Abstract
Deletion of ftsK results in the inhibition of cell division, but this inhibition can be reversed by a plasmid carrying only the first ∼17% of ftsK. The division block can be suppressed in most mutants by deletion of dacA, which codes for the d-alanine:d-alanine carboxypeptidase PBP5, or in all mutants by overexpression of ftsN. Overexpression of ftsK inhibits cell division and the formation of FtsZ rings. This division block is not due to the induction of either the SOS or the heat shock regulons.
The ftsK gene of Escherichia coli encodes a large protein (1,329 amino acids) which belongs to a family of bacterium- and plasmid-encoded proteins (2), at least some of which are required for DNA transfer between cells (12, 14, 17, 23) or between a mother cell and a spore compartment (24, 25). The FtsK protein is predicted to have an N-terminal domain (of about 200 amino acids) with several (four or five) membrane-spanning α helices, a proline-glutamine-rich region (∼660 amino acids), and a cytoplasmic domain (∼469 amino acids) with a consensus nucleotide-binding pocket (2). Two independent missense mutations (ftsK44 and ftsK3531) cause different single-amino-acid substitutions in the N-terminal domain, resulting in a temperature-dependent block of cell division (2; unpublished data). The division defect in both of these mutants can be suppressed by deletion of the dacA gene, coding for a d-alanine:d-alanine carboxypeptidase (PBP5), and can be complemented by cloned fragments of the wild-type ftsK gene that contain only the first 600 to 700 bp (2; unpublished data). The temperature sensitivity of cells carrying the ftsK44 or ftsK3531 allele is suppressed by plasmids carrying ftsN (17a).
The present report describes the effects of disruption, deletion, and overexpression of the ftsK gene. We conclude that only the N-terminal ∼200-amino-acid domain of FtsK is required for cell division and that deletion of the remainder of the protein is not lethal. The ftsK gene is preceded by an SOS-inducible promoter, PdinH (19, 20), and we show here that overexpression of FtsK blocks cell division in an SfiA-independent manner. It is therefore possible that ftsK overexpression forms part of an SfiA-independent, SOS-inducible division block.
MATERIALS AND METHODS
Strains and growth conditions.
E. coli K-12 strains used in this study are listed in Table 1. Unless otherwise stated, cells were grown with shaking in Luria-Bertani (LB) broth at the appropriate temperature. Colonies were grown on LB plates with appropriate supplements. Glucose or arabinose (0.2%) was added as required.
TABLE 1.
E. coli K-12 strains used in this work
| Strain | Genotype | Source |
|---|---|---|
| MG1655 | Prototroph | Laboratory stock |
| ME8436 | aroA::Tn10 | National Institute of Genetics, Mishima, Japan |
| MGAT | MG1655 aroA::Tn10 | This work |
| TOE44 | AB2497 leu thr proA his argE lac gal ara xyl mtl thi TsxrrpsL thyA ftsK44 | 2 |
| CDK44 | TOE44 aroA::Tn10 | This work |
| MGK44 | MGAT ftsK44 | This work |
| SP1070 | his supF dacA::kan ΔdacC | Brian Spratt |
| TP8503 | Δlac-proB | Laboratory stock |
| TP1 | TP8503 (λPsfiA::lacZ) | TP8503 lysogenized with λPsfiA::lacZ) (15) |
| GC2481 | sfiA::Tn5 | R. D’Ari (16) |
| MGS5 | MG1655 sfiA::Tn5 | This work |
| C600 | leuB6 thr-1 lacA lacY thi-1 supE44 fhuA21 | Laboratory stock |
| C6SA5 | C600 (e14− [sfiC]) sfiA::Tn5 | This work |
| JC10-240 | Hfr:PO45 ilv thr srlC::Tn10 thi recA56 relA rpsE | Laboratory stock |
| MGrecA | MG1655 recA56 srlC::Tn10 | P1 transduction of recA56 srl-300::Tn10 from JC10-240 |
| MM38 | pcnB::kan | M. Masters |
| CDK1 | aroA::Tn10 ftsK::cat-1 | This work |
| CDK2 | aroA::Tn10 ftsK::cat-Δ2 pcnB::kan/pGB101 | This work |
| CDK3 | aroA::Tn10 ftsK::cat-Δ2 dacA::kan | This work |
| CDK5 | aroA::Tn10 ftsK::cat-Δ5 pcnB::kan/pGB101 | This work |
Plasmid construction.
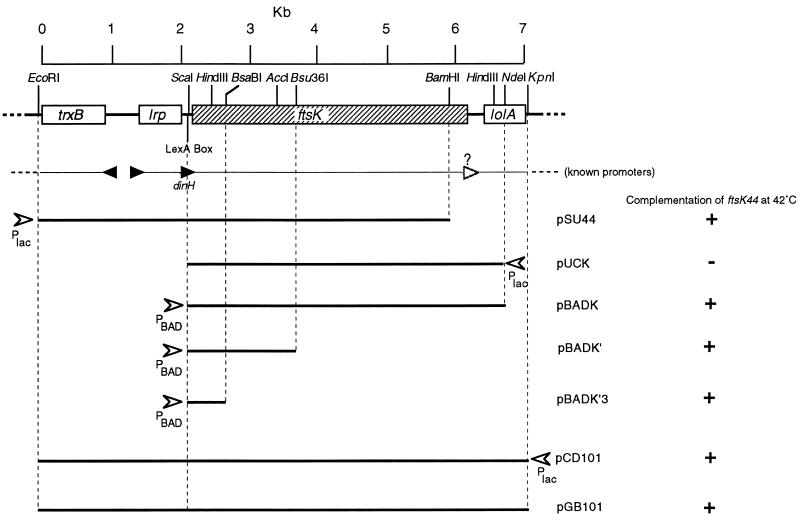
Figure 1 shows the chromosomal fragments cloned in the plasmids used in this work. The 4.4-kb ScaI/NdeI fragment from λ214 (18) was end filled and cloned into SmaI-digested pUC19 to form pUCK (in which ftsK is in the orientation opposite that of Plac). The 4.4-kb EcoRI/XbaI fragment from pUCK, containing ftsK-lolA′, was cloned into EcoRI/XbaI-digested pBAD18 (13) to produce pBADK. The 2.6-kb Bsu36I/XbaI fragment containing 2.2 kb of the 3′ end of ftsK-lolA′ was removed from pBADK, and the remaining fragment was end filled and religated to form pBADK′. pBADK′ was partially digested with BsaBI to produce linear molecules, which were purified by agarose gel electrophoresis. These linear fragments were digested with XbaI, and the plasmid backbone was purified, end filled, and religated to produce pBADK′3, containing the 5′ 677 bp of ftsK.
FIG. 1.
Cloned DNA from the trxB-lrp-ftsK-lolA region. The gene arrangement and selected restriction sites are shown at the top, with (below) the approximate locations of known promoters (filled triangles) and a putative promoter (unfilled triangle). Cloned DNA fragments are shown, together with the relative positions and orientations of controllable promoters (Plac and PBAD) (arrowheads). Plasmid designations are shown to the right of each cloned fragment, together with the ability of each plasmid to complement the ftsK44 temperature-sensitive phenotype.
The 7.2-kb EcoRI/KpnI fragment containing trxB, lrp, ftsK, and lolA from λ214 was cloned into EcoRI/KpnI-digested pUC19 to form pCD101. The 7.2-kb EcoRI/XbaI fragment from pCD101, containing trxB, lrp, ftsK, and lolA, was excised and cloned into EcoRI/XbaI-digested pGB2 (7) to yield pGB101.
The cat gene from pBR325 was amplified by PCR from primers sean2 (5′-TTACCTCCCCGGGGAGAGCC-3′) and sean3 (5′-TGTAACCCACGCGTGCACCC-3′) with Vent polymerase. This PCR product, containing the cat open reading frame (ORF) and its promoter, was cloned into the SmaI site of pUC18 to form pUCAT18.
pUCAT18 was digested with Ecl136II and HindIII to release the cat gene, which was subsequently cloned into the BsaBI site at bp 677 of the ftsK ORF in pCD101 to form pKBCAT.
The cat gene was amplified from pUCAT18 with the mutagenic primers cat-up (5′-TCAAGGATGCGGCCGCTGTTGAG-3′; the introduced NotI site is underlined) and cat-rev (5′-TCGTCAATTGTTACCTCCACGGG-3′; the introduced MfeI site is underlined). The amplification product was purified, digested with NotI and MfeI, and cloned into NotI/MfeI-digested pCD101. The resulting clone, pCDCAT, has bp 54 to 2201 of the ftsK ORF replaced with the cat gene. pCDCAT2, in which bp 54 to 3651 of the ftsK ORF is replaced with the cat gene, was created by removing the Bsu36I/NruI fragment from pCDCAT (by use of the Bsu36I site at the 3′ end of the cat gene), end filling, and religating.
Immunofluorescence.
Immunofluorescence detection of the FtsZ protein was performed as described by Addinall et al. (1).
Gene replacement.
The chromosomal copy of ftsK was replaced by a P1-based procedure (22a). Phage P1 was grown on a donor strain in which the chromosomal ftsK+ allele was linked to aroA::Tn10. This strain carries two plasmids, a pUC plasmid (Ampr) with the desired replacement ftsK allele (ftsK::cat-1, ftsK::cat-Δ2, or ftsK::cat-Δ5) and a second, compatible plasmid, pGB101, expressing ftsK+ (and conferring resistance to spectinomycin). The P1 lysate obtained was used to transduce a recipient strain (which also carried pGB101). Selection was made for chloramphenicol-resistant, tetracycline-resistant transductants, and these were screened for ampicillin-sensitive clones. P1 was grown on selected transductants and used to transduce other recipient strains to tetracycline resistance. The transductants were screened for chloramphenicol resistance. The absence of the donor plasmid and the replacement of the chromosomal ftsK allele in these clones was confirmed by Southern hybridisation.
RESULTS
Overexpression of ftsK blocks cell division.
pCD101 carries the complete trxB-, lrp-, ftsK-, and lolA-containing 7.2-kb chromosomal fragment inserted into the pUC19 vector (Fig. 1). Cells carrying pCD101 can grow at 30°C but die at temperatures higher than 34°C. Cells carrying this high-copy-number plasmid (∼200 copies/cell) are blocked in cell division and grow into long filaments at temperatures above 34°C. pCD101 was therefore transferred to a ΔpcnB strain to reduce the copy number (22). The ΔpcnB/pCD101 cells made normally sized colonies on plates at 37°C. pCD101 was shown to complement the ftsK44 and ftsK3531 temperature-sensitive phenotypes (allowing colony growth at 42°C) in a ΔpcnB background.
Plasmid pBADK (copy number, ∼50/cell) carries the ftsK gene under the control of the ara promoter, PBAD (Fig. 1). Wild-type cells carrying pBADK form colonies on broth plates containing either glucose or arabinose (although the colonies are smaller on arabinose and contain large numbers of filamentous cells). pBADK complemented ftsK44 in the presence of glucose and in the absence of arabinose, suggesting that only a very low level of expression is sufficient for FtsK function. In a ΔpcnB background, in which the plasmid copy number was reduced to about five copies/cell (22), pBADK complemented ftsK44 only in the presence of arabinose and not when glucose was substituted for arabinose.
Transfer of pBADK-containing cells from broth plus glucose to broth plus arabinose was followed by substantial inhibition of cell division (Fig. 2B); cells were typically heterogeneous in length, as shown, but approximately 80 to 90% of possible divisions failed to take place. 4′,6-Diamidino-2-phenylindole (DAPI) staining of the filaments showed normal DNA localization. Filamentous cells produced in this way were treated with anti-FtsZ antibodies and fluorescence labelled as previously described (1). The filaments showed no localization of FtsZ, in contrast to control cells, in which central rings of FtsZ were clearly visible, and also in contrast to ftsK44 filaments formed at the nonpermissive temperature, which have regularly spaced rings of FtsZ along their length (26; data not shown).
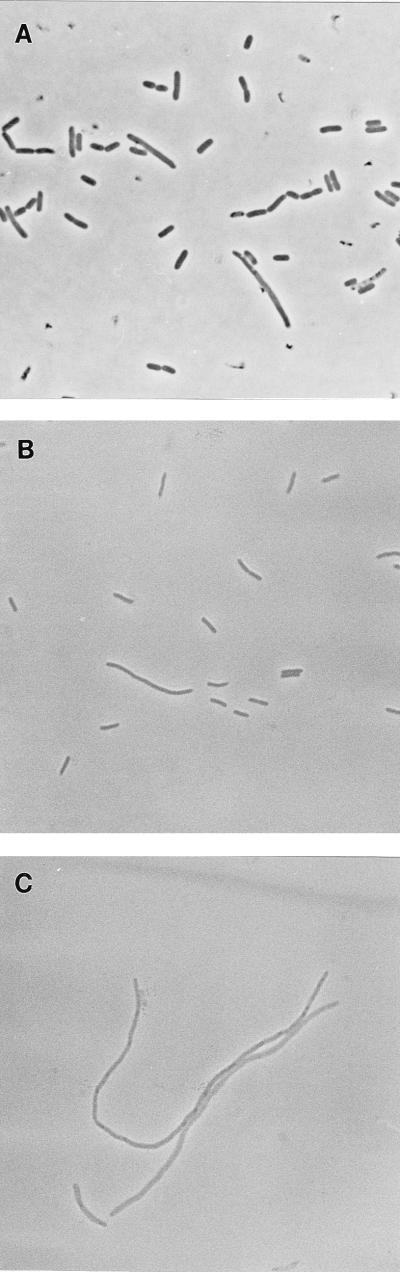
FIG. 2.
Inhibition of cell division by induction of ftsK. (A) MG1655/pBADK cells at 37°C in LB broth plus glucose. (B) MG1655/pBADK cells after 4 h at 37°C in LB broth plus arabinose.
Inhibition of cell division by excess FtsK therefore appears to result from the inhibition of FtsZ assembly into septal rings. Inhibition of FtsZ ring assembly has been reported for cells that produce the SOS-inducible inhibitor SfiA, that lack FtsW, or that have an imbalance in the ratio of FtsA to FtsZ (1, 5). To determine whether the inhibition resulting from FtsK overproduction was due to SOS induction of division inhibitors (SfiA or SfiC), the experiment was repeated with an sfiA::Tn5 mutant (MGS5) and with a recA::Tn9 (e14− [sfiC]) mutant (C6SA5). Arabinose-induced filamentation occurred to the same extent as in MG1655 (recA+ sfiA+) cells. Arabinose-induced filamentation was also monitored for a pBADK-carrying strain (TP1/pBADK) containing the reporter construct λPsfiA::lacZ. No induction of β-galactosidase occurred when filamentation was induced, although induction occurred when the same cells were treated with nalidixic acid (data not shown).
It was also possible that excess production of FtsK protein could have induced the heat shock response, which is also known to be accompanied by inhibition of cell division (6). Arabinose-induced cells were therefore assayed for the overproduction of GroEL with anti-GroEL antibodies, but there was no increase in GroEL levels (data not shown).
A new plasmid, pBADK′, in which only the first 1.8 kb of ftsK was present was produced (Fig. 1). pBADK′ was shown to complement ftsK44 (in the presence of arabinose or glucose when present in a high copy number in pcnB+ cells but only in the presence of arabinose and not in the presence of glucose when present in a low copy number in ΔpcnB cells). Arabinose induction of pBADK′ in wild-type cells did not cause filamentation (although it did cause the formation of chains of cells in <10% of the population).
We conclude that the overproduction of complete FtsK protein can prevent cell division by inhibition of FtsZ ring assembly.
Disruption of ftsK.
A cat gene (4) was inserted into the BsaBI site at bp 678 in ftsK in pCD101 to yield pKBCAT. In this plasmid, the cat gene is transcribed from its own promoter, in the same direction as ftsK. In pKBCAT, both the proximal and the distal parts of ftsK are out of frame with the cat gene and therefore cannot form fusion peptides with the chloramphenicol acetyltransferase enzyme. Nevertheless, pKBCAT is able to complement the ftsK44 mutation, probably because, as previously reported (2), the production of the N-terminal part of FtsK is sufficient to complement this mutation. (Note that the presence of pKBCAT in pcnB+ cells does not cause filamentation, unlike that of pCD101.)
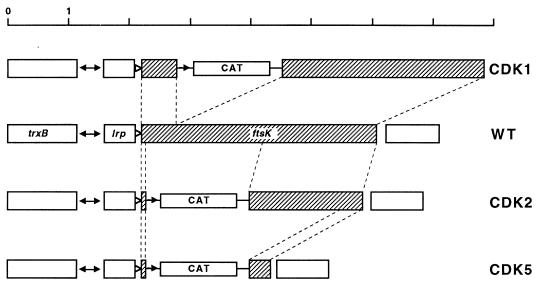
The ftsK::cat-1 mutation was used to replace the normal chromosomal copy of ftsK, as follows. pKBCAT was introduced into MG1655 (aroA::Tn10/pGB101) cells. ftsK and aroA::Tn10 are ∼70% cotransducible. Phage P1 was grown on this strain and used to transduce recipient cells containing pGB101. Selection was made for transductants that were resistant to both chloramphenicol and tetracycline, and these were then screened for transductants that were ampicillin sensitive. These transductants arise when a plasmid has first been integrated by homology into ftsK in the donor chromosome and then excised by internal recombination in such a way as to exchange the chromosomal ftsK+ allele for the plasmid-borne ftsK::cat-1 allele. The correct insertion of the disrupted ftsK gene (Fig. 3) was confirmed by PCR with primers complementary to ftsK and also by Southern blotting.
FIG. 3.
Disruptions and deletions of the chromosomal ftsK gene (shaded) in strains CDK1, CDK2, and CDK5. Broken lines connect corresponding parts of the wild-type (WT) ftsK gene in different constructions. The approximate locations of promoters are shown as triangles (the unfilled triangles represent the repressed promoter PdinH).
The ftsK::cat-1 allele was then cotransduced with aroA::Tn10 from an Amps Tetr Cmr/pGB101 (Specr) transductant into recipient cells carrying pBADK, and the Tetr Cmr (Ampr Specs) transductants were grown on plates containing either glucose or arabinose. Colonies formed well on glucose plates but were small on arabinose plates (presumably because of the deleterious effects of FtsK overproduction; see above). A wild-type strain was then used as the recipient, and Tetr Cmr (Amps Specs) transductants were again obtained at the same frequency. Cells carrying this disruption were mostly normal in appearance, although some were elongated or formed chains (Fig. 4A). The growth rate was normal. The viability and chain-forming behavior of this strain were therefore very similar to those of another strain in which Tn10 had been inserted in a similar location within ftsK (11). We conclude that the ftsK::cat-1 disruption is fully viable.
FIG. 4.
Effect of disruption or partial deletion of ftsK on cell division. (A) CDK1 (ftsK::cat-1) cells in LB broth. (B and C) CDK2 (ftsK::cat-Δ2) ΔpcnB/pBADK cells after 2 h in LB broth plus arabinose (B) or LB broth plus glucose (C).
Deletion of 54% of ftsK.
The same cat gene was substituted for bases 54 (NotI) to 2201 (MfeI) in ftsK (Fig. 3). In this construction (ftsK::cat-Δ2), almost the entire N-terminal membrane-spanning domain is deleted, and the C-terminal fragment is out of frame with the cat gene. Plasmid pCDCAT carrying this new allele had no phenotypic effects on host cells and did not complement temperature-sensitive ftsK mutants.
The ftsK::cat-Δ2 allele was then used to replace the chromosomal ftsK+ allele in a strain carrying pGB101 to yield CDK2 (Fig. 3), and the replacement was confirmed in the same way as described above. In contrast to the ftsK::cat-1 disruption, the ftsK::cat-Δ2 deletion could not be transduced into cells containing only the vector plasmid. This allele could be transduced into cells carrying either pBADK or pBADK′ but, in ΔpcnB strains, only in the presence of arabinose and in the absence of glucose.
CDK2 (ftsK::cat-Δ2) ΔpcnB/pBADK cells were grown to the mid-log phase in broth plus arabinose and then washed and transferred to broth plus glucose. The arabinose-grown population consisted of normal cells and short filaments (Fig. 4B); because this is a ΔpcnB strain, a high proportion of the cells will have lost the plasmid, which is probably the reason for the occurrence of filamentous cells. However, after 2 h of growth in glucose, almost every cell was greatly elongated, and there were many long filaments (Fig. 4C). DAPI staining showed a normal number and distribution of nucleoids. CDK2 ΔpcnB/pBADK′ and CDK2 ΔpcnB/pBADK′3 cells also grew and divided well in arabinose (although chains of cells were present). We therefore conclude that the N-terminal region of FtsK is essential only for cell division and that its function can be carried out by a truncated polypeptide consisting of as few as the first 200 amino acids (17% of the complete FtsK protein).
Deletion of 90% of ftsK.
A second deletion of the chromosomal copy of ftsK (ftsK::cat-Δ5) was constructed in the same way as described above, with plasmid pCDCAT2 as the source of the deletion. The ftsK::cat-Δ5 allele lacks 90% of the gene, from bases 54 to 3651 (Fig. 3). CDK5 (ftsK::cat-Δ5) ΔpcnB/pBADK, CDK5 ΔpcnB/pBADK′, and CDK5 ΔpcnB/pBADK′3 cells divided well in arabinose and formed filaments in glucose, like CDK2 ΔpcnB/pBADK or CDK2 ΔpcnB/pBADK′ cells (Fig. 5). There was, however, a difference between cells expressing complete FtsK (CDK5 ΔpcnB/pBADK) and those expressing only an N-terminal fragment (CDK5 ΔpcnB/pBADK′ and CDK5 ΔpcnB/pBADK′3). The first strain, making the complete FtsK protein, consisted mainly of normal cells and short filaments, whereas the strains producing only truncated FtsK polypeptides also formed chains of cells (Fig. 5). Despite this difference (which is the subject of a separate communication [21]), it is clear that the absence of the putative cytoplasmic portion (∼80%) of FtsK is nonlethal under these conditions.
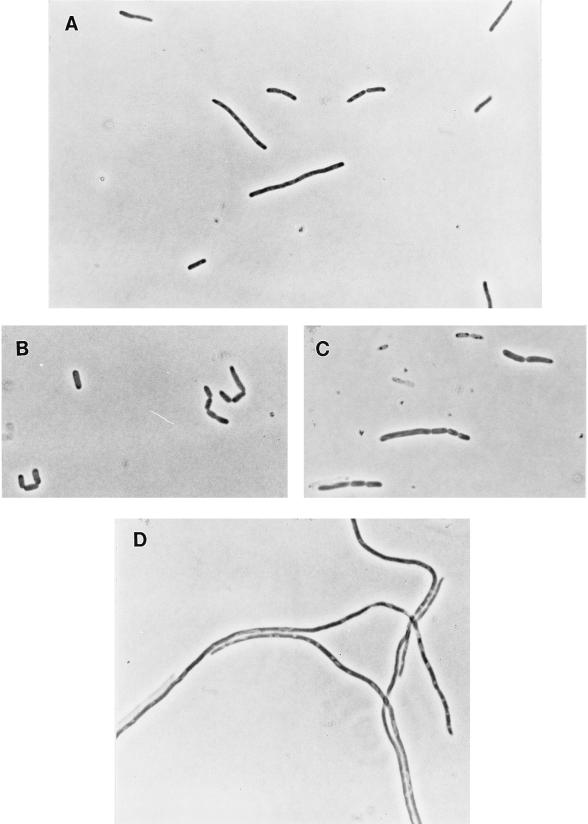
FIG. 5.
(A to C) Restoration of cell division in CDK5 (ftsK::cat-Δ5) ΔpcnB cells in LB broth plus arabinose with plasmid pBADK (A), pBADK′ (B), or pBADK′3 (C). (D) CDK5 ΔpcnB/pBADK cells in LB broth plus glucose.
Suppression of ftsK mutations.
We showed previously (2) that the missense mutation ftsK44, causing temperature-sensitive inhibition of cell division, can be suppressed by a high salt concentration (1% NaCl) in the medium or by insertional inactivation of the dacA gene, encoding PBP5. Both kinds of suppression also apply to a second missense mutation, ftsK3531, which also causes a single-amino-acid substitution in the N-terminal membrane domain (20a) and which results in the same phenotype as the ftsK44 mutation (2). We report here that both mutations are also suppressed by a plasmid (pKD140) carrying the ftsN gene, which was previously shown to suppress several temperature-sensitive ftsA, ftsI, and ftsQ alleles (9). The presence of the ftsN-carrying plasmid allowed normal growth and division of both mutants (ftsK44 and ftsK3531) at 42°C.
The ftsK::cat-Δ2 and ftsK::cat-Δ5 mutations are not salt reversible. The ftsK::cat-Δ2 dacA::kan double mutant formed small colonies on plates and grew well in liquid to produce a population of misshapen cells. In contrast, the ftsK::cat-Δ5 allele could not be transduced into a dacA::kan recipient. The presence of an ftsN-carrying plasmid (pKD140) also allowed the growth and division of cells containing either an ftsK::cat-Δ2 allele or an ftsK::cat-Δ5 allele on the chromosome, although many filaments and chains of cells were present (Fig. 6).
FIG. 6.
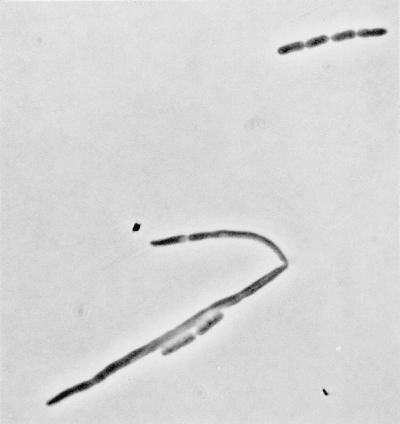
Overproduction of FtsN restores division in cells lacking ftsK. CDK5 (ftsK::cat-Δ5)/pKD140 (ftsN+) cells were grown in LB broth.
DISCUSSION
What is the role of FtsK? We have shown here that, in normal cells, only the terminal 200-amino-acid domain is required for cell division but that this requirement can be partially bypassed by inactivation of PBP5 or by increased levels of FtsN (which suppresses certain missense mutations in other fts genes [9]). Therefore, it seems likely that the membrane-spanning N-terminal part of FtsK is required indirectly for the completion of cell division, perhaps by providing an anchor point for or otherwise facilitating the function of the septum-closing enzyme(s). The role of PBP5 is not clear; perhaps, in its absence, the increased proportion of peptidoglycan chains with pentapeptide side chains can somehow partly replace the function of the FtsK N-terminal domain in cell division. Interestingly, the overproduction of PBP5 has been shown to suppress the division block in temperature-sensitive ftsI (PBP3) mutants; this effect has been ascribed to a possible preference for tripeptide side chains as acceptors in transpeptidation by PBP3 during septum synthesis (3). The present results therefore suggest the possibility that some other step in septation, perhaps septum closure (2), requires enzymes with a different substrate specificity.
There is another possible function for the intact FtsK protein. There is a promoter, PdinH, between lrp and the start of ftsK which is inactive in cells that are growing normally but which is induced as part of the SOS response (19, 20). What is the function of PdinH? There would seem to be at least two possibilities. (i) The overproduction of FtsK may be required during the period of the SOS response, because a pool of FtsK is needed to complete the wave of cell division that follows the release of FtsZ inhibition by SfiA at the end of the SOS response. Such a pool might be needed, for example, if FtsK were a very labile protein. (ii) Alternatively, since we have found that the overproduction of FtsK inhibits FtsZ ring formation, the induction of FtsK during the SOS response may result in a second, LexA-dependent but SfiA (and SfiC)-independent, inhibition of cell division (15). It remains an open question as to whether the inhibition of division by SOS induction of FtsK is functional (i.e., has been selected for this purpose) or accidental (e.g., overproduction of a protein that interacts with FtsZ can block its polymerization, as may be the case with FtsA [8, 10]). The existence of the PdinH promoter, however, strongly suggests that the induction of FtsK production during the SOS response is functional.
Since this work was submitted, it has been reported that the amino-terminal 15% of the protein is both necessary and sufficient to localize the FtsK protein at the division septum (26). That work (26) also shows that the ftsK44 mutation, which causes a single-amino-acid substitution in this part of the protein (2) and prevents cell division at 42°C, strongly reduces the localization of FtsK to the septum. One can conclude that the N-terminal ∼200-amino-acid domain, forming a number of membrane-spanning α helices (2), is as expected, necessary to anchor the protein to its site of action at the cell septum. The work reported by us also shows that this domain can provide the full function of FtsK in cell division.
This work leaves the function of the cytoplasmic domain of FtsK unclear, because a loss of this part of the protein in E. coli is not lethal. Work reported elsewhere (21), however, suggests that this domain functions in chromosome segregation.
ACKNOWLEDGMENTS
This work was supported by MRC programme grants to W.D.D. and M.M. and by an MRC postgraduate studentship to G.C.D.
We thank Kausalia Vijarayagavan and Medhat Khattar for the isolation of ftsK3531, Guowen Liu for sequencing the mutant gene, Sean McAteer for skilled technical assistance, and David Boyle for unpublished information about FtsK protein.
REFERENCES
- 1.Addinall S G, Bi E, Lutkenhaus J. FtsZ ring formation in fts mutants. J Bacteriol. 1996;178:3877–3884. doi: 10.1128/jb.178.13.3877-3884.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Begg K J, Dewar S J, Donachie W D. A new Escherichia coli cell division gene, ftsK. J Bacteriol. 1995;177:6211–6222. doi: 10.1128/jb.177.21.6211-6222.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Begg K J, Takasuga A, Edwards D H, Dewar S J, Spratt B G, Adachi H, Ohta T, Matsuzawa H, Donachie W D. The balance between different peptidoglycan precursors determines whether Escherichia coli cells will elongate or divide. J Bacteriol. 1990;172:6697–6703. doi: 10.1128/jb.172.12.6697-6703.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bolivar F. Construction and characterization of new cloning vehicles. III. Derivatives of plasmid pBR322 carrying unique EcoRI sites for selection of EcoRI-generated recombinant DNA molecules. Gene. 1978;4:121–136. doi: 10.1016/0378-1119(78)90025-2. [DOI] [PubMed] [Google Scholar]
- 5.Boyle D, Khattar M M, Addinall S G, Lutkenhaus J, Donachie W D. ftsW is an essential gene required for the onset of cell division in Escherichia coli. Mol Microbiol. 1997;24:1263–1273. doi: 10.1046/j.1365-2958.1997.4091773.x. [DOI] [PubMed] [Google Scholar]
- 6.Bukau B, Walker G C. Cellular defects caused by deletion of the Escherichia coli dnaK gene indicates roles for heat shock protein in normal metabolism. J Bacteriol. 1989;171:2337–2346. doi: 10.1128/jb.171.5.2337-2346.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churchward G, Belin D, Nagamine Y. A pSC101-derived plasmid which shows no sequence homology to other commonly used cloning vectors. Gene. 1984;31:165–171. doi: 10.1016/0378-1119(84)90207-5. [DOI] [PubMed] [Google Scholar]
- 8.Dai K, Lutkenhaus J. The proper ratio of FtsZ to FtsA is required for cell division to occur in Escherichia coli. J Bacteriol. 1992;174:6145–6151. doi: 10.1128/jb.174.19.6145-6151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dai K, Xu Y, Lutkenhaus J. Cloning and characterization of ftsN, an essential cell division gene in Escherichia coli, isolated as a multicopy suppressor of ftsA12(Ts) J Bacteriol. 1993;175:3790–3797. doi: 10.1128/jb.175.12.3790-3797.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dewar S J, Begg K J, Donachie W D. Inhibition of cell division by an imbalance in the ratio of FtsA to FtsZ. J Bacteriol. 1992;174:6314–6316. doi: 10.1128/jb.174.19.6314-6316.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Diez A A, Farewell A, Nannmark U, Nyström T. A mutation in the ftsK gene of Escherichia coli affects cell separation, stationary-phase survival, stress adaptation, and expression of the gene encoding the stress protein UspA. J Bacteriol. 1997;179:5878–5883. doi: 10.1128/jb.179.18.5878-5883.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Flannagan S E, Zitzow L A, Su Y A, Clewell D B. Nucleotide sequence of the 18 kb conjugative transposon Tn916 from Enterococcus faecalis. Plasmid. 1994;32:350–354. doi: 10.1006/plas.1994.1077. [DOI] [PubMed] [Google Scholar]
- 13.Guzman L-M, Belin D, Carson M J, Beckwith J. Tight regulation, modulation, and high-level expression by vectors containing the arabinose PBAD promoter. J Bacteriol. 1995;177:4121–4130. doi: 10.1128/jb.177.14.4121-4130.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hagège J, Pernodet J-L, Sezonov G, Gerbaud C, Friedman A, Guérineau M. Transfer functions of the conjugative integrating element pSAM2 from Streptomyces ambofaciens: characterization of a kil-kor system associated with transfer. J Bacteriol. 1993;175:5529–5538. doi: 10.1128/jb.175.17.5529-5538.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hill T M, Sharma B, Valjavec-Gratian M, Smith J. sfi-Independent filamentation in Escherichia coli is lexA dependent and requires DNA damage for induction. J Bacteriol. 1997;179:1931–1939. doi: 10.1128/jb.179.6.1931-1939.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Huisman O, D’Ari R. Effect of suppressors of SOS-mediated filamentation on sfiA operon expression in Escherichia coli. J Bacteriol. 1983;153:169–175. doi: 10.1128/jb.153.1.169-175.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kendall K J, Cohen S N. Complete nucleotide sequence of the Streptomyces lividans plasmid pIJ101 and correlation of the sequence with genetic properties. J Bacteriol. 1988;170:4634–4651. doi: 10.1128/jb.170.10.4634-4651.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17a.Khattar, M. Personal communication.
- 18.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of a new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 19.Lewis L K, Harlow G R, Gregg-Jolly L A, Mount D W. Identification of high affinity binding sites for LexA which define new DNA damage-inducible genes in Escherichia coli. J Mol Biol. 1994;241:507–523. doi: 10.1006/jmbi.1994.1528. [DOI] [PubMed] [Google Scholar]
- 20.Lewis L K, Jenkins M E, Mount D W. Isolation of DNA damage-inducible promoters in Escherichia coli: regulation of polB (dinA), dinG, and dinH by LexA repressor. J Bacteriol. 1992;174:3377–3385. doi: 10.1128/jb.174.10.3377-3385.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20a.Liu, G. Unpublished data.
- 21.Liu, G., G. C. Draper, and W. D. Donachie. FtsK is a bifunctional protein involved in cell division and chromosome localisation in Escherichia coli. Mol. Microbiol., in press. [DOI] [PubMed]
- 22.Masters M, Colloms M D, Oliver I R, He L, MacNaughton E J, Charters Y. The pcnB gene of Escherichia coli, required for ColE1 copy number maintenance, is dispensable. J Bacteriol. 1993;175:4405–4413. doi: 10.1128/jb.175.14.4405-4413.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22a.McLennan, N., and M. Masters. Unpublished data.
- 23.Tomura T, Kishino K, Doi K, Hara T, Kuhara S, Ogata S. Sporulation-inhibitory gene in pock-forming plasmid pSA1.1 of Streptomyces azureus. Biosci Biotechnol Biochem. 1993;57:438–443. doi: 10.1271/bbb.57.438. [DOI] [PubMed] [Google Scholar]
- 24.Wu L J, Errington J. Bacillus subtilis SpoIIIE protein required for DNA segregation during asymmetric cell division. Science. 1994;264:572–575. doi: 10.1126/science.8160014. [DOI] [PubMed] [Google Scholar]
- 25.Wu L J, Lewis P J, Allmansberger R, Hauser P M, Errington J. A conjugation-like mechanism for prespore chromosome partitioning during sporulation in Bacillus subtilis. Genes Dev. 1995;9:1316–1326. doi: 10.1101/gad.9.11.1316. [DOI] [PubMed] [Google Scholar]
- 26.Yu X-C, Tran A H, Sun Q, Margolin W. Localization of cell division protein FtsK to the Escherichia coli septum and identification of a potential N-terminal targeting domain. J Bacteriol. 1998;180:1296–1304. doi: 10.1128/jb.180.5.1296-1304.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]