Abstract
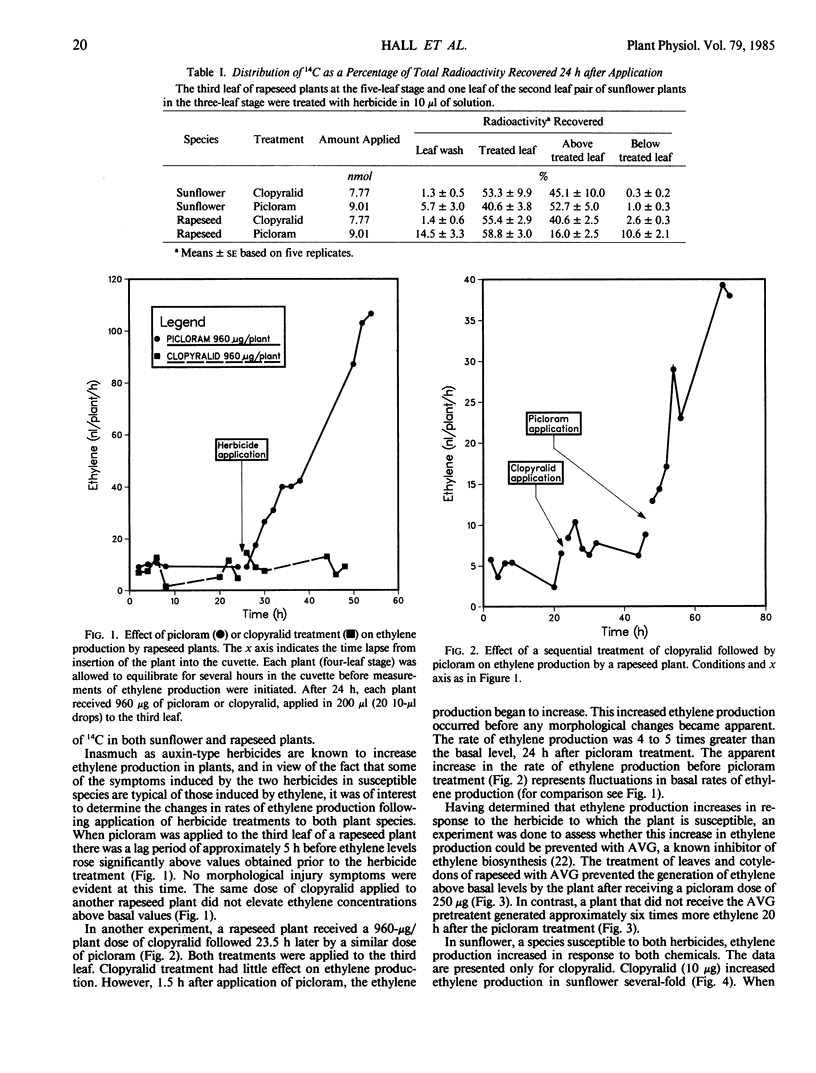
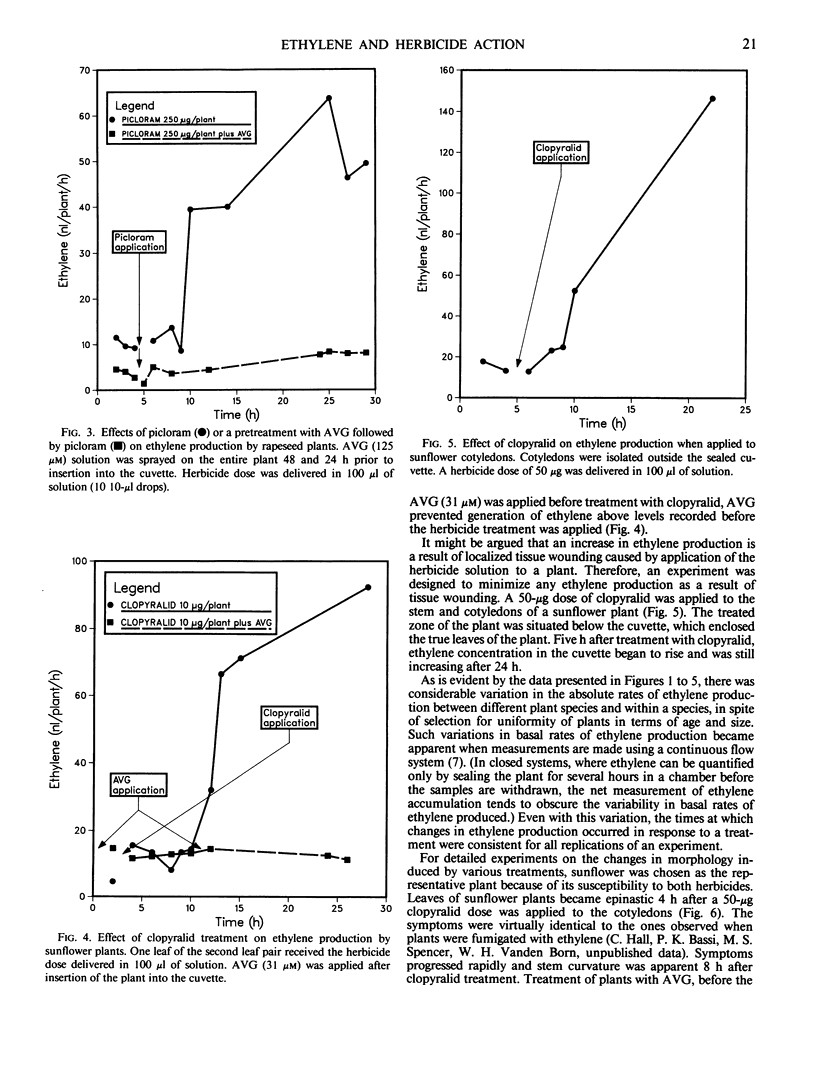
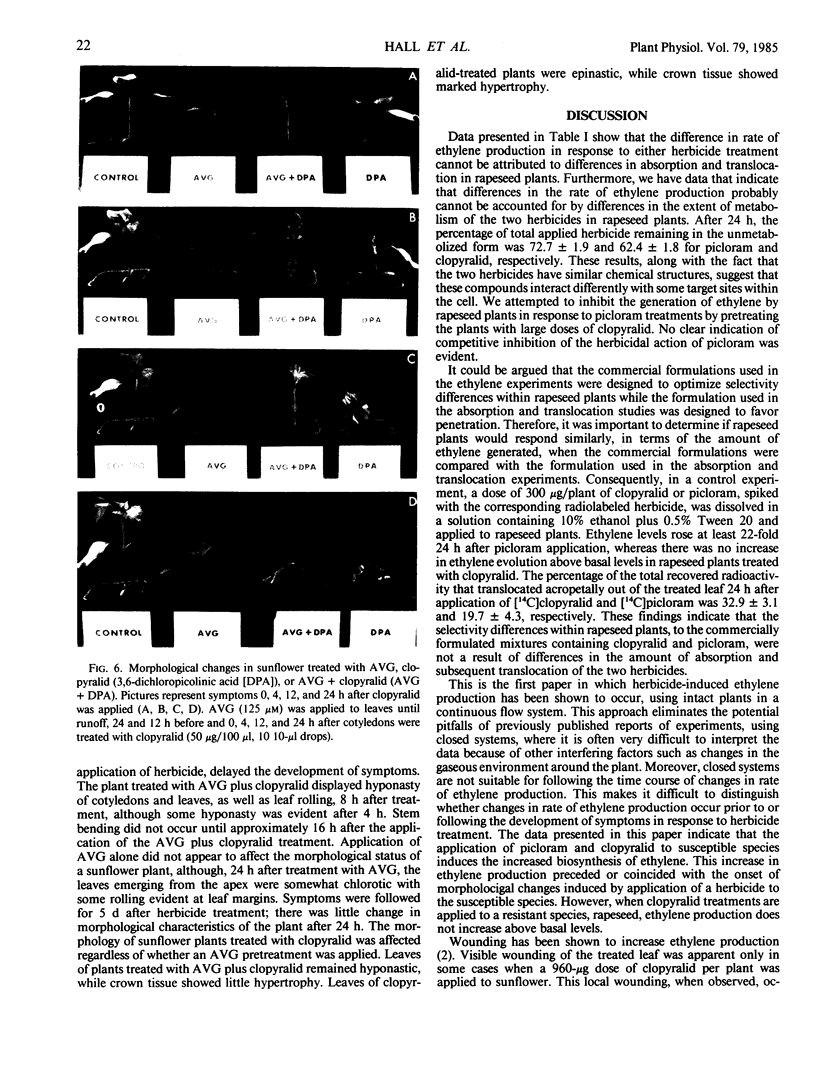
The role of ethylene in herbicidal injury induced by 4-amino-3,5,6-trichloropicolinic acid (picloram) or 3,6-dichloropicolinic acid (clopyralid) was investigated in sunflower (Helianthus annuus L.) and rapeseed (Brassica napus L. cv Altex). Picloram induces herbicide injury in both species, whereas clopyralid induces injury only in sunflower. Picloram applied to the third leaf of a rapeseed plant increased ethylene evolution several-fold. Clopyralid had no effect on ethylene production in rapeseed. In sunflower, both picloram and clopyralid elevated ethylene production. Ethylene biosynthesis induced by the herbicide treatment was not restricted to treated areas. When clopyralid was applied only to the lower stem and cotyledons of sunflower, the herbicide treatment resulted in an increase in the rate of ethylene production from the true leaves. Increased ethylene production preceded or coincided with the onset of morphological responses induced by a herbicide application to a susceptible species. The contrast in ethylene production by these two plant species cannot be accounted for by differences in absorption and translocation of clopyralid and picloram.
Treatment with aminoethoxyvinylglycine (AVG) before picloram or clopyralid application prevented an increase in ethylene production. Pretreatment with AVG also delayed the development of morphological changes induced by picloram or clopyralid. It appears that enhanced ethylene biosynthesis after application of picloram or clopyralid to the susceptible plant species was a factor involved in resulting morphological changes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Apelbaum A., Burg S. P. Effect of Ethylene on Cell Division and Deoxyribonucleic Acid Synthesis in Pisum sativum. Plant Physiol. 1972 Jul;50(1):117–124. doi: 10.1104/pp.50.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi P. K., Spencer M. S. A Cuvette Design for Measurement of Ethylene Production and Carbon Dioxide Exchange by Intact Shoots under Controlled Environmental Conditions. Plant Physiol. 1979 Sep;64(3):488–490. doi: 10.1104/pp.64.3.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassi P. K., Spencer M. S. Effect of carbon dioxide and light on ethylene production in intact sunflower plants. Plant Physiol. 1982 May;69(5):1222–1225. doi: 10.1104/pp.69.5.1222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur J. R., Morgan P. W. Effects of picloram and ethylene on leaf movement in huisache and mesquite seedlings. Plant Physiol. 1969 Jun;44(6):831–838. doi: 10.1104/pp.44.6.831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eastwell K. C., Bassi P. K., Spencer M. E. Comparison and evaluation methods for the removal of ethylene and other hydrocarbons from air for biological studies. Plant Physiol. 1978 Nov;62(5):723–726. doi: 10.1104/pp.62.5.723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee T. T., Dumas T. Effect of glyphosate on ethylene production in tobacco callus. Plant Physiol. 1983 Jul;72(3):855–857. doi: 10.1104/pp.72.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maxie E. C., Crane J. C. 2,4,5-trichlorophenoxyacetic Acid: effect on ethylene production by fruits and leaves of fig tree. Science. 1967 Mar 24;155(3769):1548–1550. doi: 10.1126/science.155.3769.1548. [DOI] [PubMed] [Google Scholar]
- Morgan P. W., Baur J. R. Involvement of Ethylene in Picloram-induced Leaf Movement Response. Plant Physiol. 1970 Nov;46(5):655–659. doi: 10.1104/pp.46.5.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stacewicz-Sapuncakis M., Marsh H. V., Vengris J., Jennings P. H., Robinson T. Participation of ethylene in common purslane response to dicamba. Plant Physiol. 1973 Nov;52(5):466–471. doi: 10.1104/pp.52.5.466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Y. B., Yang S. F. Auxin-induced Ethylene Production and Its Inhibition by Aminoethyoxyvinylglycine and Cobalt Ion. Plant Physiol. 1979 Dec;64(6):1074–1077. doi: 10.1104/pp.64.6.1074. [DOI] [PMC free article] [PubMed] [Google Scholar]



