Abstract
This review article aims to present an overview regarding the volatile compounds in different scented species of Pelargonium and their biological activities, immunomodulatory activity, cytotoxic activity, high larvicidal activity and ethnopharmacological uses. Although the Pelargonium genus includes many species, we focused only on the scented ones, with the potential to be used in different domains. Pelargonium essential oil showed great properties as antioxidant activity, antibacterial activity (against K. pneumonie, S. aureus or E. coli strains) and antifungal activity (against many fungi including Candida sp.), the responsible compounds for these properties being tannins, flavones, flavonols, flavonoids, phenolic acids and coumarins. Due to the existence of bioactive constituents in the chemical composition of fresh leaves, roots, or flowers of Pelargonium sp. (such as monoterpenoid compounds–citronellol, geraniol, linalool, and flavonoids–myricetin, quercetin and kaempferol), this species is still valuable, the bio-compounds representing the base of innovative substitutes in food processing industry, nutraceuticals, or preventive human or veterinary medicine (substitute of antibiotics). Highlighting the volatile chemical composition and properties of this scented plant aims to rediscover it and to emphasize the vast spectrum of health-promoting constituents for a sustainable approach. Future research directions should point to the application of plant biotechnology with a significant role in conservation strategy and to stimulate commercial interest.
Keywords: Pelargonium sp., bioactive compounds, sustainable approach, scented plants
1. Introduction
Pelargonium is a genus comprising approximately 230 perennial plant species [1]. This genus belongs to the family Geraniaceae and originally comes from the Cape area in South Africa. Starting from the 18th century, Pelargonium has been cultivated in Europe. The name is derived from the Greek word “pelargos” meaning stork and relates to the shape of the geranium flower, resembling a stork’s beak [2]. The Pelargonium genus can be categorized into three groups: plants with green or evergreen leaves (P. graveolens, P. quercifolium, P. tomentosum); plants with multi-colored leaves (P. graveolens “Variegatum”); and plants with flowers and fruits (P. grandiflorum-hybrid, P. peltatum) [3]. There are many cultivars of this genus, which were derived from approximately 20 species. These cultivars are known to belong to one of six horticultural groups: Angel, Ivy-leaved, Regal, Scented-leaved, Unique, and Zonal [1].
Gunes and Kahraman [4] presented the Pelargonium graveolens as being an ornamental species and, at the same time, a plant with edible flowers [4].
Plaschil et al. [5] reviewed the genetic characterization of 15 Pelargonium genotypes, resulting in the determination of their ploidy levels. Thus, the species with the highest ploidy levels are Pelargonium capitatum (2n = 66), Pelargonium graveolens, Pelargonium vitifolium, and Pelargonium radens (2n = 88). These species could be a fusion of auto allopolyploids [5]. Saraswathi et al. [6] reviewed the phytopharmacological importance of the most important species of Pelargonium: P. graveolens, P. reniforme, P. sidoides, and P. radula. The genus Pelargonium is recognized for its medicinal benefits, and rich sources of monoterpenes, tannins, phenolic acids, cinnamic acids, flavones, flavonoids, coumarins, and flavonol derivatives [6].
Van Wyk [7] presented the importance of some African medicinal plants. Pelargonium cv. Rosé leaves have the main use as fragrance, and Pelargonium sidoides roots are used in phytomedicine (bronchitis and immune stimulant) and traditional medicine (general tonic, dysentery) [7]. Additionally, Brendler and van Wyk [8] reviewed the historical, commercial, and scientific perspectives of Pelargonium species, including antibacterial, antifungal, and immunomodulatory properties [8].
The present review paper aims to present the identified components in different scented species of the Pelargonium genus, as well as their potential biological activities, as revealed by scientific papers published in the last decade.
2. Results and Discussions
2.1. Chemical Composition of Plants from Pelargonium Genus
The rose geranium’s chemical composition is influenced by various environmental elements, including climate, temperature fluctuations, sunlight duration, rainfall levels, phenological stages, harvesting periods and techniques, weed presence, and cultural practices. Pedo-climatic factors influence the quality of the essential oil (EO), in addition to the plant selection and distillation process [9].
Boukhris et al. [10] reported the chemical composition of geranium oil from P. graveolens during various phenological stages. In a separate study, Abaas et al. [11] explored the differences in essential oil composition at vegetative and flowering stages, whereas Mahboubi and Valian [12] reviewed the composition and potential applications of nine essential oils obtained from P. graveolens. Three types of geranium essential oil were classified by Couic-Marinier and Laurain-Mattar [9]. The three types are: the Chinese variant, which contains a high amount of citronellol (30–40%); the African variant, hailing from Algeria, Morocco, and Egypt, featuring 10-epi-γ-eudesmol (4–5%); and the Bourbon variant, originating from Reunion Island or Madagascar, consisting of a significant amount of guaia-6,9-diene (5–7%), geraniol (15–18%), and linalool (0.5 to 8%) [9].
Eiasu et al. [13] conducted a study on the physio-morphological response of Pelargonium plants to irrigation frequency. The results indicated that a high irrigation frequency led to an increase in the favorable ratio of citronellol and geraniol. Furthermore, modifications in essential oil distribution were observed in both glandular and non-glandular trichomes, which resulted in improved functions of plant tissues in the aerial parts (stems, leaves, and floral organs) [13]. Additionally, Lis-Balchin et al. [14] presented the chemical composition and antimicrobial properties of eight distinct Pelargonium varieties, while Mehrparvar et al. [15] reviewed the main components present in P. roseum Willd that contribute to its antifungal activity.
The composition of primary constituents detected in the essential oil from Pelargonium sp. may be impacted by different drying approaches. As a result, the research by Akçura et al. [16] investigated the impact of such methods. It revealed that the shade-drying strategy resulted in the highest concentration of linalool, citronellol, and geraniol, which recorded 7.42 ± 0.44%, 39.87 ± 0.23%, and 17.09 ± 0.12% correspondingly [16].
The featured composition varies depending on various factors, such as the value of the variety, different phenological stages, and seasonal variations. Several studies assessed species belonging to the Pelargonium genus during the analyzed period. Table 1 summarizes their primary findings, while the subsequent paragraphs detail the relevant studies.
The primary bioactive components of Pelargonium leaves comprise monoterpenoid compounds, including natural acyclic monoterpenoid citronellol (C10H20O), geraniol (C10H18O, a monoterpenoid and an alcohol), and linalool (C10H18O, a monoterpenoid and a tertiary alcohol) [10,14,17]. Additionally, root material contains flavonoids such as myricetin, quercetin, and kaempferol [18].
Another study demonstrated the biosynthesis of citronellol, the primary compound identified in Pelargonium graveolens. Banthorpe et al. [19] established that citronellol results from geraniol. This conversion can be achieved by employing a crude enzyme preparation (geraniol reductase) with the ability to reduce the double bond [19].
The chemical composition of fresh leaves of Pelargonium sp. is summarized in Table 1 based on multiple scientific studies regarding major volatile components. Geraniol, citronellol, and linalool are the most commonly occurring components.
Table 1.
Major volatile compounds in different Pelargonium species fresh leaves.
| Species | Main Identified Volatile Compounds | References |
|---|---|---|
| P. asperum | Geraniol and β-citronellol | [20] |
| P. capitatum | Citronellol, citronellyl formate, α-pinene, geraniol, geranyl formate and 6,9-guaiadiene | [21] |
| Citronellol, citronellyl formate, geranyl formate, β-caryophyllene, 6,9-guaiadiene | ||
| α-pinene, geranyl formate, β-caryophyllene, 6,9-guaiadiene | ||
| P.’Chocolate peppermint’ | Menthone (39.1%), isomenthone (22.2%), α-phellandrene (15%), ρ-cymene (4.7%) | [14] |
| P. cv. Rose | Citronellol (23.6%), geraniol (12.5%), citronellyl formate (11.1%), linalool (10%), isomenthone (2.7%) | |
| P. graveolens | Citronellol (17.74%) and geraniol (14.73%) | [17] |
| Geraniol, citronellol, citronellyl formate, geranyl formate, linalool, 10-epi-γ-eudesmol | [22] | |
| Citronellol, geraniol, citronellyl formate, L-linalool, 10-epi-γ-eudesmol and geraniol formate | [10] | |
| Geraniol (18.6–25.5%), citronellol (24.8–28.7%), citronellyl formate (7.9–10.5%), isomenthol (5.4–8.1%) and linalool (1.4–3.4%) | [23] | |
| Linalool, cis-rose oxide, trans-rose oxide, menthone, isomenthone, citronellol, geraniol, citronellyl formate, geranyl formate and 10-epi-γ-eudesmol | [24] | |
| Citronellol, geranial, geraniol, guainene, germacrene D, iso-menthone, geranyl formate | [25] | |
| Linalool, iso-menthone, citronellol, geraniol, citronellyl formate, geranyl formate, 10 epi-γ-eudesmol | [26] | |
| Citronellol (15.64%), geraniol (11.31%), citronellyl formate (10.19%), isolongifolan-7-a-ol (7.84%) | [27] | |
| Citronellol (39.9–49.19%), geraniol (6.5–14.88%), epi-γ-Eudesmol (7.6–10.49%), isomenthone (3.2–6.0%), citronellyl formate (3.6–4.9%) and linalool (1.3–4.9%) | [28] | |
| β-Citronellol, geraniol, citronellyl formate, linalool, (+)-isomenthone, σ-selinene | [29] | |
| Citronellol and geraniol | [30] | |
| Citronellol (32%), geraniol (15%), linalool (6%), isomenthone (6%), geranyl formate (2.5%), tiglate (2%), citronellyl formate (6%), guaia-6,9-diene, and 10-epi-γ eudesmol (5%) | [31] | |
| Linalool, menthone, geraniol, isomenthone, citronellyl formate, geranyl formate, cis-rose oxide, trans-rose oxide | [32] | |
| Citronellol, geraniol, citronellyl formate, iso-menthone, linalool, E-caryophyllene | [33] | |
| Citronellol (17.74%), geraniol (14.73%), 10-epi-γ-eudesmol (9.52%), citronellyl formate (5.96%), geraniol formate (3.82%), menthone (2.48%), and isomenthone (2.11%) | [34] | |
| Citronellol, linalool, geraniol, citronellyl formate, geranyl formate, geranyl acetate, limonene, trans-caryophyllene | [12] | |
| Geraniol, citronellol, β-linalool, γ-eudesmol, citronellyl formate, isomenthone, geranyl tiglate, germacrene-D, geranyl formate | [35] | |
| Isomenthone (41%), geraniol (19.1%), linalool (12.8%), citronellol (11.6%), citronellyl formate (11.3%) | [14] | |
| p-menthan-3-ol (13.31%), citronellol (27.41%), and geraniol (43.58%) | [36] | |
| Citronellol (19.22%), geraniol (14.03%) and citronellyl formate (10.02%) | [37] | |
| Citronellol, menthan-2-one, citronellyl formate, 10-epi-γ-eudesmol, rose oxide B, citronellyl propanoate and citronellyl butanoate | [38] | |
| P. graveolens cv. Bourbon | Citronellol and geraniol | [39] |
| P. graveolens L’Her | Linallol, citronellol, geraniol | [40] |
| Monoterpenic primary alcohols–citronellol and geraniol | [41] | |
| P. radens × P. capitatum | Citronellol, citronellyl formate, β-caryophyllene, germacrene D | [21] |
| P. roseum R. Br. | β-citronellol, citronellyl formate, geraniol, iso-menthone, linalool | [40] |
| P. roseum Willd. | Citronellol (34.22%), geraniol (11.67%), linalool (8.7%) | [15] |
| P. x hybridum cv. ‘Atomic snowflake’ | Citronellic acid (37%), citronellol (14.8%) + many sesquiterpenes | [14] |
| P. x hybridum cv. ‘Mabel Grey’ | Citronellal (49.9%), citronellol (37.4%), geraniol (4.1%) | |
| P. x hybridum cv. ‘Sweet Mimosa’ | Isomenthone (35.7%), β-pinene (15.9%), α-phellandrene (5.9%) | |
| P. x hybridum cv. ‘Royal Oak’ | Linalool (23%), α-terpinene (7.1%), menthol (3.5%), ρ-cymene-8-ol (2.3%) | |
| P. x hybridum cv. ‘Clorinda’ | β-pinene 20.1%, α-phellandrene 7.3%, ρ-cymene 5.1%, limonene 4.3%, fenchone 4% |
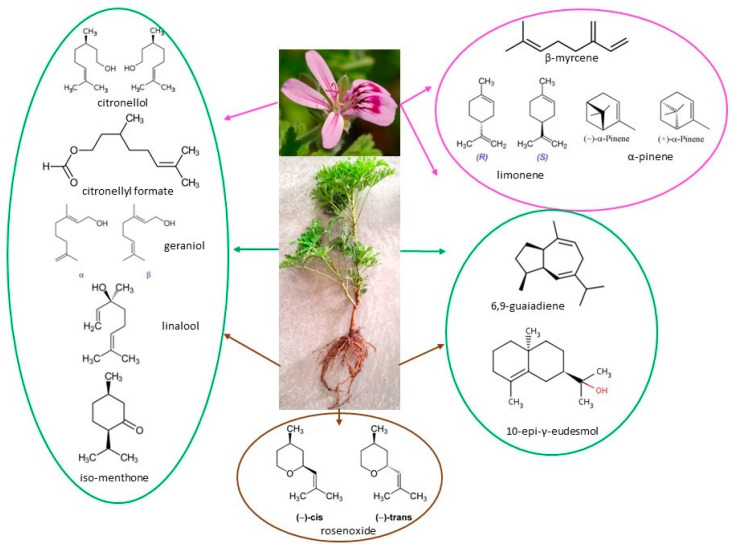
Furthermore, Figure 1 presents the primary volatile components of Pelargonium plant species, according to data from the literature.
Figure 1.
Primary volatile compounds of Pelargonium plant species.
Of the 120 phytoconstituents found in the plant, including sesquiterpenes, monoterpenes, and other important compounds, there are three key components that contribute to its scent: geraniol, citronellol, linalool, and their esters (Table 2). These compounds represent approximately 60% of the total essential oil [42].
Table 2.
The odor of different scented Pelargonium plant species.
| Species | Odor | References |
|---|---|---|
| P. betulinum | Camphoreous | [43] |
| P. blandfordianum | Scent of almonds, absinthe, and musk | [44] |
| P. capitatum | Rose | [43] |
| P. citronellum | Lemon | |
| P x Citronella hibrid | Citronella | [44] |
| P. crispum | Lemon, lime | [43] |
| P. crispum ‘Variegatum’ | Lemon fragrance | [1] |
| P. cucullatum | Spices | [43] |
| P. denticulatum | Lemongrass | |
| P. glutinosum | Balsamic | |
| P. graveolens | Mint | [43] |
| Strong, sweet, and spicy smell | [3] | |
| Rose | [44] | |
| P. ‘Graveolens’ of gardens | Pungent scent of rose lemon | [1] |
| P. hispidum | Fruity | [43] |
| P. ‘Lemon Fancy’ | A citrus fragrance | [1] |
| P. ‘Mabel Grey’ | A very strong lemon scent | |
| P. odoratissimum | Dill smell | [44] |
| A scent reminiscent of stored apples | [1] | |
| P. papilionaceum | Lemon | [43] |
| P. ‘Prince of Orange’ | Orange-scented leaves | [44] |
| P. pseudoglutinosum | Balsamic | [43] |
| P. quercifolium | Spices | |
| Strong, spicy, and hot smell, with a medicinal flavor | [3] | |
| P. radens | Mint | [43] |
| P. ‘Rober’s Lemon Rose’ | Rose-scented with lemon undertones | [1] |
| P. ‘Royal Oak’ | Exotic, spicy scent | |
| P. scabroide | Soap | [43] |
| P. scabrum | Apricot | |
| P. ‘Sweet Mimosa’ | Sweet-scented leaves and clusters | [1] |
| P. tomentosum | Mint | [43,44] |
| Strong, sweet, and menthol smell | [3] | |
| Peppermint-scented geranium | [1] | |
| P. vitifolium | Lemon balm, lemongrass | [43] |
The chemical components present in P. graveolens can be classified into several categories, including aliphatic hydrocarbons, aromatic hydrocarbons, terpene hydrocarbons, sesquiterpene hydrocarbons, aliphatic alcohols, terpene alcohols, aromatic alcohols, sesquiterpene alcohols, aliphatic esters, aromatic esters, terpene esters, aliphatic ketones, terpene ketones, sesquiterpene ketones, aliphatic aldehydes, terpene oxides, sesquiterpene oxides, aliphatic acids, terpene acids, and miscellaneous compounds [42]. Additionally, Blerot et al. [43] conducted a comprehensive analysis of Pelargonium essential oil, outlining the fatty acid derivatives, cyclic monoterpenes and derivatives, acyclic monoterpenes, and derivatives, sesquiterpenes and derivatives, and phenylpropanoid derivatives. The chemical compounds found in Pelargonium not only contribute to the flavor and aroma of plants, but also support respiratory systems when used in products. These compounds are responsible for many antibacterial, antiseptic, and antiviral properties.
Pelargonium chemical composition is influenced by the variation in environmental factors. The most common factors are related to the climate, such as high and low temperature, the sunny period, rainfalls, different phenological stages, harvesting period or harvested parts, the presence of weeds or cultural. Moreover, these pedoclimatic factors impact the quality of the P. graveolens essential oil. Thus, Table 3 illustrates a comparison of essential oil contents from various Pelargonium species, ranging from 0.11% to 4.60%.
Table 3.
Oil content in plants from Pelargonium sp.
| Plant | Part of Plant | Origin of Samples | Oil Content (%) | References |
|---|---|---|---|---|
| Pelargonium graveolens T1–T6 | fresh biomass | Bharwara Sewage Treatment Plant (STP), Gomti Nagar Lucknow, India | 0.28–0.33 | [23] |
| Pelargonium graveolens MLE1–MLE4 | leaves | Faculty of Science, Taif University, Saudi Arabia | 0.11–0.19 | [45] |
| Pelargonium graveolens L’Herit. Bourbon type | aerial parts | CSIR-Central Institute of Medicinal and Aromatic Plants (CIMAP), India | 0.20 | [30] |
| Pelargonium graveolens L’Herit. CIM-Pawan | 0.18 | |||
| Pelargonium graveolens L’Herit. CIM-Bio-G-171 | 0.22 | |||
| Pelargonium graveolens L’Herit. CIMAP Accession-21 | 0.13 | |||
| Pelargonium graveolens S1–S3 | leaves | El Maamoura (Governorate of Nabeul, North East of Tunisia) | 0.35–0.38 | [46] |
| Pelargonium graveolens S1S2 | 0.45 | |||
| Pelargonium graveolens S1S3 | 0.47 | |||
| Pelargonium graveolens S2S3 | 0.49 | |||
| Pelargonium graveolens S1S2S3 | 0.43 | |||
| Pelargonium graveolens in SDS | The mother plant native to Madagascar | 0.13 | [47] | |
| Pelargonium graveolens in BCS | 0.12 | |||
| Pelargonium graveolens in CS | 0.13 | |||
| Pelargonium graveolens (L’Hér) | Egypt | 0.31 | [48] | |
| Pelargonium graveolens NI and NCG | NA | 3.1 | [49] | |
| Pelargonium graveolens NI and MCT | 3.48 | |||
| Pelargonium graveolens LI and NCG | 3.8 | |||
| Pelargonium graveolens LI and MCT | 4.6 | |||
| Pelargonium graveolens | leaf–flowering stage | CSIR-Indian Institute of Integrative Medicine, Jammu, India | 0.16–0.18 | [50] |
| leaf–over-maturization stage | 0.09–0.10 | |||
| Pelargonium graveolens | leaves | Shanxi Agricultural University, China | 2.04 | [51] |
| Pelargonium graveolens NM-WW | Isfahan University of Technology, Iran | 0.12 | [52] | |
| Pelargonium graveolens NM-MWD | Isfahan University of Technology, Iran | 0.14 | ||
| Pelargonium graveolens | fresh plant | Burhaniye Aromatic Plants Field Station, Balıkesir Metropolitan Municipality Rural, Turkey |
1.98 | [16] |
| aerial parts | Western Himalayan region, India | 0.15 | [38] |
where: BCS—biochar growth media; CS—control growth media; LI—laser irradiation; MCT—mycorrhizal colonized treatment; MLE1 (1:40), MLE2 (1:30), MLE3 (1:20) and MLE4 (1:10) treatments—the supernatant was diluted with distilled water (v:v) to reach the required concentrations for foliar spray by adding 1 mL of supernatant into 10, 20, 30, and 40 mL of water; MWD—moderate water deficiency; NCG—non-colonized group; NI—non-irradiated; NM—Non-mycorrhizal; S1, S2, S3—inoculation using the three bacterial strains S1—Pseudomonas rhizophila S211, S2—Halomonas desertis G11, S3—Oceanobacillus iheyensis E9; SDS—solid digestate growth media; T1—100% sewage sludge, T2—80% sewage sludge + 20% soil, T3—60% sewage sludge + 40% soil, T4—40% sewage sludge + 60% soil, T5—20% sewage sludge + 80% soil; T6—soil (only soil); WW—well-watered.
Taking into consideration all the research articles mentioned in Table 4, the main components were determined using GC-MS, GC-FID, and HPLC.
Table 4.
Chemical composition of Pelargonium plant species (as presented by original works published in the period under review).
| Specie | Part of Plant | Identified Compounds | Method | References |
|---|---|---|---|---|
| P. asperum | EO | Citronellol, geraniol, citronellyl formate, 6,9-guaiadiene, isomenthone, linalool, geranyl formate, cis rose-oxide, α-pinene, trans rose-oxide, citronellyl butyrate, geranyl butyrate, geranyl tiglate, phenylethyl tiglate, menthone, isomenthol | GC | [53] |
| P. capitatum | Leaves | α-pinene, 10-epi-γ-eudesmol, citronellol, germacrene D, citronellyl formate, guaia-6,9-diene, δ-cadinene, β-caryophyllene epoxyde | GC-MS | [54] |
| P. capitatum × P. radens | Stem cutting | Linalool, citronellol, citronellyl formate, iso-menthone, geraniol, geranyl formate, guaiadiene (6,9) | GC | [13] |
| P. cv. Rosé | AP | α-pinene, linalool, isomenthone, α-terpineol, citronellol, trans-geraniol, citronellyl formate, geranyl formate, β-caryophyllene, isoledene, muurolene, guaia-6,9-diene, oleic acid,3-propyl ester, phnylethyltiglate, eudesmol, cubenol, α-cadinol, emulphor, geranyl tiglate | GC-MS | [55] |
| P. endlicherianum | EO | α-pinene, β-pinene, limonene, β-phellandrene, 2-pentyl furan, p-simen, nonanal, dimethyl tetradecane, α,p-dimetlystyrene, 2-nonly acetate, bicycloelemen, α-copaene, α-kamfolen aldehyde, pentadecane, decanal, α-burbonen, β-burbonen,trans-α-bergamoten, β-yilangen, β-elemen, β-copaene, β-caryophyllen, undecanal, 6,9-guayadien, aromadendrene, mirtenal, γ-elemen, (e)-2-decenal, γ-gurjunen, (z)-β-farnesene, heptadecane, germacrene d, (e)-2-undecanal, δ-cadenine, γ-cadenine, ar-kurkumen, tridecanal, (e)-geranyl acetone, phenyl ethyl isobutyrate, nonadecane, α-calacorene, 1,5-epoxy-salvial(4)14-en, 2-phenylethyl-2-methylbutyrate, 2-phenylethyl-3-methylbutyrate, caryophyllen oxide, pentadecanal, germacrene D-4β-ol, β-caryophyllenx alcohol, heneicosane, hexahydrofarnesyl acetone, spatulenol, 1-tetradecanol, nor-kopaonon, methylhexadecanoate, α-cadinol, cadalene, guaya-6,10(14)-dien-4β-ol, celine-11-en-4α-ol, tricosenes, farnesil acetone, pentacosenes, dodecanoic acid, phytol, tetradecanoic acid, pentadecanoic acid, nonacosan, hexadecanoic acid | GC-MS, GC-FID |
[56] |
| P. ‘Endsleigh’ | Flowers | Tartaric acid, malic acid, monogalloylhexose, methylated protocatechuic acid hexose, flavanone hexoside, methyl syringate 4-o-β-d-gentiobiose (leptosperin), myricetin 3-o-glucoside, kaempferol 3-o-di-p-coumaroylhexoside, myricetin-3-o-rhamnoside, myricetin-3-o-rhamnoside is. II, proanthocyanidin, quercetin 3-o-pentoside, kaempferol 3-o-galactoside, kaempferol 3-o-glucoside, kaempferol 3-o-pentoside | HPLC/MS | [57] |
| P. graveolens | AP | α-pinene, camphene, citronellol, citronellal, citronellic acid, geraniol, geranial, linalool, cis-linalool oxide, trans-linalool oxid, 6,9-guaiadiene, 3-carene, δ-carene, limonene, cis-ocimene, cis-β-ocimene, trans-β-ocimene, cis-sabinene, α-thujone, trans-rose oxide, cis-rose oxide, cis-limonene oxide, camphor, iso-menthone, p-menthone, p-menthene, menthol, isomenthol, isoborneol, naphthalene, cryptone, z-citral, linalyl acetate, isogeraniol, trans-anethole, azulene, geranyl-n-butyrate, geranyl formate, geranyl acetate, geranyl propanoate, geranyl hexanoate, geranyl octanoate, geranyl tiglate, citronellyl hexanoate, citronellyl acetate, citronellyl heptanoate, citronellyl-n-butyrate, citronellyl propanoate, citronellyl propionate, citronellyl formate, iso-menthyl acetate, α-cubebene, β-cubebene, isoledene, α-copaene, β-copaene, isolongifolene, junipene, α-bourbonene, β-bourbonene, trans-caryophyllene, calarene, aromadendrene, dehydroaromadendrene, α-guaiene, germacrene-d, α-caryophyllene, β-caryophyllene, seychellene, α-humulene, Ω-cadinene, δ-cadinene, ledene, valencene, δ-himachalene, eremophilene, β-bisabolene, elemol, α-calacorene, trans-longipinocarveol, cubenol, ledene oxide, humulene oxide, 10-epi-γ-eudesmol, 6-methyl-5-heptene-2-one, myrcene, α-phelleandrene, ρ-cymene, terpinen-4-ol, neoisomenthol, α-terpineol, α-cubabene, nerol, neral, piperitone, α-agarofuran, gerany valerate, γ-muurolene, α-copene, α-ylangene, terpinolene, iso-isopulegol, phenylethyl tiglate, phenylethyl propanoate, alloarmadendrene, 1,8-cineol, α-cadinol, hinesol, cis-3-hexenol, cis-calamenene, trans-a-bergamotene, α-guaiene, γ-terpinene β-elemene, 1-phenylethyl isobutanoate, caryophylla-4(12),8(13)-dien-5-ol, nonacosane, viridiflorol, α-gurjunene | GC-MS | [58,59,60,61,62,63] |
| GC-MS, LC-MS, (HPLC-MS/MS) |
[64] | |||
| HPLC | [65] | |||
| EO | Geraniol, citronellol, p-menthan-3-ol, nerol, citronellyl propionate, linalool, α-pinene, caryophyllene, menthone, rose oxide | GC | [36] | |
| α-pinene, myrcene, p-cymene, linalool, cis-rose oxide, trans-rose oxide, isomenthol, α-terpineol, citronellol, neral, geraniol, geranial, citronellyl formate, citronellyl acetate, citronellyl propionate, geranyl formate, geranyl acitate, geranyl propionate, geranyl triglate, β-bourbonene, (e)-caryophyllene, α-humulene, germacrene d, 2-phenyl ethyl tiglate, 10-epi-γ-eudesmol, geranyltiglate, 6,9-guaiadiene | GC-FID | [23,45] | ||
| Citronellol, linalool, linalool oxide, menthone, isomenthone, geraniol, geraniol formate, geranial, citronellyl acetate, citronellyl formate, citronellyl propionate, citronellyl propanoate, citronellyl tiglate, citronellyl butyrate, citronellyl butanoate, cadinene, α-terpineol, trans-rose oxide, cis-rose oxide, p-cymene, geranyl propanoate, geranyl acetate, geranyl formate, geranyl tiglate, geranyl butanoate, geranyl n-butyrate, nerol, neral, neryl formate, neryl acetate, α-pinene, β-pinene, geranic acid, (e)-cinnamaldehyde, terpinolene, α-terpinene, limonene, eugenol, borneol, coumarin, 1,8-cineole, carvacrol, α-ylangene, α-copaene, cinnamyl acetate, 10-epi-γ-eudesmol, 6,9-guaiadiene, α-salinene, phenylethanol, caryophellene, α-agarofuran, phenylethyl tiglate, decanoic acid, iso-decanoic, β-bourbonene, α-gumulene,germacrene d, α-bisabolol, β-phellandrene, cis-β-ocimene, i-menthol, β-cubebene, z-citral, trans-caryophyllene, isoledene, 2-amylfuran, alloaromadendrene, bornylene, β-phenylethyl formate, calamenene, camphene, humulene, myrcene, phenylethyl alcohol, sulcatone, tetrahydro geraniol, phenylacetaldehyde, o-tolualdehyde, trans-ocimene, neoisopulegol, citronellal, trans-menthan-3-one, neoisomenthol, rhodinol, α-cubebene, β-elemene, longifolene, β-caryophellene, β-copaene, spirolepechinene, trans-muurola-3,5-diene, trans-prenyl limonene, aromadendrene, γ-muurolene, amorphene, germacerene d, β-selinene, viridiflorene, α-muurolene, trans-β-guaiene, 11-norbourbonan-1-one, β-phenylethyl tiglate, geranyl 2-methyl butanoate, β-atlantol, 10-di-epi-cubenol, amorph-4-en-7-ol | GC-MS | [47,49,51,66,67,68,69,70,71,72,73,74,75,76,77] | ||
| Citronellol, geraniol, citronellyl formate, isomenthone, linalool, 10-epi-γ-eudesmol, menthone, geranyl formate, β-bourbonene, menthone, cis-rose oxide, β-caryophyllene, geranyl tiglate, geranyl butrate, germacrene D, phenylethyle tiglate, geranyl propionate, citronellyl propionate, geranial, α-copaene, neoisomenthol, α-pinen, α-thuyene, α-terpinol, γ-selilene, trans-rose oxide, citronellyl butrate, geranyl acetate, γ-cadinene, calemenene, 6,9-guaiadiene, geranial, 10-epi-γ-eudesmol, 2-phenyl ethyl tiglate | GC-MS GC-FID |
[6,30,78] | ||
| Citronellol (14.8–17.4%), trans-geraniol (2.10–2.60%), isomenthone (1.30–1.60%), linalool (0.60–0.96%), geranyl acetate (1.00%), γ-cadiene (0.04–0.05%), geranyl butyrate (0.70%), geranyl tiglate (0.07–1.00%), gemacrene D (0.07–0.08%), caryophyllene oxide (1.7–1.8%), geraniol (9.2–11.4%) | GC-MS HPLC |
[48] | ||
| α-pinene, 2,2,6-trimethyl-6-vinyltetrahydropyran, limonene, cis-linalool oxide (furanoid), trans-linalool oxide, linalool, cis-rose oxide, trans-rose oxide, menthone, isomenthone, menthol, α-terpineol, citronellol, neral, geraniol, geranial, citronellyl formate, neryl formate, geranyl formate, α-cubebene, citronellyl acetate, α-copaene, β-bourbonene, β-elemene, β-caryophyllene, trans-α-bergamotene, α-guaiene,6,9-guaiadiene, aromadendrene, α-humulene, allo-aromadendrene, cis-muurola-4(14),5-diene,γ-muurolene, geranyl propionate, γ-gurjunene, β-selinene, α-muurolene, γ-cadinene, geranyl isobutanoate, δ-cadinene, citronellyl butanoate, furopelargone A, geranyl butanoate, neryl isovalerate, caryophyllene oxide, 5,5,9,10-tetramethyltricyclo [7.3.0.0(1,6)]dodecan-11-one, 2-phenyl ethyl tiglate, geranyl isovalerate, humulene epoxide II, 1,10-di-epi-cubenol, 1-epi-cubenol, cubenol, α-cadinol, cis-citronellyl tiglate, geranyl tiglate | GC-MS, HPLC-UV/Vis |
[79] | ||
| Flowers | Citronellol (24.3%), geraniol (21.81%), citronellyl formate (9.94%), linalool (6.67%), 10-epi-γ-eudesmol (5.13%) and p-menthan-3-one (5.03%) | GC-FID GC-MS |
[80] | |
| α-pinene, β-myrcene, β-phellandrene, β-ocimene, linalool, linalool oxide, limonene, trans-rose oxide, citronellol, citronellal, l-menthone, l-menthol, β-citronellol, geraniol, citronellyl formate, geranyl formate, geranyl propionate, geranyl butyrate, geranyl tiglate, geranyl acetate, citronellyl acetate, copaene, nerol, lavandulyl acetate, β-bourbonene, β-cubebene, caryophyllene, trans-caryophyllene, citronellyl propionate, valencene, isoledene, α-humulene, neoalloocimene, aromadendrene, germacrene-d, β-cuvebene, ledene, α-muurolene, eremophilene, α-amorphene, δ-cadinene, epizonaren, α-agarofuran, phenylethyl tiglate, geraniol butyrate, geraniol formate, 10-epi-γ-eudesmol, mintsulfide, α-terpineol, α-gurjunene, bicyclogermacrene, cadina-1,4-diene, δ-selinene, geranyl isobutyrate, viridiflorol, γ-selinene, β-eudesmol, t-cadinol | GC-MS | [81,82,83] | ||
| Total phenolic content (tannic acid—7.7%), flavones (rutin—0.4%) | HPLC | [65] | ||
| Geranium absolute oil | Limonene (0.13%), cis-oxide rose (0.96%), trans-oxide rose (0.31%), menthone (0.88%), iso menthone (5.35%), linalool (10%), guaiadiene (0.2%), citronellyl formate (1.19%), geranyl formate (2.19%), citronellol (31.85%), geraniol (22.47%), geranyl butyrate (0.49%), epi-γ-eudesmol (3.24%) | GC-MS | [84] | |
| Geranium oil | Limonene (0.27%), linalool (4.1%), delta-selinene (9.28%), citronellol (29.76%), geraniol (12.53%), citronellyl formate (7.1%), geranyl formate (2.7%), epi-γ-eudesmol (6.25%), rose oxide trans (1.27%), menthone (5.28%), β-bourbonene (1.87%), δ-cadinene (2%), geranyl tiglate (1.46%), phenylethyl tiglate (1.16%) | |||
| Geranium stripping oil | Pentanal (7.6%), linalool (1.34%), decadienal (5.08%), citronellol (18.33%), geraniol (11.08%), geraniol formate (2.47%), 2,6-octadiene (1.42%), butanoic acid (1.31%), naphthalenemethanol (9.25%), longifolene (1.36%), 1h-cyclopropa(a) naphthalene (2.39%), geranyl tiglate (2.36%), cyclohexanone (3.15%), phenylethyl tiglate (2.39%) | |||
| Leaves | Citronellol, geraniol, linalool, rose-oxide, 10-epi-γ-eudesmol, geraniol, geranyl formate, geranyl tiglate, citronellyl formate, isomenthone | GC | [59] | |
| α-pinene, β-pinene, sabinene, myrcene, α-phellandrene, β-phellandrene, limonene, (z)-β-ocimene, (e)-β-ocimene, p-cymene, cis-3-hexenol, cis-rose oxide, trans-rose oxide, cis-linalool oxide (furanoid), menthone, trans-linalool oxide (furanoid), isomenthone, linalool, β-bourbonene, citronellyl formate, β-caryophyllene, citronellyl acetate, nerodinol, neryl formate, α-humulene, geranyl formate, citronellyl propionate, α-terpineol, germacrene-d, citronellol, geranial, geranyl acetate, piperitone, nerol, citronellyl butyrate, geraniol, geranyl-n-propionate, guaia-6,9-diene, geranyl-n-butyrate, geranyl tiglate, citronellyl tiglate, 10-epi-γ-eudesmol, 2-phenylethyl tiglate, tricyclene, β-copaene, t-elemene, t-cadiene | GC-FID | [85,86,87,88,89] | ||
| α-pinene, β-pinene, β-myrcene, phellandrene, α-phellandrene, β-phellandrene, β-ocimene, linalool, trans-rose oxide, cis-rose oxide, l-menthone, 3-p-menthanol, α-terpineol, β-citronellol, citral, citronellyl formate, citronellyl acetate, citronellyl propionate, citronellyl ester, citronellyl tiglate, citronellyl butyrate, citronellyl valerate, nerol, geraniol, geranial, geraniol formate, geranyl formate, geranyl acetate, geranyl formate, geranyl tiglate, geranyl propionate, geranyl hexanoate, geranyl butyrate, lavandulyl acetate, β-bourbonene, α-cubebene, β-cubebene, trans-caryophyllene, azulene, isoledene, α-humulene, aromadendrene, germacrene-d, ledene, α-muurolene, elemol, α-amorphene, δ-cadinene, γ-cadinene, epizonaren, α-agarofuran, neryl acetate, 2-phenylethyl tiglate, β-selinene, δ-selinene, 10-epi-γ-eudesmol, agarospirol, propanoate, mintsulfide, copaene, isomenthone, isopulegol, e,e-α-farnesene, cis-verbenol, cis-calamenene, α-agarofurane, linalyl acetate, spatulenol, ο-cymene, β-patchoulene, α-asarone, guaia-6,9-diene, propanoic acid, α-gurjunene, δ.3-carene, menthomenthol, 6-octen-1-ol, 2,6-octadien-1-ol, propionic acid, butyric acid, acide-2-butenoic, ledol, cadina-1,4-diene, valencene, α-cadinol, τ-cadinol, cis-ocismene, 3-methylpentane, neohexane, (e)-3,7-dimethylocta-2,6-dien-1-yl dodecanoate, α-iso-pentane, isohexane, trichloromethane, cyclopentane, 1-epi-cubenol, cubenol, 6-methyl-5-hepten-2-one, limonene, citronellal, methyl geranate, 2-phenylethyl isobutyrate, β-elemene, alloaromadendrene, β-selinene, bicyclogermacrene, zonarene, spathulenol, viridiflorol, 2-naphthalenemethanol, cyclohexanone, 2-pentanone, 1,3,6-octatriene, p-menthan-3-ol | GC-MS | [24,36,81,90,91,92,93,94,95,96,97,98,99] | ||
| Citronellol, geraniol, geranial, 10-epi-γ-eudesmol, citronellyl formate, linalool, nerol, neral, isomenthone, isomenthol, α-terpineol, β-citral, cyclofenchene, m-mentha-6.8-diene, cis-linalool oxide, trans-linalool oxide, geranyl butyrate, geranyl formate, geranyl tiglate, geranyl butanoate, τ-muurolol, (Z)-rose oxide, β-borbonene, caryophyllene, 6,9-guaiadiene, α−humulene, γ-muurolene, bicyclogermacrene, δ-amorphene, inalool isovalerate, 2-phenylethyl tiglate, limonene, menthone, aristolene, neryl propanoate, germacrene-D, α-calacorene, torreyol | GC-MS GC-FID |
[80,100,101,102] | ||
| Total phenolic content (tannic acid—22%), phenol carboxylic acids (chlorogenic acid—3.6%), anthocyanins (cyanine chloride—1%) | HPLC | [65] | ||
| Leaves and stems | Geraniol, β-citronellol, citronellyl formate, isomenthone, linalool, germacrene and 10-epi-γ-eudesmol | GC-MS | [103,104] | |
| Root | Iso-menthone, linalool, citronellyl formate, geranyl formate, citronellol and geraniol | GC | [105] | |
| Linalool, cis + trans rose oxide, isomenthone, citronellol, geraniol, citronellyl formate, geranyl formate, geranyl tiglate, 6,9-guadiene, 10-epi-γ-eudesmol | GC-FID | [106,107] | ||
| Sprouts and leaves | Oxygenated monoterpenes, oxygenated sesquiterpenes, sesquiterpene hydrocarbons, citronellol, geraniol, citronellyl formate, linalool | GC-MS GC-FID |
[6] | |
| Stems | β-myrcene, β-ocimene, linalool, trans-rose oxide, cis-rose oxide citronellal, l-menthone, β-citronellol, geraniol, citral, citronellyl formate, citronellyl tiglate, geranyl formate, α-copaene, β-bourbonene, β-cubebene, caryophyllene, citronellyl propionate, valencene, isoledene, α-humulene, aromadendrene, geranyl acetate, germacrene-D, viridiflorene, α-amorphene, δ-cadinene, geranyl isobutyrate, α-agarofuran, geranyl propionate, geranyl isovalerate, δ-selinene, 10-epi-γ-eudesmol, geranyl butanoate, geranyl tiglate, isomenthone, mintsulfide, cetylic acid, camphor, trans-verbenol, 2-methoxy-4-vinylphenol, β-gurjunene, β-ylangene, alloaromadendrene, ledene, cis-β-guaiene, α-bisabolene, epi-cubebol, 2-phenylethyl tiglate, isoaromadendrene epoxide, cis-phytol, α-pinene, guaia-6,9-diene, trans-calamenene | GC-MS | [46,50,81] | |
| Ethanol, limonene, linalool, phenyl ethanol, cis-rose oxide, trans-rose oxide, citronellol + nerol, geraniol, eugenol, methyl eugenol, heptadecane, farnesol, nonadecene, nonadecane, eicosane, heneicosane, tricosane, pentacosane, heptacosane | GS-FID | [108] | ||
| AP | Citronellol (24.75%), geraniol (13.99%), γ-eudesmol (11.23%), citronellyl formate (8.37%) and iso-menthone (6.82%) | GC-MS | [64] | |
| The whole herb | Linalool, isomenthone, β-citronellol, geraniol, geranial, (r)-(+)-citronellic acid, (-)-aristolene, geranyl-n-propanoate, β-cubebene, υ-moorolene, geranyl isobutyrate, phenyl ethyl tiglate, υ-eudesmol, τ-cadinol, geranyl tiglate, 2,6-octadien-1-ol, 3,7-dimethyl-acetate, neral, citronellyl formate, 10-epi-γ-eudesmol, geranyl formate, 1,8-cineole, limonene | [109,110] | ||
| P. hispidum | Dried leaves | Menthone, isomenthone, p-cimene, α-pinene, sabinene, myrcene, phellandrene, carene, terpinene and limonene | GC-MS GC-FID |
[111] |
| P. hortum | Fresh leaves | α-thujene, α-pinene, camphene, β-pinene, myrcene, α-terpinene,p-cymene, limonene, ɣ-terpinene, α-terpineol, camphor, α-fenchyl acetate, thymol, bornyl acetate, trans-β-caryophyllene, germacrene-D, δ-cadinene, and 5 more unknown components | GC-MS | [112] |
| P. odoratissimum | AP | 4-methyl-pentanol, (3E)-hexenol, α-pinene, myrcene, p-cymene, limonene, 1,8-cineole, (Z)-β-ocimene, (E)-β-ocimene, γ-terpinene, cis-linalool oxide, linalool, cis-thujone, cis-rose oxide, trans-rose oxide, camphor, neo-isopulegol, menthone, citronellal, iso-menthone, iso-menthol, α-terpineol, citronellol, neral, piperitone, cis-myrtanol, geraniol, geranial, citronellyl formate, thymol, geranyl formate, α-cubebene, citronellyl acetate, α-copaene, β-bourbonene, phenyl ethyl isobutanoate, (E)-caryophyllene, α-guaiene, 6,9-guaiadiene, cis-muurola-3,5-diene, citronellyl propanoate, cis-cadina-1(6),4-diene, germacrene-D, geranyl propanoate, ar-curcumene, phenyl ethyl 3-methyl butanoate, viridiflorene, α-muurolene, geranyl isobutanoate, δ-cadinene, trans-cadina-1,4-diene, citronellyl butanoate, furopelargone A, geranyl butanoate, spathulenol, caryophyllene oxide, 2-phenyl ethyl tiglate, geranyl isovalerate, 1,10-di-epi-cubenol, 10-epi-γ-eudesmol, 1-epi-cubenol, epi-α-cadinol, β-eudesmol, α-eudesmol, α-cadinol, (E)-citronellyl tiglate, (E)-citronellyl tiglate | GC-MS | [113] |
| EO | Linalool, citronellol, geraniol, citronellyl formate, geranyl formate, β-caryophyllene, germacrene-D, geranyl butyrate, geranyl tiglate, isomenthone, menthone, trans-rose oxide, 10-epi-γ-eudesmol | [9,77,114] | ||
| Young leaves | α-pinene, benzaldehyde, sabinene, β-pinene, myrcene, ρ-cymene, limonene, 1,8-cineole, (z)-β-ocimene, (e)-β-ocimene, γ-terpinene, fenchone, linalool, undecane, camphor, isomenthone, borneol, α-terpineol, dodecane, carveol, fenchyl acetate, pipritone, tridecane, α-cubebene, α-copaene, β-cubebene, tetradecane, methyl eugenol, β-caryophyllene, α-caryophyllene, germacrene-D, β-selinene, farnesene, γ-cadinene, germacrene b, caryophyllene oxide, citronyllyl tiglate, octadecane, nonadecane, eicosane | [115] | ||
| P. radens | Leaves | Tricyclene, α-pinene, β-pinene, myrcene, α-phellandrene, p-cymene + β-phellandrene, limonene, cis-β-ocimene, trans-β-ocimene, linalool, menthone, isomenthone, α-terpineol, citronellol, piperitone, β-copaene, β-bourbonene, guaiadiene-6,9, germacrene D, t-cadiene, nerodinol | GC-FID | [87] |
| Dried leaves | Menthone, isomenthone, p-cimene, α-pinene, rose oxides, sesquiterpenes (guajene, patchoulene, ylangene, cariophyllene oxide) | GC-MS GC-FID |
[111] | |
| P. radula | RM | Phenolic acids, phenylpropanoids, derivatives (gallic acid, gallic acid methyl ester), coumarins, coumarin glycosides/sulfates, flavan-3-ols/proanthocyanidins, miscellaneous | GC-MS | [18] |
| AP | Phenolic acids, phenylpropanoids, derivatives (gallic acid, gallic acid methyl ester, gallic acid ethyl ester, shikimic acid 3-o-gallate, protocatechuic acid, glucogallin), coumarins (scopoletin, umckalin,6,8-dihydroxy-5,7-dimethoxycoumarin, fraxetin, fraxetin-7-β-d-glucoside, magnolioside, 6,7-dihydroxycoumarin-8-sulfate), flavonoids (quercetin, dihydrokaempferol 3-o-β-d-glucoside, taxifolin-3-o-β-d-glucoside, luteolin 7-o-β-d-glucoside, vitexin, orientin, isovitexin, isoorientin, epigallocatechin-3-o-gallate), miscellaneous | |||
| P. reniforme | RM | Phenolic acids, phenylpropanoids and derivates (gallic acid, gallic acid methyl ester, p-hydroxybenzoic acid, protocatechuic acid, vanillic acid, caffeic acid, ferulic acid, p-coumaric acid, p-coumaraldehyde), coumarins, flavonoids (kaempferol-3-o-β-d-glucoside, kaempferol-3-o-β-d-galactoside, quercetin-3-o-β-d-glucoside, myricetin-3-o-β-d-glucoside), flavan-3-ols/proanthocyanidins, miscellaneous | ||
| AP | Phenolic acids, phenylpropanoids and derivates (gallic acid, gallic acid methyl ester, gallic acid ethyl ester, allic acid butyl ester, shikimic acid 3-o-gallate, shikimic acid 3,5-di-o-gallate, p-hydroxyphenylethanol, p-hydroxyphenyl acetic acid, p-hydroxyphenyl alcohol, p-coumaric acid, p-coumaroyl-4-o-β-d-glucoside, glycerol-1-gallate, glucogallin, (α,β)-3,4-di-o-galloylglucopyranoside, salidroside-6-o-gallate), coumarins (scopoletin), flavonoids (kaempferol, quercetin) | |||
| P. roseum | AP | α-pinene, linalool, cis-rose oxide, trans-rose oxide, l-menthone, citronellol, geraniol, citronellyl formate, β-bourbonene, cis-calamenene | [116,117] | |
| EO | Citronellol (44.62%), citronellyl formate (14.42%), geraniol (10.73%), linalool (5.39%), menthone (3.04%), isomenthone (0.89%) and limonene (0.35%) | [77] | ||
| Fresh leaves | α-pinene, β-pinene, β-myrcene, α-phellandrene, p-cymene, D-limonene, β-ocimene, γ-terpinene, linalool oxide, linalool, cis-rose oxide, trans-rose oxide, β-terpineol, menthone, iso-menthone, menthol, terpinene 4-ol, α-terpineol, β-citronellol, geraniol, α-cubabene, citronellyl acetate, β-bourbonene, β-elemene, α-gurjunene, β-caryophyllene, α-guaiene, β-farnesene, γ-gurjunene, germacrene-D, δ-selinene, valencene, bicyclogermacrene, epizonarene, α-muurolene, α-farnesene, γ-cadinene, δ-cadinene, α-agarofuran, β-eudesmol, cadalene, geranyl tiglate | [118] | ||
| P. sidoides | Leaf petioles | Phenolic acid compounds (gallic acid, protocatechuic acid, 4-hydroxybenzoic acid, vanillic acid, caffeic acid, p-coumaric acid, ferulic acid and salicylic acid) | UPLC-MS/MS | [119] |
| P. x hybridum cv. ‘Rosat Bourbon’ | EO | cis-3-hexanol, α-pinene, β-pinene, α-phellandrene, α-terpinene, para-cymene, β-phellandrene, limonene, terpinolene, menthone, isomenthone, menthol, α-terpineol, bois de rose oxide, myrcene, cis-β-ocimene, trans-β-ocimene, linalool oxide I, linalool oxide II, linalool, rose oxide cis, rose oxide trans, citronellol, piperitone, geraniol, geranial, citronellyl formate, citronellyl acetate, neryl acetate, geranyl acetate, citronellyl propionate, geranyl propionate, geranyl isobutyrate, citronellyl butyrate, geranyl butyrate, geranyl valerate, citronellyl caproate, citronellyl tiglate, geranyl tiglate, geranyl caproate, α-cubebene, α-copaene, β-bourbonene, β-caryophyllene, α-trans-bergamotene, guaia-1(5),11-diene, 6,9-guaiadiene, aromadendrene, α-caryophyllene, alloaromadendrene, β-cubebene, germacrene D, β-selinene, viridiflorene, γ-cadinene, calamenene, δ-cadinene, furopelargone, β-caryophyllene oxide, phenethyl propionate, β-phenethyl isobutyrate, phenethyl tiglate | GC-MS | [43] |
| P. x hybridum cv. ‘Rosat China’ | cis-3-hexanol, α-pinene, β-pinene, α-phellandrene, α-terpinene, para-cymene, β-phellandrene, limonene, terpinolene, menthone, isomenthone, menthol, α-terpineol, bois de rose oxide, myrcene, cis-β-ocimene, trans-β-ocimene, linalool oxide I, linalool oxide II, linalool, rose oxide cis, rose oxide trans, citronellol, piperitone, geraniol, geranial, citronellyl formate, citronellyl acetate, neryl acetate, geranyl acetate, citronellyl propionate, geranyl propionate, geranyl isobutyrate, citronellyl butyrate, geranyl butyrate, geranyl valerate, citronellyl caproate, citronellyl tiglate, geranyl tiglate, geranyl caproate, α-cubebene, α-copaene, β-bourbonene, β-caryophyllene, α-trans-bergamotene, guaia-1(5),11-diene, 6,9-guaiadiene, aromadendrene, α-caryophyllene, alloaromadendrene, β-cubebene, germacrene d, β-selinene, viridiflorene, γ-cadinene, calamenene, δ-cadinene, furopelargone, β-caryophyllene oxide, epi-γ-eudesmol, phenethyl propionate, β-phenethyl isobutyrate, phenethyl tiglate | |||
| P. x hybridum cv. ‘Rosat Egypt’ | cis-3-hexanol, α-pinene, β-pinene, α-phellandrene, α-terpinene, para-cymene, limonene, terpinolene, menthone, isomenthone, menthol, α-terpineol, bois de rose oxide, myrcene, cis-β-ocimene, trans-β-ocimene, linalool oxide I, linalool oxide II, linalool, rose oxide cis, rose oxide trans, citronellol, piperitone, geraniol, geranial, citronellyl formate, citronellyl acetate, geranyl acetate, citronellyl propionate, geranyl propionate, geranyl isobutyrate, citronellyl butyrate, geranyl butyrate, geranyl valerate, citronellyl caproate, citronellyl tiglate, geranyl tiglate, geranyl caproate, α-cubebene, α-copaene, β-bourbonene, β-caryophyllene, α-trans-bergamotene, guaia-1(5),11-diene, 6,9-guaiadiene, aromadendrene, α-caryophyllene, alloaromadendrene, β-cubebene, germacrene d, β-selinene, viridiflorene, γ-cadinene, calamenene, δ-cadinene, β-caryophyllene oxide, epi-γ-eudesmol, γ-eudesmol, phenethyl propionate, β-phenethyl isobutyrate, phenethyl tiglate | |||
| P. graveolens | α-pinene, β-pinene, limonene, cymol, cineol, terpinen, linalool, camphor, borneol, citronellol, geraniol, neral, geranial | GC-FID | [120] |
where: AP—aerial parts; EO—essential oil; GC-FID—gas chromatography–flame ionization detector; GC-MS—gas chromatography–mass spectrometry; HPLC—high-performance liquid chromatography; HPLC-MS—high-performance liquid chromatography with mass spectrometry; HPLC-MS/MS—liquid chromatography with tandem mass spectrometry; HPLC-UV/VIS—high-performance liquid chromatography equipped with UV/VIS detector; LC-MS—liquid chromatography–mass spectrometry; RM—root material.
2.2. Biological Activities of Plants from Pelargonium Genus
According to scientific research, Pelargonium sp. exhibited antibacterial, antifungal, antitubercular, anticancer, antioxidant, anthelmintic, insecticidal activities, as well as immunomodulatory and cytoprotective properties. All these properties are due to the quality of essential oil. The phenols, polyphenols, tannins, terpenes, ketones, aldehydes, and alcohols are responsible for other potential application [9,42,60,117,121]. Moyo and Van Staden [122] reviewed the medicinal properties and conservation of Pelargonium sidoides. In vitro studies revealed that Pelargonium sidoides extract has antiviral activity (coronavirus, influenza A viruses), antibacterial activity (Staphylococcus aureus, Escherichia coli ATCC 11775), antimycobacterial activity (Mycobacterium smegmatis), antifungal activity (Candida albicans), antiparasitic activity (Leishmania donovani) and immunomodulatory activity (Listeria monocytogenes, Leishmania donovani). Additionally, these studies demonstrated anticoagulant activity, central nervous system activity, and lipopolysaccharide-induced sickness behavior. Thus, Pelargonium sidoides extract is utilized in traditional medicine to treat dysentery, diarrhea, common cold, and respiratory infections, bronchitis, tuberculosis, acute rhinosinusitis, and asthma [122].
2.2.1. Antioxidant Properties
Mishra et al. [123] reported on the evaluation of total antioxidant activity assessed by ferric reducing antioxidant power (FRAP) and potassium ferric cyanide (PFC) assays. Regarding non-enzymatic antioxidants, younger leaves of P. graveolens showed greater flavonoid accumulation compared to mature leaves and exhibited the strongest reducing power activity. Of the four plants studied (Moringa oleifera, Pelargonium graveolens, Tagetes patula and Calotropis gigantea), mature leaves of P. graveolens were found to have the second highest phenolic content [123].
In their study, Chrysargyris et al. [124] investigated the correlation between Cu uptake from Pelargonium graveolens roots and leaves and its antioxidant activity. Using the antioxidant assays of radical scavenging activity—2,2-diphenyl-1-picrylhydrazyl (DPPH), FRAP or radical scavenging assay—2,20-azino-bis(3-thylbenzothiazoline-6-sulphonic acid (ABTS), Chrysargyris et al. [124] analyzed the effects of copper toxicity on plant growth, plant copper distribution and oxidative stress indicators. The results of the study showed that rose geranium has the ability to accumulate heavy metals in the roots even at copper concentrations of up to 100 μM, leading to an increase in both phenolic content and antioxidant activity [124].
Negro et al. [57] evaluated the antioxidant activity using of DPPH, FRAP, and SASA (Superoxide Anion Scavenging Activity) assays during three different stages of development (mature bud, full bloom, and senescing) in P. odoratissimum flowers. Although the lowest phenolic content was observed during the full bloom stage, these flowers exhibited the highest antioxidant capacity. Additionally, the levels of total phenolic compounds were found to be significant. The levels of flavonoids in the flowers of P. ‘Endsleigh’ were found to be identical to those in both flowers and stems of P. graveolens [57].
Cavar and Maksimovic [107] reported on the antioxidant activity of P. graveolens. The DPPH assay indicated IC50 values of 63.70 mg/mL in leaves and 64.88 mg/mL in stems for essential oils, and 0.19 mg/mL in stems and 0.39 mg/mL in leaves for hydrosol. Analysis of the results led to the conclusion that the EO obtained from the stems had a greater antioxidant activity than that obtained from Pelargonium leaves [107].
In addition to P. graveolens, other Pelargonium species revealed antioxidant activity. Latte and Kolodziej [125] assessed the antioxidant properties of the key components of P. reniforme (tannins and flavonoids) through DPPH radical determination and compared them with ascorbic acid, which was used as a positive control. All tested tannin compounds, including corilagin (IC50 = 2.7 μM), brevifolincarboxylic acid (IC50 = 4.6 μM), phyllanthussin C (IC50 = 5.8 μM), methyl gallate (IC50 = 6.9 μM), and glucogallin (IC50 = 9.9 μM), showed higher inhibitory activity compared to ascorbic acid (IC50 = 40.9 μM), excluding gallic acid (IC50 = 32.9 μM). Similarly, regarding flavonoids, all components showed higher inhibitory values (with IC50 ranging from 2.6 μM for orientin 2″-gallate to 23.2 μM for isoorientin) than ascorbic acid (IC50 = 40.9 μM). Therefore, this research demonstrates that P. reniforme has antioxidant activity and can potentially be used in the treatment of liver disease [125].
Krishnaiah et al. [126] reviewed the antioxidant properties of various medicinal plant species, including P. endlicherianum which is known for its numerous biological activities. In particular, the extract of P. endlicherianum exhibited a higher antioxidant activity (IC50 = 7.43 ± 0.47 μg/mL) compared to the synthetic antioxidant butylated hydroxytoluene (BTH) (IC50 = 18.0 ± 0.4 μg/mL) [126]. Meyers et al. [127] reported that Pelargonium species contain significant quantities of phenolic compounds, including hydrolysable tannins and flavonoids, that have antioxidant properties. Among the hundreds of Pelargonium subspecies, P. sidoides and P. reniforme are identified as containing methyl ester and gallic acid in their chemical composition. These two compounds have been found to enhance the immune response [127].
The reviewed articles presented in Table 5 show that various Pelargonium sp. plants have antioxidant activity attributed to responsible compounds such as phenols, flavonoids, or tannins. Ascorbic acid was frequently used as a control and in almost all Pelargonium graveolens samples the antioxidant capacity exceeded the positive control.
Table 5.
Antioxidant properties of different extracts obtained from Pelargonium plant species.
| Species | Plant Part | Responsible Compounds | Extraction Method | Antioxidant Assay | Antioxidant Potential | References |
|---|---|---|---|---|---|---|
| P. betulinum | aerial parts | flavonoids, tannins | acetone extraction | DPPH | IC50 = 4.13 μg/mL | [128] |
| P. citronellum | IC50 = 23.70 μg/mL IC50 = 84.01 μg/mL |
|||||
| P. cordifolium | IC50 = 5.01 μg/mL | |||||
| P. crispum | IC50 = 4.49 μg/mL | |||||
| P. cucullatum | IC50 = 40.18 μg/mL IC50 = 10.91 μg/mL |
|||||
| P. graveolens | leaves | phenolic compounds | methanol extraction | Phenol and flavonoids content assay, Enzyme activity assay (CAT, APX, GPX, SOD activities), MDA and H2O2 assays | The EO and total phenol and flavonoids contents increased significantly by 12.4% and 16%, respectively, when P. graveolens was underwater deficit 75% FC. The AMF inoculation treatment improved the plant enzymatic defence. Also, AMF inoculation treatment showed a lower MDA and H2O2 accumulation in plant tissue. | [129] |
| methanol extraction ethanol extraction aqueous extraction |
DPPH | IC50 = 12.24 μg/mL (methanol extraction) IC50 = 14.6 μg/mL (ethanol extraction) IC50 = 39.45 μg/mL (aqueous extraction) |
[80] | |||
| ABTS | IC50 = 241.83 μg/mL (methanol extraction) IC50 = 235.86 μg/mL (ethanol extraction) IC50 = 140.57 μg/mL (aqueous extraction) |
|||||
| heat reflux extraction | ABTS DPPH FRAP CUPRAC |
TEAC ABTS = 223.76 μM TE/g FW TEAC DPPH = 121.26 μM TE/g FW TEAC FRAP = 231.64 μM TE/g FW TEAC CUPRAC = 176.98 μM TE/g FW |
[130] | |||
| methanol extraction aqueous extraction |
DPPH | IC50 = 20.71 μg/mL (methanol extraction) IC50 = 16.59 μg/mL (aqueous extraction) |
[64] | |||
| ABTS | 0.86 mM of Trolox (methanol extraction) 0.93 mM of Trolox (aqueous extraction) |
|||||
| flowers | phenolic compounds | methanol extraction ethanol extraction aqueous extraction |
DPPH | IC50 = 16.03 μg/mL (methanol extraction) IC50 = 19.31 μg/mL (ethanol extraction) IC50 = 44.24 μg/mL (aqueous extraction) |
[80] | |
| ABTS | IC50 = 233.74 μg/mL (methanol extraction) IC50 = 227.73 μg/mL (ethanol extraction) IC50 = 131.54 μg/mL (aqueous extraction) |
|||||
| methanol extraction aqueous extraction |
DPPH | IC50 = 10.3 μg/mL (methanol extraction) IC50 = 12.85 μg/mL (aqueous extraction) |
[64] | |||
| ABTS | 1.13 mM of Trolox (methanol extraction) 0.98 mM of Trolox (aqueous extraction) |
|||||
| aerial parts | phenolic compounds | methanol extraction dichloromethane extraction hexane extraction |
DPPH | IC50 = 12.96 μg/mL (methanol extraction) IC50 = 116.91 μg/mL (dichloromethane extraction) IC50 = 37.6 μg/mL (hexane extraction) |
[131] | |
| ABTS | IC50 = 10.2 μg/mL (methanol extraction) IC50 = 10.46 μg/mL (dichloromethane extraction) IC50 = 44.46 μg/mL (hexane extraction) |
|||||
| CUPRAC | IC50 = 20.29 μg/mL (methanol extraction) IC50 = 53.36 μg/mL (dichloromethane extraction) IC50 = 89.85 μg/mL (hexane extraction) |
|||||
| flavonoids and condensed tannins | decoction | DPPH | 136.1 mg TE/g DM | [132] | ||
| flavonoids, tannins | acetone extraction | IC50 = 14.49 μg/mL | [128] | |||
| phenolic compounds and flavonoids | hydro-distillation | DPPH | IC50 = 138.23 μg/mL (vegetative stages) IC50 = 119.49 μg/mL (beginning flowering stage) IC50 = 83.26 μg/mL (full flowering stage) |
[60] | ||
| FRAP | IC50 = 151.21 μg/mL (vegetative stages) IC50 = 139.35 μg/mL (beginning flowering stage) IC50 = 116.42 μg/mL (full flowering stage) |
|||||
| ABTS | IC50 = 174.95 μg/mL (vegetative stages) IC50 = 153.39 μg/mL (beginning flowering stage) IC50 = 132.25 μg/mL (full flowering stage) |
|||||
| H2O2 | IC50 = 77.35 μg/mL (vegetative stages) IC50 = 64.81 μg/mL (beginning flowering stage) IC50 = 48.67 μg/mL (full flowering stage) |
|||||
| total phenolic content | ethanolic extraction | ABTS | IC50 = 17.53 µg/mL | [65] | ||
| FRP | IC50 = 74.43 µg/mL | |||||
| P. graveolens cv. Rosé | leaves | phenolic compounds | ethanolic extraction | DPPH | IC50 = 7.88 µg/mL | [133] |
| β-carotene bleaching assay | IC50 = 78.3 µg/mL | |||||
| FRAP | IC50 = 143 µg/mL | |||||
| H2O2 | IC50 = 2533 µg/mL | |||||
| stems | DPPH | IC50 = 10.00 µg/mL | ||||
| β-carotene bleaching assay | IC50 = 533.4 µg/mL | |||||
| FRAP | IC50 = 137.2 µg/mL | |||||
| H2O2 | IC50 = 3550.00 µg/mL | |||||
| P. glutinosum | aerial parts | flavonoids, tannins | acetone extraction | DPPH | IC50 = 16.41 μg/mL IC50 = 29.17 μg/mL |
[128] |
| P. hermanniifolium | IC50 = 13.50 μg/mL | |||||
| P. hispidum | IC50 = 12.78 μg/mL | |||||
| P. panduriforme | IC50 = 91.58 μg/mL | |||||
| P. papilionaceum | IC50 = 81.24 μg/mL | |||||
| P. pseudoglutinosum | IC50 = 52.38 μg/mL | |||||
| P. purpureum | dried leaves | phenolic compounds | infusion | FRAP | 487 μM Fe2+ | [134] |
| P. quercifolium | aerial parts | flavonoids, tannins | acetone extraction | DPPH | IC50 = 17.15 μg/mL IC50 = 61.87 μg/mL |
[128] |
| P. radula | leaves | phenols, tannins and flavonols | ultrasonic extraction–water solvent ultrasonic extraction–ethanol solvent decoction–water solvent |
DPPH | IC50 = 56.7 μg/mL (water solvent) IC50 = 70.3 μg/mL (ethanol solvent) IC50 = 28.6 μg/mL (decoction) |
[135] |
| SRP | IC50 = 0.69 μg/mL (water solvent) IC50 = 0.95 μg/mL (ethanol solvent) IC50 = 1.21 μg/mL (decoction) |
|||||
| ANT | IC50 = 56.8 μg/mL (water solvent) IC50 = 81.3 μg/mL (ethanol solvent) IC50 = 77.6 μg/mL (decoction) |
|||||
| dried leaves | DPPH | IC50 = 41.7 μg/mL (water solvent) IC50 = 54.3 μg/mL (ethanol solvent) IC50 = 38.9 μg/mL (decoction) |
||||
| SRP | IC50 = 1.00 μg/mL (water solvent) IC50 = 1.12 μg/mL (ethanol solvent) IC50 = 1.14 μg/mL (decoction) |
|||||
| ANT | IC50 = 75.2 μg/mL (water solvent) IC50 = 79.9 μg/mL (ethanol solvent) IC50 = 12.2 μg/mL (decoction) |
|||||
| P. scabrum | aerial parts | flavonoids, tannins | acetone extraction | DPPH | IC50 = 7.15 μg/mL | [135] |
| P. sidoides | NA | total phenolic content, condensend tannins | 50% methanol extraction | DPPH | IC50 = 6.64 μg/mL | [136] |
| BHT | IC50 = 2.66 μg/mL | |||||
| P. sublignosum | aerial parts | flavonoids, tannins | acetone extraction | DPPH | IC50 = 17.61 μg/mL | [128] |
where: ABTS—radical scavenging assay-2,20-azino-bis(3-thylbenzothiazoline-6-sulfonic acid; AMF—arbuscular mycorrhizal fungi; ANT—antioxidant activity in β-carotene-linoleic acid assay; APX—ascorbate peroxidase; CAT—catalase; CUPRAC—Cupric reducing antioxidant capacity; DM—dry matter; DPPH—radical scavenging activity-2,2-diphenyl-1-picrylhydrazyl; EO—essential oil; FC—field capacity; FRP—ferric reducing power; FRAP—ferric reducing antioxidant power assay; FW—fresh weight; GPX—glutathione peroxidase; IC50—half maximal inhibitory concentration; LP—lipid peroxidation assay; MDA—malondialdehyde; NA—not available; SOD—superoxide dismutase; SRP—slope of trend line in reducing power assay; TE—Trolox equivalent; TEAC—Trolox equivalent antioxidant capacity.
2.2.2. Antimicrobial Activity
The volatile oil of P. graveolens was analyzed for chemical composition and assessed for its anti-Helicobacter activity using GG-MS. Ninety-two chemical compounds were identified in the oil sample. Among them, citronellol, geraniol, citronellyl-formate, and isolongifolan-7-a-ol were found to be the predominant components, representing 15.64%, 11.31%, 10.19%, and 7.84% of the total, respectively. The EO showed good activity against H. pylori with a minimum inhibitory concentration (MIC) of 15.63 mg/mL. Combining the volatile oil with clarithromycin (CLR) resulted in a significant synergistic effect, with a fractional inhibitory concentration index (FICI) of 0.38 mg/mL. The in vitro interaction between P. graveolens oil and CLR augmented the antimicrobial activity of the latter, indicating the need for further studies to determine formulations for potential antimicrobial uses [27].
Choi et al. [83] conducted a study on the antimicrobial activity of P. graveolens in combination with antibiotics against S. pneumonia. The study employed three antibiotics, erythromycin, norfloxacin, and oxacillin, combined with three main compounds identified in P. graveolens, citronellol, geraniol, and linalool. The combination of norfloxacin and citronellol demonstrated the strongest synergistic effect with a FICI of 0.16 against four strains of S. pneumonia (0.38, 0.31, 0.16 and 0.28, respectively).
Gâlea and Hâncu [137] demonstrated the antiseptic properties of P. roseum extract by studying its antibacterial and antifungal effects. They tested the antimicrobial activity on three Gram-negative bacteria, two Gram-positive bacteria, and a fungus, with P. graveolens EO exhibiting varying degrees of sensitivity for each bacterial strain. The growth of C. albicans was inhibited by 100% in less than 48 h [137].
Antibacterial Activity
In most cases, P. graveolens EO was obtained through the process of hydro-distillation. Using the MIC and MBC (minimum bactericidal concentrations) assays, P. graveolens EO demonstrated an average MIC value of 1%. Furthermore, the average MIC for Gram-positive bacteria was approximately 0.3%, whereas Gram-negative bacteria had an average MIC of 1.5%. P. aeruginosa strains were found to be more resistant, with 2% MIC. Concerning Gram-positive cocci, each tested strain demonstrated a low MIC percentage (<0.85%), apart from S. saprophyticus, which had a MIC of 1%. The essential oil of P. graveolens showed an MBC average of 0.4% against Gram-positive bacteria and more than 4% against Gram-negative bacteria, except for A. baumannii strains, for which it was nearly 2%. Citronellol and geraniol were identified as the active compounds responsible for these MIC and MBC values [37].
Extracts of P. reniforme and P. sidoides have been prepared using ethanol and acetone. They have demonstrated activity at 5 × 103 mg/L against H. influenzae, M. catarrhalis and S. pneumoniae. However, they are not as effective as streptomycin sulphate, which demonstrated activity at 10.0 mg/L and showed complete inhibition activity against these three bacteria [138].
The essential oil extracted from P. endlicherianum had a MIC of 5 g/L against H. influenzae and 20 g/L against Neisseria meningitidis. Pelargonium EO combined with ciprofloxacin or ampicillin demonstrated a synergistic effect on N. meningitidis and an additive effect on H. influenzae. Additionally, the combination of Pelargonium EO and gentamicin had a synergistic effect against both meningitis causative pathogens (FICI 0.5). The combinations were tested on human leukocyte cells to determine their effects. The responsible components, determined by MIC and time–kill assays [139], were phenols.
The reviewed literature shows that Pelargonium sp. can have comparable results to antibiotic controls. The combination of Pelargonium sp. and antibiotics (such as norfloxacin, ciprofloxacin or cefepime) resulted in a synergistic effect on both Gram-positive bacteria (S. aureus, B. cereus) and Gram-negative bacteria (E. coli). This was attributed to the presence of compounds such as phenols, flavonoids, or tannins. The results are shown in Table 6.
Table 6.
Antibacterial properties of different extracts obtained from Pelargonium plant species.
| Plant | Extraction Method | Assay | Results | Responsible Compound | References |
|---|---|---|---|---|---|
| P. endlicherianum Fenzl. | Hydro-distillation | MIC, ADD, MBC, time–kill assay, PAE, SEM | The antibiotics used in combination with the EO can be effective in the treatment against Klebsiella pneumoniae. The time–kill assay detected the bactericidal effects of that combination. Considering the obtained results, cefepime and essential oil presented a synergistic effect against K. pneumoniae. | β-bourbonene, α-pinene, β-pinene |
[56] |
| P. graveolens | Hydro distillation | MIC and MBC | The MICs values ranging from 0.15 to 2.5 μg/mL, showed that essential oils are effective as antimicrobial agents, and the MIC/MBC ratio are very close to 1, confirming their bactericidal activity. | β-citronellol | [10] |
| Disk diffusion assay; vapor diffusion assay | Among the Gram-negative bacteria, the EO was more effective against E. coli and E. aerogenes. Among the Gram-positive bacteria, S. aureus ATCC 6538 and E. faecalis ATCC 29212 (DIZ 21.17 mm) were the most sensitive strains to the EO. | Citronellol, geraniol and their esters | [59] | ||
| MIC | Good activity against H. pylori at a MIC of 15.63 mg/mL. Once combined the volatile oil with CLR, a significant synergistic effect appeared at a FICI of 0.38%. | Citronellol, geraniol, citronellyl formate, isolongifolan-7-a-ol | [27] | ||
| MIC, MBC | The most active was citronellol and the lowest MIC was found against E. coli (0.007 ± 0.0003 mg/mL). | Citronellol | [140] | ||
| ADM | P. graveolens has the most limited spectrum of activity, comparing with the other studied plants (T. vulgaris, O. vulgare, S. aromaticum, M. fragrans, P. nigrum). | Phenolic compounds |
[141] | ||
| MIC ADM |
The effect increased when the P. graveolens EO was combined with Norfloxacin. The results showed a synergism between them, against B. cereus ATCC 11778, S. aureus ATCC 6538 and S. aureus ATCC 29213, with FICI of 0%, 50%, 0.37%, 0.38%, respectively. | NA | [41] | ||
| Steam distillation | ADM, MIC, MFC | The Gram-positive bacteria were resistant to EO, with one exception, S. aureus, which was the most sensitive. The high antimicrobial activity is associated with the high contents of oxygenated monoterpenes. | Oxygenated monoterpene | [93] | |
| MIC, MBC | The highest antibacterial activity was obtained for S. tiphi: MIC = 7 mg/mL; MBC > 14 mg/mL. The lowest antibacterial activity was obtained for E. coli: MIC = 0.870 mg/mL; MBC 0.878 mg/mL. |
Hexadecanonic acid | [11] | ||
| MIC, FICI | P. graveolens EO exerts strong activity against all clinical isolates of S. aureus with MIC values from 0.25 to 2.50 µL/mL. The FICI for K. pneumoniae and P. mirabilis was 0.375%, while for S. aureus FICI was 0.5%. The FICI values for the tested microorganisms were <0.5%, indicating synergy between P. graveolens EO and ciprofloxacin. | β-citronellol | [99] | ||
| EP-SFME and HD |
MIC, MBC, MFC | The GEOs from the EP-SFME and HD methods had the best antimicrobial effect on Escherichia coli with MIC and MBC of 6.25 and 12.5 mg/mL, respectively. | Citronellol, geraniol | [24] | |
| Methanolic extract | ADD, MIC | Inhibitory effect was exerted by the extracts against urease and tyrosinase, with IC50 values of 31.05 ± 3.76 μg/mL and 21.11 ± 0.38 μg/mL, respectively. | Phenolic, flavonoids, flavonols, tannins | [130] | |
| Aqueous extract | Microdilution method | Inhibition activity against the COX-1 enzyme. | Citronellol | [73] | |
| Decoction, infusion, heat reflux | ADM | The P. graveolens antimicrobial activity was evaluated against four bacteria species (S. aureus, L. monocytogenes, E. coli, S. enterica). The highest inhibitory effect of P. graveolens was against L. monocytogenes. | Phenolic compounds |
[131] | |
|
P. reniforme and P. sidoides |
Methanolic extracts | PRB, BMD, ADM | P. sidoides showed high inhibitory activity (96%) against Mycobacterium tuberculosis. P. reniforme was inactive. | Phenols and coumarins | [121] |
| P. sidoides | NA | DDT | P. sidoides had significant anti-adhesive activity agains H. pylori and it can be a useful choice in avoiding the first steps of a bacterial infection. | Polymeric proanthocyanidins |
[142] |
| P. zonale | Ethanol and acetone extracts | MIC | The P. zonale leaves were the most effective against R. pseudosolanacearum. The MIC of P. zonale, started at 6.25 mg/mL. P. zonale had similar results/values like controls (KOBE 1.2 SL-Chrysophanol 12 g/L and ENRICH VM—Bronopol 27% w/w). | NA | [143] |
where: ADD—agar disk diffusion; ADM—agar diffusion method; BMD—broth microdilution; DDT—disk diffusion test; EO—essential oil; EP-SFME—enzymatic pretreatment combined with solvent-free microwave extraction; FICI—fractional inhibitory concentration index; GEO—geranium essential oil; IC50 the half-inhibition concentration; MBC—minimum bactericidal concentration; MDA—malondialdehyde; MFC—minimum fungicidal concentration; MIC—minimum inhibitory concentration; NA—not available; PAE—Determination of post-antibiotic effect; PRB—primary radiorespirometric bioassay; SEM—scanning electron microscopy.
Antifungal Activity
In their review, Hamidpour et al. [117] state that P. graveolens EO has antioxidant, antibacterial, antifungal, and medicinal properties and has been shown to be effective against Gram-negative bacteria such as E. coli, P. vulgaris and E. aerogenes when compared to controls such as chloramphenicol and amoxicillin. In addition, the review specifies that the essential oil extract is more effective in inhibiting the yeast than the bacteria, discussing the effectiveness of P. graveolens EO against Candida tropicalis and Candida albicans yeasts, as well as Staphylococcus aureus bacteria [117]. According to Rosato et al. [144], P. graveolens EO was the most effective oil among the tested ones. With a minimum inhibitory concentration of a single sample (MIC A) ranging from 0.18 to 0.70 mg/mL, and a minimum inhibitory concentration of a single sample of the most effective combination (MIC B) ranging from 0.04 to 0.28 mg/mL, alongside with FICI values ranging from 0.13 to 0.40 mg/mL, P. graveolens oil demonstrated superior efficacy against five strains of Candida species (C. albicans ATCC 14053, C. albicans NRRL y-869, C. albicans ATCC 10231, C. albicans NRRL y-22077 and C. guilliermondii NRRL y-324). Additionally, P. graveolens oil demonstrated a strong degree of synergism with amphotericin B [144].
Pelargonium asperum EO, together with two other essential oils, has been used at a high concentration of 30% for anti-infective purposes in cases of bacterial, viral, or parasitic dermatitis. The main aim in treating fungal infections was to rapidly eliminate pruritus within 1–2 days using the essential oil’s potent anti-inflammatory and antihistamine properties. P. asperum EO has been shown to be effective in stopping progression and initiating regression of various types of allergic conditions such as eczema, psoriasis and dyshidrosis, while also repairing the skin barrier [53].
Table 7 illustrates that all Pelargonium species inhibited the growth of several fungal agents through citronellol and geraniol compounds. These results had been obtained via MIC, MFC, ADM and other types of assays.
Table 7.
Antifungal properties of different extracts obtained from Pelargonium plant species.
| Plant | Extraction Method | Assay | Results | Responsible Compound | References |
|---|---|---|---|---|---|
| P. graveolens | NA | Microdilution method and macrodilution method, MIC, MFC | The Pelargonium EO showed high antifungal activity for C. albicans, C. fulvum, P. macdonaldii, T. menthagrophytes than the bifonazole (used as control) | Citronellol and geraniol | [79] |
| HS extraction-stirring time of 10 min., heating temperature of 70 °C, under 500 rpm | MIC | The most active EO was the sample from South Africa, with MIC between 128 and 256 μg/mL. MIC values obtained for HCNPG were lower for the most part of isolates tested, reaching 8 μg/mL for C. albicans and C. glabrata | [145] | ||
| NA | Crystal violet, total protein, and ATP-bioluminescence assays | Reduction in antibiofilm treated with GO and NEG; reduction in protein on the plates and catheters; GO and NEG showed lower MIC for C. albicans and C. tropicalis. | [17] | ||
| hydro-distillation | MIC, MFC | cis-menthone was the most active against selected fungi (MIC: from 0.07 ± 0.01 to 0.17 ± 0.01 mg/mL); linalool was active against oral C. albicans | cis-menthone, linalool | [140] | |
| SFE, hydro-distillation, maceration | Insecticidal tests, antifungal tests (MIC) | P. graveolens essential oil obtained by hydro-distillation had the highest acute toxicity; thus, it can be used as botanical pesticides |
trans-nerolidol, geraniol and citronellol |
[146] | |
| P. odoratissimum | NA | ADM | P. odoratissimum EO showed higher inhibition effect against fungal species growth. P. odoratissimum inhibited the growth of 3 fungal agents at 1 μL/mL (O. yallundae, Z. tritici, P. teres) by 100%. | Phenolic compounds | [114] |
| P. reniforme and P. sidoides | Ethanol and acetone extracts | PDA, ANOVA and Duncan’s multiple range test | The P. sidoides ethanol extract and P. reniforme ethanol and acetone extracts showed activity against fungal pathogens at a concentration of 5 × 103 mg/L. Amphotericin B was active at 0.5 mg/L on each fungus. | NA | [138] |
where: ADM—agar diffusion method; ANOVA—analysis of variance; ATP—Adenosine Triphosphate; EO—essential oil; GO—geranium oil; HCNPG—hydrogel-thickened nano-emulsion; HS—conditions of headspace MBC—minimum bactericidal concentration; MDA—malondialdehyde; MFC—minimum fungicidal concentration; MIC—minimum inhibitory concentration; NA—not available; NEG—nano-emulsions containing geranium; PDA—pile driving analysis; SFE—supercritical fluid extraction.
2.2.3. Other Potential Applications
In their study, Brendler and Van Wyk [8] reviewed the medicinal uses of Pelargonium species. Therefore, Pelargonium species are acknowledged to aid in the treatment of diarrhea and dysentery (Pelargonium antidysentericum), amenorrhea, anemias, and weaknesses (Pelargonium grossularioides), animal liver diseases, colic, fever, dysenteries, and diarrheas (Pelargonium reniforme), human and cattle dysentery, colic, gonorrhea, worms in calves, and intisila-stomach ailments in babies (Pelargonium sidoides) [8]. Referring to Pelargonium sidoides, Rachel Wynberg presented its commercial use in the treatment of bronchitis and in South Africa as a traditional medicine [147]. Additionally, Wopker et al. [148] discussed the use of Pelargonium sidoides root extract as an alternative medicine for bronchitis treatment in children.
Meyers et al. [127] described the various uses of Pelargonium in their book, including culinary, craft, cosmetic, medicinal, ethnobotanical, aromatherapy, and gardening applications (Table 8). Additionally, Pelargonium species can be used as insect repellents, agents with a preservative role, tobacco substitutes, or in nanotechnology (Table 9).
Table 8.
Main Pelargonium uses (from Meyers et al., 2006 [127]).
| Plant | Product Obtained |
|---|---|
| CULINARY USE | |
| P. acetosum | Salads or cooked into soups and stews |
| P. bowkeri | Salad herb |
| P. citronellum | Lemon liqueur |
| P. ‘Ginger’ (syn. P. ‘Torento’) | Cakes, jellies, beverages, desserts, and sandwiches |
| P. graveolens | Baked goods, gelatin, pudding, candy, frozen dairy desserts, and alcoholic and non-alcoholic beverages |
| P. ‘Nutmeg’ | Cakes, pâté, stuffing, potato salad and coffee |
| P. odoratissimum | Fruit drinks, syrups, sauces, and desserts |
| COSMETIC USE | |
| P. capitatum | Perfumery |
| P. inquinans | Deodorant |
| MEDICINAL, ETHNOBOTANICAL USES AND AROMATHERAPY | |
| P. alchemilloides | Root infusion in treating diarrhea Leaf juice in treating the eyes Root decoction in treating fever |
| P. antidysentericum | Decoction used in treating diarrhea Leaf tea used in treating nausea, diarrhea and dysentery |
| P. bowkeri | Treatment for colic, diarrhea |
| P. botulinum | Leaf decoction treatment of dermatological conditions, colds, respiratory infections, sinusitis |
| P. capitatum | Leaf infusion use in the treatment of urinary bladder and kidney diseases |
| P. cucullatum | The crushed leaves used in treating insect bites, bruises, boils, wounds The leaf infusion used in treating fever, diarrhea, abdominal pain |
| P. graveolens | The leaf infusion used in treating insomnia, dysentery, diarrhea, vomiting Inhalation used to treat asthma |
| P. luridum | Root infusion used in abdominal pains, dysentery, diarrhea, backache Leaf infusion or powdered root used in fever, abdominal pains |
| P. quercifolium | Remedy for rheumatism, heart disease |
| P. reniforme | The decoction used in dysentery and diarrhea |
| P. sidoides | The decoction used in different parasitic zoonoses |
Swanepoel [149] specified that Pelargonium sp. has numerous potential applications and is currently being utilized in food, cosmetic, and pharmaceutical product compositions [149].
Abdel Rahman et al. [150] investigated the potential effects of Pelargonium graveolens essential oil on the toxic impacts of profenofos in common carp. Their findings suggest that the oil could be used as a dietary supplement in aquaculture [150]. The article regarding the effect of Pelargonium sidoides extract on growth of crayfish (Astacus leptodactylus) also falls in the same field. After 105 days of diets containing P. sidoides extract (0, 0.5, 1 and 2 mL × 100 g−1), there was an increase in the parameters of weight gain, survival rate, Food Conversion Ratio and Protein Efficiency Ratio. Additionally, the advantages of this experimental diet were observed in the increase in moisture, protein content, as well as the decrease in lipid content [151]. Can et al. [6] proposed that Pelargonium graveolens EO exhibited anesthetic properties for two fish species, Sciaenochromis fryeri and Labidochromis caeruleus, with an optimal concentration of 75 μL × L−1. These findings suggest potential use of the EO as an agent for anesthesia and sedation in aquaculture [6].
Naveenkumar et al. [152] identified a method of obtaining an eco-friendly biofungicide used in the treatment of rice seed diseases. The researchers utilized three plant oils—C. citratus, C. martini, and P. graveolens—to create a highly effective emulsifiable concentrate (EC) against C. lunata, F. moniliforme, B. oryzae, and S. oryzae. The results indicated that these three oils possess the capacity to suppress mycelial growth of rice seed pathogens. The formula containing 30EC P. graveolens essential oil was found to be effective against C. lunata, F. moniliforme, B. oryzae, and S. oryzae, inhibiting their growth by 89.8%, 90.7%, 86.6%, and 94.1%, respectively [152].
Lozano-Navarro et al. [153] presented a method for viscosity modification of Mexican superheavy crude oil using an aqueous extract of Pelargonium hortorum, a common geranium species. The extract showed efficient dispersion of asphaltenes.
Upadhyaya et al. [154] studied a novel agrotechnology for producing high-quality planting material of Pelargonium graveolens. They prepared stem cuttings and planted them below three trees (Putranjiva roxburghii, Bischofia javanica, Ficus religiosa), with necessary irrigation. The raising of cutting in root trainer placed under Putranjiva roxburghii showed good results regarding plant height, leaves per plant, and survival rate [154].
Loto et al. [155] studied the electrochemical effects of Pelargonium oil concentrates on the corrosion of 1018 carbon steel (high-manganese carbon alloy) in an anionic solution. This study investigated the corrosion inhibition in media containing H2SO4 0.5 M and HCl 0.5 M. The electrochemical polarization assay demonstrated that Pelargonium oil was highly effective, inhibiting corrosion by 91.56% at a high concentration in H2SO4, and by 87.32% at 2.5% concentration. ATR-FTIR spectroscopy (Attenuated Total Reflection with Fourier Transform Infrared Spectroscopy) determined an increase in the transmittance of reactive groups in Pelargonium concentrates after corrosion. In addition, the inhibition mechanism of Pelargonium was revealed by ATR-FTIR spectroscopy. X-ray diffractometry detected corrosive precipitate on the steel, but without concentrate addition [155].
Numerous scientific articles have reported studies on the antioxidant, antibacterial and antifungal properties of Pelargonium species. In addition, it was found that rose geranium essential oil (RGEO) possessed anti-inflammatory effects. The application of RGEO at a dose of 200 mL/kg resulted in a reduction in edema by 73%, whereas a dose of 400 mL/kg produced an 88% decrease in edema. These effects were compared to those of the positive control, diclofenac (40 mg/kg), which produced an 85% inhibition of inflammation [66].
Anheyer et al. [156] reviewed Pelargonium sidoides as a treatment option for symptoms of respiratory tract infections (RTIs) compared to placebo. The results indicate that P. sidoides may be a viable option for treating RTIs in children. Further meta-analyses demonstrate moderate efficacy and safety of the use of P. sidoides [156].
Pelargonium asperum oil exhibited significant effects when administered either cutaneous or intraperitoneally to mice in response to curdlan intradermal injection-induced inflammation. Geranium oil (GO) was administered intraperitoneally, and the results indicate that GO suppressed neutrophil accumulation. The same result was seen in the use of prednisolone. Maruyama et al. [20] observed a sedative effect and a loss of normal movement following the second administration, indicating that GO suppresses the activity of MPO (human myeloperoxidase) in a dose-dependent manner.
The acaricidal properties of P. graveolens extract were observed against mites. First identified research study demonstrates the mite-control activity against Dermatophagoides farina and Dermatophagoides pteronyssinus. The activity of P. graveolens EO was compared with that of commercial acaricides, namely benzyl benzoate and N,N-diethyl-m-toluamide (DEET). The findings showed that the major components of P. graveolens were more toxic than the commercial acaricide. In the case of D. farina, the most toxic compound was geraniol (LD50 of 0.26 µg/cm2), followed by other P. graveolens compounds, and ultimately benzyl benzoate (LD50 of 10.03 μg/cm2) and DEET (LD50 of 37.12 μg/cm2). Similarly, in the case of D. pteronyssinus, the most toxic compound was geraniol (LD50 of 0.28 µg/cm2), followed by benzyl benzoate (LD50 of 9.58 μg/cm2) and DEET (LD50 of 18.23 μg/cm2) [102]. In a separate study, it was found that P. graveolens EO contains compounds that exhibit acaricidal activity against Tyrophagus putrescentiae, a type of food mite. Consequently, P. graveolens oil was compared to a commercial acaricide, and the results demonstrated that geraniol (LD50 of 1.95 μg/cm3), nerol (LD50 of 2.21 μg/cm3) and citral (LD50 of 9.65 μg/cm3) were more effective than the positive control, benzyl benzoate (LD50 of 11.27 μg/cm3) [157].
Fillipova et al. [95] developed a technique to produce toothpaste named “SPLAT Medical Herbs” with essential oils from Pelargonium graveolens. Gas chromatography analysis confirmed the presence of geraniol, validating the use of this essential oil. Consequently, the resultant toothpaste has anti-inflammatory, hemostatic and cleaning properties [95].
Pelargonium graveolens has been studied, revealing its extract waste as a viable natural dye for wool fabrics. The study analyzed variables such as temperature, pH, and extraction time, which had an impact on the flavonoid, condensed tannin, and polyphenol content, as well as the potassium sulfur ratio (K/S), ultimately affecting the color strength. The most effective results were obtained at pH = 11, a temperature of 100 °C, and an extraction period of approximately 65 min. Based on the findings mentioned above, the optimal K/S value was 115.15. Thus, the hydro-distillation of solid waste produced by P. graveolens is a viable solution for coloring wool fabrics naturally [158].
Apart from its medicinal use and other various biological properties, P. graveolens is a beneficial plant in sustainable urban horticulture. A SWOT Analysis conducted on the Aloysia citrodora plant in co-cultivation with P. graveolens demonstrated twelve advantages, including consistent and uniform crop management, pest control, and enhancement of the food chain [159].
Mazeed et al. [160] reviewed the primary objectives of geranium cultivation in India, which include supplying the aroma, pharmaceutical, and cosmetic industries, serving as a potential phyto-accumulator of heavy metals or bioremediation agent, employing distilled waste in vermiculture, and stimulating the economy and employment. To ensure high-quality rose-geranium, the main macronutrients, including phosphorus, nitrogen, potassium, and sulphur, as well as micronutrients such as iron, manganese, and zinc, are essential [160].
Table 9.
Other biological activities of Pelargonium plants, presented in the literature.
| Plant | Action | Extraction Method | Assay | Results | Responsible Compound | References |
|---|---|---|---|---|---|---|
| P. graveolens | Antagonistic activity | DNA extraction | TSA, King’s B BOX-PCR |
In P. graveolens roots were found Aerococcus, Agrococcus, Bhargavaea, Dietzia, Klebsiella and Solibacillus species. In P. graveolens rhizosphere and root samples, were found Bacillus, Paenibacillus and Streptomyces species. The genus Bacillus was found in 56.2% of isolates. Thus, 14 Bacillus sp. isolates had antagonistic activity against Colletotrichum acutatum, being able to produce indolic compounds, siderophores and mineralized organic phosphate. | NA | [161] |
| Anti-dermatophyte activity | NA | mycelium growth inhibition method, micro-broth dilution assay, MFC, MIC | Inhibitory effect of mycelium growth. The main compounds of GO, geraniol and citronellol are useful in cell membrane interference of dermatophytes and in level decreasing of ergosterol content of cells. | Geraniol and citronellol | [12] | |
| Anti-Inflammatory | Ethanolic extract | MTT assay | Potential level of inhibition of prostanoid production. | Flavonoids (rutin, myricetin, and kaempferol) | [162] | |
| Antitumor (Anticancer) activity | NA | Trypan Blue assay | The Pelargonium EO showed anticancer activity: LC50 = 62.50/86.5 µg/mL in NB4/HL-60, thus the using in cancer treatments. Another study revealed that P. graveolens has antitumor activity against uterine cervical neoplasia. | Citronellol, trans-geraniol | [60] | |
| Cytotoxicity | Aqueous extract | Cell viability assay-MTT assay | PdNPs synthesis using P. graveolens as reducing, capping agent confirmed by FTIR analysis and zeta potential measurements MTT assay showed that the synthesized PdNPs obtained using P. graveolens extract exhibited a significant dose-dependent cytotoxicity towards K562 cells. It is found that cell viability of K562 cells is significantly reduced to 57% when exposed to PdNPs of 10 μg/mL. | Polyphenols | [163] | |
| MTS assay; COX inhibitor screening assay | Cytotoxicity for HeLa, MCF-7, and Hep3B tumor cell lines; reduced tumor cells viability | Citronellol | [73] | |||
| Insecticidal activity | Steam distillation | Area preference method | The 3 tested EOs had a repellent effect against T. castaneum and R. dominica. For both tested insects, at concentrations 0.24 mg/cm2 the repellent activity was 100% for the 3 tested EOs. For R. dominica, at the lowest concentration, 0.03%, the repellent activity was 50.5% for the geranium stripping oil, 20% for the geranium oil and 10% for the geranium absolute oil. For T. castaneum, at the lowest concentration, 0.03%, the repellent activity was 66.7% for geranium stripping oil and geranium absolute, and 60% for the geranium oil | NA | [84] | |
| Hydro-distillation | NA | Pelargonium graveolens EO acts on fungi such as C. neoformans, C. albicans. The results of experiments showed that P. graveolens essential oil exerts strong activity against all clinical isolates of S. aureus, including multidrug-resistant strains, MRSA strains and MLS (B)-positive with values MIC from 0.25 to 2.50 μL/mL | 10-epi-γ-eudesmol | [99] | ||
| DBM | The P. graveolens EO showed the most toxic values against larvae (LC50 = 0.75 μg/μL after 24 h, LC 50 = 0.49 μg/μL after 48 h, and LC50 = 0.36 μg/μL after 72 h), stronger than the positive control (matrine) and then the other 12 plant’s essential oils. | β-citronellol, linalool, and geraniol |
[51] | |||
| NA | Bioassays | P. graveolens EO showed a high treatment in tick reproduction, but not to inhibit hatchability: Geranium 1% = 85.9%; Geranium 5% = 92.6%; Geranium 10% = 97.0% The other EO (C. martini, C. citratus, C. atlantica) have demonstrated 100% efficacy regardless the concentration. | Citronellol | [164] | ||
| Larval immersion test, adulticidal tests, repulsion test |
The different concentration of geranium oil does not show larval mortality (for M. domestica and L. cuprina) considering Diazinon (1%), the positive control. On the other side, for the adulticidal activity, all the treatments showed in 93–100% mortality. | Citronellol and geraniol (trans-geraniol) |
[34] | |||
| Phytoremediation activity | Hydro-distillation | ICP-OES, TF, BCF, BAF | P. graveolens had the capacity to accumulate high concentrations of heavy metals (chromium 6.6–49.1%, cadmium 40.2–78.9%, lead 20.5–67.6% and nickel 19.3–76.4%) contaminated sludge. | NA | [23] | |
| Treatments for infertility | Sperm Motility Assay, Hormonal Analysis (ELISA test), Histopathological Investigations | GEO prevents male reproductive disorders by increasing antioxidant capacity, regulates steroidogenesis and mitochondrial biogenesis-related genes. GEO protects against testicular tissue damage caused by TiO2 NPs. | Citronellol and geraniol | [91] | ||
| P. graveolens cv. Rosé | Anti-inflammatory activity | Ethanolic extract, water extract, ethyl acetate extract, chloroforms extract. | Albumin denaturation and heat-induced hemolysis | All extracts showed high inhibition of protein denaturation. The highest activity was from the stem chloroform extract, IC50 = 0.86 mg/mL (higher than the positive control, diclofenac IC50 = 3.77 mg/mL) and the lowest activity was the leaf aqueous extract, IC50 = 5.63 mg/mL. For the heat-induced hemolysis, the best results was obtained using the leaf extractions than the stem extractions. The highest result was in the leaves chloroform extract (IC50 = 0.21 mg/mL). | Flavonoids | [133] |
| Cytotoxic activity | Ethanolic, water, ethyl acetate, chloroforms extracts | WST-1 cell proliferation assay | The leaves chloroform extract presents the most cytotoxic potential activity with IC50 = 0.4 mg/mL | Gallic acid, rutin, quercetin, phenolic compounds and flavonoids | ||
| P. reniforme and P. sidoides | Antitubercular activity | extracted three times with 1 L of acetone, chloroform, and ethanol. | BACTEC radiometric system | The P. reniforme acetone, chloroform and ethanol extracts from roots were active at 5 × 103 mg/L. The positive controls, like streptomycin, ethambutol, rifampicin, and isoniazid showed stronger antitubercular activity than those of the extracts. | NA | [138] |
| P. roseum | Cytotoxic activity | hydro-distillation | Larvicidal bioassay and adulticidal bioassay | Comparing with Juniperus virginiana, Pelargonium roseum and its components showed higher larvicidal activity against population of An. gambiae, in laboratory conditions: LC50 = 7.13 ppm (24 h); 1.26 ppm (48 h); 0.90 ppm (72 h) | Sabinene, β-myrcene, bornyl acetate terpinen-4-ol |
[116] |
| Insecticidal activity | Mosquito rearing, larvicidal assay, ovicidal assay, adulticidal bioassay, ANOVA | P. roseum showed mosquito larvicidal activity against Culex pipiens species having as mode of action stomach poison. | The lethal concentrations: −7.64 μg/mL (β-citronellol) −6.86 μg/mL (geraniol −14.87 μg/mL (linalool)) |
[118] | ||
| P. sidoides | Immune-modulatory or antiviral treatment for SARS-CoV-2 infection | Four extracts: Methanolic, ethyl acetate, n-butanol, and water | ADM | Pelargonium sidoides showed immune-modulatory and antiviral properties and it inhibits replication of HCov-229E coronavirus. | Anthocyanins, coumarins, gallic acid, flavonoids, tannins, phenols and hydroxycinnamic acid derivatives | [165] |
where: ADM—agar dilution method; ANOVA—analysis of variance; BAF—bio-accumulation factor; BCF—bio-concentration factor; CLR—clarithromycin; COX—cyclooxygenase; DBM—the diamondback moth; DIZ—diameter of the inhibition zone; DMSO—dimethyl sulfoxide; DNA—deoxyribonucleic acid; ELISA—enzyme-linked immunosorbent assay; EO—essential oil; EP-SFME—enzymatic pretreatment combined with solvent-free microwave extraction; EtOAcE—ethyl acetate extract; FICI—fractional inhibitory concentration index; HCov-229E—human coronavirus 229E; HeLa—human cervical; Hep3B-liver; GO—geranium oil; HCNPG—a chitosan hydrogel thickened-nano-emulsion containing P. graveolens essential oil; HS—conditions of headspace; IFN—interferon; ICP—OES—inductively coupled plasma-optical emission spectrometry; MBC—minimum bactericidal concentration; MCF—7-breast; MeOHE—methanol extract; MFC—minimum fungicidal concentration; MIC—minimum inhibitory concentration; MLS (B)—Macrolide-lincosamide-streptogramin B; MRSA—methicillin-resistant Staphylococcus aureus; MTS-3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium; MTT-[3-(4,5-2-yl)-2,diphenyltetrazoliumbromide]; NB4/HL-60—two human promyelocytic leukemia cell lines; NEG—nano-emulsions containing geranium; PAE—determination of postantibiotic effect; PdNPs—palladium nanoparticles; SEM—scanning electron microscope; SFE—supercritical fluid extraction; TF—translocation factor; TLC—thin-layer chromatography; TNF—tumor necrosis factor; TSA—tryptic soy agar; UV—ultraviolet; WE—water extract.
3. Materials and Methods
The selection of the articles included in this review was performed based on well-known databases (Scopus, Web of Science, ScienceDirect), using specific keywords (“Pelargonium”, “Pelargonium graveolens”, “geranium”, “composition”, “anti*”, -returning results for “antibacterial”, “antifungal”, “antioxidant activity”).
The validation of the articles was performed manually, inserting only relevant articles with significant contributions to the field of research, resulting in fulfilling this review in its final form.
4. Conclusions
The scientific literature presents Pelargonium sp.’s biological properties as a potential candidate for employment of rose geranium compounds in alternative medicine, ethnobotanical, plant decoration, and diverse horticultural farming practices. In addition, the pharmacological utility of Pelargonium sp. implies the need for friendly conservation approaches within its use. In this sense, applications of plant biotechnology can play a significant role in holistic conservation strategy. Exploring and researching the bioactive principles of interest, including proof-of-concept studies, is necessary to stimulate commercial interest. The identified phytochemicals and their derivatives could thus serve as the foundation for innovative substitutes in various fields, such as the food processing industry, nutraceuticals, or preventive medicine (both human and veterinary).
Acknowledgments
We appreciate the institutional support from the University of Agronomic Sciences and Veterinary Medicine of Bucharest.
Author Contributions
Conceptualization, S.R., C.V. and N.B.; methodology, S.R. and N.B.; investigation S.R., C.V. and N.B.; resources, S.R., C.V. and N.B.; writing—original draft preparation, S.R., C.V. and N.B.; writing—review and editing, S.R., C.V. and N.B.; supervision, N.B.; project administration, N.B.; funding acquisition C.V. and N.B. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Data sharing not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
The authors gratefully acknowledge the financial support obtained by CNFIS, grant number CNFIS-FDI-2023-F-0715 “Sustaining and consolidating excellence research in USAMV Bucharest through optimal capitalization and consistent promotion of inter-and multidisciplinary research”. The APC was funded by CNFIS-FDI-2023-F-0715.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Brickell C. A–Z Encyclopedia of Garden Plants. Volume 2. Dorling Kindersley Limited; London, UK: 1996. p. 1080. [Google Scholar]
- 2.Vinereanu M. Redescoperă Mușcata: Ghid Practic Pentru Cultura Mușcatelor. Ceres Press; Bucharest, Romania: 2011. p. 198. [Google Scholar]
- 3.Courtier J. Plante de Apartament. Aquila’93 Press; Oradea, Romania: 2003. p. 175. [Google Scholar]
- 4.Gunes Z., Kahraman O. Edible ornamental plants used in landscaping areas: The case of Canakkale city center. AgroLife Sci. J. 2022;11:66–72. doi: 10.17930/AGL202228. [DOI] [Google Scholar]
- 5.Plaschil S., Budahn H., Wiedemann M., Olbricht K. Genetic characterization of Pelargonium L’Hér. germplasm. Genet. Resour. Crop Evol. 2017;64:1051–1059. doi: 10.1007/s10722-016-0424-x. [DOI] [Google Scholar]
- 6.Can E., Kizak V., Can Ș.S., Özçiçek E. Anesthetic potential of geranium (Pelargonium graveolens) oil for two cichlid species, Sciaenochromis fryeri and Labidochromis caeruleus. Aquaculture. 2018;491:59–64. doi: 10.1016/j.aquaculture.2018.03.013. [DOI] [Google Scholar]
- 7.Van Wyk B.E. A review of commercially important African medicinal plants. J. Ethnopharmacol. 2015;176:118–134. doi: 10.1016/j.jep.2015.10.031. [DOI] [PubMed] [Google Scholar]
- 8.Brendler T., Van Wyk B.E. A historical, scientific and commercial perspective on the medicinal use of Pelargonium sidoides (Geraniaceae) J. Ethnopharmacol. 2008;119:420–433. doi: 10.1016/j.jep.2008.07.037. [DOI] [PubMed] [Google Scholar]
- 9.Couic-Marinier F., Laurain-Mattar D. Huile essentielle de Géranium rosat. Actual. Pharm. 2018;57:57–59. doi: 10.1016/j.actpha.2018.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boukhris M., Hadrich F., Chtourou H., Dhouib A., Bouaziz M., Sayadi S. Chemical composition, biological activities and DNA damage protective effect of Pelargonium graveolens L’Hér. essential oils at different phenological stages. Ind. Crops Prod. 2015;74:600–606. doi: 10.1016/j.indcrop.2015.05.051. [DOI] [Google Scholar]
- 11.Abaas S.I., Jasim A., Ali J.A. Variation of essential oil quantity of geranium leaves (Pelargonium graveolens L.) at different growth stages, with preliminary evaluation of antibacterial activity. Int. J. Pharm. Pharm. Sci. 2013;5:280–281. [Google Scholar]
- 12.Mahboubi M., Valian M. Anti-dermatophyte activity of Pelargonium graveolens essential oils against dermatophytes. Clin. Phytosci. 2019;5:25. doi: 10.1186/s40816-019-0121-3. [DOI] [Google Scholar]
- 13.Eiasu B.K., Steyn J.M., Soundy P. Physiomorphological response of rose-scented geranium (Pelargonium spp.) to irrigation frequency. S. Afr. J. Bot. 2012;78:96–103. doi: 10.1016/j.sajb.2011.05.013. [DOI] [Google Scholar]
- 14.Lis-Balchin M., Steyrl H., Krenn E. The comparative effect of novel Pelargonium essential oils and their corresponding hydrosols as antimicrobial agents in a model food system. Phytother. Res. 2003;17:60–65. doi: 10.1002/ptr.1086. [DOI] [PubMed] [Google Scholar]
- 15.Mehrparvar M., Goltapeh E.M., Safaie N., Ashkani S., Hedesh R.M. Antifungal activity of essential oils against mycelial growth of Lecanicillium fungicola var. fungicola and Agaricus bisportus. Ind. Crops Prod. 2016;84:391–398. doi: 10.1016/j.indcrop.2016.02.012. [DOI] [Google Scholar]
- 16.Akçura S., Çakmakçi R., Ürüşan Z. Changes in the essential oil content and composition of Pelargonium graveolens with different drying methods. Grasas Aceites. 2023;74:e497. doi: 10.3989/gya.0226221. [DOI] [Google Scholar]
- 17.Giongo J.L., De Almeida Vaucher R., Pedroso-Fausto V., Quatrin P.M., Quintana Soares Lopes L., Vianna Santos R.C., Gündel A., Gomes P., Steppe M. Anti-Candida activity assessment of Pelargonium graveolens oil free and nanoemulsion in biofilm formation in hospital medical supplies. Microb. Pathogen. 2016;100:170–178. doi: 10.1016/j.micpath.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 18.Kolodziej H. Fascinating metabolic pools of Pelargonium sidoides and Pelargonium reniforme, traditional and phytomedicinal sources of the herbal medicine Umckaloabo. Phytomedicine. 2007;14:9–17. doi: 10.1016/j.phymed.2006.11.021. [DOI] [PubMed] [Google Scholar]
- 19.Banthorpe D., Long D., Pink C. Biosynthesis of geraniol and related monoterpenes in Pelargonium graveolens. Phytochemistry. 1983;22:2459–2463. doi: 10.1016/0031-9422(83)80140-X. [DOI] [Google Scholar]
- 20.Maruyama N., Sekimoto Y., Ishibashi H., Inouye S., Oshima H., Yamaguchi H., Abe S. Suppression of neutrophil accumulation in mice by cutaneous application of geranium essential oil. J. Inflamm. 2005;2:1. doi: 10.1186/1476-9255-2-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demarne F., Van Der Walt J.J.A. Origin of the rose-scented Pelargonium cultivar grown on Réunion Island. S. Afr. J. Bot. 1989;55:184–191. doi: 10.1016/S0254-6299(16)31205-4. [DOI] [Google Scholar]
- 22.Singh M., Singh U.B., Ram M., Yadav A., Chanotiya C.S. Biomass yield, essential oil yield and quality of geranium (Pelargonium graveolens L. Her.) as influenced by intercropping with garlic (Allium sativum L.) under subtropical and temperate climate of India. Ind. Crops Prod. 2013;46:234–237. doi: 10.1016/j.indcrop.2013.01.032. [DOI] [Google Scholar]
- 23.Mazeed A., Lothe N.B., Kumar A., Sharma S.K., Srivastav S., Verma R.K. Evaluation of phytoaccumulation potential of toxic metals from sewage sludge by high-value aromatic plant geranium. J. Environ. Biol. 2019;41:761–769. doi: 10.22438/jeb/41/4/MRN-1210. [DOI] [Google Scholar]
- 24.Ravindra N.S., Kulkarni R.N. Essential oil yield and quality in rose-scented geranium: Variation among clones and plant parts. Sci. Hortic. 2015;184:31–35. doi: 10.1016/j.scienta.2014.12.023. [DOI] [Google Scholar]
- 25.Ríos N., Stashenko E.E., Duque E.J. Evaluation of the insecticidal activity of essential oils and their mixtures against Aedes aegypti (Diptera: Culicidae) Rev. Bras. Entomol. 2017;61:307–311. doi: 10.1016/j.rbe.2017.08.005. [DOI] [Google Scholar]
- 26.Patel A., Patra D.D. Phytoextraction capacity of Pelargonium graveolens L’Hér. grown on soil amended with tannery sludge—Its effect on the antioxidant activity and oil yield. Ecol. Eng. 2015;74:20–27. doi: 10.1016/j.ecoleng.2014.10.013. [DOI] [Google Scholar]
- 27.Ibrahim A.M., Sallem W.O., Abdelhassib R.M., Eldahshan A.O. Potentiation of anti-Helicobacter pylori activity of clarithromycin by Pelargonium graveolens oil. Arab J. Gastroenterol. 2021;22:224–228. doi: 10.1016/j.ajg.2021.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Kumar N., Ghosh D., Chaudhary N., Chanotiya C.S. Rainfall-induced premature senescence modulates biochemical and essential oils profiles in Pelargonium graveolens L’Hér. under sub-tropical climate. Ind. Crops Prod. 2022;178:114630. doi: 10.1016/j.indcrop.2022.114630. [DOI] [Google Scholar]
- 29.Elansary O.H., Abdelgaleil A.M.S., Mahmoud A.E., Yessoufou K., Elhindi K., El-Hendawy S. Effective antioxidant, antimicrobial and anticancer activities of essential oils of horticultural aromatic crops in northern Egypt. BMC Complement. Altern. Med. 2018;18:214. doi: 10.1186/s12906-018-2262-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Upadhyay R., Lothe B.N., Bawitlung L., Singh S., Singh M.K., Kumar P., Verma R.K., Tandon S., Pal A., Verma R.S. Secondary metabolic profile of rose-scented geranium: A tool for characterization, distinction and quality control of Indian genotypes. Ind. Crops Prod. 2022;187:115487. doi: 10.1016/j.indcrop.2022.115487. [DOI] [Google Scholar]
- 31.Al-Jumaili A., Alancherry S., Bazaka K., Jacob V.M. The electrical properties of plasma-deposited thin films derived from Pelargonium graveolens. Electronics. 2017;6:86. doi: 10.3390/electronics6040086. [DOI] [Google Scholar]
- 32.Amiri R., Nikbakht A., Rahimmalek M., Hosseini H. Variation in the Essential Oil Composition, Antioxidant Capacity, and Physiological Characteristics of Pelargonium graveolens L. Inoculated with Two Species of Mycorrhizal Fungi Under Water Deficit Conditions. J. Plant Growth Regul. 2017;36:502–515. doi: 10.1007/s00344-016-9659-1. [DOI] [Google Scholar]
- 33.Chorbanpour M., Hatami M. Changes in growth, antioxidant defense system and major essential oil constituents of Pelargonium graveolens plant exposed to nano-scale silver and thidiazuron. Indian J. Plant Physiol. 2015;20:116–123. doi: 10.1007/s40502-015-0145-8. [DOI] [Google Scholar]
- 34.Saraiva L.C., Magalhães De Matos A.F.I., Filippin Cossetin L., Martins Couto J.C., Dos Santos Petry L., Gonzáles Monteiro S. Insecticida land repellent activity of geranium essential oil against Musca domenstica and Lucilia cuprina. Int. J. Trop. Insect Sci. 2020;40:1093–1098. doi: 10.1007/s42690-020-00137-4. [DOI] [Google Scholar]
- 35.Stegmayer M.I., Álvarez N.H., Sager N.G., Buyatti M.A., Derita M.G. Evaluation of Pelargonium graveolens essential oil to prevent gray mold in rose flowers. J. Plant Prot. Res. 2022;62:145–152. doi: 10.24425/jppr.2022.141352. [DOI] [Google Scholar]
- 36.Rajesh Y., Khan N., Shaikh A.R., Mane V., Daware G., Dabhade G. Investigation of geranium oil extraction performance by using Soxhlet extraction. Mater. Today Proc. 2023;72:2610–2617. doi: 10.1016/j.matpr.2022.07.276. [DOI] [Google Scholar]
- 37.Atailia I., Djahoudi A. Composition chimique et activité antibactérienne de l’huile essentielle de géranium rosat (Pelargonium graveolens L’Hér.) cultivé en Algérie. Phytothérapie. 2015;13:156–162. doi: 10.1007/s10298-015-0950-2. [DOI] [Google Scholar]
- 38.Rathore S., Mukhia S., Kumar R., Kumar R. Essential oil composition and antimicrobial potential of aromatic plants grown in the mid-hill conditions of the Western Himalayas. Sci. Rep. 2023;13:4878. doi: 10.1038/s41598-023-31875-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dharni S., Srivastava A.K., Samad A., Patra D.D. Impact of plant growth promoting Pseudomonas monteilii PsF84 and Pseudomonas plecoglossicida PsF610 on metal uptake and production of secondary metabolite (monoterpenes) by rose-scented geranium (Pelargonium graveolens cv. bourbon) grown on tannery sludge amended soil. Chemosphere. 2014;117:433–439. doi: 10.1016/j.chemosphere.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Adenubi O.T., Fasina F.O., Mcgaw L.J., Eloff J.N., Naidoo V. Plant extracts to control ticks of veterinary and medical importance: A review. S. Afr. J. Bot. 2016;105:178–193. doi: 10.1016/j.sajb.2016.03.010. [DOI] [Google Scholar]
- 41.Rosato A., Vitali C., De Laurentis N., Armenise D., Milillo M.A. Antibacterial effect of some essential oils administered alone or in combination with Norfloxacin. Phytomedicine. 2007;14:727–732. doi: 10.1016/j.phymed.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 42.Natnoliya L.K., Jadaun J.S., Singh S.P. The Phytochemical Composition, Biological Effects and Biotechnological Approaches to the Production of High-Value Essential Oil from Geranium. In: Malik S., editor. Essential Oil Research. Springer; Cham, Switzerland: 2019. pp. 327–352. [DOI] [Google Scholar]
- 43.Blerot B., Baudino S., Prunier C., Demarne F., Toulemonde B., Caissard J.C. Botany, agronomy and biotechnology of Pelargonium used for essential oil production. Phytochem. Rev. 2016;15:935–960. doi: 10.1007/s11101-015-9441-1. [DOI] [Google Scholar]
- 44.Mioulane P., Delavie A., Delvaux C. Grădini și Plante de Interior. Enciclopedia RAO Press; Bucharest, Romania: 2004. p. 511. [Google Scholar]
- 45.Ali E.F., Hassan F.A.S., Elgimabi M. Improving the growth, yield and volatile oil content of Pelargonium graveolens L. Herit by foliar application with moringa leaf extract through motivating physiological and biochemical parameters. S. Afr. J. Bot. 2018;119:383–389. doi: 10.1016/j.sajb.2018.10.003. [DOI] [Google Scholar]
- 46.Riahi L., Cherif H., Miladi S., Neifar M., Bejaoui B., Chouchane H., Masmoudi A.S., Cherif A. Use of plant growth promoting bacteria as an efficient biotechnological tool to enhance the biomass and secondary metabolites production of the industrial crop Pelargonium graveolens L’Hér. under semi-controlled conditions. Ind. Crops Prod. 2020;154:112721. doi: 10.1016/j.indcrop.2020.112721. [DOI] [Google Scholar]
- 47.Calamai A., Pachetti E., Masoni A., Marini L., Chiaramonti D., Dibari C., Brilli L. The influence of biochar and solid digestate on rose-scented geranium (Pelargonium graveolens L’Hér.) Productivity and essential oil quality. Agronomy. 2019;9:260. doi: 10.3390/agronomy9050260. [DOI] [Google Scholar]
- 48.Mohamed El-Shafey N., Marzouk A.M., Yasser M.M., Shaban A.S., Beemster T.S.G., Abdelgawad H. Harnessing Endophytic Fungi for Enhancing Growth, Tolerance and Quality of Rose-Scented Geranium (Pelargonium graveolens (L’Hér) Thunb.) Plants under Cadmium Stress: A Biochemical Study. J. Fungi. 2021;7:1039. doi: 10.3390/jof7121039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Okla K.M., Rubnawaz S., Dawoud M.T., Al-Amri S., El-Tayeb A.M., Abdel-Maksoud A.M., Akhtar N., Zrig A., Abdelgayed G., Abdelgawad H. Laser light treatment improves the mineral composition, essential oil production and antimicrobial activity of mycorrhizal treated Pelargonium graveolens. Molecules. 2022;27:1752. doi: 10.3390/molecules27061752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Pandith S., Dhar N., Wani A.T., Razdan S., Bhat W.W., Rana S., Khan S., Verma K.M., Lattoo K.S. Production dynamics in relation to ontogenetic development and induction of genetic instability through in vitro approaches in Pelargonium graveolens: A potential essential oil crop of commercial significance. Flavour. Fragr. J. 2017;32:376–387. doi: 10.1002/ffj.3390. [DOI] [Google Scholar]
- 51.Song C., Zhao J., Zheng R., Hao C., Yan X. Chemical composition and bioactivities of thirteen non-host plant essential oils against Plutella xylostella L. (Lepidoptera: Plutellidae) J. Asia-Pac. Entomol. 2022;25:101881. doi: 10.1016/j.aspen.2022.101881. [DOI] [Google Scholar]
- 52.Amiri R., Nikbakht A., Etemadi N., Sabzalian M.R. Nutritional status, essential oil changes and water-use efficiency of rose geranium in response to arbuscular mycorrhizal fungi and water deficiency stress. Symbiosis. 2017;73:15–25. doi: 10.1007/s13199-016-0466-z. [DOI] [Google Scholar]
- 53.Pidoux M., Harilalarisoa H., Iharilanto R., Rabenoavy M., Rakotondramanana R., Raharisoa I., Ravaoarinirina S., Rakotoariniaina N. Traitements topiques d’affections dermatologiques par les huiles essentielles de géranium, saro, niaouli, à Madagascar (2e partie) Phytothérapie. 2015;13:214–222. doi: 10.1007/s10298-015-0930-6. [DOI] [Google Scholar]
- 54.Viljoen A.M., Van Der Walt J.J.A., Demarn F.E., Swart J.P.J. A study of the variation in the essential oil and morphology of Pelargonium capitatum (L.) L’Hérit. (Geraniaceae). Part III. Geographical variation in essential oil composition and floral structure. S. Afr. J. Bot. 1995;61:105–113. doi: 10.1016/S0254-6299(15)30496-8. [DOI] [Google Scholar]
- 55.Fayoumi L., Khalil M., Ghareeb D., Chokr A., Bouaziz M., El-Dakdouki M. Phytochemical constituents and therapeutic effects of the essential oil of rose geranium (Pelargonium hybrid) cultivated in Lebanon. S. Afr. J. Bot. 2022;147:894–902. doi: 10.1016/j.sajb.2022.03.039. [DOI] [Google Scholar]
- 56.Dumlupinar B., Seker Karatoprak G., Damar Celik D., Soyoğul Gürer Ü., Demirci B., Gürbüz B., Rayaman P., Merve Kurtulus E. Synergic potential of Pelargonium endlicherianum Fenzl. Essential oil and antibiotic combinations against Klebsiella pneumoniae. S. Afr. J. Bot. 2020;135:117–126. doi: 10.1016/j.sajb.2020.08.022. [DOI] [Google Scholar]
- 57.Negro C., Dimita R., Allah S.M., Miceli A., Luvisi A., Blando F., De Bellis L., Accogli R. Phytochemicals and Volatiles in Developing Pelargonium ‘Endsleigh’ Flowers. Horticulturae. 2021;7:419. doi: 10.3390/horticulturae7110419. [DOI] [Google Scholar]
- 58.Benelli G., Pavela R., Canale A., Cianfaglione K., Ciaschetti G., Conti F., Nicoletti M., Senthil-Nathan S., Mehlhorn H., Maggi F. Acute larvicidal toxicity of five essential oils (Pinus nigra, Hyssopus officinalis, Satureja montana, Aloysia citrodora and Pelargonium graveolens) against the filariasis vector Culex quinquefasciatus: Synergistic and antagonistic effects. Parasitol. Int. 2017;66:166–171. doi: 10.1016/j.parint.2017.01.012. [DOI] [PubMed] [Google Scholar]
- 59.Boukhatem M.N., Kameli A., Saidi F. Essential oil of Algerian rose-scented geranium (Pelargonium graveolens): Chemical composition and antimicrobial activity against food spoilage pathogens. Food Control. 2013;34:208–213. doi: 10.1016/j.foodcont.2013.03.045. [DOI] [Google Scholar]
- 60.Al-Mijalli S., Mrabti H.N., Assaggaf H., Attar A.A., Hamed M., El Baaboua A., El Omari N., El Menyiy N., Hazzoumi Z., Sheikh A.R., et al. Chemical Profiling and biological activities of Pelargonium graveolens essential oil at three different phenological stages. Plants. 2022;11:2226. doi: 10.3390/plants11172226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Saraswathi J., Venkatesh K., Nirmala B., Majid H.H., Roja Rani A. Phytopharmacological importance of Pelargonium species. J. Med. Plants Res. 2011;5:2587–2598. [Google Scholar]
- 62.Sharopov S.F., Zhang H., Setzer N.W. Composition of geranium (Pelargonium graveolens) essential oil from Tajikistan. Am. J. Essent. Oil. Nat. Prod. 2014;2:13–16. [Google Scholar]
- 63.Essaid R., Rahali F.Z., Msaada K., Sghair I., Hammami M., Bouratbine A., Aoun K., Limam F. Antileishmanial and cytotoxic potential of essential oils from medicinal plants in Northen Tunisia. Ind. Crops Prod. 2015;77:795–802. doi: 10.1016/j.indcrop.2015.09.049. [DOI] [Google Scholar]
- 64.Boukhris M., Simmonds S.J.M., Sayadi S., Bouaziz M. Chemical composition and biological activities of polar extracts and essential oil of rose-scented Geranium, Pelargonium graveolens. Phytother. Res. 2013;27:1206–1213. doi: 10.1002/ptr.4853. [DOI] [PubMed] [Google Scholar]
- 65.Neagu A.F., Costea T., Nencu I., Duțu L.E., Popescu M.L., Olaru O.T., Gîrd C.E. Obtaining and characterization of a selective Pelargonium graveolens L’Hér. Dry extract with potential therapeutic activity in metabolic diseases. Farmacia. 2018;66:4. doi: 10.31925/farmacia.2018.4.5. [DOI] [Google Scholar]
- 66.Boukhatem M.N., Kameli A., Ferhat M.A., Saidi F., Mekarnia M. Rose geranium essential oil as a source of new and safe anti-inflammatory drugs. Libyan J. Med. 2013;8 doi: 10.3402/ljm.v8i0.22520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xie Y., Wang Z., Huang Q., Zhang D. Antifungal activity of several essential oils and major components against wood-rot fungi. Ind. Crops Prod. 2017;108:278–285. doi: 10.1016/j.indcrop.2017.06.041. [DOI] [Google Scholar]
- 68.Naeni A.R., Nazeri M., Shokri H. Antifungal activity of Zataria multiflora, Pelargonium graveolens and Cuminum cyminum essential oils towards three species of Malassezia isolated from patients with pityriasis versicolor. J. Mycol. Med. 2011;21:87–91. doi: 10.1016/j.mycmed.2011.01.004. [DOI] [Google Scholar]
- 69.Kujur A., Kumar A., Yadav A., Prakash B. Antifungal and aflatoxin B1 inhibitory efficacy of nanoencapsulated Pelargonium graveolens L. essential oil and its mode of action. LWT. 2020;130:109619. doi: 10.1016/j.lwt.2020.109619. [DOI] [Google Scholar]
- 70.Han X., Beaumont C., Stevens N. Chemical composition analysis and in vitro biological activities of ten essential oils in human skin cells. Biochim. Open. 2017;5:1–7. doi: 10.1016/j.biopen.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Rosato A., Vitali C., Piarulli M., Mazzotta M., Argentieri M.P., Mallamaci R. In vitro synergic efficacy of the combination of Nystatin with the essential oils of Origanum vulgare and Pelargonium graveolens against some Candida species. Phytomedicine. 2009;16:972–975. doi: 10.1016/j.phymed.2009.02.011. [DOI] [PubMed] [Google Scholar]
- 72.Gucwa K., Milewski S., Dymerski T., Szweda P. Investigation of the Antifungal Activity and Mode of Action of Thymus vulgaris, Citrus limonum, Pelargonium graveolens, Cinnamomum cassia, Ocimum basilicum, and Eugenia caryophyllus Essential Oils. Molecules. 2018;23:1116. doi: 10.3390/molecules23051116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jaradat N., Hawash M., Qadi M., Abualhasan M., Odetallah A., Qasim G., Awayssa R., Abdullah I., Al-Maharik N. Chemical markers and pharmacological characters of Pelargonium graveolens essential oil from Palestine. Molescules. 2022;25:5721. doi: 10.3390/molecules27175721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Prasad A., Kumar S., Pandey A., Chand S. Microbial and chemical sources of phosphorus supply modulate the yield and chemical composition of essential oil of rose-scented geranium (Pelargonium species) in sodic soils. Biol. Fertil. Soils. 2012;48:117–122. doi: 10.1007/s00374-011-0578-9. [DOI] [Google Scholar]
- 75.Gupta R., Sastry K.P., Banerjee S., Mallavarapu G.R., Kumar S. Genetic resource enhancement by isolation of diverse genotypes from seed progeny in predominantly sterile rose scented geranium Pelargonium graveolens. Genet. Resour. Crop Evol. 2001;48:629–636. doi: 10.1023/A:1013869025606. [DOI] [Google Scholar]
- 76.Ponomareva E.I., Molohova E.I. Evaluation of the Efficiency of Supercritical Carbon Dioxide Extraction for Pelargonium graveolens L’Her. Essential Oil Production. Russ. J. Phys. Chem. B. 2017;11:1270–1275. doi: 10.1134/S1990793117080097. [DOI] [Google Scholar]
- 77.Szutt A., Dołhańczuk-Śródka A., Sporek M. Evaluation of chemical composition of essential oils derived from different pelargonium species leaves. Ecol. Chem. Eng. 2019;26:807–816. doi: 10.1515/eces-2019-0057. [DOI] [Google Scholar]
- 78.Sandasi M., Kamatou G.P.P., Gavaghan C., Baranska M., Viljoen A.M. A quality control method for geranium oil based on vibrational spectroscopy and chemometric data analysis. Vib. Spectrosc. 2011;57:242–247. doi: 10.1016/j.vibspec.2011.08.002. [DOI] [Google Scholar]
- 79.Džamić M.A., Soković D.M., Ristić S.M., Grujić M.S., Mileski S.K., Marin D.P. Chemical composition, antifungal and antioxidant activity of Pelargonium graveolens essential oil. J. Appl. Pharm. Sci. 2014;4:001–005. doi: 10.7324/JAPS.2014.40301. [DOI] [Google Scholar]
- 80.Ben Elhadj Ali I., Tajini F., Boulila A., Jebri M.A., Boussaid M., Messaoud C., Sebaï H. Bioactive compounds from Tunisian Pelargonium graveolens (L’Hér.) essential oils and extracts: α-amylase and acethylcholinesterase inhibitory and antioxidant, antibacterial and phytotoxic activities. Ind. Crops Prod. 2020;158:112951. doi: 10.1016/j.indcrop.2020.112951. [DOI] [Google Scholar]
- 81.Boukhris M., Nasri-Ayachi M.B., Mezghani I., Bouaziz M., Boukhris M., Sayadi S. Trichomes morphology structure and essential oils of Pelargonium graveolens L’Hér. (Geraniaceae) Ind. Crops Prod. 2013;50:604–610. doi: 10.1016/j.indcrop.2013.08.029. [DOI] [Google Scholar]
- 82.Peterson A., Machmudah S., Roy C.B., Goro M., Sasaki M., Hirose T. Extraction of essential oil from geranium (Pelargonium graveolens) with supercritical carbon dioxide. J. Chem. Technol. Biotechnol. 2006;81:167–172. doi: 10.1002/jctb.1375. [DOI] [Google Scholar]
- 83.Choi S.H., Lim S., Shin S. Combined Effects of the Essential Oil from Pelargonium graveolens with Antibiotics against Streptococcus pneumoniae. Nat. Prod. Sci. 2007;13:342–346. [Google Scholar]
- 84.Abouelatta A., Keratum A., Ahmed S., El-Zun H. Repellent, contact and fumigant activities of geranium (Pelargonium graveolens L.’Hér) essential oils against Tribolium castaneum (Herbst) and Rhyzopertha dominica (F.) Int. J. Trop. Insect Sci. 2020;40:1021–1030. doi: 10.1007/s42690-020-00161-4. [DOI] [Google Scholar]
- 85.Kumar A., Verma N., Kaur P., Kumar D., Ghosh D., Singh A., Siddiqui A., Kumar N., Singh A.K., Khare P., et al. Physiological and chemical changes induced by transparent polythene + green net shed on Pelargonium graveolens L. mother plants during monsoon season. Pt BInd. Crops Prod. 2022;188:115686. doi: 10.1016/j.indcrop.2022.115686. [DOI] [Google Scholar]
- 86.Pandey V., Patra D.D. Crop productivity, aroma profile and antioxidant activity in Pelargonium graveolens L’Hér. under integrated supply of various organic and chemical fertilizers. Ind. Crops Prod. 2015;67:257–263. doi: 10.1016/j.indcrop.2015.01.042. [DOI] [Google Scholar]
- 87.Van Der Walt J.J.A., Demarne F. Pelargonium graveolens and P. radens: A comparison of their morphology and essential oils. S. Afr. J. Bot. 1988;54:617–622. doi: 10.1016/S0254-6299(16)31263-7. [DOI] [Google Scholar]
- 88.Babu G.D.K., Kaul V.K. Variation in essential oil composition of rose-scented geranium (Pelargonium sp.) distilled by different distillation techniques. Flavour. Fragr. J. 2005;20:222–231. doi: 10.1002/ffj.1414. [DOI] [Google Scholar]
- 89.Ganesan P., Samuel R., Mutheeswaran S., Pandikumar P., Reegan A.D., Aremu A.O., Pgnacimuthu S. Phytocompounds for mosquito larvicidal activity and their modes of action: A review. S. Afr. J. Bot. 2023;152:19–49. doi: 10.1016/j.sajb.2022.11.028. [DOI] [Google Scholar]
- 90.Gomes B.P., Mata G.V., Rodrigues E.A. Production of rose geranium oil using supercritical fluid extraction. J. Supercrit. Fluids. 2007;41:50–60. doi: 10.1016/j.supflu.2006.08.018. [DOI] [Google Scholar]
- 91.Said A.A., Nasr Y., Galal A.A.A., Abdelhamid E.A., Mohamed A.H., Metwally M.M.M., Said A.M., Nassan A.M., Dahran N., Abdel-Rahman Mohamed A. Concerns with Male Infertility Induced by Exposure to Titanium Nanoparticles and the Supporting Impact of Pelargonium graveolens Essential Oil: Morphometric Records in Male-Wistar Rats. Life. 2022;12:639. doi: 10.3390/life12050639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Boukhris M., Bouaziz M., Feki I., Jemei H., El Feki A., Sayadi S. Hypoglycemic and antioxidant effects of leaf essential oil of Pelargonium graveolens L’Hér. in alloxan induced diabetic rats. Lipids Health Dis. 2012;11:81. doi: 10.1186/1476-511X-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hsouna A.B., Hamdi N. Phytochemical composition and antimicrobial activities of the essential oils and organic extracts from Pelargonium graveolens growing in Tunisia. Lipids Health Dis. 2012;11:167. doi: 10.1186/1476-511X-11-167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Slima A.B., Ali M.B., Barkallah M., Traore A.I., Boudawara T., Allouche N., Gdoura R. Antioxidant properties of Pelargonium graveolens L’Her. essential oil on the reproductive damage induced by deltamethrin in mice as compared to alpha-tocopherol. Lipids Health Dis. 2013;12:30. doi: 10.1186/1476-511X-12-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Fillipova A.A., Szhenova T.M., Bokov D.O., Golovina N.V., Garnova N.Y., Dobrokhotov D.A. Gas Chromatography Quantification of Geraniol in a Dental Hydrogel Containing the Essential Oil of Pelargonium graveolens. Mosc. Univ. Chem. Bull. 2021;76:137–146. doi: 10.3103/S0027131421020036. [DOI] [Google Scholar]
- 96.Al-Sagheer A.A., Mahmoud H.K., Reda F.M., Mahgoub S.A., Ayyat M.S. Supplementation of diets for Oreochromis niloticus with essential oil extracts from lemongrass (Cymbopogon citratus) and geranium (Pelargonium graveolens) and effects on growth, intestinal microbiota, antioxidant and immune activities. Aquacult. Nutr. 2017;24:1006–1014. doi: 10.1111/anu.12637. [DOI] [Google Scholar]
- 97.Nejad A.R., Ismaili A. Changes in growth, essential oil yield and composition of geranium (Pelargonium graveolens L.) as affected by growing media. J. Sci. Food Agric. 2013;94:905–910. doi: 10.1002/jsfa.6334. [DOI] [PubMed] [Google Scholar]
- 98.Jeon J.H., Kim H.W., Kim M.G., Lee H.S. Mite-control activities of active constituents isolated from Pelargonium graveolens against house dust mites. J. Microbiol. Biotechnol. 2008;18:1666–1671. [PubMed] [Google Scholar]
- 99.Ghedira K., Goetz P. Géranium rosat: Pelargonium graveolens L’Hér. (Géraniaceae) Phytothérapie. 2015;13:197–201. doi: 10.1007/s10298-015-0955-x. [DOI] [Google Scholar]
- 100.De Silva Santana A., Lopes Baldin E.L., Braga Dos Santos T.L., Baptista Y.A., Dos Santos M.C., Santana Lima A.P., Stenico Tanajura L., Manzini Vieira T., Miller Crotti A.E. Synergism between essential oil: A promising alternative to control Sitophiluz zeamais (Coleoptera: Curculionidae) Crop Prot. 2022;153:105882. doi: 10.1016/j.cropro.2021.105882. [DOI] [Google Scholar]
- 101.Dos Santos Niculau E., Alves P.B., De Lima Nogueira P.C., Pimenta Crus Romão L., De Costa Cunha G., Fitzgerald Blank A., De Carvalho Silva A. Chemical Profile and Use of the Peat as an Adsorbent for Extraction of Volatile Compounds from Leaves of Geranium (Pelargonium graveolens L’Herit) Molecules. 2020;25:4923. doi: 10.3390/molecules25214923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Baldin L.L.E., Aguiar P.G., Fanela L.M.T., Soares C.E.M., Froppo M., Crotti E.M.A. Bioactivity of Pelargonium graveolens essential oi land related monoterpenoids against sweet potato whitefly, Bemisia tabaci biotype B. Pest. Sci. 2015;88:191–199. doi: 10.1007/s10340-014-0580-8. [DOI] [Google Scholar]
- 103.Cavar S., Maksimović M. Antioxidant activity of essential oil and aqueous extract of Pelargonium graveolens L’Her. Food Control. 2012;23:263–267. doi: 10.1016/j.foodcont.2011.07.031. [DOI] [Google Scholar]
- 104.Jalali-Heravi M., Zekavat B., Sereshti H. Characterization of essential oil components of Iranian geranium oil using gas chromatography–mass spectrometry combined with chemometric resolution techniques. J. Chromatogr. A. 2006;1114:154–163. doi: 10.1016/j.chroma.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 105.Rajeswara Rao B.R., Singh K., Bhattacharya A.K., Naqvi A.A. Effect of prilled urea and modified urea materials on yield and quality of geranium (Pelargonium graveolens L. Her.) Fertil. Res. 1990;23:81–85. doi: 10.1007/BF01063334. [DOI] [Google Scholar]
- 106.Saxena G., Rahman L., Verma P.C., Banerjee S., Kumar S. Field performance of somaclones of rose scented geranium (Pelargonium graveolens L’Her. Ex Ait.) for evaluation of their essential oil yield and composition. Ind. Crops Prod. 2008;27:86–90. doi: 10.1016/j.indcrop.2007.08.001. [DOI] [Google Scholar]
- 107.Verma R.K., Chauhan A., Verma R.S., Rahman L.U., Bisht A. Improving production potential and resources use efficiency of peppermint (Mentha piperita L.) intercropped with geranium (Pelargonium graveolens L. Herit ex Ait) under different plant density. Ind. Crops Prod. 2013;44:577–582. doi: 10.1016/j.indcrop.2012.09.019. [DOI] [Google Scholar]
- 108.Badzhelova V. Main parameters of essential oil of two species from genus Pelargonium, cultivated in laboratory conditions. J. Agric. Sci. Technol. 2021;13:76–78. doi: 10.15547/ast.2021.01.014. [DOI] [Google Scholar]
- 109.Swamy Gowda M.R., Hirtemath C., Singh S., Verma R.S. The influence of NaCl salt stress on the yield and quality of the essential oil from two varieties of rose-scented geranium (Pelargonium graveolens L’Hér.) Biochem. Syst. Ecol. 2022;105:104532. doi: 10.1016/j.bse.2022.104532. [DOI] [Google Scholar]
- 110.Juárez Z.N., Bahc H., Sánchez-Arreola E., Bach H., Hernández L.R. Protective antifungal activity of essential oils extracted from Buddleja perfoliata and Pelargonium graveolens against fungi isolated from stored grains. J. Appl. Microbiol. 2016;120:1264–1270. doi: 10.1111/jam.13092. [DOI] [PubMed] [Google Scholar]
- 111.Iancu C.E., Cioancă O., Hăncianu M., Mircea C. Phytochemical profile of two cultivated Pelargonium (Geraniaceae) species. Farmacia. 2016;64:6. [Google Scholar]
- 112.Ganbarianzade-Mahabadi A., Mirzakhani A., Azizi A., Chavoshi S., Khaghani S. Extracts of Pelargonium hortorum: A natural and efficient fluid for fast and eco-friendly biosynthesis of CeO2 nanoparticles for antioxidant and photocatalytic applications. Inorg. Chem. Commun. 2021;127:108553. doi: 10.1016/j.inoche.2021.108553. [DOI] [Google Scholar]
- 113.Benelli G., Pavela R., Giordani C., Casettari L., Curzi G., Cappellacci L., Petrelli R., Maggi F. Acute and sub-letal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crops Prod. 2018;112:668–680. doi: 10.1016/j.indcrop.2017.12.062. [DOI] [Google Scholar]
- 114.Matusinsky P., Zouhar M., Pavela R., Novy P. Antifungal effect of five essential oils against important pathogenic fungi of cereals. Ind. Crops Prod. 2015;67:208–215. doi: 10.1016/j.indcrop.2015.01.022. [DOI] [Google Scholar]
- 115.Khalid A.K., Teixeira Da Silva A.J., Cai W. Water deficit and polyethylene glycol 6000 affects morphological and biochemical characters of Pelargonium odoratissimum (L.) Sci. Hortic. 2010;125:159–166. doi: 10.1016/j.scienta.2010.03.009. [DOI] [Google Scholar]
- 116.Yohana R., Chisulumi S.P., Kidima W., Tahghighi A., Maleki-Ravasan N., Kweka J.E. Anti-mosquito properties of Pelargonium roseum (Geraniaceae) and Juniperus virginiana (Cupressaceae) essential oils against dominant malaria vectors in Africa. Malar. J. 2022;21:219. doi: 10.1186/s12936-022-04220-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Tabari M.A., Youssefi M.R., Esfandiari A., Benelli G. Toxicity of β-citronellol, geraniol and linalool from Pelargonium roseum essential oil against the West Nile and filariasis vector Culex pipiens (Diptera: Culicidae) Res. Vet. Sci. 2017;114:36–40. doi: 10.1016/j.rvsc.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 118.Moyo M., Aremu A., Gruz J., Šubrtová M., Szüčová L., Doležal K., Van-Staden J. Conservation strategy for Pelargonium sidoides DC: Phenolic profile and pharmacological activity of acclimatized plants derived from tissue culture. J. Ethnopharmacol. 2013;149:557–561. doi: 10.1016/j.jep.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 119.Filippova A.A., Szhenova T.M., Golovina N.V., Garnova N.Y., Bokov D.O. Standardization of Geranium Essential Oil. Mosc. Univ. Chem. Bull. 2020;75:200–206. doi: 10.3103/S0027131420030037. [DOI] [Google Scholar]
- 120.Kolodziej H., Kiderlen A. In vitro evaluation of antibacterial and immunomodulatory activities of Pelargonium reniforme, Pelargonium sidoides and the related herbal drug preparation EPs® 7630. Phytomedicine. 2007;14:18–26. doi: 10.1016/j.phymed.2006.11.020. [DOI] [PubMed] [Google Scholar]
- 121.Hamidpour R., Hamidpour S., Hamidpour M., Marshall V., Hamidpour R. Pelargonium graveolens (Rose Geranium)—A Novel Therapeutic Agent for Antibacterial, Antioxidant, Antifungal and Diabetics. Arch. Can. Res. 2017;5:134. doi: 10.21767/2254-6081.1000134. [DOI] [Google Scholar]
- 122.Moyo M., Van Staden J. Medicinal properties and conservation of Pelargonium sidoides DC. J. Ethnopharmacol. 2014;152:243–255. doi: 10.1016/j.jep.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Mishra B., Chandra M., Pant D. Genome-mining for stress-responsive genes, profiling of antioxidants and radical scavenging metabolism in hyperaccumulator medicinal and aromatic plants. Ind. Crops Prod. 2021;173:114102. doi: 10.1016/j.indcrop.2021.114107. [DOI] [Google Scholar]
- 124.Chrysargyris A., Maggini R., Incrocci L., Pardossi A., Tzortzakis N. Cooper Tolerance and Acumulation on Pelargonium graveolens L’Hér. Grown in Hydroponic Culture. Plants. 2021;10:1663. doi: 10.3390/plants10081663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Latte K.P., Kolodziej H. Antioxidant Properties of Phenolic Compounds from Pelargonium reniforme. J. Agric. Food. Chem. 2004;52:4899–4902. doi: 10.1021/jf0495688. [DOI] [PubMed] [Google Scholar]
- 126.Krishnaiah D., Sarbatly R., Nithyanandam R. A review of the antioxidant potential of medicinal plant species. Food Bioprod. Process. 2011;89:217–233. doi: 10.1016/j.fbp.2010.04.008. [DOI] [Google Scholar]
- 127.Meyers M., Adams J., Amidon C., Barclay G., Barrow J., Belsinger S., Brobst J., Cole J., England K., Hill M., et al. Pelargonium, An Herb Society of America Guide. The Herb Society of America; Kirtland, OH, USA: 2006. p. 73. [Google Scholar]
- 128.Lalli J.Y.Y., Van-Zyl R.L., Van-Vuuren S.F., Viljoen A.M. In vitro biological activities of South African Pelargonium (Geraniaceae) species. S. Afr. J. Bot. 2008;74:153–157. doi: 10.1016/j.sajb.2007.08.011. [DOI] [Google Scholar]
- 129.Amiri R., Nikbakht A., Etemadi N. Alleviation of drought stress on rose geranium [Pelargonium graveolens (L.) Herit.] in terms of antioxidant activity and secondary metabolites by mycorrhizal inoculation. Sci. Hortic. 2015;197:373–380. doi: 10.1016/j.scienta.2015.09.062. [DOI] [Google Scholar]
- 130.Dimitrova M., Mihaylova D., Popova A., Alexieva J., Sapundzhierva T., Fidan H. Phenolic profile, antibacterial and antioxidant activity of Pelargonium graveolens leaves’ extracts. Sci. Bulletin. Ser. F. Biotechnol. 2015;XIX:130–135. doi: 10.13140/RG.2.1.2368.7128. [DOI] [Google Scholar]
- 131.El Aanachi S., Gali L., Nacer S.N., Bensouici C., Dari K., Aassila H. Phenolic contents and in vitro investigation of the antioxidant, enzyme inhibitory, photoprotective, and antimicrobial effects of the organic extracts of Pelargonium graveolens growing in Morocco. Biocatal. Agric. Biotechnol. 2020;29:101819. doi: 10.1016/j.bcab.2020.101819. [DOI] [Google Scholar]
- 132.Ennaifer M., Bouzaiene T., Chouaibi M., Hamdi M. Pelargonium graveolens Aqueous Decoction: A New Water-Soluble Polysaccharide and Antioxidant-Rich Extract. Biomed. Res. Int. 2018;2018:11. doi: 10.1155/2018/2691513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Fayoumi L., Khalil M., Ghareeb D., El-Dakdouki H.M. Chemical composition and therapeutic activity of lebanese rose geranium (Pelargonium hybrid) extracts. Farmacia. 2022;70:3. doi: 10.31925/farmacia.2022.3.13. [DOI] [Google Scholar]
- 134.Koutelidakis E.A., Serafini M., Komaitis M., Kapsokefalou M. Oxidative activity of some iron compounds on colon tissue homogenates from mice after administration of green tea, white tea and Pelargonium purpureum. Food Chem. 2010;120:895–901. doi: 10.1016/j.foodchem.2009.10.064. [DOI] [Google Scholar]
- 135.Petlevski R., Flajs D., Kalođera Z., Zovko- Končić M. Composition and antioxidant activity of aqueous and ethanolic Pelargonium radula extracts. S. Afr. J. Bot. 2013;85:17–22. doi: 10.1016/j.sajb.2012.11.009. [DOI] [Google Scholar]
- 136.Sithole N.T., Kulkarni M.G., Finnie J.F., Van-Staden J. Potential nematicidal properties of plant extracts against Meloidogyne incognita. S. Afr. J. Bot. 2021;139:409–417. doi: 10.1016/j.sajb.2021.02.014. [DOI] [Google Scholar]
- 137.Gâlea C., Hancu G. Antimicrobial and Antifungal Activity of Pelargonium roseum Essential Oil. Adv. Pharm. Bull. 2015;4:511–514. doi: 10.5681/apb.2014.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Mativandlela S.P.N., Lall N., Meyer J.J.M. Antibacterial, antifungal and antitubercular activity of (the roots of) Pelargonium reniforme (CURT) and Pelargonium sidoides (DC) (Geraniaceae) root extracts. S. Afr. J. Bot. 2006;72:232–237. doi: 10.1016/j.sajb.2005.08.002. [DOI] [Google Scholar]
- 139.Dumlupinar B., Celik D.D., Karatoprak G.Ș., Gürer U.S. Synergy between Pelargonium endlicherianum essential oil and conventional antibiotics against Neisseria meningitidis and Haemophilus influenzae. S. Afr. J. Bot. 2022;146:243–253. doi: 10.1016/j.sajb.2021.10.006. [DOI] [Google Scholar]
- 140.Abd El-Kareem S.M.M., Rabbih A.M., Elansary O.H., Al-Mana A.F. Mass Spectral Fragmentation of Pelargonium graveolens Essential Oil Using GC–MS Semi-Empirical Calculations and Biological Potential. Processes. 2020;8:128. doi: 10.3390/pr8020128. [DOI] [Google Scholar]
- 141.Dorman H.J.D., Deans S.G. Antimicrobial agents from plants: Antibacterial activity of plant volatile oils. J. Appl. Microbiol. 2000;88:308–316. doi: 10.1046/j.1365-2672.2000.00969.x. [DOI] [PubMed] [Google Scholar]
- 142.Wittschier N., Faller G., Hensel A. An extract of Pelargonium sidoides (EPs 7630) inhibits in situ adhesion of Helicobacter pylori to human stomach. Phytomedicine. 2006;14:285–288. doi: 10.1016/j.phymed.2006.12.008. [DOI] [PubMed] [Google Scholar]
- 143.Okeyo G.O., Charimbu M., Nyaanga J., Mendes T. Antibacterial activity of guava, moringa, camphor bush and Pelargonium extracts against bacterial wilt (Ralstonia pseudosolanacearum sp. nov.) of Potato. Saudi J. Biol. Sci. 2022;29:103438. doi: 10.1016/j.sjbs.2022.103438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Rosato A., Vitali C., Gallo D., Balenzano L., Mallamaci R. The inhibition of Candida species by selected essential oils and their synergism with amphotericin B. Phythomedicine. 2008;15:635–638. doi: 10.1016/j.phymed.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 145.Kerpel Dos Santos M., Kreutz T., Jacobi-Danielli L., Barreto De Marchi J.G., Pippi B., Scherer-Koester L., Fuentefria A.M., Pereira Limberger R. A chitosan hydrogel-thickened nanoemulsion containing Pelargonium graveolens essential oil for treatment of vaginal candidiasis. Pt AJ. Drug Deliv. Sci. Technol. 2020;56:101527. doi: 10.1016/j.jddst.2020.101527. [DOI] [Google Scholar]
- 146.Machalova Z., Sajfrtova M., Pavela R., Topiar M. Extraction of botanical pesticides from Pelargonium graveolens using supercritical carbon dioxide. Ind. Crops Prod. 2015;67:310–317. doi: 10.1016/j.indcrop.2015.01.070. [DOI] [Google Scholar]
- 147.Wynberg R. Biopiracy: Crying wolf or a lever for equity and conservation? Res. Policy. 2023;52:104674. doi: 10.1016/j.respol.2022.104674. [DOI] [Google Scholar]
- 148.Wopker P.M., Schwermer M., Sommer S., Längler A., Fetz K., Ostermann T., Zuzak T.J. Complementary and alternative medicine in the treatment of acute bronchitis in children: A systematic review. Complement. Ther. Med. 2020;49:102217. doi: 10.1016/j.ctim.2019.102217. [DOI] [PubMed] [Google Scholar]
- 149.Swanepoel K.M. Essential oil from Pelargonium sp. as alternative crops. S. Afr. J. Bot. 2008;74:379. doi: 10.1016/j.sajb.2008.01.110. [DOI] [Google Scholar]
- 150.Abdel Rahman N.A., Abdel-Rahman M.A., Mohammed H.H., Elseddawy M.N., Salem A.G., El-Ghareeb W.R. The ameliorative role of geranium (Pelargonium graveolens) essential oil against hepato-renal toxicity, immunosuppression, and oxidative stress of profenofos in common carp, Cyprinus carpio (L.) Aquaculture. 2020;517:734777. doi: 10.1016/j.aquaculture.2019.734777. [DOI] [Google Scholar]
- 151.Turan F., Mazlum Y., Yildirim Y.B., Gezer A. Use of Dietary Pelargonium sidoides Extract to Improve Growth and Body Composition of Narrow-Clawed Crayfish Astacus leptodactylus Eschscholtz, 1823 Juveniles. Turk. J. Fish. Aquat. Sci. 2012;12:233–238. [Google Scholar]
- 152.Naveenkumar R., Muthukumar A., Sangeetha G., Mohanapriya R. Developing eco-friendly biofungicide for the management of major seed borne diseases of rice and assessing their physical stability and storage life. Comptes Rendus Biol. 2017;340:214–225. doi: 10.1016/j.crvi.2017.03.001. [DOI] [PubMed] [Google Scholar]
- 153.Lozano-Navarro J., Palacio-Pérez A., Suárez-Domínguez E., Pérez-Sánchez J., Díaz-Zavala N., Melo-Banda J. Modification of the viscosity of extra-heavy crude oil using aqueous extracts of common geranium (Pelargonium hortorum) Pt AJ. Pet. Sci. Eng. 2022;215:110583. doi: 10.1016/j.petrol.2022.110583. [DOI] [Google Scholar]
- 154.Upadhyay R.K., Verma R.S., Singh V.R., Bahl J.R., Sharma S.K., Tewari S.K. New agrotechnology for quality planting material production of rose-scented geranium (Pelargonium graveolens L. Herit.) J. Appl. Res. Med. Aromat. Plants. 2016;3:128–130. doi: 10.1016/j.jarmap.2016.05.004. [DOI] [Google Scholar]
- 155.Loto R.T., Ikuerowo T., Ifezue S. Electrochemical action of Citrus reticulata and Pelargonium oil concentrates on 1018 carbon steel corrosion in anionic solution. J. Mater. Res. Technol. 2022;16:1305–1323. doi: 10.1016/j.jmrt.2021.12.067. [DOI] [Google Scholar]
- 156.Anheyer D., Cramer H., Lauche R., Saha F.J., Dobos G. Herbal Medicine in Children With Respiratory Tract Infection: Systematic Review and Meta-Analysis. Acad. Pediatr. 2018;18:8–19. doi: 10.1016/j.acap.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 157.Jeon J.H., Lee C.H., Lee H.S. Food Protective Effect of Geraniol and Its Congeners against Stored Food Mites. J. Food Prot. 2009;72:1468–1471. doi: 10.4315/0362-028X-72.7.1468. [DOI] [PubMed] [Google Scholar]
- 158.Moussa I., Ghezal I., Sakli F. Valorization of Pelargonium graveolens L’Hér. Hydrodistillation Solid Waste as Natural Dye for Wool Fabrics. J. Nat. Fibers. 2023;20:2156966. doi: 10.1080/15440478.2022.2156966. [DOI] [Google Scholar]
- 159.Maknea K.I., Asănica A., Fabian C., Peticilă A., Tzortzi J.N., Popescu D. The use of co-cultivation of aromatic, medicinal plants and vegetables in sustainable urban horticulture. AgroLife Sci. J. 2022;11:111–120. doi: 10.17930/AGL2022113. [DOI] [Google Scholar]
- 160.Mazeed A., Maurya P., Kumar D., Yadav S.S., Suryavanshi P. Efficient nutrient management for rose scented geranium (Pelargonium graveolens L′Herit ex Ait) J. Appl. Res. Med. Aromat. Plants. 2022;31:100409. doi: 10.1016/j.jarmap.2022.100409. [DOI] [Google Scholar]
- 161.Freitas Da Silva T., Estebanez Vollú R., Do Carmo Dias B., Rossetti Mateus De Lacerda K., Montezano Marques J., Martins Nishikawa M., De Vasconcelos Goulart F.R., Sales Alviano C., Seldin L. Cultivable bacterial communities associated with roots of rose-scented geranium (Pelargonium graveolens) with the potential to contribute to plant growth. Appl. Soil. Ecol. 2017;111:123–128. doi: 10.1016/j.apsoil.2016.12.002. [DOI] [Google Scholar]
- 162.Martins C.A.F., Campos M.L., Irioda A.C., Stremel D.P., Trindade A.C.L.B., Pontarolo R. Anti-inflammatory effect of Malva sylvestris, Sida cordifolia, and Pelargonium graveolens Is Related to Inhibition of Prostanoid Production. Molecules. 2017;22:1883. doi: 10.3390/molecules22111883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Li Y., Wang H., Zhang R., Zhang G., Yang Y., Liu Z. Biofabrication of polyphenols coated Nano palladium and its in-vitro cytotoxicity against human leukemia cell lines (K562) J. Photochem. Photobiol. B. 2017;175:173–177. doi: 10.1016/j.jphotobiol.2017.07.025. [DOI] [PubMed] [Google Scholar]
- 164.Pazinato R., Volpato A., Baldissera D.M., Santos C.V.R., Baretta D., Vaucher A.R., Giongo L.J., Boligon A.A., Stefani L.M., Schafer Da Silva A. In vitro effect of seven essential oils on the reproduction of the cattle tick Rhipicephalus microplus. J. Adv. Res. 2016;7:1029–1034. doi: 10.1016/j.jare.2016.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Gajewski A., Kosmider A., Nowacka A., Puk O., Wicinski M. Potential of herbal products in prevention and treatment of COVID-19. Literature review. Biomed. Pharmacother. 2021;143:112150. doi: 10.1016/j.biopha.2021.112150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data sharing not applicable.