Abstract
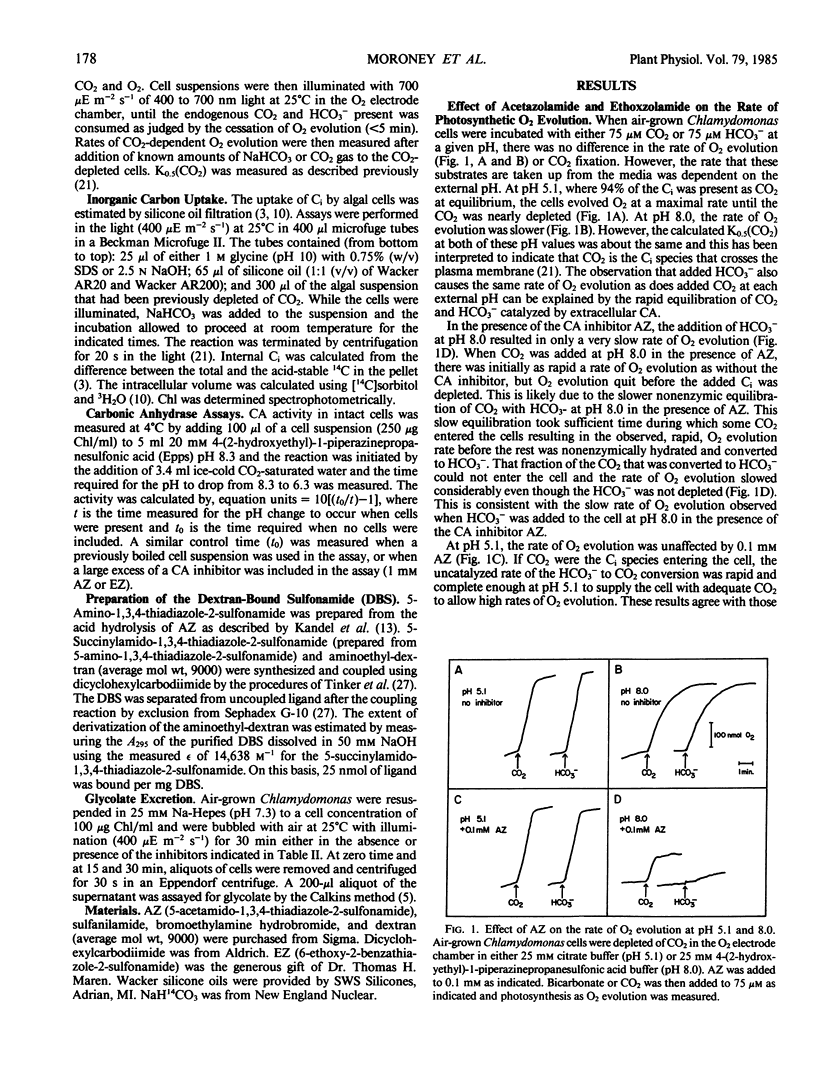
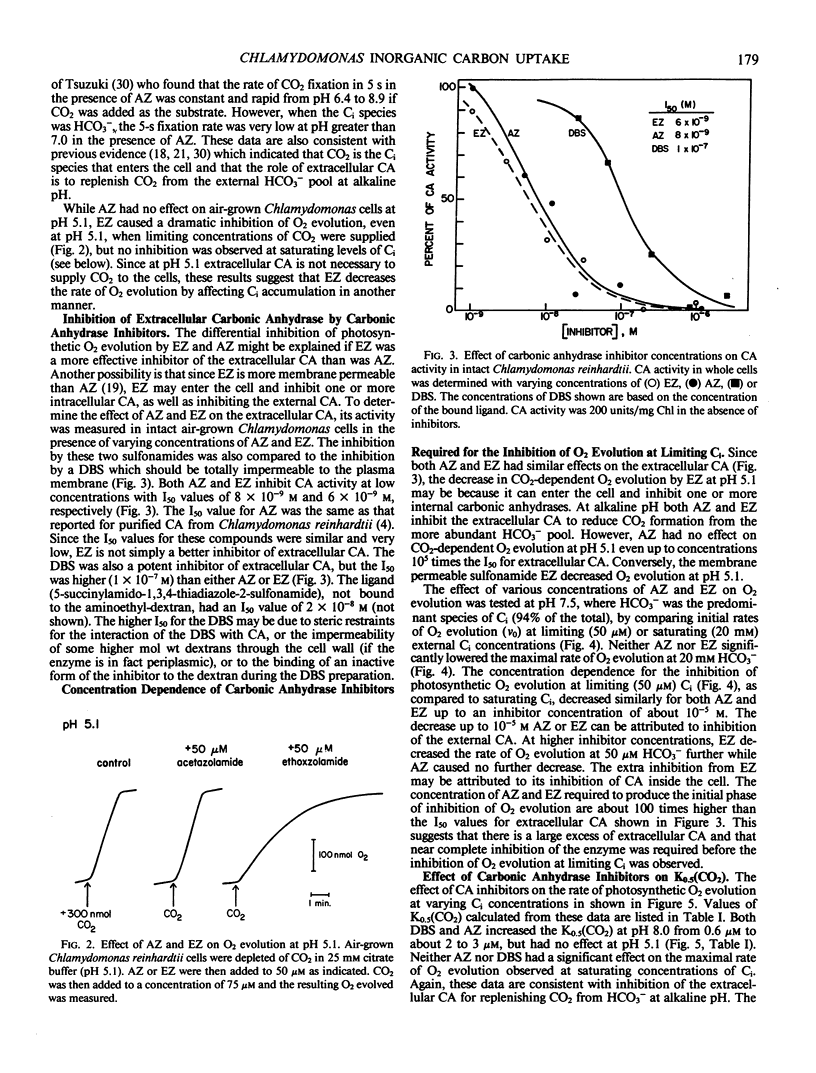
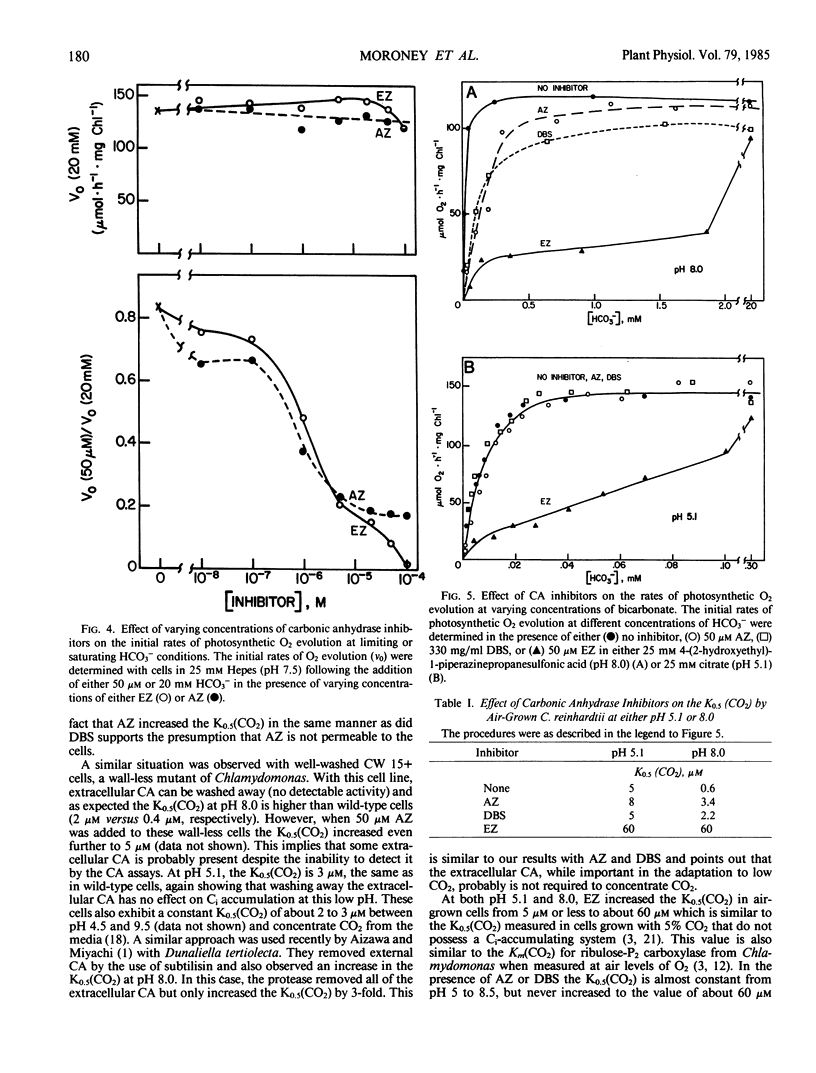
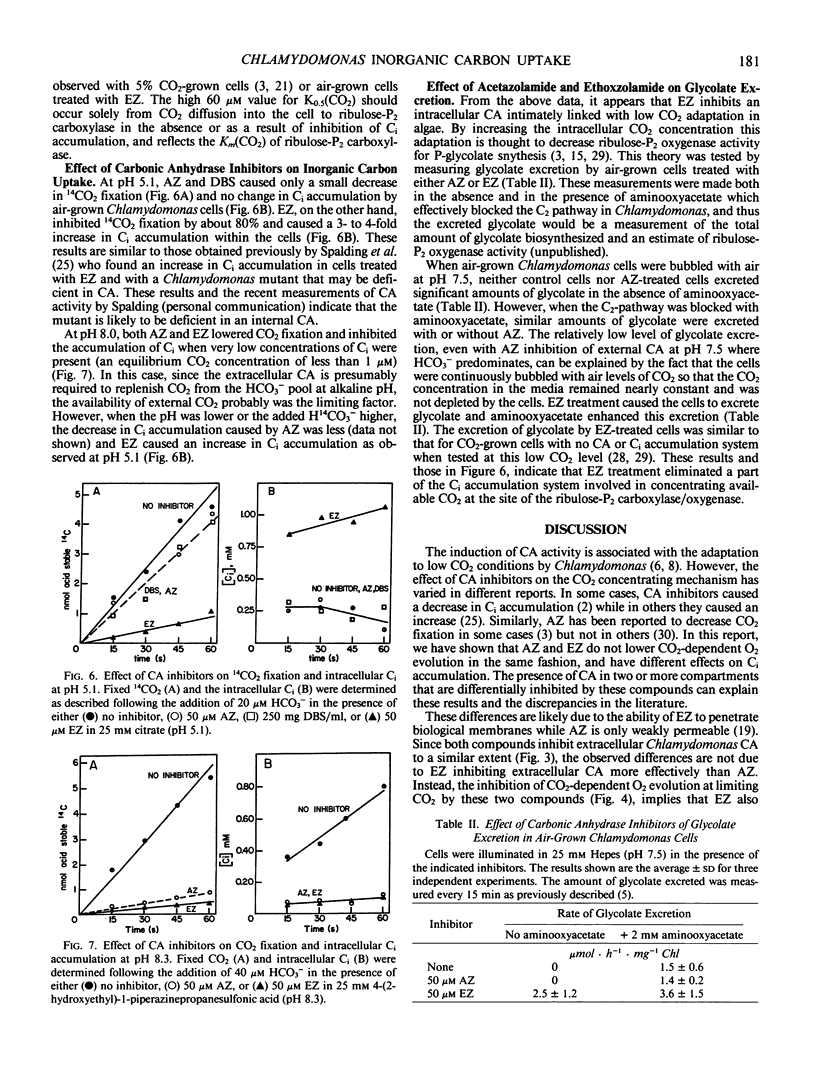
Membrane-permeable and impermeable inhibitors of carbonic anhydrase have been used to assess the roles of extracellular and intracellular carbonic anhydrase on the inorganic carbon concentrating system in Chlamydomonas reinhardtii. Acetazolamide, ethoxzolamide, and a membrane-impermeable, dextran-bound sulfonamide were potent inhibitors of extracellular carbonic anhydrase measured with intact cells. At pH 5.1, where CO2 is the predominant species of inorganic carbon, both acetazolamide and the dextran-bound sulfonamide had no effect on the concentration of CO2 required for the half-maximal rate of photosynthetic O2 evolution (K0.5[CO2]) or inorganic carbon accumulation. However, a more permeable inhibitor, ethoxzolamide, inhibited CO2 fixation but increased the accumulation of inorganic carbon as compared with untreated cells. At pH 8, the K0.5(CO2) was increased from 0.6 micromolar to about 2 to 3 micromolar with both acetazolamide and the dextran-bound sulfonamide, but to a higher value of 60 micromolar with ethoxzolamide. These results are consistent with the hypothesis that CO2 is the species of inorganic carbon which crosses the plasmalemma and that extracellular carbonic anhydrase is required to replenish CO2 from HCO3− at high pH. These data also implicate a role for intracellular carbonic anhydrase in the inorganic carbon accumulating system, and indicate that both acetazolamide and the dextran-bound sulfonamide inhibit only the extracellular enzyme. It is suggested that HCO3− transport for internal accumulation might occur at the level of the chloroplast envelope.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Badger M. R., Kaplan A., Berry J. A. Internal Inorganic Carbon Pool of Chlamydomonas reinhardtii: EVIDENCE FOR A CARBON DIOXIDE-CONCENTRATING MECHANISM. Plant Physiol. 1980 Sep;66(3):407–413. doi: 10.1104/pp.66.3.407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Berry J. A., Togasaki R. K., Grossman A. R. Identification of Extracellular Carbonic Anhydrase of Chlamydomonas reinhardtii. Plant Physiol. 1984 Oct;76(2):472–477. doi: 10.1104/pp.76.2.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coleman J. R., Colman B. Inorganic Carbon Accumulation and Photosynthesis in a Blue-green Alga as a Function of External pH. Plant Physiol. 1981 May;67(5):917–921. doi: 10.1104/pp.67.5.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutknecht J., Bisson M. A., Tosteson F. C. Diffusion of carbon dioxide through lipid bilayer membranes: effects of carbonic anhydrase, bicarbonate, and unstirred layers. J Gen Physiol. 1977 Jun;69(6):779–794. doi: 10.1085/jgp.69.6.779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kandel S. I., Wong S. C., Kandel M., Gornall A. G. Irreversible inactivation of bovine carbonic anhydrase B by bromoacetazolamide. J Biol Chem. 1968 May 10;243(9):2437–2439. [PubMed] [Google Scholar]
- Kaplan A., Berry J. A. Glycolate Excretion and the Oxygen to Carbon Dioxide Net Exchange Ratio during Photosynthesis in Chlamydomonas reinhardtii. Plant Physiol. 1981 Feb;67(2):229–232. doi: 10.1104/pp.67.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd N. D., Canvin D. T., Culver D. A. Photosynthesis and photorespiration in algae. Plant Physiol. 1977 May;59(5):936–940. doi: 10.1104/pp.59.5.936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maren T. H. The general physiology of reactions catalyzed by carbonic anhydrase and their inhibition by sulfonamides. Ann N Y Acad Sci. 1984;429:568–579. doi: 10.1111/j.1749-6632.1984.tb12389.x. [DOI] [PubMed] [Google Scholar]
- Moroney J. V., Tolbert N. E. Inorganic Carbon Uptake by Chlamydomonas reinhardtii. Plant Physiol. 1985 Feb;77(2):253–258. doi: 10.1104/pp.77.2.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson E. B., Tolbert N. E. The regulation of glycolate metabolism in Chlamydomonas reinhardtii. Biochim Biophys Acta. 1969 Jul 30;184(2):263–270. doi: 10.1016/0304-4165(69)90028-2. [DOI] [PubMed] [Google Scholar]
- Pocker Y., Sarkanen S. Carbonic anhydrase: structure catalytic versatility, and inhibition. Adv Enzymol Relat Areas Mol Biol. 1978;47:149–274. doi: 10.1002/9780470122921.ch3. [DOI] [PubMed] [Google Scholar]
- Spalding M. H., Spreitzer R. J., Ogren W. L. Carbonic Anhydrase-Deficient Mutant of Chlamydomonas reinhardii Requires Elevated Carbon Dioxide Concentration for Photoautotrophic Growth. Plant Physiol. 1983 Oct;73(2):268–272. doi: 10.1104/pp.73.2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sueoka N. MITOTIC REPLICATION OF DEOXYRIBONUCLEIC ACID IN CHLAMYDOMONAS REINHARDI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):83–91. doi: 10.1073/pnas.46.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker J. P., Coulson R., Weiner I. M. Dextran-bound inhibitors of carbonic anhydrase. J Pharmacol Exp Ther. 1981 Sep;218(3):600–607. [PubMed] [Google Scholar]
- Tolbert N. E., Harrison M., Selph N. Aminooxyacetate stimulation of glycolate formation and excretion by chlamydomonas. Plant Physiol. 1983 Aug;72(4):1075–1083. doi: 10.1104/pp.72.4.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]


