Abstract
Although it has been 10 years since the discovery that the Escherichia coli UmuD protein undergoes a RecA-mediated cleavage reaction to generate mutagenically active UmuD′, the function of UmuD′ has yet to be determined. In an attempt to elucidate the role of UmuD′ in SOS mutagenesis, we have utilized a colorimetric papillation assay to screen for mutants of a hydroxylamine-treated, low-copy-number umuD′ plasmid that are unable to promote SOS-dependent spontaneous mutagenesis. Using such an approach, we have identified 14 independent umuD′ mutants. Analysis of these mutants revealed that two resulted from promoter changes which reduced the expression of wild-type UmuD′, three were nonsense mutations that resulted in a truncated UmuD′ protein, and the remaining nine were missense alterations. In addition to the hydroxylamine-generated mutants, we have subcloned the mutations found in three chromosomal umuD1, umuD44, and umuD77 alleles into umuD′. All 17 umuD′ mutants resulted in lower levels of SOS-dependent spontaneous mutagenesis but varied in the extent to which they promoted methyl methanesulfonate-induced mutagenesis. We have attempted to correlate these phenotypes with the potential effect of each mutation on the recently described structure of UmuD′.
While it has been known for some time that SOS mutagenesis in Escherichia coli is not a passive process (see references 12 and 40 for recent reviews), the complexity of the protein-protein interactions necessary to facilitate error-prone translesion DNA synthesis is only just being comprehended. Both genetic and biochemical experiments have demonstrated that UmuD is functionally inactive until it undergoes a posttranslational cleavage reaction to UmuD′ (4, 25, 33). Once generated, UmuD′ associates with UmuC, RecA, and DNA polymerase III proteins to form the so-called “mutasome” (30). It was previously thought that this mutasome facilitated only translesion replication, but recent data suggest that it can also promote extension from mispairs generated during normal DNA synthesis (8).
The likelihood of formation of the mutasome depends upon a series of sequential protein-protein interactions that determine the stability of the Umu mutagenesis proteins (5, 10). For example, both the UmuD and UmuC proteins are labile in vivo and are rapidly degraded by the Lon protease (9). Although UmuD can autodigest (4), an interaction with a RecA nucleoprotein filament greatly enhances the efficiency of the conversion of UmuD to UmuD′ (33, 39). As a consequence, UmuD′ is able to form a complex with UmuC that appears to be much more stable than the corresponding UmuDC complex (10, 18).
Despite advances in the understanding of the regulation of SOS mutagenesis, little is known about the function of UmuD′ in the mutagenic process. Potential insights into such activities can be gained from the structural analysis of UmuD′. The UmuD′ monomer consists of a compact globular head (residues 46 to 135) with an unusual antiparallel β-structure composed of seven β-strands which associate to form three β-sheets (27). The remaining residues (25 to 45 and 136 to 139) form long N-terminal and short C-terminal extensions from the body of the protein. Within the crystallized UmuD′ structure, three discrete but interrelated structural forms are observed. The first, termed the “molecular dimer,” is primarily hydrophobic and consists of parts of residues Tyr52, Val54, Ile87, Gly92, Glu93, Phe94, and Phe128, with the Phe94 residues from each monomer stacked on top of each other. The second form is the “filament dimer.” The filament dimer interface involves hydrogen bonds between the two short carboxyl-terminal extensions from each monomer as well as several hydrophobic interactions between the overlapping extended amino termini. The two dimers were so named because of additional molecular and biochemical data which suggested that the molecular dimer predominates over the filament dimer in dilute solution (28). Using nuclear magnetic resonance (NMR), Ferentz et al. have reported strong intersubunit nuclear overhauser effects at the C terminus of UmuD′ (6) and suggest that only the crystal filament dimer may, in fact, exist in solution. Finally, again within the UmuD′ crystal, UmuD′ molecular and filament dimers associate together such that they form an “extended UmuD′ filament” (27, 28) that, based upon mutational analysis, is hypothesized to be important for the biological function of UmuD′ (28).
Experiments that will, hopefully, resolve the differences in interpretation of dimeric UmuD′ structures as defined by crystallography and NMR spectroscopy are currently in progress. In this work, we continue to use the terms “molecular dimer” to indicate the protein-protein interactions between the two globular domains of each UmuD′ promoter and “filament dimer” to indicate the interactions between the long and short N- and C-terminal extensions (28).
As part of our continuing studies into the molecular mechanisms of damage-inducible mutagenesis, we were interested in isolating novel loss-of-function umuD′ mutants that might provide structure-function insights into the activities of the UmuD′ protein. To do so, we have taken advantage of a powerful colorimetric papillation assay to screen for randomly mutagenized plasmid-encoded UmuD′ proteins that are unable to promote SOS-dependent spontaneous mutagenesis. Similar to a previous study in which loss-of-function UmuC proteins were identified (41), this strategy resulted in the isolation of a number of novel umuD′ mutants. While all of the novel umuD′ mutants that we have isolated result in a reduced capacity to promote spontaneous SOS mutagenesis, they vary in the extent to which they promote mutagenesis in damaged cells; we have attempted to explain these phenotypes based upon the predicted structural changes between the mutant and wild-type UmuD′ proteins (6, 27, 28).
MATERIALS AND METHODS
Plasmids and bacterial strains.
To facilitate the specific identification of E. coli umuD′ mutants, we have utilized two compatible plasmids that separately encode the genetically engineered umuD′ and umuC genes (41). pRW66 is a low-copy-number spectinomycin resistance plasmid that expresses UmuD′. pRW124 is a medium-copy-number ampicillin resistance plasmid that expresses UmuC. Both vectors express the Umu proteins from the native umu promoter, but pRW124 lacks an obvious ribosome binding site. As a consequence, the level of UmuC protein expressed from the medium-copy-number plasmid is comparable to that of UmuD′ from the low-copy-number plasmid and is only three- to fivefold higher than that expressed chromosomally (41).
Three E. coli K-12 strains were utilized to identify and characterize the novel plasmid-encoded umuD′ mutants. Strain RW126 [relevant genotype, Δ(umuDC)595::cat recA718 srlC300::Tn10 lexA71(Def)::Tn5 hisG4(Oc) galK2(Oc)] was used for the initial screening and principle characterization of the plasmid-encoded umuD′ mutants. This strain is extremely useful in identifying plasmids that carry either a gain (15) or loss (41) of Umu-like activity, as it measures both Umu-dependent spontaneous mutator activity and damage-induced Umu-dependent mutagenesis. Strain JL852 [relevant genotype, recA730 srlC300::Tn10 lexA71(Def)::Tn5 hisG4(Oc)] (22) was used to determine if any of the plasmid-encoded mutants were phenotypically dominant negative. This strain expresses the chromosomally encoded UmuD and UmuC proteins, but since the RecA730 protein constitutively mediates cleavage of UmuD (33, 39) the strain is, in fact, phenotypically UmuD′C. Strain TK614 [relevant genotype, recA+ lexA+ uvrA6 umuD77 hisG4(Oc)] (19) was used to isolate plasmid DNA for sequencing (see below).
In vitro mutagenesis of pRW66 and screening for umuD′ mutants.
Randomly mutagenized pRW66 was obtained following the hydroxylamine mutagenesis procedure described previously (16, 41). Briefly, 2 μg of purified plasmid DNA was incubated in 100 μl of 800 mM hydroxylamine HCl (Sigma), 50 mM sodium phosphate (pH 6.0), and 1 mM EDTA for 48 h at 37°C. The mutagenized DNA was recovered in TE (10 mM Tris-HCl [pH 8.0], 1 mm EDTA) by repeated dilution and concentration cycles in a Centricon 30 ultrafiltration cartridge (Amicon). Potential umuD′ mutants were identified by introducing the mutagenized plasmid DNA into the umu tester strain RW126 harboring the medium-copy-number umuC plasmid pRW124. Transformants were selected on Luria-Bertani plates supplemented with spectinomycin (50 μg/ml) and ampicillin (100 μg/ml) and then restreaked with a sterile toothpick onto MacConkey galactose (1%) indicator plates supplemented with spectinomycin and ampicillin. After 10 days of incubation at 37°C, colonies that produced less than half of the number of Gal+ papillae seen with the control strain were analyzed further by using the qualitative His+ mutagenesis assay.
Qualitative mutagenesis assay.
Assays were performed essentially as described previously (41). Aliquots (1 ml) from a fresh overnight culture were centrifuged and resuspended in an equal volume of SM buffer (23). The ability of particular plasmid-bearing strains to promote Umu-dependent SOS mutator activity in the absence of exogenous DNA damage was judged by plating 100-μl aliquots on Davis and Mingioli minimal agar plates supplemented with a trace amount of histidine (1 μg/ml) (3). Umu-dependent, chemically induced mutagenesis was determined essentially as described above, except that 1 μl of methyl methanesulfonate (MMS) (Sigma) was applied to a small sterile disk in the center of the plate. Both spontaneously arising and MMS-induced His+ mutants were scored after 4 days of incubation at 37°C.
Steady-state levels of wild-type and mutant UmuD′ proteins.
UmuD′ protein was detected in whole-cell E. coli extracts by the Western-light chemiluminescence assay (Tropix, Bedford, Mass.), essentially as described previously (39). Briefly, whole-cell extracts from ∼108 cells were separated by electrophoresis in an 18% acrylamide-sodium dodecyl sulfate gel. Proteins were transferred to an Immobilon P (Millipore) support membrane and incubated overnight with a 1:10,000 dilution of affinity-purified polyclonal UmuD′ antiserum (10). After appropriate washes, the membranes were incubated with a 1:10,000 dilution of a goat anti-rabbit antibody conjugated with alkaline phosphatase (Bio-Rad). UmuD′ was visualized after the membrane was incubated with the CPD-Star chemiluminescent substrate (Tropix) and exposed to X-ray film for an appropriate period of time. The relative level of UmuD′ protein was obtained by image analysis of films using NIH Image, version 1.59. In most cases, values were obtained from multiple exposures so that the desired bands were in the linear range of the film (39).
DNA sequence analysis.
Plasmid DNA (pRW66 derivative and pRW124) was isolated from RW126 and transformed into TK614 [relevant genotype, recA+ lexA+ uvrA6 umuD77 hisG4(Oc)] (19), selecting for spectinomycin-resistant (pRW66) colonies and subsequently screening for ampicillin-sensitive (lacking pRW124) colonies. The pRW66 mutant phenotype was verified by using the qualitative His+ reversion assay described above. In all cases, plasmids that failed to promote any spontaneous or damage-induced mutagenesis in the recA718 strain (RW126) were unable to complement the chromosomally encoded umuD77 allele in the recA+ background. By comparison, those plasmids that were able to promote modest levels of Umu-dependent spontaneous or damage-induced mutagenesis did complement the umuD77 allele (data not shown). The entire umuD′ gene (including regulatory elements) in plasmid pRW66 (and mutant derivatives) was sequenced by Lark Sequencing Technologies Inc. (Houston, Tex.), using standard dideoxy sequencing protocols and four unique oligonucleotide primers spaced about 150 bp apart.
Subcloning of the previously identified chromosomal umuD1, umuD44, and umuD77 mutations into umuD′.
The umuD44 and umuD77 mutations (2, 21) were subcloned into umuD′ by isolating a ClaI-HindIII fragment from pGW2055 and pGW2051, respectively, and cloning the fragment into the similarly digested umuD′ vector pRW66. The umuD1 mutation (2, 21) was generated in umuD′ by synthesizing four synthetic oligonucleotides that, when annealed together, generated approximately 100 bp of double-stranded DNA with EcoRI and ClaI ends. This fragment encodes the wild-type umuDC promoter-operator regulatory sequences but also introduces a unique NdeI restriction enzyme site at the 5′ end of the mutant umuD′1 gene. Plasmid pRW368, which carries the entire umuD′1 gene, was constructed by ligating the synthetic oligonucleotides into the EcoRI–ClaI-digested vector pRW66.
Yeast two-hybrid interactions.
The wild-type umuD′ gene was obtained from plasmid pEC42 (11) and subcloned as an EcoRI-to-BglII fragment into the yeast two-hybrid vectors pGAD424 and pGBT9 (1) in such a way as to create in-frame fusions with the GAL4 activating domain and the GAL4 binding domain. These plasmids were designated pJM8 (pGAD424 derivative) and pJM10 (pGBT9 derivative), respectively. Various mutant umuD′ genes were subcloned from pRW66 into pJM10 by using the unique ClaI and AgeI sites in the plasmid. The umuD′ mutant derivatives of pJM10 were designated pJM50 to pJM60, and each was sequenced to ensure that it contained the correct allele of umuD′. These mutant umuD′ genes were then subcloned from the pJM10-derivative plasmids, by utilizing the unique restriction enzymes sites for EcoRI and PstI, into pGAD424 and were designated pJM61-71. The various wild-type and mutant pGAD424-umuD′ derivatives were then cotransformed with the corresponding pGBT9-umuD′ derivatives into Saccharomyces cerevisiae SFY526 (1, 14, 17, 31). Transformants were selected on synthetic medium lacking both leucine and tryptophan (−leu −trp) (32). These transformants, designated T13 (wild-type umuD′) or T125 to T135 (mutant umuD′), were inoculated into 5-ml cultures of liquid −leu −trp medium, supplemented with 40 μl of YPD (2% peptone, 1% yeast extract, and 2% dextrose) per ml to enhance growth, and grown to mid-log phase (optical density at 600 nm [OD600], approximately 1.0). These cultures were then used to perform liquid β-galactosidase assays. Briefly, 0.1 ml of culture, 0.7 ml of Z buffer (60 mM Na2HPO4, 40 mM NaH2PO4, 10 mM KCl, and 1 mM MgSO4 [pH 7.0]), 50 μl of CHCl3, and 50 μl of 0.1% sodium dodecyl sulfate were vortexed for 30 s in a 1.5-ml microcentrifuge tube. One hundred sixty microliters of ONPG solution (4 mg of o-nitrophenylgalactoside per ml in 0.1 M phosphate buffer, pH 7.0) was added, and the tube was vortexed. After 1 h of incubation at 30°C, the reactions were quenched by adding 0.4 ml of 1 M Na2CO3. The microcentrifuge tubes were then centrifuged for 10 min at 14,000 rpm. The absorbance of 1 ml of the supernatant was then determined at 420 nm. β-Galactosidase units were calculated with the following equation: 1,000(OD420/t × V × OD600) where t is the time (in minutes) of incubation and V is the volume (in milliliters) of culture added to Z buffer (23).
RESULTS
Isolation of novel plasmid-encoded E. coli umuD′ mutants.
recA718 lexA(Def) strains that carry functional chromosomally encoded or plasmid-encoded umu genes normally exhibit modest Umu-dependent spontaneous mutator activity (15, 37, 41). This spontaneous mutator activity can be conveniently monitored by assaying for the reversion of either the hisG4(Oc) or galK2(Oc) (26) mutations, the latter being monitored by a colorimetric papillation assay. Since we were interested in specifically obtaining umuD′ mutants, we utilized the compatible two-plasmid system that we have previously employed to identify umuC mutants (41). In this case, however, the umuD′ plasmid, pRW66, was mutagenized in vitro with hydroxylamine and introduced into the tester strain harboring the wild-type umuC plasmid pRW124.
Approximately 7,000 transformants were screened for reduced abilities to promote Umu-dependent spontaneous mutagenesis. A number of potential mutants were identified, of which 17 were chosen for further characterization. Subsequent DNA analysis revealed that 3 of the mutants had been independently identified twice (Table 1), leaving 14 novel mutants. Of these mutants, two carried promoter mutations, three were nonsense mutations, and the remaining nine were missense mutations (Table 1). As would be expected with hydroxylamine mutagenesis, most of these changes correlated with a C→T transition in either the coding or noncoding DNA strand. The exception was a G→T transversion mutation found in the promoter region of pRW66-42, which presumably arose spontaneously.
TABLE 1.
Promoter, nonsense, and missense umuD′ mutants
| umuD′ plasmid | Mutagenesis function (His+ mutants per plate)a
|
Mutation in umuD′
|
umuD′ allele | Relative amt of UmuD′c | ||
|---|---|---|---|---|---|---|
| Spontaneous | MMS-induced | Nucleotide | Amino acidb | |||
| Noned | 3 | 6 | NAe | NA | NA | NA |
| pRW66f | 170 | 425 | NA | NA | NA | 1.00 |
| pRW66-17 | 6 | 102 | −60G→A | NA | NA | ND |
| pRW66-42 | 38 | 349 | −60G→T | NA | NA | 0.02g |
| pRW66-11 | 6 | 87 | 106C→Th | Q36X(Am) | umuD′309 | ND |
| pRW66-48 | 3 | 3 | 124C→T | Q42X(Oc) | umuD′310 | ND |
| pRW66-31 | 3 | 4 | 298C→T | Q100X(Oc) | umuD′311 | ND |
| pRW368 | 79 | 256 | 79C→T | P27S | umuD′1 | 0.56 |
| pRW66-1 | 22 | 223 | 148G→A | A50T | umuD′312 | 0.82 |
| pRW66-52 | 6 | 50 | 149C→T | A50V | umuD′313 | 0.58 |
| pRW66-38 | 3 | 28 | 152C→Th | T51I | umuD′314 | 0.38 |
| pRW66-13 | 43 | 435 | 183G→A | M61I | umuD′315 | 0.29 |
| pRW66-19 | 80 | 507 | 190G→A | G64D | umuD′316 | 0.44 |
| pRW358 | 46 | 120 | 193G→A | G65R | umuD′44 | 0.50 |
| pRW66-30 | 21 | 275 | 244C→T | H82Y | umuD′305 | 0.37 |
| pRW356 | 101 | 462 | 274G→A | G92D | umuD′77 | 1.00 |
| pRW66-44 | 7 | 74 | 385G→A | G129S | umuD′317 | 0.64 |
| pRW66-20 | 2 | 6 | 386G→Ah | G129D | umuD′318 | 0.30 |
| pRW66-18 | 19 | 200 | 388G→A | V130M | umuD′319 | 0.04 |
Data presented are means from at least three separate cultures, with three plates per culture. Strain RW126 (15) was used to determine the abilities of plasmids to promote spontaneous and MMS-induced mutagenesis.
Amino acid changes are noted in single-letter code for the original residue, followed by the residue in the mutant protein. X, termination codon; (Am), amber codon; (Oc), ochre codon. The numbering system is based upon that used by Perry et al. (29). In this system, the codon for the N-terminal Gly residue of UmuD′ is located at residues 72 to 75 and the amino-terminal Gly residue of UmuD′ is numbered 25 (not 1). Such numbering makes it easy to compare substitutions at the exact same residues in both UmuD and the shorter UmuD′ protein.
Data shown were obtained from strain RW126/pRW124, harboring the mutant pRW66 derivatives. The values reported are representative and are based upon densitometric analysis of multiple exposures of the gel in Fig. 2. ND, not detected.
Data presented are for spontaneous and MMS-induced mutagenesis in the presence of the medium-copy-number umuC plasmid pRW124.
NA, not applicable.
Wild type.
Based upon experiments in which ∼5 times the normal amount of whole-cell extract was assayed (data not shown).
This mutation was independently isolated twice.
Effects of plasmid-encoded umuD′ mutations on the ability to promote SOS mutagenesis.
Most of the plasmid-encoded umuD′ mutations generated by the hydroxylamine treatment resulted in a strain that phenotypically exhibited 5 to 10% of the level of spontaneous SOS mutagenesis of the wild-type counterpart. The exceptions were plasmids that exhibited approximately 20% (pRW66-13 [M61I] and pRW66-42 [−35 promoter mutation]) and approximately 50% (pRW66-19 [G64D]) of the spontaneous mutagenesis activity promoted by the wild-type protein. In contrast, many of the umuD′ mutants were able to promote MMS-induced mutagenesis, albeit to varying degrees (Table 1), indicating that under experimental conditions in which cells are exposed to continuous exogenous DNA damage, the mutant UmuD′ proteins are at least partially active. Such observations were confirmed by the fact that these mutants were able to complement a umuD77 allele in a damage-induced recA+ lexA+ background (data not shown). The notable exceptions were plasmids with ochre nonsense mutations {pRW66-31 [Q100X(Oc)] and pRW66-48 [Q42X(Oc)]} and a plasmid with a missense mutation resulting in G129D (pRW66-20) that failed to promote any Umu-dependent mutagenesis (Table 1). The latter observation correlates with the fact that the Gly129 residue is critical to UmuD and UmuD′ structure, as it is absolutely conserved in 14 of the UmuD/LexA homologs identified to date (28).
By comparison, the three chromosomal umuD alleles, umuD1 (P27S), umuD44 (G65R), and umuD77 (G92D) (which normally render UmuD noncleavable and the strain concomitantly nonmutable [2, 19, 21, 35]), all promoted significant levels of spontaneous and MMS-induced mutagenesis when cloned into umuD′ (Table 1). It would appear, therefore, that these mutations affect the ability of UmuD to undergo posttranslational processing but do not seem to have a major role in any subsequent activity of UmuD′.
Ability of mutant UmuD′ proteins to form dimers: the two-hybrid assay.
Since we believe that the mutagenically active form of UmuD′ protein is a dimer, we were interested in determining if we could correlate the level of mutagenesis promoted by each mutant protein with the extent to which it dimerized. One way to assess the strength of such protein-protein interactions is to use the yeast two-hybrid assay (7). Indeed, such an approach has been useful in determining the strength of UmuD-UmuD′ interactions in vivo (18). We therefore subcloned many of the umuD′ missense mutations into the appropriate two-hybrid vectors and determined the ability of the mutant proteins to interact with themselves. The two-hybrid fusion is at the N-terminal end of UmuD′ (and mutant derivatives). Since the N-terminal tail of UmuD′ makes extensive protein-protein contacts within the filament dimer (28), as well as additional UmuD′ promoters within the crystallized extended UmuD′ filament (28), we believe that simple steric hindrance of the fusion protein will preclude such N-terminal interactions. Any detectable protein-protein interactions are, therefore, thought to represent either molecular dimer (27) or filament dimer interactions through the short C-terminal extension (6, 28).
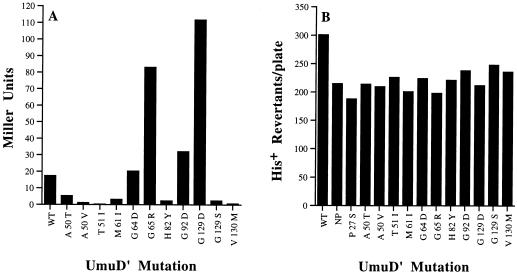
Based upon the levels of β-galactosidase expressed by these plasmids (Fig. 1A), it appeared that in the heterologous S. cerevisiae background, the mutational changes in 7 of the 11 mutant proteins resulted in a protein with a reduced ability to interact with itself (Fig. 1A). In many cases, this inability to interact did indeed correlate with the level of spontaneous SOS mutagenesis promoted by the mutant proteins (Table 1). In general, those mutants that produced β-galactosidase levels of ≤1 Miller units (A50V, T51I) were generally the most deficient in spontaneous and damage-induced mutagenesis. One exception was the V130M mutation, which also produced ≤1 Miller units but resulted in intermediate levels of mutagenesis. We believe that the lower level of β-galactosidase produced by this mutant most likely reflects the inherent instability of the mutant protein (see below) rather than its inability to dimerize. Mutants that produced ∼2.3 to 5.3 Miller units of β-galactosidase (A50T, M61I, H82Y, G129S) all gave intermediate levels of spontaneous and induced mutagenesis, indicating that dimerization of these mutants is impaired but not completely abolished. Further comparison revealed that two of the mutants, G64D and G92D, exhibited levels of β-galactosidase somewhat similar to the wild-type construct (Fig. 1A) and both of these mutants gave high levels of spontaneous and induced mutagenesis. The G65R mutant and especially the G129D mutant produced approximately four- to fivefold-higher levels of β-galactosidase than the wild-type control. We interpret these results to indicate that both the G65R and G129D mutations increase the ability of the mutant protein to dimerize. While the G65R mutant was moderately proficient for mutagenesis, the G129D mutant was completely defective for both spontaneous and damage-induced mutagenesis.
FIG. 1.
Abilities of various missense mutants to interact with each other and wild-type UmuD′C. (A) Abilities of missense mutants to interact with themselves when expressed as two-hybrid fusion proteins in a heterologous S. cerevisiae background. This fusion is at the N-terminal end of UmuD′ (and mutant derivatives) and presumably precludes any filament dimer interactions via the N-terminal extension of UmuD′. Any interactions are, therefore, thought to represent either molecular dimer or filament dimer interactions through the short C-terminal extension. The amount of β-galactosidase produced by each construct is expressed in Miller units, and the amino acid substitution in each mutant protein is indicated in single-letter amino acid code. (B) Effects on cellular mutagenesis of missense UmuD′ mutants in JL852, a strain that constitutively expresses UmuD′C. As in panel A, the amino acid substitution in each mutant protein is indicated in single-letter amino acid code. WT is the level of mutagenesis in the presence of pRW66, while NP is the level of mutagenesis in its absence. Since none of the mutants appear to exhibit a dramatic dominant-negative phenotype, we assume that they are all recessive to the wild-type UmuD′ protein.
Dominant/recessive nature of umuD′ mutants.
All of our initial mutational studies were performed in a ΔumuDC background in which the mutant protein can interact only with itself. To determine if any of the missense mutants might, in fact, be dominant mutations, we introduced each of the missense mutations into E. coli JL852 (22). This strain expresses the chromosomally encoded UmuD and UmuC proteins, but since the RecA730 protein constitutively mediates cleavage of UmuD (33, 39) the strain is, in fact, phenotypically UmuD′C. As a consequence, the strain exhibits a greatly elevated spontaneous mutation rate compared to recA+ lexA(Def) cells, with approximately 210 His+ revertants appearing on each plate (Fig. 1B). Introduction of the wild-type UmuD′ plasmid pRW66 into the cells increased the level to approximately 300 His+ mutants per plate, suggesting that the normal cellular levels of UmuD′(C) are, in fact, limiting for optimal SOS-dependent spontaneous mutagenesis. Strictly speaking, dominance of a particular allele over the wild-type gene should be determined when the cellular levels of both proteins are approximately equal. In this experiment, however, the mutant proteins are expressed from a low-copy-number plasmid while the wild-type UmuD′(C) proteins are chromosomally expressed. If any of the missense mutants are dominant negative, one would expect that they would reduce the level of spontaneous mutagenesis promoted by the chromosomally expressed UmuD′C complex. Despite the disparity in the levels of protein expression, analysis of the missense mutants identified here, including the G129D and G65R mutants (which we predict to at least homodimerize more efficiently that the wild-type protein), revealed that they failed to significantly reduce the level of spontaneous mutagenesis (Fig. 1B). These observations suggest that under our experimental conditions, all of the mutants are, in fact, recessive to the wild-type protein.
Steady-state levels of plasmid-encoded UmuD′ mutants.
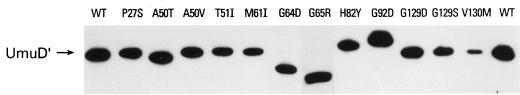
Another obvious explanation for the inability of various umuD′ mutants to promote mutagenesis is that the mutant protein is unstable and rapidly degraded in vivo. We have previously shown that analysis of the steady-state levels of the Umu proteins is often an accurate reflection of the relative stability of the protein in vivo (10). In a ΔumuDC background (as employed here), the wild-type UmuD′ protein is relatively stable in vivo (10). Analysis of the steady-state levels of the missense mutant UmuD′ proteins (with UmuC coexpressed in trans from pRW124) revealed that most mutant proteins exhibited 30 to 100% of the steady-state level of the wild-type protein (Table 1). These observations suggest that the various missense mutations have only negligible to moderate effects on the stability of the mutant UmuD′ protein or that if they do affect its stability, such a phenotype is suppressed by coexpression with UmuC (Fig. 2). One obvious exception was the V130M protein, which was judged to be present at only 4% of the level of the wild-type protein.
FIG. 2.
Steady-state levels of missense mutants. The levels of UmuD′ protein expressed from pRW66 and missense derivatives were analyzed in whole-cell E. coli extracts (RW126/pRW124) by chemiluminescence immunoassay. The amino acid substitution in each mutant protein is indicated in single-letter code above the individual lanes. The position of wild-type UmuD′ is indicated on the left. As noted previously (21), some of the missense mutations result in a protein that migrates anomalously compared to the wild-type UmuD′ protein.
Plasmids with nonsense mutations {pRW66-11 [Q36X(Am)], pRW66-31 [Q42X(Oc)], and pRW66-48 [Q100X(Oc)]} failed to yield a detectable UmuD′ protein under a variety of conditions (data not shown).
We were also unable to detect the wild-type UmuD′ protein expressed from plasmid pRW66-17 (which contains the −35 promoter mutation TTGATA→TTAATA). By comparison, however, we were able to detect steady-state levels of the wild-type UmuD′ protein expressed from the TTGATA→TTTATA −35 promoter mutation in plasmid pRW66-42, but only if we increased the amount of cell extract normally applied to the gel from 40 to 200 μg per track (data not shown).
DISCUSSION
Molecular basis for the phenotypes of E. coli umuD′ mutants. (i) Promoter mutations.
Two mutations that caused G→A or G→T changes in the −35 region of the umu promoter (TTGATA) were recovered. Previous studies have suggested that this guanine residue within the −35 promoter region is critical for efficient promoter activity (20). Consistent with this suggestion, both mutations reduced the expression of UmuD′ (based upon the steady-state levels of UmuD′ protein). Our studies demonstrated that the G→A mutation caused the greatest phenotypic defect, while the G→T mutation resulted in levels of UmuD′ that were apparently suboptimal for both spontaneous and MMS-induced mutagenesis (Table 1). Such observations are therefore consistent with mutational studies of the lacUV5 −35 promoter region (20).
(ii) Nonsense mutations.
Three nonsense mutations were recovered. Two were ochre stop codons that resulted in truncated UmuD′ proteins of 16 and 74 amino acids. Neither truncated protein was detected by Western analysis, suggesting either that the peptide fragments were not recognized by the polyclonal UmuD′ antiserum or that the peptide produced was perhaps more rapidly degraded in vivo. In addition, neither mutant protein possessed any ability to promote either spontaneous or MMS-induced mutagenesis. The third nonsense mutation occurred when the Glu36 codon was changed to an amber codon (Table 1). As previously noted (41), strain RW126 carries the amber-suppressing mutation supE44, which inserts a Glu residue instead of terminating protein synthesis. Thus, if suppressed, the Q36X(Am) mutation should, in fact, produce a full-length wild-type protein. Similar to previous results obtained with an amber mutation in umuC (41), the levels of wild-type UmuD′ produced by the suppressed Q36X(Am) mutation were insufficient to promote spontaneous mutagenesis and were obviously limiting for MMS-induced mutagenesis (Table 1).
(iii) Missense mutations.
Most of the loss-of-function mutations identified here were missense mutations. We have also cloned the three previously identified chromosomal missense umuD mutations into umuD′. We were therefore interested in determining the molecular basis for the observed phenotypes of these novel umuD′ mutants. This goal has been greatly facilitated by the recent determination of the three-dimensional structure of UmuD′ (6, 27, 28). As noted in the introduction, based upon the crystallized structure of UmuD′, we have previously suggested that the UmuD′ protein might exist in several forms (27, 28). In this article we use the terms “molecular dimer” to indicate the protein-protein interactions between the two globular domains of each UmuD′ promoter, “filament dimer” for the interactions between the long and short N- and C-terminal extensions, respectively, and “extended filaments” for the higher-order protein-protein interactions observed between molecular and filament dimers.
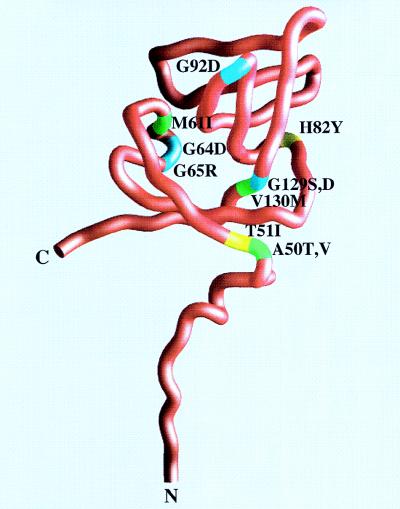
The phenotypes of all of these missense umuD′ mutants are therefore discussed in light of the effects that the changes might have on the structural integrity of the UmuD′ protein. A worm diagram of a UmuD′ monomer and the position of the various missense mutations are shown in Fig. 3. Over half of the missense mutations (A50T, A50V, G64D, G65R, H82Y, G92D, and probably P27S) resulted in amino acid substitutions at residues that are solvent accessible in a UmuD′ monomer. The remaining mutations were at residues normally buried within the body of the protein.
FIG. 3.
Worm diagram of a UmuD′ promoter with mutations labeled. The N and C termini are labeled, as well as each of the mutations described in this study. Wild-type amino acids are identified as follows: hydrophobic residues are colored green, hydrophilic residues are colored yellow, and glycines are colored cyan. Each residue is labeled along with the change found in the mutant protein. This figure was generated with the program GRASP (24).
P27S(pRW368/umuD′1).
The P27S mutant was generated by subcloning the umuD1 mutation into umuD′. The Pro27 residue is absolutely conserved in all 10 UmuD-like proteins identified to date but is somewhat more variable in the structurally related repressor proteins (28, 40). While this mutation blocks cleavage of UmuD and results in a nonmutable phenotype (2, 21, 35), when cloned into umuD′ the mutant was moderately proficient at promoting both SOS-dependent spontaneous and MMS-induced mutagenesis (Table 1). Unfortunately, the P27S mutation is in a region of the UmuD′ protein that is disordered within the UmuD′ crystal and cannot be accurately determined (27, 28). We speculate, however, that the phenotypic differences seen when the mutation is expressed in UmuD′ compared to UmuD support the hypothesis that the amino terminus of UmuD undergoes a conformational change during its conversion to the mutagenically active UmuD′ protein (6, 13, 28). We hypothesize that the P27S mutation somehow prevents the amino terminus of UmuD from entering the catalytic active site of UmuD yet is less important for the subsequent activity of UmuD′.
A50T(pRW66-1/umuD′312), A50V(pRW66-52/umuD′313), and T51I(pRW66-38/umuD′314).
The Ala50 residue is highly conserved in the UmuD-like mutagenesis proteins and is invariant with the exceptions of the Salmonella typhimurium UmuD protein (which has a serine) (34, 38) and RulA (which has a histidine at residue 50) (36). Thr51 is also highly conserved in 9 of the 10 UmuD-like proteins. Interestingly, isoleucine is naturally found at this position in RulA (36). Ala50 and Thr51 are located at the N-terminal end of the first β-strand of UmuD′ (Fig. 3). The Ala50 residue is about 3.5 to 4.5 Å from Ala77, Ser76, and His47. It is relatively exposed to solvent in the UmuD′ monomer and is at the start of the turn that comes from the extended N terminus. Substituting a Val or Thr at this position puts a larger side chain in the space. Thr51 points toward the N-terminal extended strand and is relatively close to Ile38 of the symmetrically related molecule in the UmuD′ filament dimer (6, 28). Based upon the two-hybrid assay (Fig. 1A), mutational changes at both Ala50 and Thr51 appear to affect the ability of UmuD′ to dimerize, the T51I mutation being more severe than the A50V or A50T mutation. One hypothesis that explains these observations is that Thr51 may be important, along with Ala50, in keeping the N terminus in an appropriate orientation for filament dimer formation (6, 28). While not at the filament dimer interface per se, we hypothesize that the defects associated with the T51I, A50V, and A50T mutations result from a reduced ability of the mutant proteins to form filament dimers.
M61I(pRW66-13/umuD′315).
The Met61 residue is conserved in virtually all UmuD-like mutagenesis proteins and repressor proteins (13 of 14 homologs), with the only exception being the bacteriophage P1 HumD protein, which has a histidine at the corresponding position (40). Met61 is juxtaposed to Ser60 of the catalytic active site and is normally buried within the body of the protein at the end of the catalytic crevice (Fig. 4). Interestingly, the M61I substitution would create a hole, which we speculate that Ile85 may move to fill. This, in turn, could pull on the β-strand to which it is attached and thereby affect the filament dimer interface (Fig. 5) (6, 28). The M61I protein did not appear to dimerize quite as efficiently as the wild-type protein and exhibited intermediate levels of spontaneous SOS mutagenesis but normal levels of damage-induced mutagenesis. Thus, the mild phenotype of the mutant appears to be suppressed in damaged cells, presumably because of interactions between the M61I mutant protein and activated RecA in vivo.
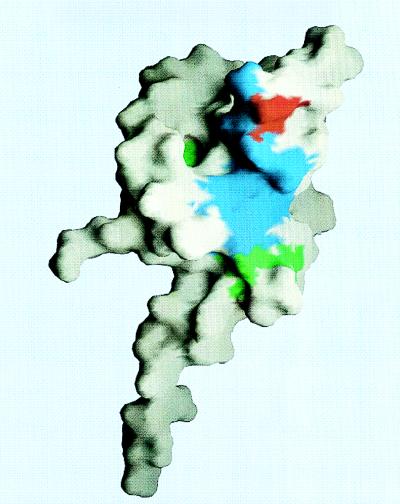
FIG. 4.
Surface representation of the UmuD′ promoter. This orientation is approximately the same as that of the worm diagram of Fig. 3. The surface areas of residues involved in the molecular dimer interface (27, 28) are colored blue, except Gly92, which is colored red. The surface areas of residues that were mutated, other than Gly92, are colored green. Also seen in this view are Met61, Ala50, and Thr51. This figure was generated with the program GRASP (24).
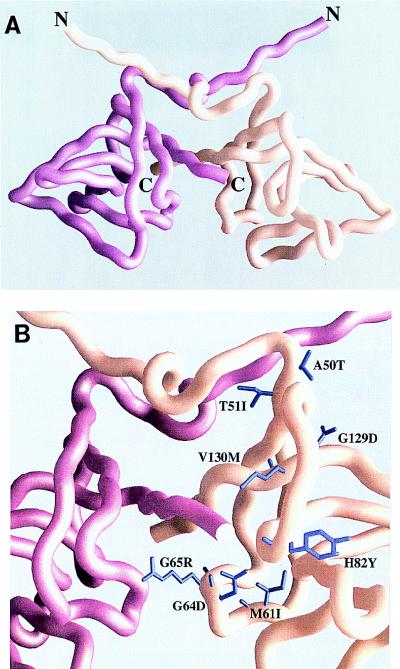
FIG. 5.
Position of UmuD′ mutants in a filament dimer. (A) Overall view of a UmuD′ filament dimer. The view presented here is similar to that previously reported (28) and is shown for orientation purposes. One promoter is colored magenta, while the other is peach. (B) Close-up of the positions of the various UmuD′ mutants and their locations with respect to the filament dimer interface. This view is identical to that of panel A. The side chains of the mutant residues are colored violet. Although the mutant residues are found in each protomer, for the sake of clarity only the mutant residues in the peach-colored protomer are identified. This figure was generated with the program GRASP (24).
G64D(pRW66-19/umuD′316).
The G64D mutation also resulted in a mild phenotype, with approximately half the normal levels of spontaneous and MMS-induced mutagenesis, and appeared to interact with itself to the same extent as the wild-type protein. Gly64 is located in the short 310 helix of UmuD′ and is variable among UmuD-like proteins. This residue is on the surface of the UmuD′ protein and would be predicted to be in close proximity to the carboxyl-terminal tail of another promoter in the filament dimer (Fig. 5). Presumably, the G64D mutation moderately affects filament dimer formation, but as with the M61I mutation, this effect is suppressed in a damaged cell, where we hypothesize that activated RecA acts as a scaffold to correctly position UmuD′ for its subsequent role in translesion replication (28).
G65R(pRW356/umuD′44).
The G65R mutation was originally identified on the basis of its nonmutable phenotype when expressed in UmuD (2, 19, 21). In contrast, however, when subcloned into umuD′, G65R can apparently promote significant levels of spontaneous and damage-induced mutagenesis (Table 1). Clearly, such a mutational change has dramatic and specific effects on the ability of E. coli UmuD to undergo posttranslational processing but much less effect on the subsequent mutagenic function of UmuD′. The G65R residue is solvent exposed and is located in the short 310 helix of UmuD′ (Fig. 3 and 5). The Cα of Gly65 is ∼4 Å from the Nζ of Lys136 in the same molecule and ∼5 Å from the Nζ of Lys136 of the symmetrically related filament dimer molecule (Fig. 5). Thus, one hypothesis to explain the defect in mutagenesis of the G65R UmuD′ substitution is that it results in a positively charged area that subsequently affects efficient filament dimer formation.
H82Y(pRW66-30/umuD′305).
The His82 residue is highly conserved in 7 of the 10 UmuD-like proteins. This residue is located on a ridge on the surface of UmuD′, and we have previously hypothesized that it could make contact with the amino-terminal tail of another promoter in the extended UmuD′ filament (28). His82, like Gly64 and Gly65, is also in the vicinity of the C terminus of a filament dimer mate (Fig. 5), but at its closest approach of 9 to 10 Å, it would be less likely to affect filament dimer formation. Either way, the H82Y substitution appears to result in a more severe defect than G64D, as both spontaneous and MMS-induced mutagenesis rates were lower than those seen with the G64D substitution.
G92D(pRW358/umuD′77).
Like the G65R mutation, the G92D mutation was originally identified on the basis of its nonmutable phenotype when expressed in UmuD (2, 19, 21). The molecular basis for this phenotype is not immediately clear, as two structurally related proteins (LexA and bacteriophage 434 CI) have an aspartate at a position equivalent to residue 92 (21, 28). The G92D residue is found at the crystallized molecular dimer interface of UmuD′ (Fig. 4). The G92D mutant was phenotypically indistinguishable from wild-type UmuD′, and had we not subcloned the mutation from umuD77, this change would not have been identified in our colorimetric screen for loss-of-function umuD′ mutants. If, however, UmuD and UmuD′ have the same general structure, as is now hypothesized (6), one could easily explain the nonmutable phenotype of UmuD G92D by the fact that it might favor molecular dimer formation. Such an interaction is predicted to occlude the catalytic active site of UmuD and thereby exclude the possibility of UmuD cleavage (27).
G129D(pRW66-20/umuD′318), G129S(pRW66-44/umuD′317), and V130M(pRW66-18/umuD′319).
Residues 129 and 130 are highly conserved in all of the structurally related proteins. Gly129 is invariant in both the UmuD-like mutagenesis proteins and the LexA and CI repressor proteins (28, 40); Val130 is strictly conserved in the mutagenesis protein but not the repressor proteins. Interestingly, two mutations were recovered at residue 129. Both substitutions dramatically reduce the ability of the mutant protein to perform both spontaneous and MMS-induced mutagenesis (Table 1). The G129D substitution is also unique in that the same substitution renders UmuD noncleavable (2). Both the Gly129 and Val130 residues are found in a tightly packed area in the last β-strand of UmuD′ (Fig. 3). Indeed, Gly129 is packed closely (less than 4Å away) to the backbone of residues Asp75 and Ser76, and any side chain would impinge on these residues (Fig. 5). Glycine is normally invariant at position 129, as it is presumably the only amino acid small enough to fit into the available space. Substitution with either serine or aspartate presumably affects the overall structure of UmuD′, but these changes lead only to a modest increase in susceptibility to proteolysis. In contrast, they clearly have dramatic effects on the mutant protein’s ability to perform mutagenesis functions. Based upon the two-hybrid assay, it appeared that the G129D mutant protein is very efficient at forming dimers, presumably because the mutation somehow affects filament dimer formation, but we cannot exclude the possibility that it also affects the molecular dimer. As noted for the G92D mutation, if G129D does promote molecular dimerization, an inability of the UmuDG129D molecular dimer to dissociate might explain why the UmuDG129D protein is rendered noncleavable (2).
The V130M mutant performs mutagenesis functions moderately efficiently, but this mutation dramatically increases the susceptibility of the protein to proteolysis (Fig. 2). Val130 is close to the very C terminus of the symmetrically related filament dimer molecule and is about 4 Å away from Asp84, Ala80, and Ile78 (Fig. 5). In the case of the V130M mutant protein, we hypothesize that the defect in mutagenesis functions is more likely attributed to the instability of the mutant protein than to any gross defect in function.
Concluding remarks.
Based upon the crystal structure of UmuD′ (27, 28), we hypothesized that UmuD′ might interact with itself in two ways: by the formation of a molecular dimer and by the formation of a filament dimer. The filament dimer gained its name from the extended filament structures seen in the UmuD′ crystal (27, 28) as well as the multimeric (dimer, tetramer, hexamer) complexes seen at physiological concentrations in solution (28). These observations suggested to us that both dimer interactions occur in the crystal and in solution. Very recently, Ferentz et al. (6) reported that they see strong nuclear overhauser effects across the carboxyl termini of UmuD′, concluding that only the filament dimer exists in solution. Indeed, filament dimer interaction appears to be important for the in vivo mutagenesis-promoting activity of UmuD′, as N-terminal and C-terminal deletion mutants of UmuD′ that exhibit reduced abilities to form multimeric complexes are also rendered phenotypically nonmutable (28). At present, we cannot rule out the possibility that a molecular dimer also exists, however, as the C-terminal deletion mutant (28) which, based upon the NMR-derived structure of UmuD′ would be predicted to be unable to form a filament dimer, still apparently forms a chemically cross-linkable dimer (unpublished observations). What is clear, however, is that our screen for loss-of-function umuD′ mutants appears to yield predominantly filament dimer mutants. Based upon these observations, it would seem that mutations which disrupt the UmuD′ filament dimer have a significant effect on the ability of the mutant protein to promote mutagenesis, whereas those at the putative molecular dimer interface are much less pronounced.
We previously hypothesized that, as part of the mutagenic process, a RecA nucleoprotein filament would act as a scaffold for the assembly of UmuD′ filaments (28). Further support for such a model comes from our observations that many of the loss-of-function mutants were defective for spontaneous mutagenesis but that under conditions of DNA damage, where one would predict more extensive RecA nucleoprotein filament formation, the phenotypes of many UmuD′ mutants were much less pronounced. To explain such observations, we favor the hypothesis that the various UmuD′ mutants have reduced abilities to form UmuD′ filament dimers per se, but by binding to a RecA nucleoprotein filament the UmuD′ filament dimer (and perhaps the higher-order filament structures) is sufficiently stabilized for error-prone repair to occur with roughly normal efficiency.
Further experiments with site-directed mutants of UmuD′ are currently in progress to address the role of the molecular and filament UmuD′ dimers in damage-induced mutagenesis and how such structures might affect the ability of UmuD′ to interact with UmuC, RecA, and, potentially, DNA polymerase III holoenzyme.
ACKNOWLEDGMENT
We thank Brian Meffle for help with the yeast two-hybrid assays.
REFERENCES
- 1.Bartel P L, Fields S. Analyzing protein-protein interactions using two-hybrid system. Methods Enzymol. 1995;254:241–263. doi: 10.1016/0076-6879(95)54018-0. [DOI] [PubMed] [Google Scholar]
- 2.Battista J R, Ohta T, Nohmi T, Sun W, Walker G C. Dominant negative umuD mutations decreasing RecA-mediated cleavage suggest roles for intact UmuD in modulation of SOS mutagenesis. Proc Natl Acad Sci USA. 1990;87:7190–7194. doi: 10.1073/pnas.87.18.7190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bridges B A, Woodgate R. Mutagenic repair in Escherichia coli: products of the recA gene and of the umuD and umuC genes act at different steps in UV-induced mutagenesis. Proc Natl Acad Sci USA. 1985;82:4193–4197. doi: 10.1073/pnas.82.12.4193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Burckhardt S E, Woodgate R, Scheuermann R H, Echols H. UmuD mutagenesis protein of Escherichia coli: overproduction, purification and cleavage by RecA. Proc Natl Acad Sci USA. 1988;85:1811–1815. doi: 10.1073/pnas.85.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Donnelly C E, Walker G C. Coexpression of UmuD′ with UmuC suppresses the UV mutagenesis deficiency of groE mutants. J Bacteriol. 1992;174:3133–3139. doi: 10.1128/jb.174.10.3133-3139.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ferentz A E, Opperman T, Walker G C, Wagner G. Dimerization of the UmuD′ protein in solution and its implications for regulation of SOS mutagenesis. Nat Struct Biol. 1997;4:979–983. doi: 10.1038/nsb1297-979. [DOI] [PubMed] [Google Scholar]
- 7.Fields S, Song O K. A novel genetic system to detect protein-protein interactions. Nature. 1989;340:245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- 8.Fijalkowska I J, Dunn R L, Schaaper R M. Genetic requirements and mutational specificity of the Escherichia coli SOS mutator activity. J Bacteriol. 1997;179:7435–7445. doi: 10.1128/jb.179.23.7435-7445.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Frank E G, Ennis D G, Gonzalez M, Levine A S, Woodgate R. Regulation of SOS mutagenesis by proteolysis. Proc Natl Acad Sci USA. 1996;93:10291–10296. doi: 10.1073/pnas.93.19.10291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frank E G, Gonzalez M, Ennis D G, Levine A S, Woodgate R. In vivo stability of the Umu mutagenesis proteins: a major role for RecA. J Bacteriol. 1996;178:3550–3556. doi: 10.1128/jb.178.12.3550-3556.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Frank E G, Hauser J, Levine A S, Woodgate R. Targeting of the UmuD, UmuD′ and MucA′ mutagenesis proteins to DNA by RecA protein. Proc Natl Acad Sci USA. 1993;90:8169–8173. doi: 10.1073/pnas.90.17.8169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Friedberg E C, Walker G C, Siede W. DNA repair and mutagenesis. Washington, D.C: American Society for Microbiology; 1995. [Google Scholar]
- 13.Guzzo A, Lee M H, Oda K, Walker G C. Analysis of the region between amino acids 30 and 42 of intact UmuD by a monocysteine approach. J Bacteriol. 1996;178:7295–7303. doi: 10.1128/jb.178.24.7295-7303.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hill J, Donald K A G, Griffiths D E. DMSO-enhanced whole cell yeast transformation. Nucleic Acids Res. 1991;19:5791. doi: 10.1093/nar/19.20.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho C, Kulaeva O I, Levine A S, Woodgate R. A rapid method for cloning mutagenic DNA repair genes: isolation of umu-complementing genes from multidrug resistance plasmids R391, R446b, and R471a. J Bacteriol. 1993;175:5411–5419. doi: 10.1128/jb.175.17.5411-5419.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Isackson P J, Bertrand K P. Dominant negative mutations in the Tn10 tet repressor: evidence for use of the conserved helix-turn-helix motif in DNA binding. Proc Natl Acad Sci USA. 1985;82:6226–6230. doi: 10.1073/pnas.82.18.6226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ito H, Fukuda Y, Murata K, Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983;153:163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jonczyk P, Nowicka A. Specific in vivo protein-protein interactions between Escherichia coli SOS mutagenesis proteins. J Bacteriol. 1996;178:2580–2585. doi: 10.1128/jb.178.9.2580-2585.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kato T, Shinoura Y. Isolation and characterization of mutants of Escherichia coli deficient in induction of mutations by ultraviolet light. Mol Gen Genet. 1977;156:121–131. doi: 10.1007/BF00283484. [DOI] [PubMed] [Google Scholar]
- 20.Kobayashi M, Nagata K, Ishihama A. Promoter selectivity of Escherichia coli RNA polymerase: effect of base substitutions in the promoter −35 region on promoter strength. Nucleic Acids Res. 1990;18:7367–7372. doi: 10.1093/nar/18.24.7367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Koch W H, Ennis D G, Levine A S, Woodgate R. Escherichia coli umuDC mutants: DNA sequence alterations and UmuD cleavage. Mol Gen Genet. 1992;233:443–448. doi: 10.1007/BF00265442. [DOI] [PubMed] [Google Scholar]
- 22.Lin L-L, Little J W. Isolation and characterization of non-cleavable (Ind−) mutants of the LexA repressor of Escherichia coli K-12. J Bacteriol. 1988;170:2163–2173. doi: 10.1128/jb.170.5.2163-2173.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 24.Nicholls A, Sharp K A, Honig B. Protein folding and association: insights from the interfacial and thermodynamic properties of hydrocarbons. Proteins. 1991;11:281–296. doi: 10.1002/prot.340110407. [DOI] [PubMed] [Google Scholar]
- 25.Nohmi T, Battista J R, Dodson L A, Walker G C. RecA-mediated cleavage activates UmuD for mutagenesis: mechanistic relationship between transcriptional derepression and posttranslational activation. Proc Natl Acad Sci USA. 1988;85:1816–1820. doi: 10.1073/pnas.85.6.1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oller A R, Fijalkowska I J, Schaaper R M. The Escherichia coli galK2 papillation assay: its specificity and application to seven newly isolated mutator strains. Mutat Res. 1993;292:175–185. doi: 10.1016/0165-1161(93)90145-p. [DOI] [PubMed] [Google Scholar]
- 27.Peat T S, Frank E G, McDonald J P, Levine A S, Woodgate R, Hendrickson W A. Structure of the UmuD′ protein and its regulation in response to DNA damage. Nature. 1996;380:727–730. doi: 10.1038/380727a0. [DOI] [PubMed] [Google Scholar]
- 28.Peat T S, Frank E G, McDonald J P, Levine A S, Woodgate R, Hendrickson W A. The UmuD′ protein filament and its potential role in damage induced mutagenesis. Structure. 1996;4:1401–1412. doi: 10.1016/s0969-2126(96)00148-7. [DOI] [PubMed] [Google Scholar]
- 29.Perry K L, Elledge S J, Mitchell B, Marsh L, Walker G C. umuDC and mucAB operons whose products are required for UV light and chemical-induced mutagenesis: UmuD, MucA, and LexA products share homology. Proc Natl Acad Sci USA. 1985;82:4331–4335. doi: 10.1073/pnas.82.13.4331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rajagopalan M, Lu C, Woodgate R, O’Donnell M, Goodman M F, Echols H. Activity of the purified mutagenesis proteins UmuC, UmuD′ and RecA in replicative bypass of an abasic DNA lesion by DNA polymerase III. Proc Natl Acad Sci USA. 1992;89:10777–10781. doi: 10.1073/pnas.89.22.10777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schiestl R H, Gietz R D. High efficiency transformation of intact yeast cells using single stranded nucleic acids as a carrier. Curr Genet. 1989;16:339–346. doi: 10.1007/BF00340712. [DOI] [PubMed] [Google Scholar]
- 32.Sherman F, Fink G R, Hicks J B. Methods in yeast genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1986. [Google Scholar]
- 33.Shinagawa H, Iwasaki H, Kato T, Nakata A. RecA protein-dependent cleavage of UmuD protein and SOS mutagenesis. Proc Natl Acad Sci USA. 1988;85:1806–1810. doi: 10.1073/pnas.85.6.1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Smith C M, Koch W H, Franklin S B, Foster P L, Cebula T A, Eisenstadt E. Sequence analysis of the Salmonella typhimurium LT2 umuDC operon. J Bacteriol. 1990;172:4964–4978. doi: 10.1128/jb.172.9.4964-4978.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Steinborn G. Uvm mutants of Escherichia coli K12 deficient in UV mutagenesis. I. Isolation of uvm mutants and their phenotypical characterization in DNA repair and mutagenesis. Mol Gen Genet. 1978;165:87–93. doi: 10.1007/BF00270380. [DOI] [PubMed] [Google Scholar]
- 36.Sundin G W, Kidambi S P, Ullrich M, Bender C L. Resistance to ultraviolet light in Pseudomonas syringae: sequence and functional analysis of the plasmid-encoded rulAB genes. Gene. 1996;177:77–81. doi: 10.1016/0378-1119(96)00273-9. [DOI] [PubMed] [Google Scholar]
- 37.Sweasy J B, Witkin E M, Sinha N, Roegner-Maniscalco V. RecA protein of Escherichia coli has a third essential role in SOS mutator activity. J Bacteriol. 1990;172:3030–3036. doi: 10.1128/jb.172.6.3030-3036.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thomas S M, Crowne H M, Pidsley S C, Sedgwick S G. Structural characterization of the Salmonella typhimurium LT2 umu operon. J Bacteriol. 1990;172:4979–4987. doi: 10.1128/jb.172.9.4979-4987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Woodgate R, Ennis D G. Levels of chromosomally encoded Umu proteins and requirements for in vivo UmuD cleavage. Mol Gen Genet. 1991;229:10–16. doi: 10.1007/BF00264207. [DOI] [PubMed] [Google Scholar]
- 40.Woodgate R, Levine A S. Damage inducible mutagenesis: recent insights into the activities of the Umu family of mutagenesis proteins. In: Lindahl T, editor. Cancer surveys: genetic instability in cancer. 28th ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1996. pp. 117–140. [PubMed] [Google Scholar]
- 41.Woodgate R, Singh M, Kulaeva O I, Frank E G, Levine A S, Koch W H. Isolation and characterization of novel plasmid-encoded umuC mutants. J Bacteriol. 1994;176:5011–5021. doi: 10.1128/jb.176.16.5011-5021.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]