Abstract
Tuberculous bronchopleural fistula (BPF) is a rare and potentially life-threatening complication of pulmonary tuberculosis, in which abnormal connections form between the bronchial tree and the pleural space. These abnormal connections allow air and secretions to pass from the lungs into the pleural space, causing a range of symptoms from benign cough to acute tension pneumothorax. The management of tuberculous BPF requires an individualized approach based on the patient’s condition and response to treatment. Anti-tuberculosis therapy is essential for controlling the active tuberculosis infections. Intercostal drainage and suction are also commonly used to drain air and fluid from the pleural space, providing relief from the symptoms. For some patients, more invasive surgeries, such as decortication, thoracoplasty or pleuropneumonectomy are required to definitively close the fistula when medical management alone is insufficient. Herein, we describe a rare case of tuberculous BPF in a young adult female, who was treated with anti-tuberculosis medications and open thoracotomy.
Keywords: bronchopleural fistula, pulmonary tuberculosis, pneumothorax, video-assisted thoracoscopic surgery, thoracotomy
Introduction
Bronchopleural fistula (BPF) is an abnormal communication between the bronchial tree and pleural space. 1 Its causes include surgical resection of lung tissue, lung tissue necrosis from infection, trauma, and lung cancers. Tuberculous BPF is a rare but serious complication of pulmonary tuberculosis (TB) that requires prompt diagnosis and management. 2 Tuberculous BPF is often caused by the rupture of a tuberculous cavity in the lung or erosion of caseating mediastinal lymph nodes into adjacent structures, such as the esophagus or bronchus. Symptoms are often inconspicuous; therefore, a high degree of clinical suspicion is imperative for a timely diagnosis. Imaging plays an integral role in diagnosing and guiding further management. 3 We report a rare case of tuberculous BPF and large pneumothorax in an immunocompetent young female patient.
Case Report
A 29-year-old female with no significant medical history presented to the emergency department (ED) complaining of a dry cough of two weeks duration. The cough started with no obvious trigger, and the patient tried over-the-counter cough suppressant with little to no success. Two days before her ED visit, the patient experienced intermittent chest pains and shortness of breath on exertion. Chest pain was reproducible, nonradiating and worse on the left side, but was not associated with diaphoresis, palpitations, nausea, or vomiting. The patient denied any recent falls, thoracic surgery, or blunt chest trauma. She also denied headache, fever, chills, heartburn, hemoptysis, night sweats, recent weight loss, diarrhea, dysuria, or sick contacts. The patient denied any personal or family history of pulmonary tuberculosis.
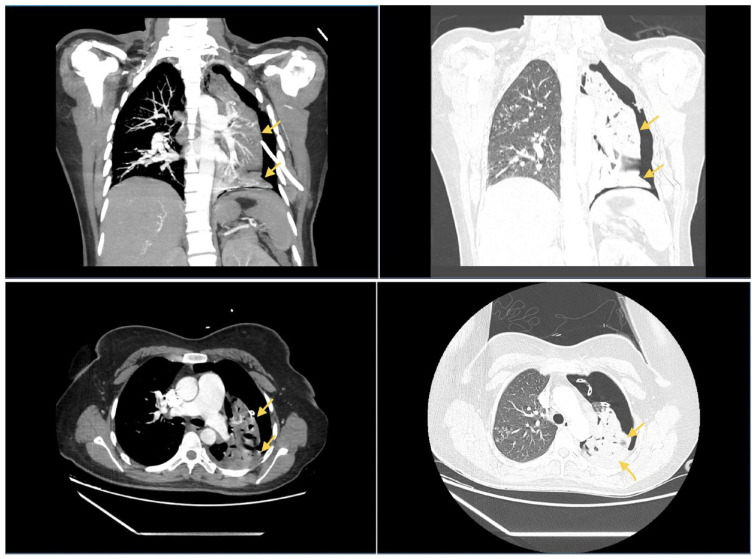
In the ED, the patient appeared fatigued, but was in no acute distress. She was afebrile and saturated at 96% on room air. She was tachycardic at 126 beats per minute, but her blood pressure and respiratory rate were within the normal ranges. Physical examination revealed decreased breath sounds and hyperresonant sounds in the left hemithorax, but no use of accessory muscles, wheezing, or stridor. The rest of the physical examination was unremarkable. Chest radiography showed a large left pneumothorax, and a left pigtail catheter was emergently placed in the ED (Figure 1). Repeat chest X-ray confirmed good positioning of the catheter but with incomplete reexpansion of the left lung. Bubbling was also noted on the chest drainage unit connected to suction, and there was concern for a bronchopleural fistula (BPF). As part of her workup, she underwent a chest computed tomography (CT) with a pulmonary embolism protocol to rule out a pulmonary embolism. Imaging revealed the presence of a significant pneumothorax on the left side with multiple apparent cavitary lesions in the left upper lobe and a possible BPF (Figure 2).
Figure 1.
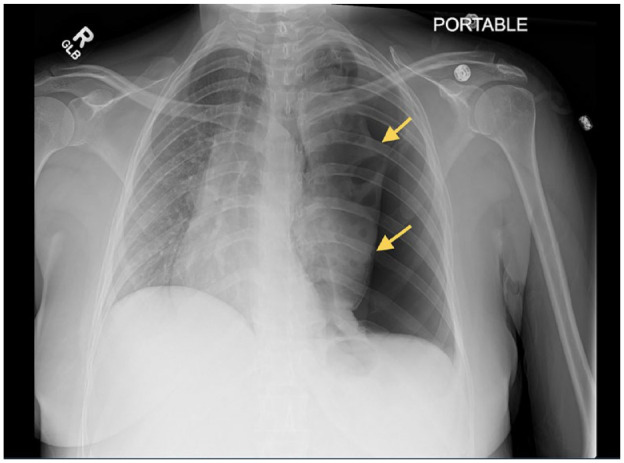
Chest X-ray (frontal view) showing a large left-sided pneumothorax. The trachea and heart are deviated to the right.
Figure 2.
CT angiography of the chest showing a large left pneumothorax with a probable bronchopleural fistula. Also seen are tree-in-bud infiltrates in the right upper lobe concerning for tuberculosis, fungal pneumonia, or bacterial pneumonia.
The patient was placed on airborne isolation precautions because of concerns about pulmonary tuberculosis (TB), and an infectious workup was ordered. Cardiothoracic surgery service was consulted while the patient was still in the ED to evaluate the persistent air leak, and they decided on continued observation with daily chest X-rays pending the infectious workup results. Mycobacterium tuberculosis (MTB) PCR was positive on day two of admission, whereas the first acid-fast bacilli (AFB) culture was negative. AFB cultures were repeated, and once again sent to an outside laboratory for processing. Blood cultures, TB QuantiFERON, bacterial pneumonia panel, respiratory viral panel including Covid-19 virus, urine Legionella antigen, urine Streptococcus pneumoniae antigen, Methicillin-Resistant Staphylococcus aureus nasal screening, HIV, fungal culture, and Fungitell-BD glucan were all negative.
The patient was continued on Azithromycin 250 mg, daily and ceftriaxone 1000 mg, daily as empiric coverage for community acquired pneumonia. She was, however, taken to the operating room on day four of admission due to the persistent left-sided pneumothorax despite good-positioning of the pigtail chest tube. Left video-assisted thoracoscopic surgery (VATS) was attempted but subsequently converted to an open thoracotomy due to the surgeon’s inability to properly palpate the extent of the left upper lobe involvement. The procedure entailed left upper lobectomy, left wedge resection, lysis of intrapleural adhesions, and decortication of the left lower lobe. The left upper lobe appeared to exhibit several areas of abscess with an active BPF in the lateral aspect of the upper lobe and anteriorly.
On postoperative day 1 (day 5 of admission), sputum cultures returned positive for AFB smear and it was presumed to be MTB given the patient’s positive MTB PCR test. TB Spot came back negative. Azithromycin and ceftriaxone were discontinued and the patient was started on anti-tuberculosis medications, including levofloxacin (substituting INH due to national shortage), rifampin, pyrazinamide, ethambutol, and pyridoxine. AFB culture identification confirmed MTB, which was susceptible to streptomycin, isoniazid, rifampin, ethambutol, and pyrazinamide. The pathology of the lung specimens was significant for acute fibrinous and chronic pleuritis and lung parenchyma with multiple caseating granulomas consistent with active tuberculosis. Multiple lymph nodes with granulomatous lymphadenopathy were observed. The patient was diagnosed with pulmonary tuberculosis with BPF. Chest drains were removed on postoperative day 7, but the patient was discharged 2 weeks later with anti-tuberculosis medications and plans for outpatient follow-up at the pulmonology and surgical clinics. The patient presented to the ED 2 weeks after discharge for repeat imaging. Chest X-ray showed postoperative changes with no evidence of infiltrates, consolidations, or pneumothorax.
Discussion
Bronchopleural fistula (BPF) is a rare pathology characterized by an anomalous communication between the bronchi and the pleural cavity.1,4-6 Surgical and bronchoscopic interventions such as pneumonectomy and lobectomy carry a high risk of BPF, which can be acute or subacute.4,7-9 Nonsurgical causes of include infections, neoplastic lesions, trauma, and chemoradiation.10-15 Complications from BPF include contamination of the pleural space, causing inflammation and empyema. This can lead to reduced lung expansion and repeated pneumonia of the opposite lung due to aspiration of the contaminated pleural fluid. 7
Pulmonary tuberculosis (TB) is a global health challenge and a leading cause of infection-related deaths. Mycobacterium tuberculosis (MBT) infects 10 million individuals every year resulting in 1.5 million deaths per annum. 16 Patients with TB often present with a productive cough, shortness of breath, fever, weight loss, night sweats or hemoptysis. Less common presentations include back pain from Pott’s disease, headaches, and seizures with meningitis. MBT causes cavitation in the bronchial tree, which can rupture, and release contents into the pleural cavity, causing further inflammation and damage to the pleura. Chronic inflammation can lead to fibrosis and the formation of sinus tracts between the bronchial tree and the pleural space. Air escapes into the pleural space and causes pneumothorax, resulting in atelectasis on the affected side. Tuberculous BPF is rare and often a diagnostic challenge due to its subtle and indolent presentation.11,14 Symptoms range from overt to covert, and include productive cough, subcutaneous emphysema, fever, sudden dyspnea, low oxygen saturation, low blood pressure and pneumothorax. 17
Due to its rarity, a BPF can be a diagnostic conundrum. The diagnosis is made using a combination of clinical and imaging findings that confirm an air leak from the bronchial tree to the pleural space. 5 There are no specific laboratory tests for BPFs, but elevated erythrocyte sedimentation rate (ESR) and leukocytosis have been reported in some cases. Wali reported a unique case of mycobacterium chelonae empyema with a BPF in an immunocompetent patient. 18 The patient’s bloodwork was significant for an elevated ESR level and leukocytosis suggestive of an infectious process. 18 In patients with a suspected infectious cause, workup including HIV test, acid fast bacilli cultures, TB PCR, interferon gamma assay, fungal culture, Fungitell-BD glucan, and pneumonia panel can be revealing. Chest radiography can help rule out malignancies, pneumonia, pneumothorax and BPF. CT scans of the thorax, however, allow localization and characterization of the pathology or size of the BPF. 6 Padmanaban et al 19 reported a rare case of central BPF from pulmonary mucormycosis in a diabetic patient. CT scan of the chest, bronchoscopy, and histopathology were used to make the diagnosis. Similarly, our patient was noted to have a large pneumothorax and BPF on imaging, which prompted an immediate intervention.
BPFs are a rare but serious complication of tuberculosis that requires prompt treatment and management. Surgical options include thoracomyoplasty, in which the chest wall is reconstructed to close the fistula. 20 However, surgery is risky, especially in high-risk patients, so less invasive options are ideal. Surgical therapy is effective for larger bronchopleural fistulas because of its ability to create and amplify negative pressure in the pleural cavity. The accumulation of negative pressure produces a cascade of mechanisms that aid in closure of the fistula, including development of granulation tissue, refined oxygenation of tissues, evacuation of any surplus interstitial fluid, and decreased residual pleural space volume. 7 If a patient presents with an empyema, however, open window thoracostomy for treatment is preferred. 16
Bronchoscopic procedures, like using endobronchial Watanabe spigots (EWS) to plug the fistula, have been shown to effectively reduce air leakage and promote healing, even in intensive care settings. 21 EWS are removable, providing a reversible solution. Similarly, Gogia et al 22 reported the successful use of bronchoscopic cyanoacrylate glue to block a persistent BPF. Conservative medical management is an alternative treatment approach. With chest tube drainage, ventilation adjustments, and treatment of the underlying tuberculosis, BPFs have resolved in 5 to 36 days. 23 Patients with untreated BPFs may develop respiratory compromise, subcutaneous emphysema, or even death.
Conclusion
Bronchopleural fistulas (BPFs) are abnormal connections between the bronchus and the pleural space that allow air to enter the pleural cavity. Although surgery may be required in some cases, nonsurgical bronchoscopic procedures and conservative medical management are viable and often preferable options for treating tuberculous BPFs. Prompt diagnosis and appropriate treatment are necessary to prevent BPF, a rare but serious BPF complication of pulmonary tuberculosis.
Footnotes
Author Contributions: LB, AS, TW, DA, and JS conceived the idea for this case report and drafted the manuscript. MA, JS, and RS fact-checked, edited, and proofread the final manuscript.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics Approval: Our institution does not require ethical approval to report individual cases or case series.
Informed Consent: The patient consented for the publication of this case report.
ORCID iD: Lefika Bathobakae  https://orcid.org/0000-0002-2772-6085
https://orcid.org/0000-0002-2772-6085
Data Availability Statement: Further enquiries can be directed to the corresponding author
References
- 1. Mazzolini KJ, Dzubnar JM, Velotta JB. A step-up approach to management of complex bronchopleural fistula. J Surg Case Rep. 2022;2022(10):rjac490. doi: 10.1093/jscr/rjac490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Hosna AU, Miller D. A case of bronchopleural fistula and hydropneumothorax in a patient with necrotizing pneumonia complicated by Mycobacterium avium complex. Cureus. 2022; 14(10):e30280. doi: 10.7759/cureus.30280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Marques P, Andrade G, Granadas J, et al. Iatrogenic bronchopleural fistula. Cureus. 2020;12:e12187. doi: 10.7759/cureus.12187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Khaitan PG. Incidence of bronchopleural fistula after pneumonectomy in an era of revolution. J Thorac Dis. 2023;15(2): 226-228. doi: 10.21037/jtd-22-1545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Pasetyo KW, Rosihaningsih JJ, Kusuma Wardhana K, et al. Case report: bronchopleural fistula dextra with pyopneu-mothorax dextra as complication of lung tuberculosis. Malang Resp J. 2023;5:e00501. [Google Scholar]
- 6. Duarte-Ribeiro F, Dias C, Mota M. Bronchopleural and pleurocutaneous fistula in HIV patient with pulmonary tuberculosis. Idcases. 2017;9:82-84. doi: 10.1016/j.idcr.2017.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Gritsiuta AY, Eguchi T, Jones DR, Rocco G. A stepwise approach for postlobectomy bronchopleural fistula. Oper Tech Thorac Cardiovasc Surg. 2020;25(2):85-104. doi: 10.1053/j.optechstcvs.2019.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Menezes V, Soder S, Kadadah S, Masson JB, Lafontaine E, Liberman M. Bronchoscopic treatment of a bronchopleural fistula after pneumonectomy. JTCVS Tech. 2020;4:345-348. doi: 10.1016/j.xjtc.2020.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Cusumano G, Alifano M, Lococo F. Endoscopic and surgical treatment for bronchopleural fistula after major lung resection: an enduring challenge. J Thorac Dis. 2019;11(suppl 9): S1351-S1356. doi: 10.21037/jtd.2019.03.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Wada H, Suzuki H, Tanaka K, Sakairi Y, Yoshino I. Postoperative bronchopleural fistula after induction therapy with bevacizumab. Thorac Cancer. 2023;14(22):2229-2232. doi: 10.1111/1759-7714.15008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Shen L, Jiang Y-H, Dai X-Y. Successful surgical treatment of bronchopleural fistula caused by severe pulmonary tuberculosis: a case report and review of literature. World J Clin Cases. 2023;11(10):2282-2289. doi: 10.12998/wjcc.v11.i10.2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Madhusudhan KS, Das P. Mesenchymal tumors of the stomach: radiologic and pathologic correlation. Abdom Radiol (NY). 2022;47(6):1988-2003. doi: 10.1007/s00261-022-03498-1. [DOI] [PubMed] [Google Scholar]
- 13. Nakiyingi L, Baluku JB, Ssengooba W, et al. Recurrent pneumothorax in a human immunodeficiency virus-positive patient with multidrug-resistant tuberculosis: a rare case of bronchopleural fistula: a case report. J Med Case Rep. 2022; 16(1):e03436. doi: 10.1186/s13256-022-03436-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Stewart AGA, Boyd SC. Tuberculous bronchopleural fistula. Respirol Case Rep. 2021;9(4):e740. doi: 10.1002/rcr2.740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. van de Pas JM, van Roozendaal LM, Wanders SL, Custers FL, Vissers YLJ, de Loos ER. Bronchopleural fistula after concurrent chemoradiotherapy. Adv Radiat Oncol. 2020;5(3): 511-515. doi: 10.1016/j.adro.2019.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Al-Neyadi M, Alghfeli S, Dukandar M. Hydropneumothorax with bronchopleural fistula following the activation of Mycobacterium tuberculosis: a case report. Cureus. 2023;15(6): e40844. doi: 10.7759/cureus.40844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zhao P. Progress report on interventional treatment for bronchopleural fistula. Emerg Med Int. 2023;2023:8615055. doi: 10.1155/2023/8615055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wali S. Mycobacterium chelonae empyema with bronchopleural fistula in an immunocompetent patient case report. Ann Thorac Med. 2009;4:213-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Padmanaban E, Kannan P, Amirthalingam U, et al. Pulmonary mucormycosis presenting as central bronchopleural fistula: a case report with review of literature. Egypt J Radiol Nucl Med. 2021;52(1):e00546. doi: 10.1186/s43055-021-00546-6. [DOI] [Google Scholar]
- 20. Divisi D, Barone M, Zaccagna G, Di Francescantonio W, Crisci R. Management of bronchopleural fistula complicated by skin wound necrosis after thoracomyoplasty. Plast Reconstr Surg Glob Open. 2017;5(1):e1193. doi: 10.1097/GOX.0000000000001193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Dalar L, Kosar F, Eryuksel E, Karasulu L, Altin S. Endobronchial watanabe spigot embolisation in the treatment of bronchopleural fistula due to tuberculous empyema in intensive care unit. Ann Thorac Cardiovasc Surg. 2013;19(2):140-143. doi: 10.5761/atcs.cr.11.01760. [DOI] [PubMed] [Google Scholar]
- 22. Gogia P, Gupta S, Goyal R. Bronchoscopic management of bronchopleural fistula. Indian J Chest Dis Allied Sci. 2010; 53:161–163. [PubMed] [Google Scholar]
- 23. Naranjo Gómez JM, Carbajo Carbajo M, Valdivia Concha D, Campo-Cañaveral de la Cruz JL. Conservative treatment of post-lobectomy bronchopleural fistula. Interact Cardiovasc Thorac Surg. 2012;15(1):152-154. doi: 10.1093/icvts/ivs078. [DOI] [PMC free article] [PubMed] [Google Scholar]