Abstract
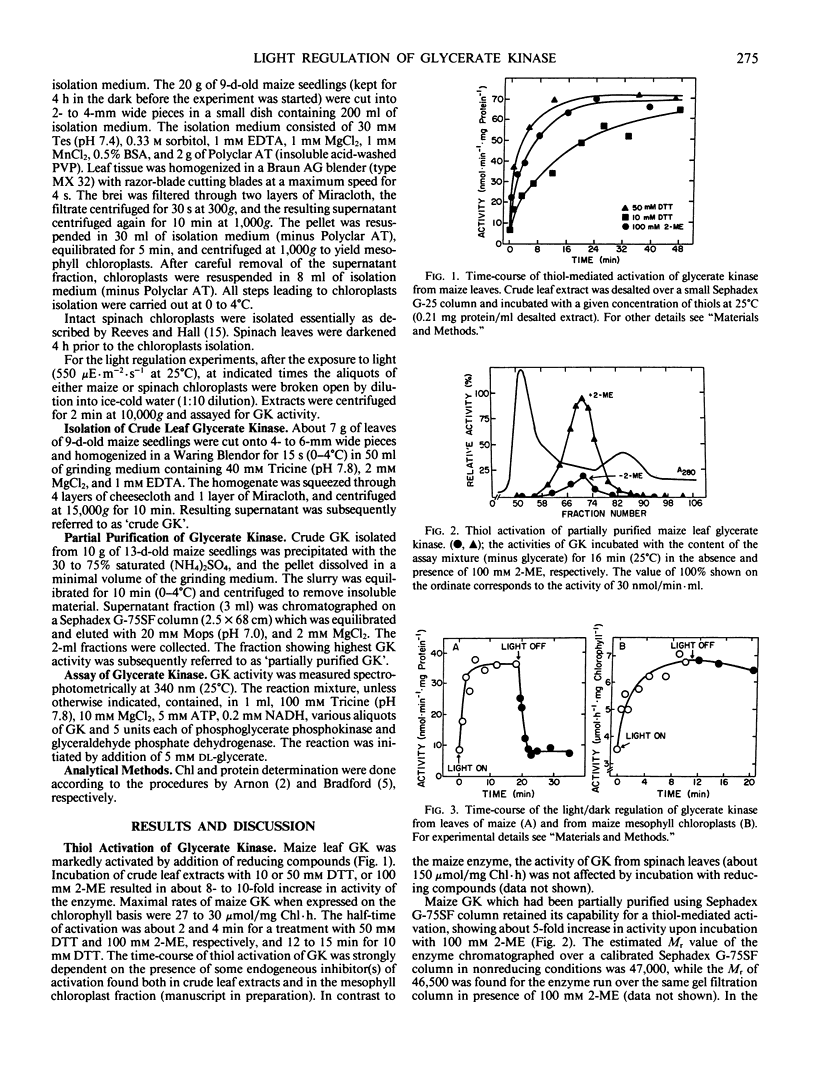
Glycerate kinase (EC 2.7.1.31) from maize (Zea mays) leaves was shown to be regulated by light/dark transition. The enzyme more than doubled in activity after either the leaves or isolated mesophyll chloroplasts were illuminated with white light for 10 minutes. Rate of inactivation in the dark was faster in leaves than in the isolated chloroplast fraction. The stimulating effect of light could be mimicked in crude preparations by addition of 10 or 50 millimolar dithiothreitol or 100 millimolar 2-mercaptoethanol. The thiol treatment resulted in 8- to 10-fold activation of glycerate kinase, with the highest rates in the range of 27 to 30 micromoles per mg chlorophyll per hour. Activation was not accompanied by any changes in the apparent Mr value of glycerate kinase as determined by gel filtration (Mr = 47,000). In contrast to maize glycerate kinase, the enzyme from spinach was not affected by either light or thiol exposure.
Partially purified maize glycerate kinase was activated up to 3-fold upon incubation with a mixture of spinach thioredoxins m and f and 5 millimolar dithiothreitol. The thioredoxin and dithiothreitol-treated glycerate kinase could be further stimulated by addition of 2.5 millimolar ATP. The results suggest that glycerate kinase from maize leaves is capable of photoactivation by the ferredoxin/thioredoxin system. The synergistic effect of ATP and thioredoxins in activation of the enzyme supports the earlier expressed view that the ferredoxin/thioredoxin system functions jointly with effector metabolites in light-mediated regulation during photosynthesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M., Boardman N. K., Spencer D. Phosphorylation by intact bundle sheath chloroplasts from maize. Biochim Biophys Acta. 1971 Aug 6;245(1):253–258. doi: 10.1016/0005-2728(71)90032-6. [DOI] [PubMed] [Google Scholar]
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashton A. R., Hatch M. D. Regulation of C4 photosynthesis: regulation of activation and inactivation of NADP-malate dehydrogenase by NADP and NADPH. Arch Biochem Biophys. 1983 Dec;227(2):416–424. doi: 10.1016/0003-9861(83)90471-x. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Buchanan B. B. The ferredoxin/thioredoxin system: a key element in the regulatory function of light in photosynthesis. Bioscience. 1984 Jun;34(6):378–383. [PubMed] [Google Scholar]
- Davis G. H., MacIvor J., Freeman M. Changes in the vaginal and rectal carriage of group B streptococci during pregnancy. Aust N Z J Obstet Gynaecol. 1980 Feb;20(1):32–34. doi: 10.1111/j.1479-828x.1980.tb00892.x. [DOI] [PubMed] [Google Scholar]
- Kleczkowski L. A., Randall D. D., Zahler W. L. The substrate specificity, kinetics, and mechanism of glycerate-3-kinase from spinach leaves. Arch Biochem Biophys. 1985 Jan;236(1):185–194. doi: 10.1016/0003-9861(85)90618-6. [DOI] [PubMed] [Google Scholar]
- Nishizawa A. N., Buchanan B. B. Enzyme regulation in C4 photosynthesis. Purification and properties of thioredoxin-linked fructose bisphosphatase and sedoheptulose bisphosphatase from corn leaves. J Biol Chem. 1981 Jun 25;256(12):6119–6126. [PubMed] [Google Scholar]
- Randall D. D., Tolbert N. E., Gremel D. 3-Phosphoglycerate Phosphatase in Plants: II. Distribution, Physiological Considerations, and Comparison with P-Glycolate Phosphatase. Plant Physiol. 1971 Oct;48(4):480–487. doi: 10.1104/pp.48.4.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitt M. R., Edwards G. E. Glycerate kinase from leaves of C3 plants. Arch Biochem Biophys. 1983 Jul 1;224(1):332–341. doi: 10.1016/0003-9861(83)90217-5. [DOI] [PubMed] [Google Scholar]
- Usuda H., Edwards G. E. Localization of glycerate kinase and some enzymes for sucrose synthesis in c(3) and c(4) plants. Plant Physiol. 1980 May;65(5):1017–1022. doi: 10.1104/pp.65.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Usuda H., Ku M. S., Edwards G. E. Activation of NADP-Malate Dehydrogenase, Pyruvate,Pi Dikinase, and Fructose 1,6-Bisphosphatase in Relation to Photosynthetic Rate in Maize. Plant Physiol. 1984 Sep;76(1):238–243. doi: 10.1104/pp.76.1.238. [DOI] [PMC free article] [PubMed] [Google Scholar]


