Abstract
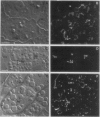
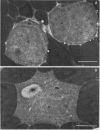
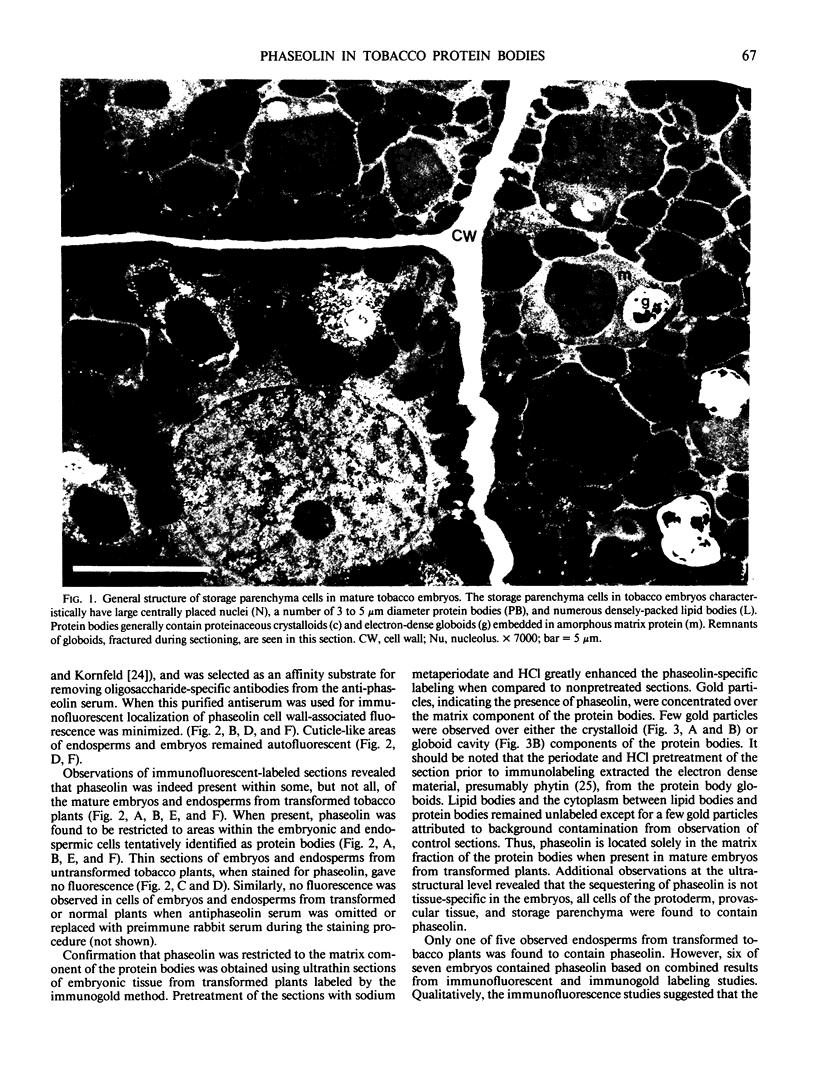
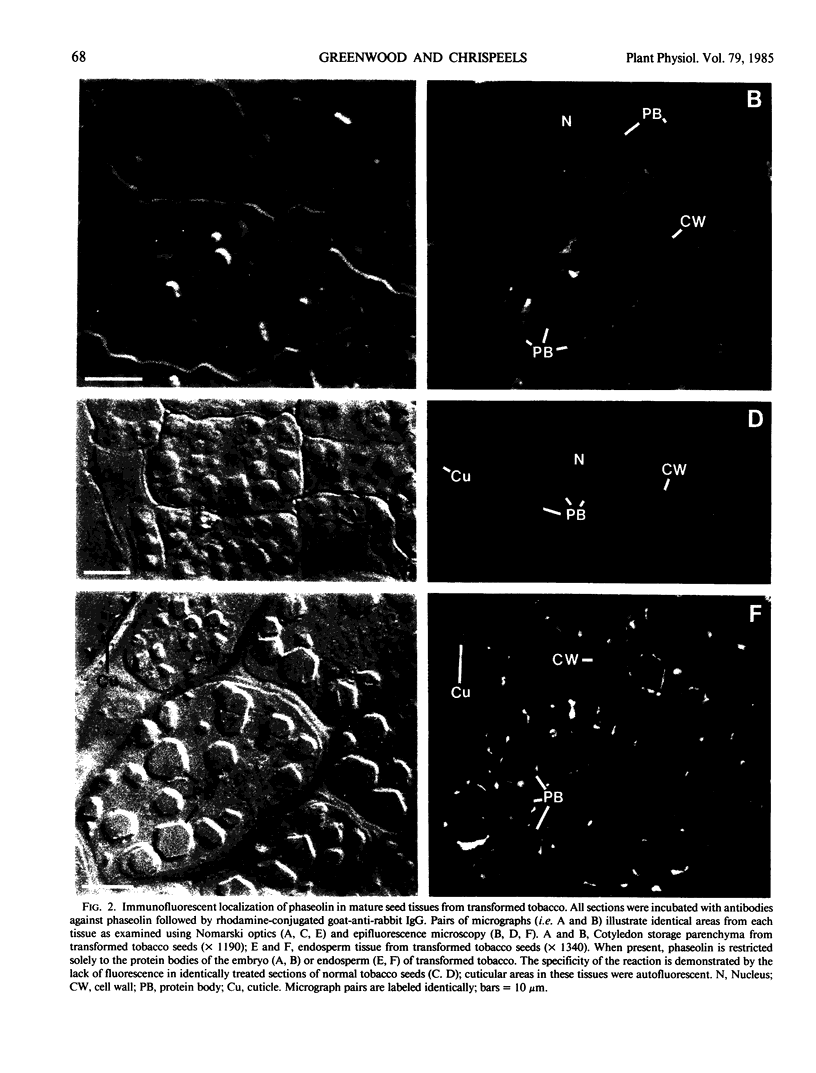
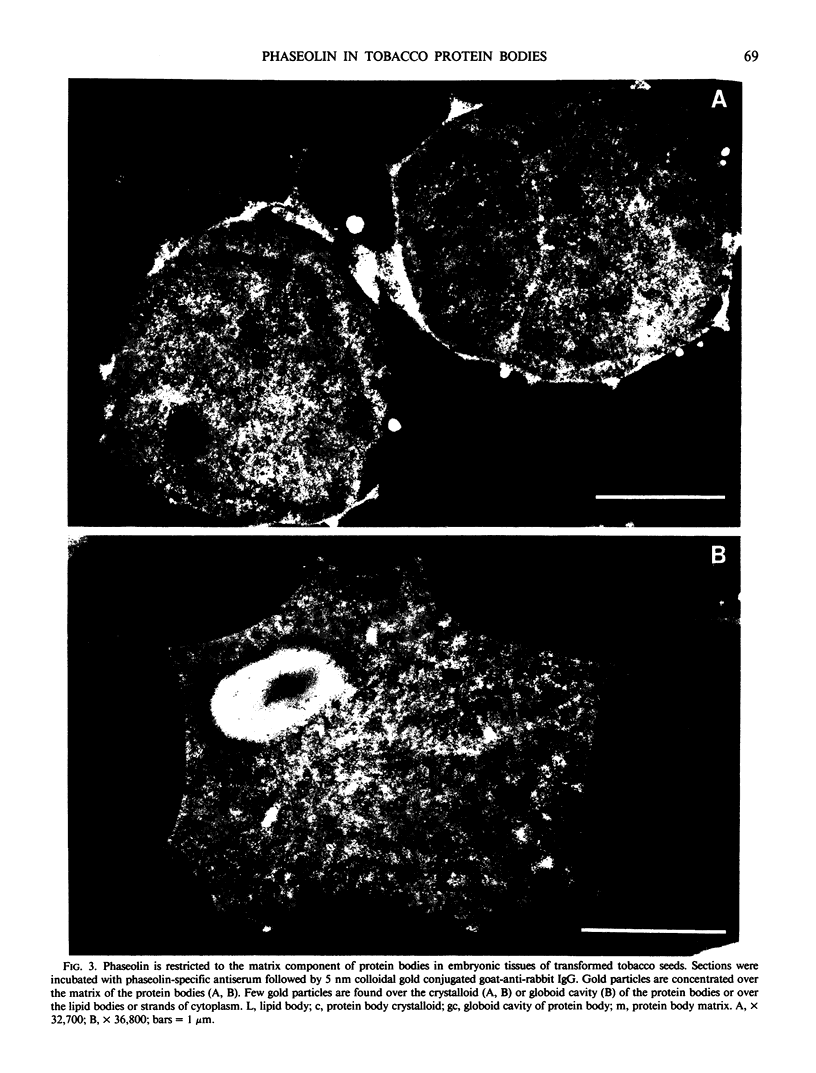
The storage protein phaseolin accumulates during seed development in protein bodies in cotyledons of the common bean Phaseolus vulgaris. Hall et al. (In L Van Vloten-Doting, TC Hall, eds, Molecular Form and Function of the Plant Genome, 1985 Plenum Press, In press) recently reported the expression of a gene coding for phaseolin and the accumulation of phaseolin protein in developing seeds of tobacco plants regenerated from transformed callus cells. The protein did not accumulate in other organs of the plants. Mature seeds from normal and transformed tobacco plants were obtained and the subcellular distribution of phaseolin in the seeds was examined using both light and electron microscopic immunocytochemical methods. Phaseolin was found in six of seven transformed tobacco embryos examined, but was present in only one endosperm of five. When present, phaseolin was located exclusively in the protein bodies of the embryonic and endospermic cells. Furthermore, phaseolin was restricted solely to the amorphous matrix of the protein bodies and was excluded from the globoid and proteinaceous crystalloid components of these organelles. The subcellular location of phaseolin in seeds from transformed tobacco plants is similar to that seen in mature seeds of the common bean indicating that in the transformed cells the protein is targeted to the right subcellular compartment.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baumgartner B., Tokuyasu K. T., Chrispeels M. J. Localization of vicilin peptidohydrolase in the cotyledons of mung bean seedlings by immunofluorescence microscopy. J Cell Biol. 1978 Oct;79(1):10–19. doi: 10.1083/jcb.79.1.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendayan M., Zollinger M. Ultrastructural localization of antigenic sites on osmium-fixed tissues applying the protein A-gold technique. J Histochem Cytochem. 1983 Jan;31(1):101–109. doi: 10.1177/31.1.6187796. [DOI] [PubMed] [Google Scholar]
- Bollini R., Vitale A., Chrispeels M. J. In vivo and in vitro processing of seed reserve protein in the endoplasmic reticulum: evidence for two glycosylation steps. J Cell Biol. 1983 Apr;96(4):999–1007. doi: 10.1083/jcb.96.4.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandtzaeg P. Conjugates of immunoglobulin G with different fluorochromes. I. Characterization by anionic-exchange chromatography. Scand J Immunol. 1973;2(3):273–290. doi: 10.1111/j.1365-3083.1973.tb02037.x. [DOI] [PubMed] [Google Scholar]
- Clarke A. E., Knox R. B., Jermyn M. A. Localization of lectins in legume cotyledons. J Cell Sci. 1975 Oct;19(1):157–167. doi: 10.1242/jcs.19.1.157. [DOI] [PubMed] [Google Scholar]
- Craig S., Goodchild D. J., Millerd A. Immunofluorescent localization of pea storage proteins in glycol methacrylate embedded tissue. J Histochem Cytochem. 1979 Oct;27(10):1312–1316. doi: 10.1177/27.10.390032. [DOI] [PubMed] [Google Scholar]
- Craig S., Goodchild D. J. Post-embedding immunolabelling. Some effects of tissue preparation on the antigenicity of plant proteins. Eur J Cell Biol. 1982 Oct;28(2):251–256. [PubMed] [Google Scholar]
- Craig S., Miller C. LR white resin and improved on-grid immunogold detection of vicilin, a pea seed storage protein. Cell Biol Int Rep. 1984 Oct;8(10):879–886. doi: 10.1016/0309-1651(84)90072-9. [DOI] [PubMed] [Google Scholar]
- Davies H. M., Delmer D. P. Two Kinds of Protein Glycosylation in a Cell-Free Preparation from Developing Cotyledons of Phaseolus vulgaris. Plant Physiol. 1981 Aug;68(2):284–291. doi: 10.1104/pp.68.2.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Mey J., Moeremans M., Geuens G., Nuydens R., De Brabander M. High resolution light and electron microscopic localization of tubulin with the IGS (immuno gold staining) method. Cell Biol Int Rep. 1981 Sep;5(9):889–899. doi: 10.1016/0309-1651(81)90204-6. [DOI] [PubMed] [Google Scholar]
- Keller G. A., Tokuyasu K. T., Dutton A. H., Singer S. J. An improved procedure for immunoelectron microscopy: ultrathin plastic embedding of immunolabeled ultrathin frozen sections. Proc Natl Acad Sci U S A. 1984 Sep;81(18):5744–5747. doi: 10.1073/pnas.81.18.5744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray D. R., Knox R. B. Immunofluorescent localization of urease in the cotyledons of jack bean, Canavalia ensiformis. J Cell Sci. 1977 Aug;26:9–18. doi: 10.1242/jcs.26.1.9. [DOI] [PubMed] [Google Scholar]
- Sengupta-Gopalan C., Reichert N. A., Barker R. F., Hall T. C., Kemp J. D. Developmentally regulated expression of the bean beta-phaseolin gene in tobacco seed. Proc Natl Acad Sci U S A. 1985 May;82(10):3320–3324. doi: 10.1073/pnas.82.10.3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tokuyasu K. T., Singer S. J. Improved procedures for immunoferritin labeling of ultrathin frozen sections. J Cell Biol. 1976 Dec;71(3):894–906. doi: 10.1083/jcb.71.3.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- zur Nieden U., Manteuffel R., Weber E., Neumann D. Dictyosomes participate in the intracellular pathway of storage proteins in developing Vicia faba cotyledons. Eur J Cell Biol. 1984 May;34(1):9–17. [PubMed] [Google Scholar]
- zur Nieden U., Neumann D., Manteuffel R., Weber E. Electron microscopic immunocytochemical localization of storage proteins in Vicia faba seeds. Eur J Cell Biol. 1982 Feb;26(2):228–233. [PubMed] [Google Scholar]