Abstract
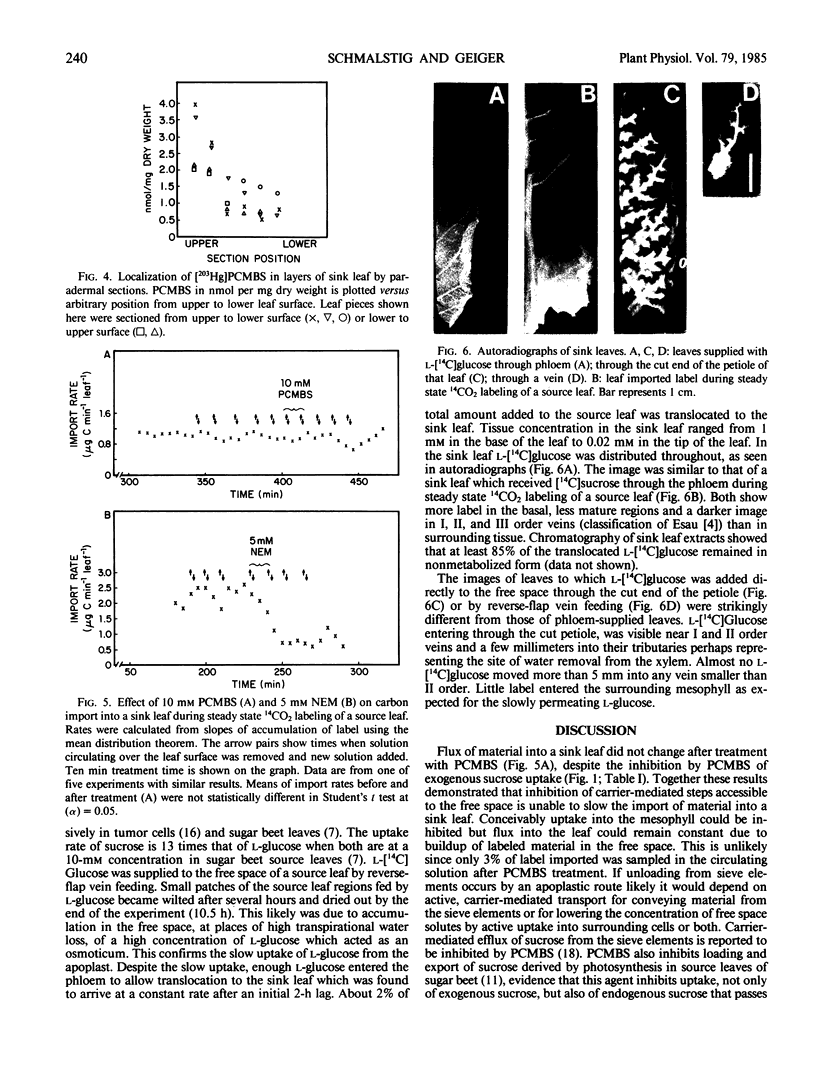
Physiological and transport data are presented in support of a symplastic pathway of phloem unloading in importing leaves of Beta vulgaris L. (`Klein E multigerm'). The sulfhydryl reagent p-chloromercuribenzene sulfonic acid (PCMBS) at concentration of 10 millimolar inhibited uptake of exogenous [14C]sucrose by sink leaf tissue over sucrose concentrations of 0.1 to 5.0 millimolar. Inhibited uptake was 24% of controls. The same PCMBS treatment did not affect import of 14C-label into sink leaves during steady state labeling of a source leaf with 14CO2. Lack of inhibition of import implies that sucrose did not pass through the free space during unloading. A passively transported xenobiotic sugar, l-[14C]glucose, imported by a sink leaf through the phloem, was evenly distributed throughout the leaf as seen by whole-leaf autoradiography. In contrast, l-[14C]glucose supplied to the apoplast through the cut petiole or into a vein of a sink leaf collected mainly in the vicinity of the major veins with little entering the mesophyll. These patterns are best explained by transport through the symplast from phloem to mesophyll.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Felker F. C., Shannon J. C. Movement of C-labeled Assimilates into Kernels of Zea mays L: III. AN ANATOMICAL EXAMINATION AND MICROAUTORADIOGRAPHIC STUDY OF ASSIMILATE TRANSFER. Plant Physiol. 1980 May;65(5):864–870. doi: 10.1104/pp.65.5.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fellows R. J., Geiger D. R. Structural and Physiological Changes in Sugar Beet Leaves during Sink to Source Conversion. Plant Physiol. 1974 Dec;54(6):877–885. doi: 10.1104/pp.54.6.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fondy B. R., Geiger D. R. Sugar Selectivity and Other Characteristics of Phloem Loading in Beta vulgaris L. Plant Physiol. 1977 May;59(5):953–960. doi: 10.1104/pp.59.5.953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Christy A. L. Effect of sink region anoxia on translocation rate. Plant Physiol. 1971 Feb;47(2):172–174. doi: 10.1104/pp.47.2.172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger D. R., Fondy B. R. A method for continuous measurement of export from a leaf. Plant Physiol. 1979 Sep;64(3):361–365. doi: 10.1104/pp.64.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. T., Lin W., Sadler N. L., Franceschi V. R. Pathway of Phloem unloading of sucrose in corn roots. Plant Physiol. 1983 Jun;72(2):362–367. doi: 10.1104/pp.72.2.362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giaquinta R. Evidence for Phloem loading from the apoplast: chemical modification of membrane sulfhydryl groups. Plant Physiol. 1976 Jun;57(6):872–875. doi: 10.1104/pp.57.6.872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gifford R. M., Thorne J. H., Hitz W. D., Giaquinta R. T. Crop productivity and photoassimilate partitioning. Science. 1984 Aug 24;225(4664):801–808. doi: 10.1126/science.225.4664.801. [DOI] [PubMed] [Google Scholar]
- Hatanaka M. Transport of sugars in tumor cell membranes. Biochim Biophys Acta. 1974 Apr 29;355(1):77–104. doi: 10.1016/0304-419x(74)90008-0. [DOI] [PubMed] [Google Scholar]
- M'batchi B., Delrot S. Parachloromercuribenzenesulfonic Acid : a potential tool for differential labeling of the sucrose transporter. Plant Physiol. 1984 May;75(1):154–160. doi: 10.1104/pp.75.1.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maynard J. W., Lucas W. J. Sucrose and Glucose Uptake into Beta vulgaris Leaf Tissues : A Case for General (Apoplastic) Retrieval Systems. Plant Physiol. 1982 Nov;70(5):1436–1443. doi: 10.1104/pp.70.5.1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNeil D. L., Atkins C. A., Pate J. S. Uptake and Utilization of Xylem-borne Amino Compounds by Shoot Organs of a Legume. Plant Physiol. 1979 Jun;63(6):1076–1081. doi: 10.1104/pp.63.6.1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne J. H. Morphology and ultrastructure of maternal seed tissues of soybean in relation to the import of photosynthate. Plant Physiol. 1981 May;67(5):1016–1025. doi: 10.1104/pp.67.5.1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon R. Termination of nutrient import and development of vein loading capacity in albino tobacco leaves. Plant Physiol. 1984 Sep;76(1):45–48. doi: 10.1104/pp.76.1.45. [DOI] [PMC free article] [PubMed] [Google Scholar]