Abstract
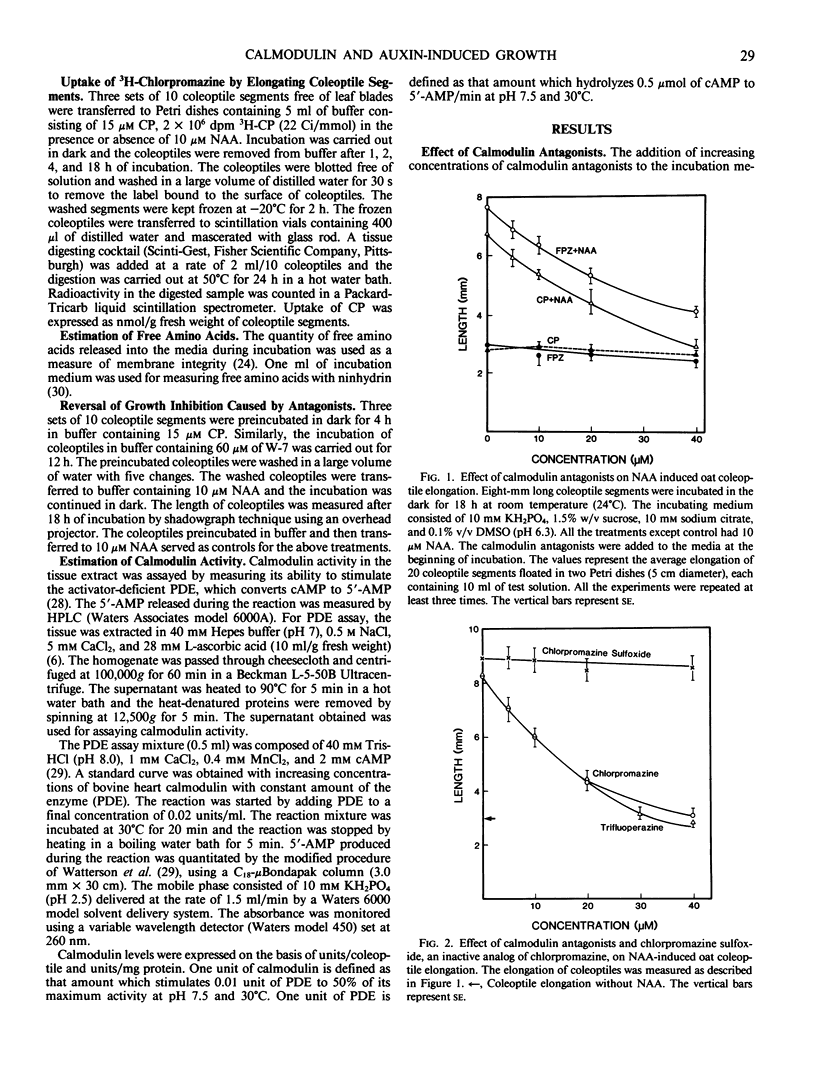
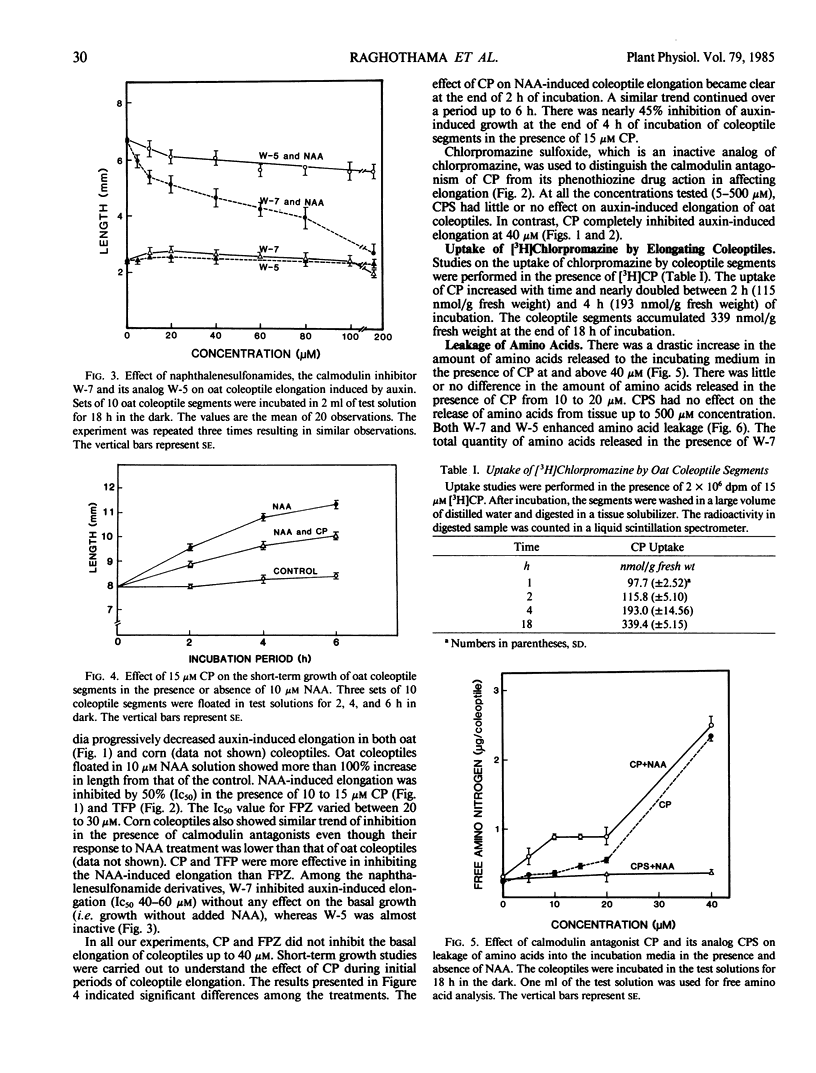
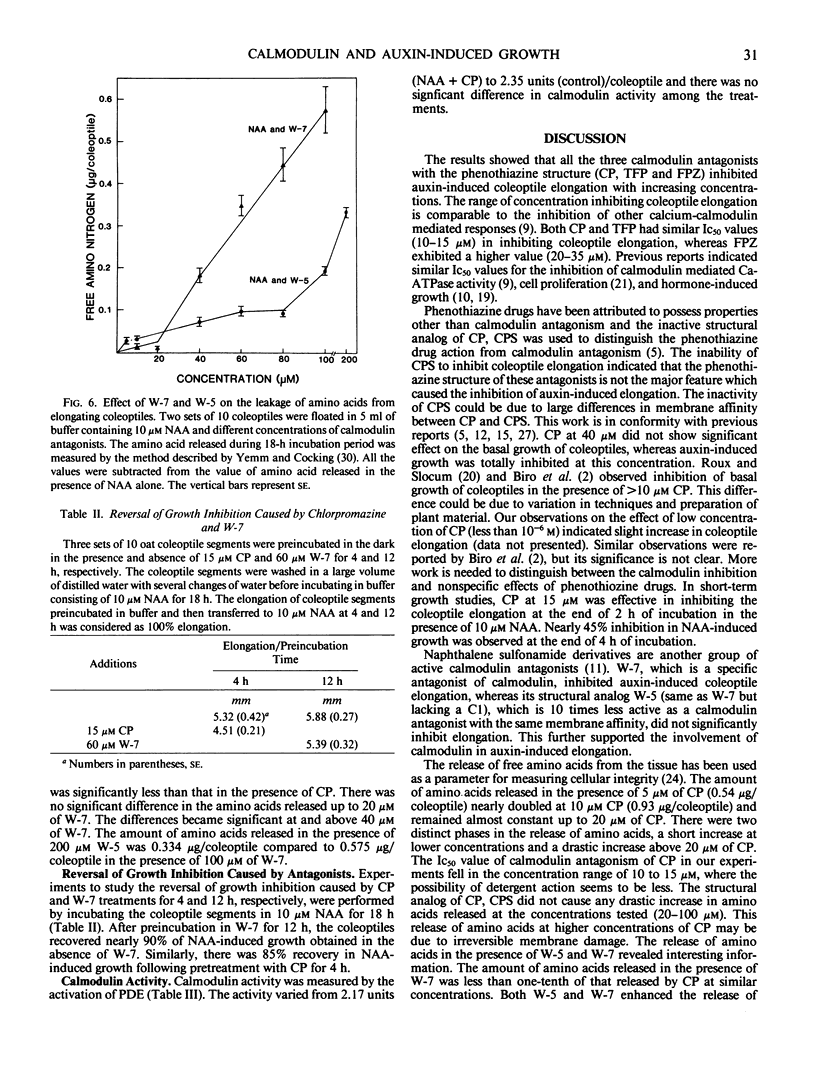
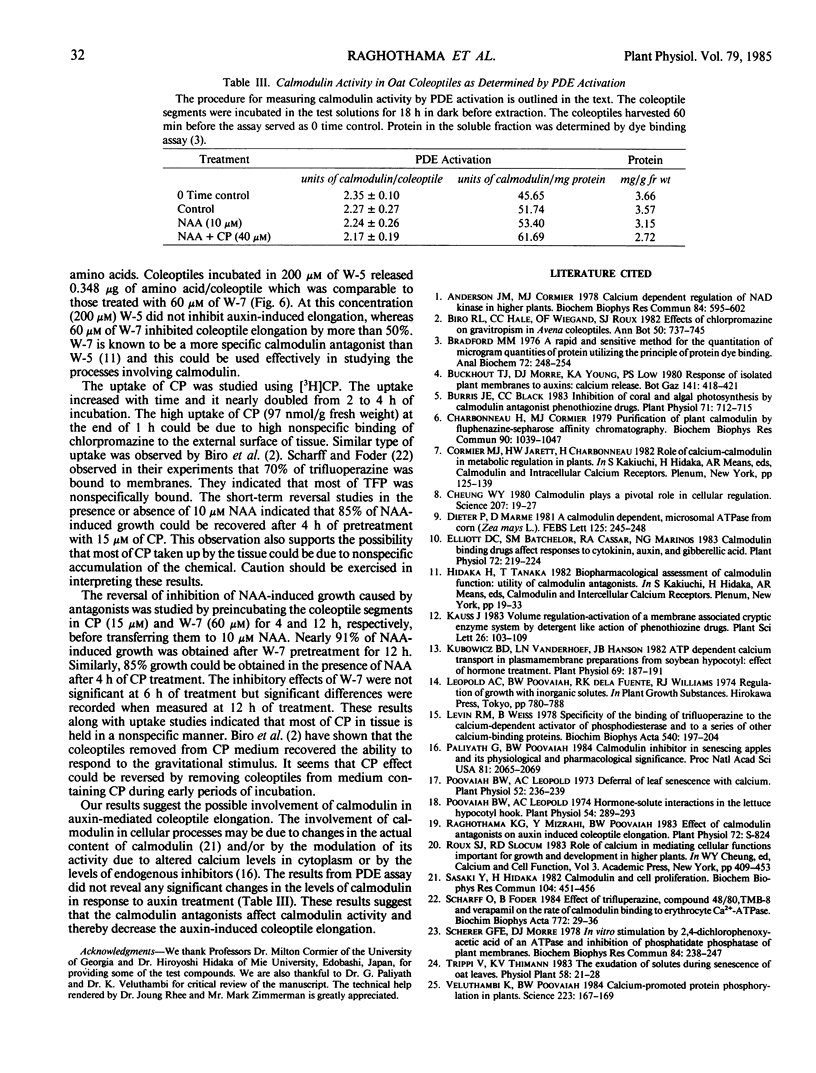
Coleoptile segments of oat (Avena sativa var Cayuse) and corn (Zea mays L. var Patriot) were incubated in different concentrations of calmodulin antagonists in the presence and absence of α-naphthaleneacetic acid. The calmodulin antgonists (chlorpromazine (CP), trifluoperazine, and fluphenazine) inhibited the auxin-induced elongation at 5 to 50 micromolar concentrations. Chlorpromazine sulfoxide, an analog of chlorpromazine, did not have significant effect on the elongation of oat and corn coleoptiles. A specific inhibitor of calmodulin N-(6-aminohexyl)5-chloro-1-naphthalenesulfonamide hydrochloride (W-7, a naphthalenesulfonamide derivative) inhibited coleoptile elongation, while its inactive analog N-(6-aminohexyl)-1-naphthalenesulfonamide hydrochloride (W-5) was ineffective at similar concentrations. During a 4-hour incubation period, coleoptile segments accumulated significant quantities of 3H-CP. About 85 to 90% of auxin-induced growth was recovered after 4 hours of preincubation with CP or 12 hours with W-7 and transferring coleoptiles to buffer containing NAA. Leakage of amino acids from coleoptiles increased with increasing concentration of CP, showing a rapid and significant increase above 20 micromolar CP. The amount of amino acids released in the presence of W-7 and W-5 was significantly lower than the amount released in the presence of CP. Both W-5 and W-7 increased amino acid release but only W-7 inhibited auxin-induced growth. Calmodulin activity measured by phosphodiesterase activation did not differ significantly between auxin-treated and control coleoptile segments. These results suggest the possible involvement of calmodulin in auxin-induced coleoptile elongation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Cormier M. J. Calcium-dependent regulation of NAD kinase. Biochem Biophys Res Commun. 1978 Oct 16;84(3):595–602. doi: 10.1016/0006-291x(78)90747-7. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burris J. E., Black C. C. Inhibition of coral and algal photosynthesis by ca-antagonist phenothiazine drugs. Plant Physiol. 1983 Apr;71(4):712–715. doi: 10.1104/pp.71.4.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Cormier M. J. Purification of plant calmodulin by fluphenazine-Sepharose affinity chromatography. Biochem Biophys Res Commun. 1979 Oct 12;90(3):1039–1047. doi: 10.1016/0006-291x(79)91931-4. [DOI] [PubMed] [Google Scholar]
- Cheung W. Y. Calmodulin plays a pivotal role in cellular regulation. Science. 1980 Jan 4;207(4426):19–27. doi: 10.1126/science.6243188. [DOI] [PubMed] [Google Scholar]
- Elliott D. C., Batchelor S. M., Cassar R. A., Marinos N. G. Calmodulin-binding drugs affect responses to cytokinin, auxin, and gibberellic Acid. Plant Physiol. 1983 May;72(1):219–224. doi: 10.1104/pp.72.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubowicz B. D., Vanderhoef L. N., Hanson J. B. ATP-Dependent Calcium Transport in Plasmalemma Preparations from Soybean Hypocotyls : EFFECT OF HORMONE TREATMENTS. Plant Physiol. 1982 Jan;69(1):187–191. doi: 10.1104/pp.69.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin R. M., Weiss B. Specificity of the binding of trifluoperazine to the calcium-dependent activator of phosphodiesterase and to a series of other calcium-binding proteins. Biochim Biophys Acta. 1978 May 3;540(2):197–204. doi: 10.1016/0304-4165(78)90132-0. [DOI] [PubMed] [Google Scholar]
- Paliyath G., Poovaiah B. W. Calmodulin inhibitor in senescing apples and its physiological and pharmacological significance. Proc Natl Acad Sci U S A. 1984 Apr;81(7):2065–2069. doi: 10.1073/pnas.81.7.2065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Deferral of leaf senescence with calcium. Plant Physiol. 1973 Sep;52(3):236–239. doi: 10.1104/pp.52.3.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poovaiah B. W., Leopold A. C. Hormone-solute interactions in the lettuce hypocotyl hook. Plant Physiol. 1974 Sep;54(3):289–293. doi: 10.1104/pp.54.3.289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki Y., Hidaka H. Calmodulin and cell proliferation. Biochem Biophys Res Commun. 1982 Jan 29;104(2):451–456. doi: 10.1016/0006-291x(82)90658-1. [DOI] [PubMed] [Google Scholar]
- Scharff O., Foder B. Effect of trifluoperazine, compound 48/80, TMB-8 and verapamil on the rate of calmodulin binding to erythrocyte Ca2+-ATPase. Biochim Biophys Acta. 1984 Apr 25;772(1):29–36. doi: 10.1016/0005-2736(84)90514-5. [DOI] [PubMed] [Google Scholar]
- Scherer G. F., Morré D. J. In vitro stimulation by 2,4-dichlorophenoxyacetic acid of an ATPase and inhibition of phosphatidate phosphatase of plant membranes. Biochem Biophys Res Commun. 1978 Sep 14;84(1):238–247. doi: 10.1016/0006-291x(78)90288-7. [DOI] [PubMed] [Google Scholar]
- Veluthambi K., Poovaiah B. W. Calcium-promoted protein phosphorylation in plants. Science. 1984 Jan 13;223(4632):167–169. doi: 10.1126/science.223.4632.167. [DOI] [PubMed] [Google Scholar]
- Watterson D. M., Iverson D. B., Van Eldik L. J. Rapid separation and quantitation of 3',5'-cyclic nucleotides and 5'-nucleotides in phosphodiesterase reaction mixtures using high-performance liquid chromatography. J Biochem Biophys Methods. 1980 Mar;2(3):139–146. doi: 10.1016/0165-022x(80)90021-4. [DOI] [PubMed] [Google Scholar]


