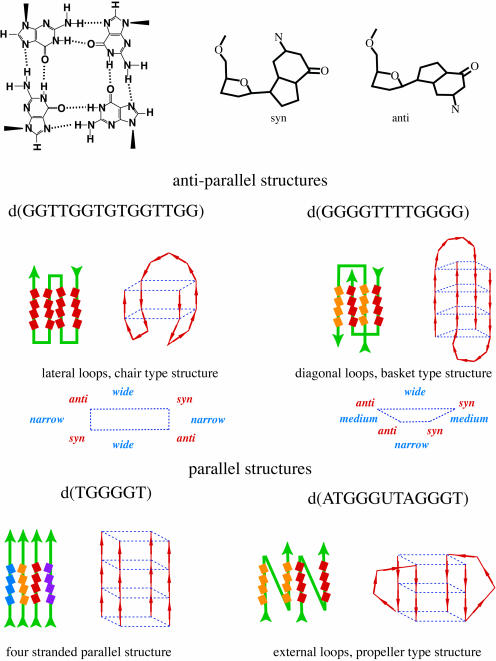
Telomere repeat sequences have been shown to form a variety of quadruplex, also referred to as G4 or tetrahelical, DNA structures (8). Some of the types of quadruplex structures that have been observed are depicted in Figure 1. There is also evidence that potassium may play a significant role in determining which, if any, quadruplex structure is formed by a telomere repeat sequence (9–15).
Figure 1.
Depictions of the anti-parallel structures formed by d(GGTTGGTGTGGTTGG) and dimers of d(GGGGTTTTGGGG) are shown as they are the parallel structures formed by tetramers of d(TGGGGT) and by dimers of d(TAGGGUTAGGGT). Also shown is a depiction of a dG quartet and dG residues in syn and anti configurations. The dG residues that are in quartets are depicted as boxes. Intramolecular quadruplex structures are shown with one color for the strands, dimers are depicted with a separate color for each strand as is the parallel quadruplex structure formed from four strands.
A motivation for the investigation of quadruplex DNA is to understand the transformation and to identify targets for chemotherapy (16–24). The inhibition of telomerase is thought to be a potential route to cancer therapy and ∼85% of human tumor cells have elevated telomerase activity (25). Molecules that bind to quadruplex DNA have been shown to inhibit telomerase and to be selectively toxic to tumor cells (26–28) and at least some of the cytotoxic activity of quadruplex binding ligands may arise from the disruption of telomere structure. Thus, it is of interest to gain more information about the interplay between the potassium dependence of the structures of telomere DNAs and the ligand binding to quadruplex DNAs.
Quadruplex DNA structures may be important in regions other than the telomere. Proteins with high affinity for quadruplex DNAs have been found, and defects in these proteins can lead to errors in replication, transcription and recombination as well as to increases in the rates of tumor formation and aging (7). Quadruplex DNAs have been shown to inhibit telomerase, HIV integrase and thrombin (7). There is evidence that the presence of quadruplex DNA can lead to errors in transcription, replication and recombination, while quadruplex DNA may play roles in recombination, the formation of the synaptonemal complex and regulation of the c-myc gene (29–33).
In this study, we have focused on the effects of potassium on repeats of the telomere sequences of vertebrates, Oxytricha and Tetrahymena. The circular dichroism, CD, spectra of a number of repeat DNAs have been obtained in the presence of high and low concentrations of sodium and potassium. DNAs of known anti-parallel and parallel structure were used as reference samples. NMR spectra have also been obtained on some of these DNAs.
The results indicate that high concentrations of potassium can induce the formation of external loop parallel-strand structures by repeats of the vertebrate telomere DNA sequence. A high concentration of sodium, in the absence of potassium, appears to induce the formation of anti-parallel structures. External loop structures have been previously observed for vertebrate telomere repeat DNAs in solution (34) and in crystals (35). In low salt conditions, the vertebrate telomere DNAs tend to be either in disordered single-stranded states or four-stranded parallel structures. The effects of potassium on the vertebrate telomere repeat DNAs appear to be distinctly different from those previously observed for the thrombin binding aptamer as well as for DNAs that contain repeats of the telomere sequences of either Tetrahymena or Oxytricha.
MATERIALS AND METHODS
Circular dichroism experiments
The CD spectra were obtained using a Jasco J-810 spectropolarimeter equipped with a six cell, programmable Peltier junction temperature controlled cell holder. The DNA samples used in the CD experiments were at a concentration of 1.1 × 10−5 M. The buffers used for the CD experiments were 20 mM phosphate, 140 mM total Na and 0.1 mM EDTA at pH 7.0; 2.5 mM phosphate, 5 mM total Na and 0.1 mM EDTA at pH 7.0. The KCl additions increased the volume of the samples <10%. The DNA samples were annealed by heating to 363 K and then cooled to room temperature over a period of 8 h before obtaining the CD spectra. The CD measurements used a 0.2 cm path cell and were carried out at 293 K. The CD spectra were averaged over three scans and the data were obtained with a 1 nm slit width from 350 to 200 nm at 0.1 nm intervals. The CD spectrum of the buffer solution was subtracted from that of the sample containing DNA.
The solution conditions for the four reference DNAs all contained 0.1 mM EDTA at pH 7.0. The d(GGTTGGTGTGGTTGG) solution also contained 100 mM sodium and 140 mM potassium. The d(GGGGTTTTGGGG) solution contained 140 mM sodium. The d(TGGGGT) solution contained 140 mM sodium and 100 mM potassium. The d(TAGGGUTAGGGT) solution contained 5 mM sodium and 100 mM potassium.
NMR spectra
The NMR spectra were obtained using a Varian Unity Inova 500 spectrometer operating at a magnetic field of 11.75 T. The spectra were acquired with the samples at 298 K with a repetition rate of 0.17 s. The water solvent signal suppression was accomplished by using the watergate pulse sequence. The samples were prepared in 95% H2O/5% 2H2O and were annealed for ∼10 min at 373 K followed by slow cooling to room temperature. The DNA concentrations were 0.5–1.0 mM.
DNA samples
The DNAs were obtained from Integrated DNA Technologies, Inc. (Coralville, Iowa). The DNA samples were supplied as HPLC-purified samples and desalted by ethanol precipitation before use. The concentration of each DNA sample was determined from the absorbance value at 260 nm at 293 K of a sample prepared in distilled H2O by using the nearest neighbor extinction coefficients. The hypochromicity of each DNA was determined by melting and the hypochromicities were found to be in the range of 9–14 %. The extinction coefficients of the vertebrate repeat DNA samples studied here and the naming convention used are listed below.
| d(GGGTTAGGG) | 92 800 | Vet 1.5 |
| d(TTAGGGTTAGGG) | 122 400 | Vet T2 |
| d(GGGTTAGGGTTA) | 124 100 | Vet 2 |
| d(GGGTTAGGGTTAGGG) | 153 900 | Vet 2.5 |
| d(GGGTTAGGGTTAGGGTTAGGG) | 215 000 | Vet 3.5 |
| d(TTAGGGTTAGGGTTAGGGTTAGGG) | 244 600 | Vet T4 |
| d(GGGTTAGGGTTAGGGTTAGGGTTA) | 246 300 | Vet 4 |
| d(TGGGGTTGGGGT) | 115 000 | |
| d(GGGGTTTTGGGG) | 110 000 | |
| d(GGTTGGTGTGGTTGG) | 143 300 | |
| d(TGGGGT) | 57 800 | |
| d(TAGGGUTAGGGT) | 121 500 |
RESULTS AND DISCUSSION
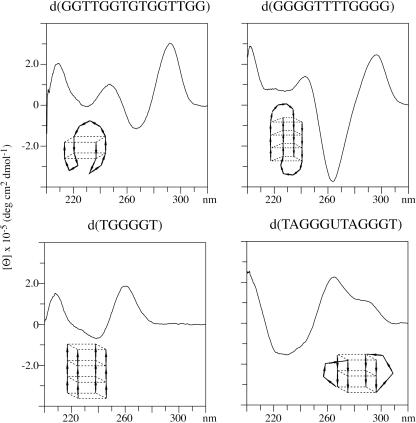
CD has been used to examine the structures of vertebrate repeat DNAs by comparing their spectra with those of quadruplex DNAs of known structure. All of the CD spectra were obtained on annealed samples. The reference spectrum for a four-stranded parallel quadruplex structure is that of d(TGGGGT) (36). This DNA has a positive band near 260 nm that has been previously attributed to parallel-strand quadruplex structures (37,38). The thrombin binding aptamer, d(GGTTGGTGTGGTTGG), adopts a chair type anti-parallel structure in solution (39,40). The CD spectrum is shown in Figure 2 and this DNA has a positive band near 290 nm and a negative band near 260 nm that have been found to be characteristic of an anti-parallel structure (41–43). A symmetric basket type anti-parallel structure is formed by d(GGGGTTTTGGGG) as determined by using NMR (44) and crystallography (45). This DNA also has a CD spectrum characteristic of an anti-parallel structure (46) as shown in Figure 2. The CD spectrum of d(GGGGTTTTGGGG) has a more pronounced negative band near 260 nm than does d(GGTTGGTGTGGTTGG).
Figure 2.
The CD spectra of four quadruplex DNAs of known structure are shown. Depictions of the structures of each of the DNAs are also shown.
The other reference sample is d(TAGGGUTAGGGT), which adopts an external loop type structure in the presence of 100 mM potassium (34). The CD spectrum of this sample is distinctly different from that of the four-stranded parallel structures or the anti-parallel structures as shown from the data in Figure 2. This external loop DNA has a CD spectrum that has positive maxima near 260 nm and near 290 nm. As discussed below, the 260 nm band is assigned to the parallel-strand quartets and the 290 nm band to the external loop residues. In the presence of 100 mM potassium, d(TAGGGTTAGGGT) is present in nearly equal percentages of an anti-parallel structure and an external loop structure (47).
CD spectra of d(TTAGGGTTAGGG)
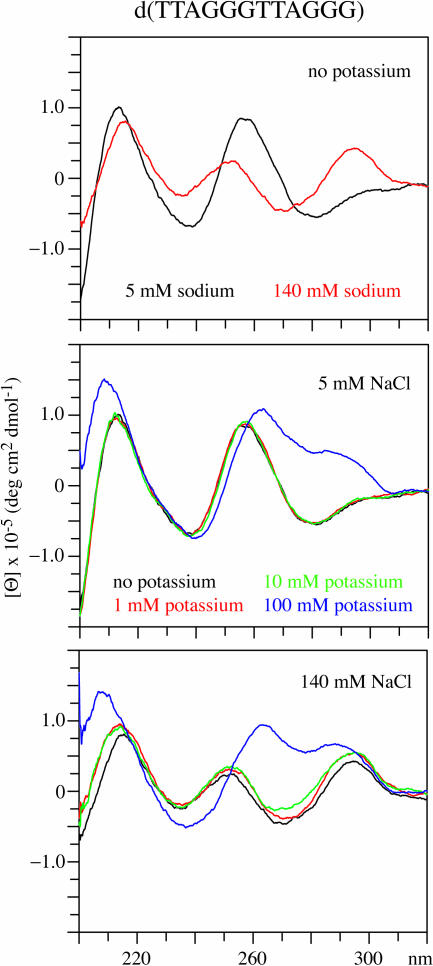
The CD spectra of d(TTAGGGTTAGGG), Vet T2, in the presence of 5 and 140 mM sodium are shown in Figure 3. In the presence of 5 mM sodium, the CD spectrum is similar to that of a single-stranded DNA with a positive band near 250 nm. The proton NMR spectrum of this DNA, shown in Supplementary Material, indicates that this DNA is disordered in 5 mM sodium. When the DNA is in 140 mM sodium the spectrum is characteristic of an anti-parallel structure. The spectrum in 140 mM sodium does not contain a large negative peak near 260 nm and this may be owing to the presence of a mixture of both four-stranded parallel and anti-parallel structures. The NMR spectrum (data not shown) of the sample in 140 mM sodium indicates that quartets and also multiple conformers of the DNA are present.
Figure 3.
The CD spectrum of d(TTAGGGTTAGGG) in the presence of 5 and 140 mM sodium is shown in the upper panel. The CD spectrum of this DNA in the presence of 5 mM sodium with 0, 1, 10 or 100 mM potassium is shown in the middle panel. In the lower panel, the CD spectra of this DNA in the presence of 140 mM sodium with 0, 1, 10 or 100 mM potassium are shown.
The CD spectrum changes to that of an external loop quadruplex structure in the presence of 100 mM potassium with either 5 or 140 mM sodium as shown in the results in Figure 3. The CD spectrum is essentially the same with 5 or 140 mM sodium when 100 mM potassium is present. There are small changes in the CD spectrum upon the addition of 10 mM potassium to the DNA in the presence of 5 or 140 mM sodium. The Supplementary Material contains the NMR spectrum of this DNA in the presence of 100 mM potassium. The NMR results are consistent with an external loop structure formed by a symmetric dimer.
The CD and NMR results for d(TTAGGGTTAGGG) indicate that in low salt conditions, 5 mM sodium with up to 10 mM potassium, the DNA is primarily in a disordered, single-strand state. In the presence of 140 mM sodium the DNA has a CD spectrum characteristic of an anti-parallel structure. In the presence of 100 mM potassium, the CD spectrum indicates that an external loop parallel-strand structure is present with sodium concentrations in the range of 5–140 mM.
CD spectra of vertebrate telomere repeats
The CD spectra of DNAs with 2.5, 3.5 and 4 repeats of the vertebrate telomere sequence have been examined under the same conditions as described above for d(TTAGGGTTAGGG), Vet T2. In the presence of 140 mM sodium these DNAs have CD spectra characteristic of anti-parallel structures as shown in Figures 4 and 5. All of the spectra contain positive bands near 290 nm and negative bands near 260 nm. These results indicate that these vertebrate telomere repeats adopt anti-parallel structures in the presence of 140 mM sodium.
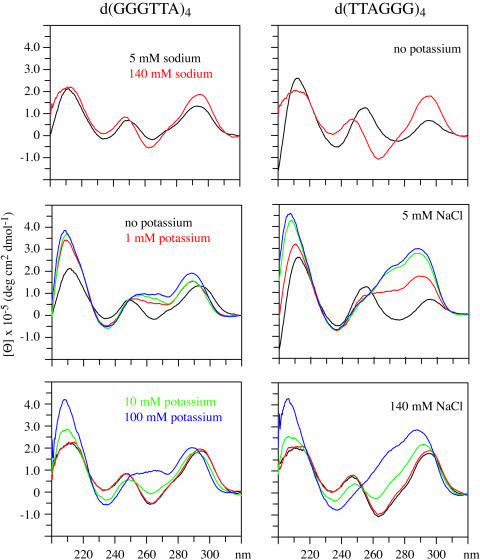
Figure 4.
The CD spectra of d(GGGTTA)4 and d(TTAGGG)4 in the presence of 5 and 140 mM sodium are shown in the upper panels. The CD spectra of these DNAs in the presence of 5 mM sodium with 0, 1, 10 or 100 mM potassium are shown in the middle panels. In the lower panels, the CD spectra of these DNAs in the presence of 140 mM sodium with 0, 1, 10 or 100 mM potassium are shown.
Figure 5.
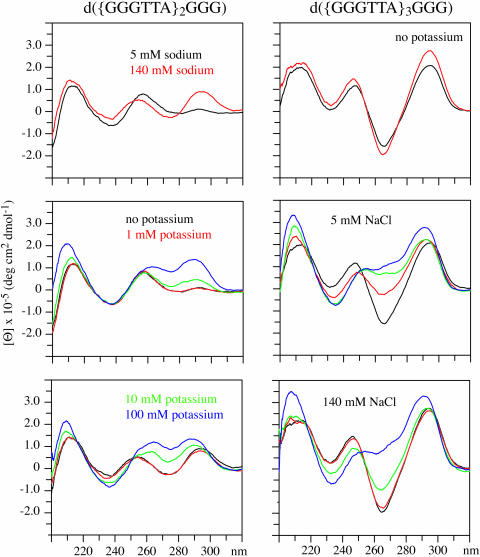
The CD spectra of d({GGGTTA}2GGG) and d({GGGTTA}3GGG) in the presence of 5 and 140 mM sodium are shown in the upper panels. The CD spectra of these DNAs in the presence of 5 mM sodium with 0, 1, 10 or 100 mM potassium are shown in the middle panels. In the lower panels, the CD spectra of these DNAs in the presence of 140 mM sodium with 0, 1, 10 or 100 mM potassium are shown.
The spectra of the vertebrate telomere repeat DNAs in the presence of 5 mM sodium vary depending on the number of repeats as shown in the results in Figures 4 and 5. The spectra of the Vet 3.5 and Vet 4 are of the anti-parallel type whereas those of the Vet 2.5 and Vet T4 samples appear to indicate that more than one structural type is present when the DNAs are in the presence of 5 mM sodium.
The presence of 100 mM potassium induces the formation of structures that have CD spectra characteristic of external loop structures both in the presence of 5 mM and in the presence of 140 mM sodium for Vet 2.5, Vet 3.5, Vet 4 and Vet T4, as the results demonstrate in Figures 4 and 5. The changes induced by potassium are analogous to those presented above for Vet T2. The presence of 100 mM potassium induces the formation of structures with CD spectra that are characteristic of external loop structures whether 5 or 140 mM sodium is present.
The CD spectra indicate that for Vet 2.5 10 mM potassium induces more change when in the presence of 140 mM sodium than in the presence of 5 mM sodium whereas the opposite is the case for Vet 4, Vet T4, Vet 3.5 and Vet T2. The potassium effect for each of these vertebrate repeat DNAs is not an ionic strength effect since 140 mM sodium gives distinctly different CD spectra than does the combination of 100 mM potassium and 5 mM sodium.
The ratio of the intensity of the maxima near 260 and 290 nm varies between the vertebrate telomere repeat DNAs but the overall pattern is the same for each of the DNAs in the presence of 100 mM potassium. The CD results indicate that for this set of vertebrate telomere repeat DNAs the presence of 100 mM potassium favors the formation of external loop structures.
Crystal structures of the external loop quadruplex structures of d(AGGG{TTAGGG}3) and d(TAGGGTTAGGGT) have been obtained (8,35). The DNAs were crystallized from a solution that contained 50 mM potassium. The initial conditions for the hanging drops contained 100 mM potassium for the d(TAGGGTTAGGGT) crystals and 300 mM potassium for the d{AGGG(TTAGGG)3} crystals.
Effect of the dA residue
The CD spectra of d(GGGTTAGGG) and d(GGGTTTGGG) were compared in order to gain information about the effect of the dA residue. The CD spectra of both samples show similar potassium sensitivity as shown in the Supplementary Material. There are some differences in the intensities of the bands near 290 and 260 nm but the general patterns of the spectral changes are similar in the two cases.
Tentative assignments of the 260 and 290 nm bands of external loop structures
DNAs containing portions of the c-myc promoter sequence have been shown to form a parallel-strand external loop structure in the presence of 100 mM potassium (29,30). The CD spectra of these c-myc promoter DNAs have a positive band at 260 nm but do not exhibit the positive band at 290 nm observed from the vertebrate telomere DNAs (29). The external loops of the vertebrate telomere DNAs contain three residues, d(TTA), while those of the c-myc DNAs contain a single dA residue. This suggests that the 290 nm band is due to the loop residues and the 260 nm band is due to the parallel quartet dG residues. The differences observed between d(GGGTTAGGG) and d(GGGTTTGGG) is also in the relative intensities of the 260 and 290 nm bands.
A comparison of the crystal structures of four-strand parallel and external loop structures indicated that the quartet regions have very similar structures in the two cases (8,35). This is consistent with the quartets of both external loop and four-stranded parallel-strand structures giving rise to the positive 260 nm band and the loop residues at the 290 nm band.
CD spectra of Tetrahymena and Oxytricha telomere repeats
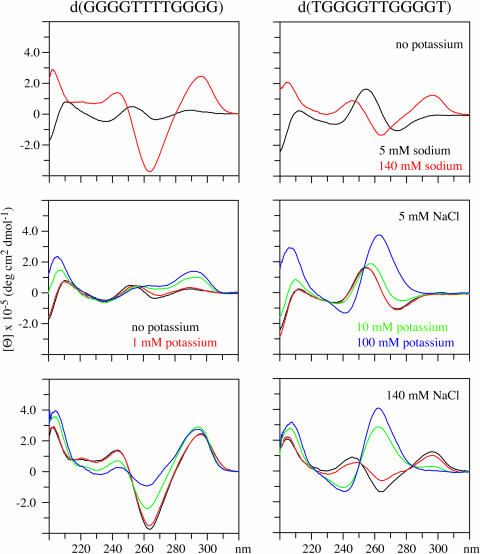
In contrast to the behavior of the DNAs containing vertebrate telomere repeats the CD spectrum of the Oxytricha repeat DNA, d(GGGGTTTTGGGG), is of the anti-parallel type under all the conditions examined, as shown in the results in Figure 6. An NMR-based study showed that the addition of potassium to this DNA alters the loop structure but not the basket type anti-parallel quartet structure (13). The structure of a DNA containing four repeats of the Oxytricha sequence has been shown to form an anti-parallel structure in the presence of 100 mM sodium (48).
Figure 6.
The CD spectra of d(GGGGTTTTGGGG) and d(TGGGGTTGGGGT) in the presence of 5 and 140 mM sodium are shown in the upper panels. The CD spectra of these DNAs in the presence of 5 mM sodium with 0, 1, 10 or 100 mM potassium are shown in the middle panels. In the upper panels, the CD spectra of these DNAs in the presence of 140 mM sodium with 0, 1, 10 or 100 mM potassium are shown.
The Tetrahymena repeat DNA, d(TGGGGTTGGGGT), has been shown to be a mixture of two anti-parallel structures in the presence of 100 mM sodium (47). The CD data for this DNA shown in Figure 6 are in accordance with the NMR derived structure. The CD spectrum of d(TGGGGTTGGGGT) in 5 mM sodium indicates that some four-stranded parallel structure may be present. These results indicate that high sodium concentration is needed for this DNA to adopt anti-parallel structures. The addition of 100 mM potassium to this DNA, in either 5 or 100 mM sodium, suggests that potassium induces the formation of parallel type structures as indicated by the positive band near 260 nm.
The behavior of the CD spectra of both the Oxytricha and the Tetrahymena repeat DNAs are different from that observed for any of the vertebrate telomere repeats. These results indicate that the Oxytricha and Tetrahymena repeat DNAs form different types of structures in the presence of physiological levels of potassium than do the vertebrate telomere repeats.
CD of the thrombin binding aptamer
The CD spectrum of the thrombin binding aptamer d(GGTTGGTGTGGTTGG) remains characteristic of an anti-parallel structure over the range of potassium concentrations from 1–100 mM. The CD spectrum in the presence of 100 mM potassium is shown in Figure 2 and the CD spectra obtained in the presence of other concentrations of potassium have been reported previously. The thrombin binding aptamer requires ∼1 mM potassium, or a suitable substitute, for the formation of the chair type anti-parallel structure (9,10). This behavior was not observed for any of the telomere repeat DNAs examined here and indicates that the thrombin binding aptamer, similar to the Tetrahymena and Oxytricha repeats, is not a good model system for the quadruplex structures formed by the vertebrate telomere repeat.
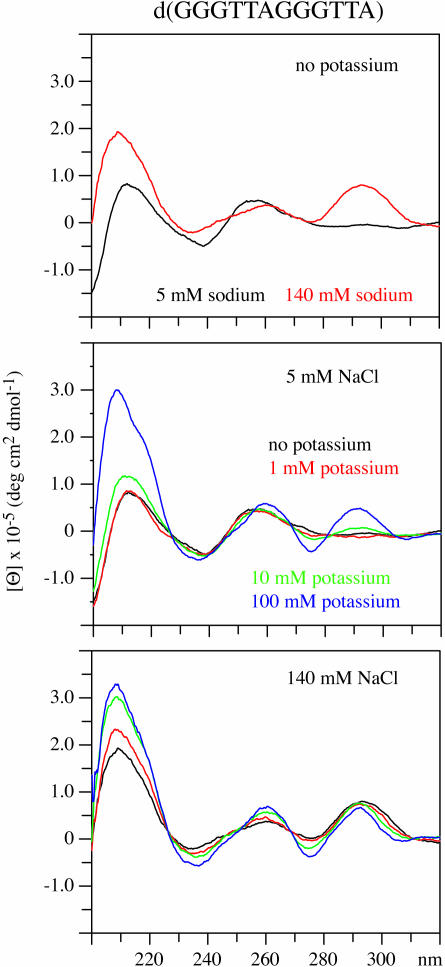
d(GGGTTAGGGTTA) behaves differently from that of the other vertebrate telomere repeats
The CD spectra of d(GGGTTAGGGTTA), Vet 2, shown in Figure 7 do not show the characteristic pattern of external loop structures when the DNA is in the presence of 100 mM potassium. The CD spectrum of Vet 2 is largely unchanged upon the addition of potassium whether the DNA is in the presence of 5 or 140 mM sodium. The CD spectrum does not appear to be comparable with any of the four reference types.
Figure 7.
The CD spectrum of d(GGGTTAGGGTTA) in the presence of 5 and 140 mM sodium are shown in the upper panel. The CD spectrum of this DNA in the presence of 5 mM sodium with 0, 1, 10 or 100 mM potassium is shown in the middle panel. In the lower panel, the CD spectra of this DNA in the presence of 140 mM sodium with 0, 1, 10 or 100 mM potassium are shown.
The proton NMR spectrum of Vet 2 has been obtained in the presence and in the absence of 100 mM potassium and these spectra are included in the Supplementary Material. The imino proton spectrum in the absence of potassium is consistent with a quadruplex structure formed by asymmetric dimers as there are at least 12 dG imino resonances. The imino proton spectrum of the DNA in the presence of 100 mM potassium indicates that quartets are present and that there are also resonances downfield of 13 p.p.m. that are indicative of the presence of A–T base pairs. It is noted that A–T base pairs are consistent with the DNA adopting a four-stranded parallel structure but other quadruplex structural types have been observed to allow A–T pairs to form (49). The structure of d(GGGTTAGGGTTA) in the presence of potassium is under investigation and may be of a novel type.
Comparison with selected prior results
A prior study of Vet T4 and Vet 3.5 found that the reactivity of the N7 positions of the dA residues are different for the samples in 70 mM sodium when compared with the samples in 70 mM potassium as are the gel mobilities (50). Their gel results showed one predominant band for Vet T4 in 70 mM potassium and for both Vet T4 and Vet 3.5 in 70 mM sodium. We have obtained one predominant band for these and other vertebrate telomere repeat DNAs in 100 mM potassium (data not shown). The CD spectra of Vet T4 and Vet 3.5 that they obtained (50) are quite similar to the ones shown here. External loop structures had not been discovered when this prior study was published and hence CD spectra of these types of structures were not available for comparison. They assigned the CD spectra of the vertebrate telomere repeats obtained in the presence of sodium to anti-parallel structures as is performed in this study.
The reactions of d(AGGG{TTAGGG}3) with platinum (51) and 125I degradation (52) have been studied previously (52). An NMR study of d(AGGG{TTAGGG}3) showed that in 100 mM sodium the structure is an anti-parallel basket type that has low stability for a quadruplex structure requiring the NMR data to be acquired with the sample at 280 K (53). This DNA exhibited imino proton exchange rates with water on the timescale of seconds, even at 280 K, indicating that the structure has low kinetic stability (53). Other quadruplex DNAs have been shown to have imino proton exchange rates on the timescale of days to weeks for the dG residues in the quartets. When crystallized in the presence of high levels of potassium d(AGGG{TTAGGG}3) was found to have an external loop type structure (8,35). The NMR spectrum of this DNA in the presence of potassium is not interpretable. The low thermal and kinetic stabilities of d(AGGG{TTAGGG}3) may reduce the utility of this DNA in chemical-based structural studies.
The results of the platinum reactions d(AGGG{TTAGGG}3) were taken to indicate that a basket type structure is present in both 50 mM sodium and 50 mM potassium (51). The reaction of a dG residue with platinum requires that the N7 is accessible. If a dG residue is present in a quartet this means that the platinum reaction may be predominantly occurring from a disrupted or partially disrupted state. The platination experiments were carried out under conditions, 310 K, at which the structure of the DNA is likely to be significantly disrupted.
The structure of modified forms of d(AGGG{TTAGGG}3) that contained extra residues on the 3′ and/or 5′ ends have also been investigated using 125I. The decay of 125I cleaves the DNA at a rate inversely proportional to the distance from the radiolabel. The 125I cleavage reactions were carried out on frozen samples and the results in the presence of sodium are consistent with the NMR anti-parallel basket type quadruplex structure obtained in the presence of sodium. Significant differences in the 125I cleavage pattern obtained with 100 mM potassium and 100 mM sodium were observed. The observed cleavage pattern for the modified forms of d(AGGG{TTAGGG}3) are not consistent with either an anti-parallel basket type structure or an external loop type structure.
CONCLUSION
These results indicate that vertebrate telomere repeat DNAs favor the formation of external loop parallel-strand structures in the presence of potassium and sodium concentrations similar to those that are present in vivo. The structures formed by the vertebrate telomere repeat DNAs are distinctly different from those of repeats of the Oxytricha or Tetrahymena telomere and from that of the thrombin binding aptamer. The cation dependence of the vertebrate telomere repeat DNAs are also distinctly different from that of the Oxytricha or Tetrahymena telomere repeats and from that of the thrombin binding aptamer. The formation of extended structures that contain more than one quadruplex unit by vertebrate telomere repeat DNAs may have three external loops per unit rather than two. The structures of DNAs with more than four repeats of the vertebrate telomere sequence are now being investigated.
SUPPLEMENTARY MATERIAL
Supplementary Material is available at NAR Online.
Supplementary Material
Acknowledgments
This work was supported by NIH grant GM06581. Funding to pay the Open Access publication charges for this article was provided by NIH grant GM06581.
Conflict of interest statement. None declared.
REFERENCES
- 1.Wai L.K. Telomeres, telomerase, and tumorigenesis—a review. MedGenMed. 2004;6:19. [PMC free article] [PubMed] [Google Scholar]
- 2.Smogorzewska A., de Lange T. Regulation of telomerase by telomeric proteins. Annu. Rev. Biochem. 2004;73:177–208. doi: 10.1146/annurev.biochem.73.071403.160049. [DOI] [PubMed] [Google Scholar]
- 3.Harrington L. Those dam-aged telomeres! Curr. Opin. Genet Dev. 2004;14:22–28. doi: 10.1016/j.gde.2003.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Cech T.R. Beginning to understand the end of the chromosome. Cell. 2004;116:273–279. doi: 10.1016/s0092-8674(04)00038-8. [DOI] [PubMed] [Google Scholar]
- 5.Colgin L., Reddel R. Telomere biology: a new player in the end zone. Curr. Biol. 2004;14:R901–R902. doi: 10.1016/j.cub.2004.09.075. [DOI] [PubMed] [Google Scholar]
- 6.de Lange T. T-loops and the origin of telomeres. Nature Rev. Mol. Cell Biol. 2004;5:323–329. doi: 10.1038/nrm1359. [DOI] [PubMed] [Google Scholar]
- 7.Arthanari H., Bolton P.H. Functional and dysfunctional roles of quadruplex DNA in cells. Chem. Biol. 2001;8:221–230. doi: 10.1016/s1074-5521(01)00007-2. [DOI] [PubMed] [Google Scholar]
- 8.Neidle S., Parkinson G.N. The structure of telomeric DNA. Curr. Opin. Struct. Biol. 2003;13:275–283. doi: 10.1016/s0959-440x(03)00072-1. [DOI] [PubMed] [Google Scholar]
- 9.Marathias V.M., Bolton P.H. Determinants of DNA quadruplex structural type: sequence and potassium binding. Biochemistry. 1999;38:4355–4364. doi: 10.1021/bi982604+. [DOI] [PubMed] [Google Scholar]
- 10.Marathias V.M., Bolton P.H. Structures of the potassium-saturated, 2:1, and intermediate, 1:1, forms of a quadruplex DNA. Nucleic Acids Res. 2000;28:1969–1977. doi: 10.1093/nar/28.9.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Oka Y., Thomas C.A., Jr The cohering telomeres of Oxytricha. Nucleic Acids Res. 1987;15:8877–8898. doi: 10.1093/nar/15.21.8877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patel D.J. Structural biology: a molecular propeller. Nature. 2002;417:807–808. doi: 10.1038/417807a. [DOI] [PubMed] [Google Scholar]
- 13.Schultze P., Hud N.V., Smith F.W., Feigon J. The effect of sodium, potassium and ammonium ions on the conformation of the dimeric quadruplex formed by the Oxytricha nova telomere repeat oligonucleotide d(G(4)T(4)G(4)) Nucleic Acids Res. 1999;27:3018–3028. doi: 10.1093/nar/27.15.3018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sen D., Gilbert W. A sodium–potassium switch in the formation of four-stranded G4-DNA. Nature. 1990;344:410–414. doi: 10.1038/344410a0. [DOI] [PubMed] [Google Scholar]
- 15.Takenaka S., Ueyama H., Nojima T., Takagi M. Comparison of potassium ion preference of potassium-sensing oligonucleotides, PSO-1 and PSO-2, carrying the human and Oxytricha telomeric sequence, respectively. Anal. Bioanal. Chem. 2003;375:1006–1010. doi: 10.1007/s00216-003-1799-z. [DOI] [PubMed] [Google Scholar]
- 16.Callen E., Surralles J. Telomere dysfunction in genome instability syndromes. Mutat. Res. 2004;567:85–104. doi: 10.1016/j.mrrev.2004.06.003. [DOI] [PubMed] [Google Scholar]
- 17.von Zglinicki T. Telomeres, telomerase and the cancer cell: an introduction. Cancer Lett. 2003;194:137–138. doi: 10.1016/s0304-3835(02)00700-0. [DOI] [PubMed] [Google Scholar]
- 18.Stampfer M.R., Yaswen P. Human epithelial cell immortalization as a step in carcinogenesis. Cancer Lett. 2003;194:199–208. doi: 10.1016/s0304-3835(02)00707-3. [DOI] [PubMed] [Google Scholar]
- 19.Saretzki G. Telomerase inhibition as cancer therapy. Cancer Lett. 2003;194:209–219. doi: 10.1016/s0304-3835(02)00708-5. [DOI] [PubMed] [Google Scholar]
- 20.Neidle S., Parkinson G. Telomere maintenance as a target for anticancer drug discovery. Nature Rev. Drug Discov. 2002;1:383–393. doi: 10.1038/nrd793. [DOI] [PubMed] [Google Scholar]
- 21.Incles C.M., Schultes C.M., Neidle S. Telomerase inhibitors in cancer therapy: current status and future directions. Curr. Opin. Investig. Drugs. 2003;4:675–685. [PubMed] [Google Scholar]
- 22.Gowan S.M., Harrison J.R., Patterson L., Valenti M., Read M.A., Neidle S., Kelland L.R. A G-quadruplex-interactive potent small-molecule inhibitor of telomerase exhibiting in vitro and in vivo antitumor activity. Mol. Pharmacol. 2002;61:1154–1162. doi: 10.1124/mol.61.5.1154. [DOI] [PubMed] [Google Scholar]
- 23.Hurley L.H. DNA and its associated processes as targets for cancer therapy. Nature Rev. Cancer. 2002;2:188–200. doi: 10.1038/nrc749. [DOI] [PubMed] [Google Scholar]
- 24.Rezler E.M., Bearss D.J., Hurley L.H. Telomeres and telomerases as drug targets. Curr. Opin. Pharmacol. 2002;2:415–423. doi: 10.1016/s1471-4892(02)00182-0. [DOI] [PubMed] [Google Scholar]
- 25.Brunori M., Luciano P., Gilson E., Geli V. The telomerase cycle: normal and pathological aspects. J. Mol. Med. 2005;4:4. doi: 10.1007/s00109-004-0616-2. [DOI] [PubMed] [Google Scholar]
- 26.Shammas M.A., Shmookler Reis R.J., Li C., Koley H., Hurley L.H., Anderson K.C., Munshi N.C. Telomerase inhibition and cell growth arrest after telomestatin treatment in multiple myeloma. Clin. Cancer Res. 2004;10:770–776. doi: 10.1158/1078-0432.ccr-0793-03. [DOI] [PubMed] [Google Scholar]
- 27.Kim M.Y., Gleason-Guzman M., Izbicka E., Nishioka D., Hurley L.H. The different biological effects of telomestatin and TMPyP4 can be attributed to their selectivity for interaction with intramolecular or intermolecular G-quadruplex structures. Cancer Res. 2003;63:3247–3256. [PubMed] [Google Scholar]
- 28.Kim M.Y., Vankayalapati H., Shin-Ya K., Wierzba K., Hurley L.H. Telomestatin, a potent telomerase inhibitor that interacts quite specifically with the human telomeric intramolecular G-quadruplex. J. Am. Chem. Soc. 2002;124:2098–2099. doi: 10.1021/ja017308q. [DOI] [PubMed] [Google Scholar]
- 29.Seenisamy J., Rezler E.M., Powell T.J., Tye D., Gokhale V., Joshi C.S., Siddiqui-Jain A., Hurley L.H. The dynamic character of the G-quadruplex element in the c-MYC promoter and modification by TMPyP4. J. Am. Chem. Soc. 2004;126:8702–8709. doi: 10.1021/ja040022b. [DOI] [PubMed] [Google Scholar]
- 30.Phan A.T., Modi Y.S., Patel D.J. Propeller-type parallel-stranded G-quadruplexes in the human c-myc promoter. J. Am. Chem. Soc. 2004;126:8710–8716. doi: 10.1021/ja048805k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Han H., Langley D.R., Rangan A., Hurley L.H. Selective interactions of cationic porphyrins with G-quadruplex structures. J. Am. Chem. Soc. 2001;123:8902–8913. doi: 10.1021/ja002179j. [DOI] [PubMed] [Google Scholar]
- 32.Huber M.D., Lee D.C., Maizels N. G4 DNA unwinding by BLM and Sgs1p: substrate specificity and substrate-specific inhibition. Nucleic Acids Res. 2002;30:3954–3961. doi: 10.1093/nar/gkf530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Muniyappa K., Anuradha S., Byers B. Yeast meiosis-specific protein Hop1 binds to G4 DNA and promotes its formation. Mol. Cell. Biol. 2000;20:1361–1369. doi: 10.1128/mcb.20.4.1361-1369.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phan A.T., Patel D.J. Two-repeat human telomeric d(TAGGGTTAGGGT) sequence forms interconverting parallel and antiparallel G-quadruplexes in solution: distinct topologies, thermodynamic properties, and folding/unfolding kinetics. J. Am. Chem. Soc. 2003;125:15021–15027. doi: 10.1021/ja037616j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Parkinson G.N., Lee M.P., Neidle S. Crystal structure of parallel quadruplexes from human telomeric DNA. Nature. 2002;417:876–880. doi: 10.1038/nature755. [DOI] [PubMed] [Google Scholar]
- 36.Read M.A., Neidle S. Structural characterization of a guanine–quadruplex ligand complex. Biochemistry. 2000;39:13422–13432. doi: 10.1021/bi001584k. [DOI] [PubMed] [Google Scholar]
- 37.Hardin C.C., Watson T., Corregan M., Bailey C. Cation-dependent transition between the quadruplex and Watson–Crick hairpin forms of d(CGCG3GCG) Biochemistry. 1992;31:833–841. doi: 10.1021/bi00118a028. [DOI] [PubMed] [Google Scholar]
- 38.Hardin C.C., Henderson E., Watson T., Prosser J.K. Monovalent cation induced structural transitions in telomeric DNAs: G-DNA folding intermediates. Biochemistry. 1991;30:4460–4472. doi: 10.1021/bi00232a013. [DOI] [PubMed] [Google Scholar]
- 39.Schultze P., Macaya R.F., Feigon J. Three-dimensional solution structure of the thrombin-binding DNA aptamer d(GGTTGGTGTGGTTGG) J. Mol. Biol. 1994;235:1532–1547. doi: 10.1006/jmbi.1994.1105. [DOI] [PubMed] [Google Scholar]
- 40.Wang K.Y., Krawczyk S.H., Bischofberger N., Swaminathan S., Bolton P.H. The tertiary structure of a DNA aptamer which binds to and inhibits thrombin determines activity. Biochemistry. 1993;32:11285–11292. doi: 10.1021/bi00093a004. [DOI] [PubMed] [Google Scholar]
- 41.Shafer R.H. Stability and structure of model DNA triplexes and quadruplexes and their interactions with small ligands. Prog. Nucleic Acid Res. Mol. Biol. 1998;59:55–94. doi: 10.1016/s0079-6603(08)61029-6. [DOI] [PubMed] [Google Scholar]
- 42.Shafer R.H., Smirnov I. Biological aspects of DNA/RNA quadruplexes. Biopolymers. 2000;56:209–227. doi: 10.1002/1097-0282(2000/2001)56:3<209::AID-BIP10018>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- 43.Smirnov I., Shafer R.H. Effect of loop sequence and size on DNA aptamer stability. Biochemistry. 2000;39:1462–1468. doi: 10.1021/bi9919044. [DOI] [PubMed] [Google Scholar]
- 44.Schultze P., Smith F.W., Feigon J. Refined solution structure of the dimeric quadruplex formed from the Oxytricha telomeric oligonucleotide d(GGGGTTTTGGGG) Structure. 1994;2:221–233. doi: 10.1016/s0969-2126(00)00023-x. [DOI] [PubMed] [Google Scholar]
- 45.Haider S., Parkinson G.N., Neidle S. Crystal structure of the potassium form of an Oxytricha nova G-quadruplex. J. Mol. Biol. 2002;320:189–200. doi: 10.1016/S0022-2836(02)00428-X. [DOI] [PubMed] [Google Scholar]
- 46.Krafft C., Benevides J.M., Thomas G.J., Jr. Secondary structure polymorphism in Oxytricha nova telomeric DNA. Nucleic Acids Res. 2002;30:3981–3991. doi: 10.1093/nar/gkf517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Phan A.T., Modi Y.S., Patel D.J. Two-repeat Tetrahymena telomeric d(TGGGGTTGGGGT) sequence interconverts between asymmetric dimeric G-quadruplexes in solution. J. Mol. Biol. 2004;338:93–102. doi: 10.1016/j.jmb.2004.02.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gilbert D.E., Feigon J. Multistranded DNA structures. Curr. Opin. Struct. Biol. 1999;9:305–314. doi: 10.1016/S0959-440X(99)80041-4. [DOI] [PubMed] [Google Scholar]
- 49.Zhang N., Gorin A., Majumdar A., Kettani A., Chernichenko N., Skripkin E., Patel D.J. Dimeric DNA quadruplex containing major groove-aligned A-T-A-T and G-C-G-C tetrads stabilized by inter-subunit Watson–Crick A-T and G-C pairs. J. Mol. Biol. 2001;312:1073–1088. doi: 10.1006/jmbi.2001.5002. [DOI] [PubMed] [Google Scholar]
- 50.Balagurumoorthy P., Brahmachari S.K. Structure and stability of human telomeric sequence. J. Biol. Chem. 1994;269:21858–21869. [PubMed] [Google Scholar]
- 51.Redon S., Bombard S., Elizondo-Riojas M.A., Chottard J.C. Platinum cross-linking of adenines and guanines on the quadruplex structures of the AG3(T2AG3)3 and (T2AG3)4 human telomere sequences in Na+ and K+ solutions. Nucleic Acids Res. 2003;31:1605–1613. doi: 10.1093/nar/gkg259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.He Y., Neumann R.D., Panyutin I.G. Intramolecular quadruplex conformation of human telomeric DNA assessed with 125I-radioprobing. Nucleic Acids Res. 2004;32:5359–5367. doi: 10.1093/nar/gkh875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang Y., Patel D.J. Solution structure of the human telomeric repeat d[AG3(T2AG3)3] G-tetraplex. Structure. 1993;1:263–282. doi: 10.1016/0969-2126(93)90015-9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.