Abstract
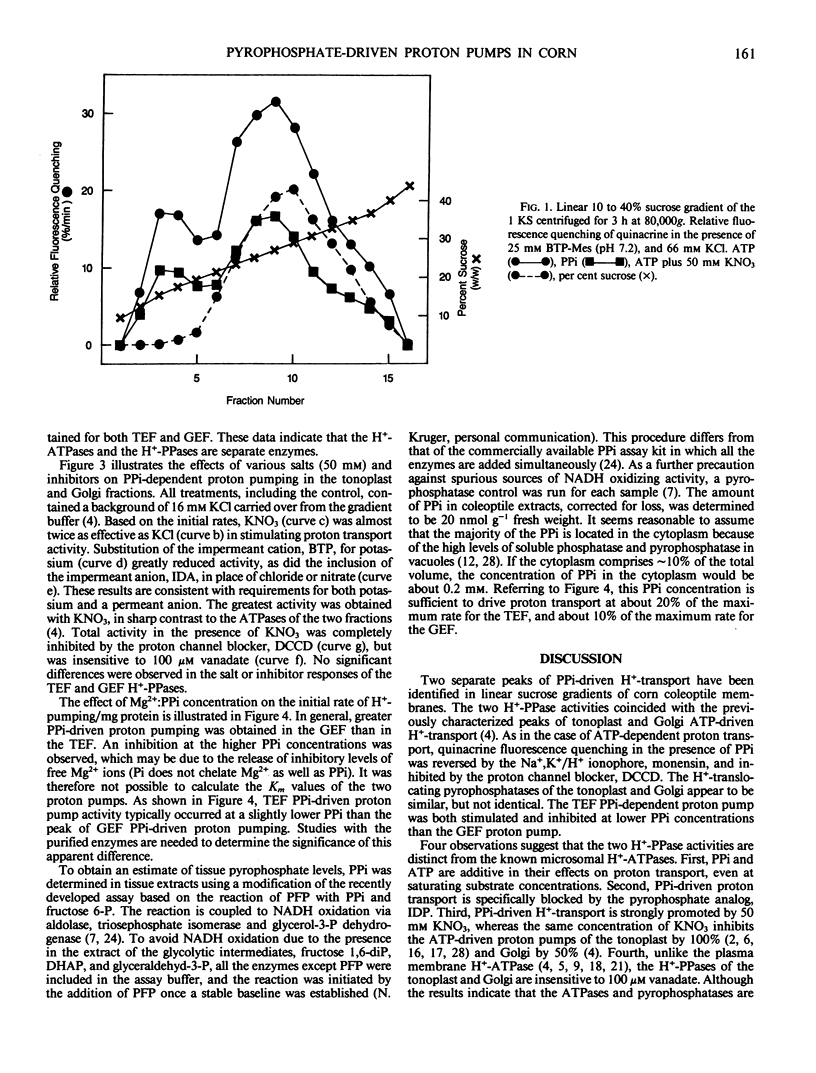
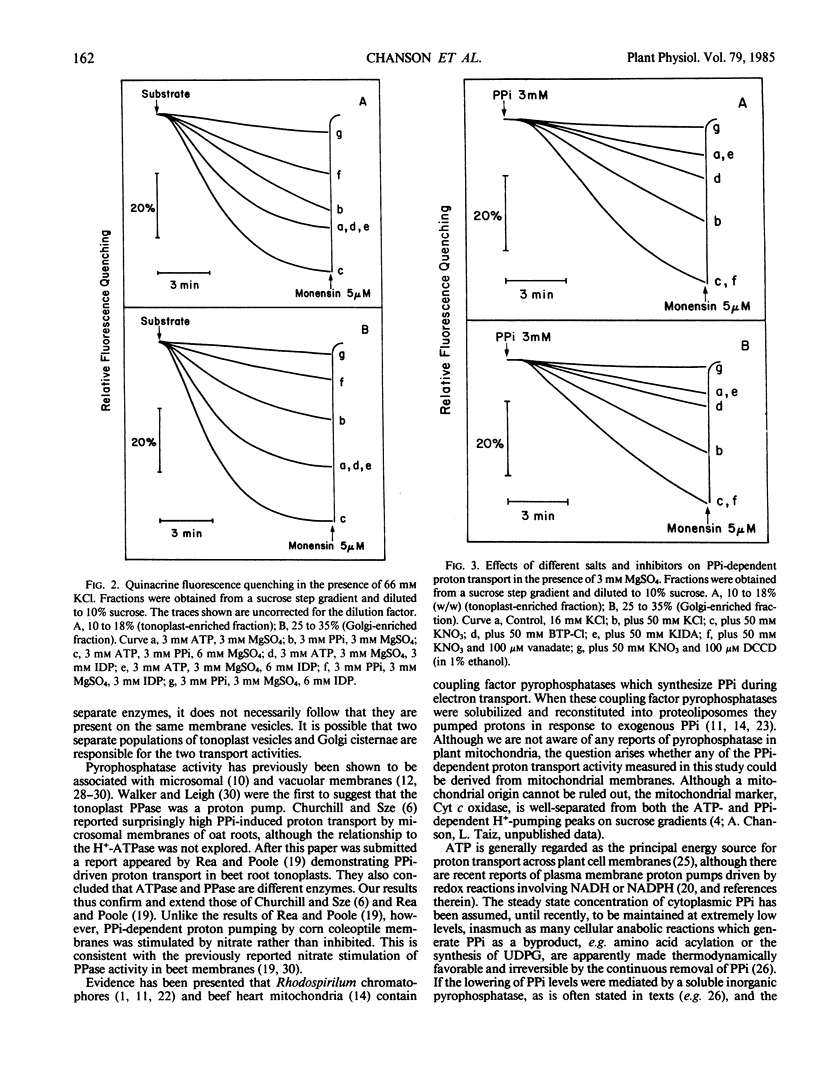
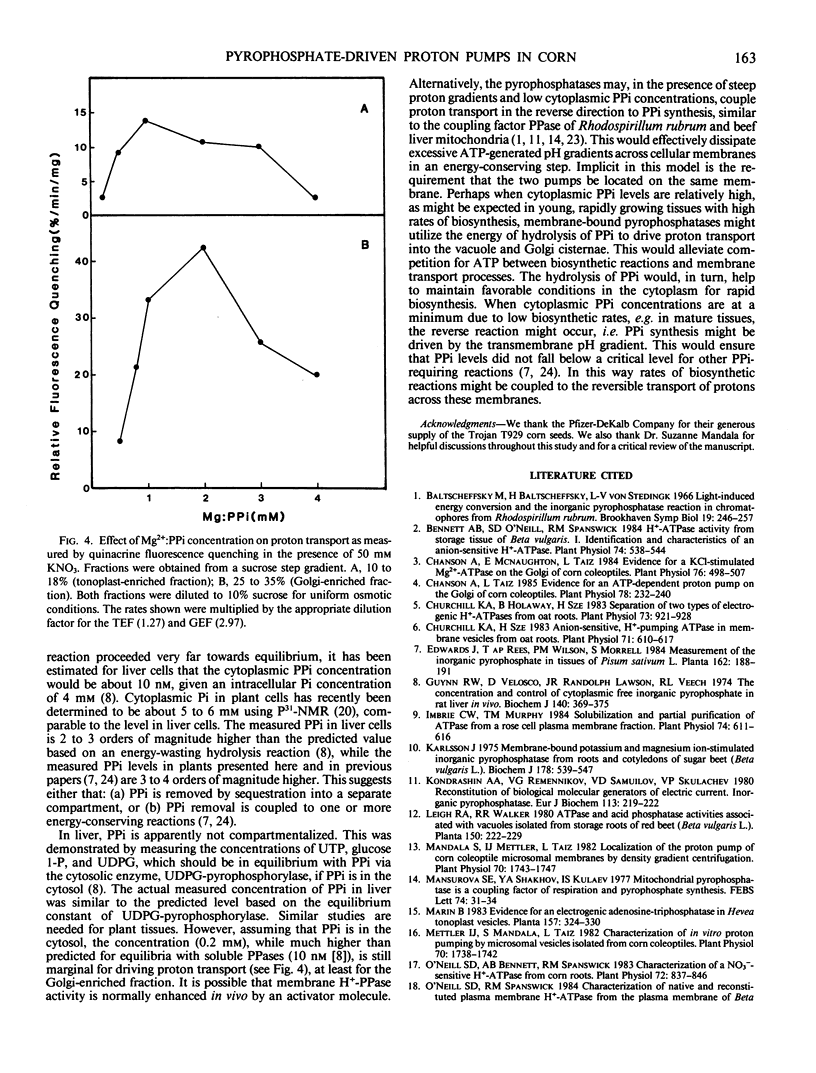
Corn (Zea mays L. cv Trojan T929) coleoptile membranes were fractionated on isopycnic sucrose density gradients. Two peaks of ATP-driven H+-transport activity, corresponding to the previously characterized tonoplast (1.07 grams per cubic centimeter) and Golgi (1.13 grams per cubic centimeter) fractions (Chanson and Taiz, Plant Physiol 1985 78: 232-240) were localized. Coincident with these were two peaks of inorganic pyrophosphate (PPi)-driven H+-transport. At saturating (3 millimolar) concentrations of Mg2+:ATP, the rate of proton transport was further enhanced by the addition of 3 millimolar PPi, and the stimulation was additive, i.e. equal to the sum of the two added separately. The specific PPi analog, imidodiphosphate, antagonized PPi-driven H+-transport, but had no effect on ATP-driven transport. Moreover, PPi-dependent proton transport in both tonoplast-enriched and Golgi-enriched fractions was strongly promoted by 50 millimolar KNO3, unlike the ATP-dependent H+-pumps of the same membranes. Taken together, the results indicate that PPi-driven proton transport is mediated by specific membrane-bound H+-translocating pyrophosphatases. Both potassium and a permanent anion (NO3− > Cl−), were required for maximum activity. The PPi-driven proton pumps were totally inhibited by N,N′-dicyclohexylcarbodiimide, but were insensitive to 100 millimolar vanadate. The PPi concentration in coleoptile extracts was determined using an NADH oxidation assay system coupled to purified pyrophosphate:fructose 6-phosphate 1-phosphotransferase (EC 2.7.1.90). The total pyrophosphate content of corn coleoptiles was 20 nanomoles/gram fresh weight. Assuming a cytoplasmic location, the calculated PPi concentration is sufficient to drive proton transport at 20% of the maximum rate measured in vitro for the tonoplast-enriched fraction, and 10% of the maximum rate for the Golgi-enriched fraction.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baltscheffsky M., Baltscheffsky H., von Stedingk L. V. Light-induced energy conversion and the inorganic pyrophosphatase reaction in chromatophores from Rhodospirillum rubrum . Brookhaven Symp Biol. 1966;19:246–257. [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson A., McNaughton E., Taiz L. Evidence for a KCl-Stimulated, Mg-ATPase on the Golgi of Corn Coleoptiles. Plant Physiol. 1984 Oct;76(2):498–507. doi: 10.1104/pp.76.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanson A., Taiz L. Evidence for an ATP-Dependent Proton Pump on the Golgi of Corn Coleoptiles. Plant Physiol. 1985 Jun;78(2):232–240. doi: 10.1104/pp.78.2.232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Holaway B., Sze H. Separation of two types of electrogenic h-pumping ATPases from oat roots. Plant Physiol. 1983 Dec;73(4):921–928. doi: 10.1104/pp.73.4.921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill K. A., Sze H. Anion-sensitive, h-pumping ATPase in membrane vesicles from oat roots. Plant Physiol. 1983 Mar;71(3):610–617. doi: 10.1104/pp.71.3.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guynn R. W., Veloso D., Lawson J. W., Veech R. L. The concentration and control of cytoplasmic free inorganic pyrophosphate in rat liver in vivo. Biochem J. 1974 Jun;140(3):369–375. doi: 10.1042/bj1400369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbrie C. W., Murphy T. M. Solubilization and partial purification of ATPase from a rose cell plasma membrane fraction. Plant Physiol. 1984 Mar;74(3):611–616. doi: 10.1104/pp.74.3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondrashin A. A., Remennikov V. G., Samuilov V. D., Skulachev V. P. Reconstitution of biological molecular generators of electric current. Inorganic pyrophosphatase. Eur J Biochem. 1980 Dec;113(1):219–222. doi: 10.1111/j.1432-1033.1980.tb06159.x. [DOI] [PubMed] [Google Scholar]
- Mandala S., Mettler I. J., Taiz L. Localization of the proton pump of corn coleoptile microsomal membranes by density gradient centrifugation. Plant Physiol. 1982 Dec;70(6):1743–1747. doi: 10.1104/pp.70.6.1743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansurova S. E., Shakhov Y. A., Kulaev I. S. Mitochondrial pyrophosphatase is a coupling factor of respiration and pyrophosphate synthesis. FEBS Lett. 1977 Feb 15;74(1):31–34. doi: 10.1016/0014-5793(77)80745-x. [DOI] [PubMed] [Google Scholar]
- Mettler I. J., Mandala S., Taiz L. Characterization of in vitro proton pumping by microsomal vesicles isolated from corn coleoptiles. Plant Physiol. 1982 Dec;70(6):1738–1742. doi: 10.1104/pp.70.6.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'neill S. D., Bennett A. B., Spanswick R. M. Characterization of a NO(3)-Sensitive H-ATPase from Corn Roots. Plant Physiol. 1983 Jul;72(3):837–846. doi: 10.1104/pp.72.3.837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea P. A., Poole R. J. Proton-Translocating Inorganic Pyrophosphatase in Red Beet (Beta vulgaris L.) Tonoplast Vesicles. Plant Physiol. 1985 Jan;77(1):46–52. doi: 10.1104/pp.77.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rebeille F., Bligny R., Douce R. Is the cytosolic pi concentration a limiting factor for plant cell respiration? Plant Physiol. 1984 Feb;74(2):355–359. doi: 10.1104/pp.74.2.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B., Stern A. I., Stout R. G. Redox activity at the surface of oat root cells. Plant Physiol. 1984 Oct;76(2):386–391. doi: 10.1104/pp.76.2.386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shakhov Y. A., Nyrén P., Baltscheffsky M. Reconstitution of highly purified proton-translocating pyrophosphatase from Rhodospirillum rubrum. FEBS Lett. 1982 Sep 6;146(1):177–180. doi: 10.1016/0014-5793(82)80730-8. [DOI] [PubMed] [Google Scholar]
- Smyth D. A., Black C. C. Measurement of the pyrophosphate content of plant tissues. Plant Physiol. 1984 Jul;75(3):862–864. doi: 10.1104/pp.75.3.862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vara F., Serrano R. Partial purification and properties of the proton-translocating ATPase of plant plasma membranes. J Biol Chem. 1982 Nov 10;257(21):12826–12830. [PubMed] [Google Scholar]


