Abstract
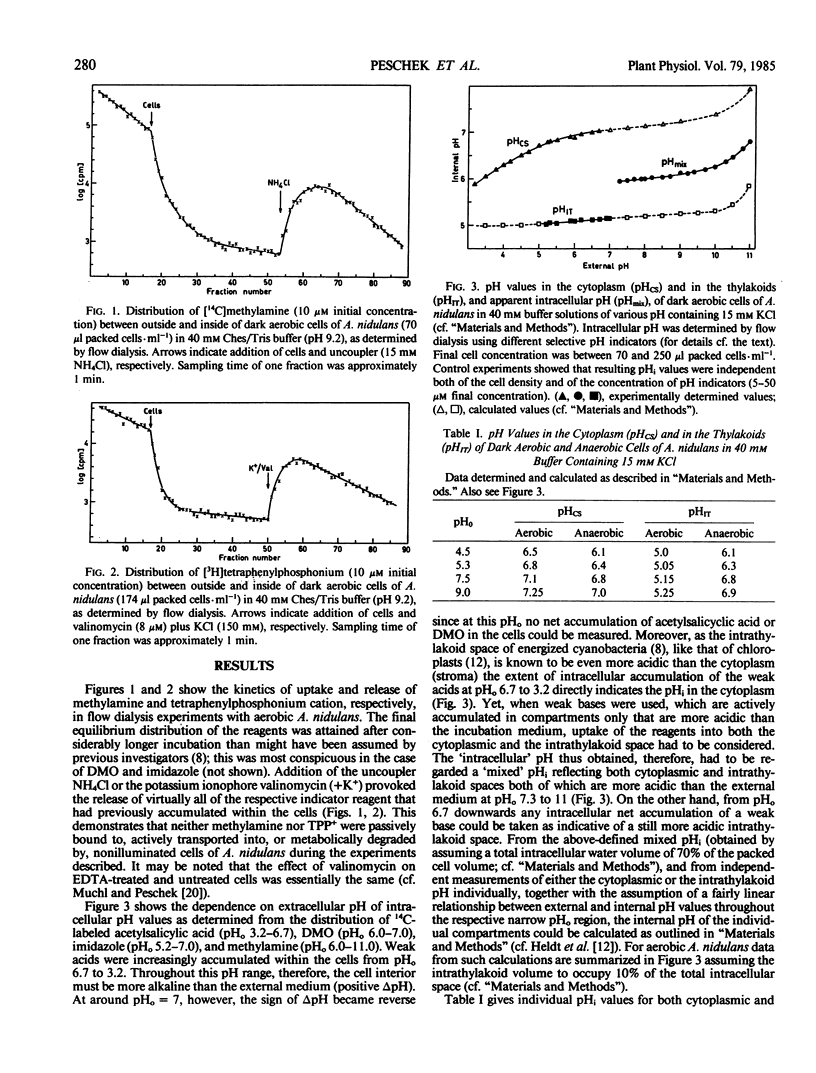
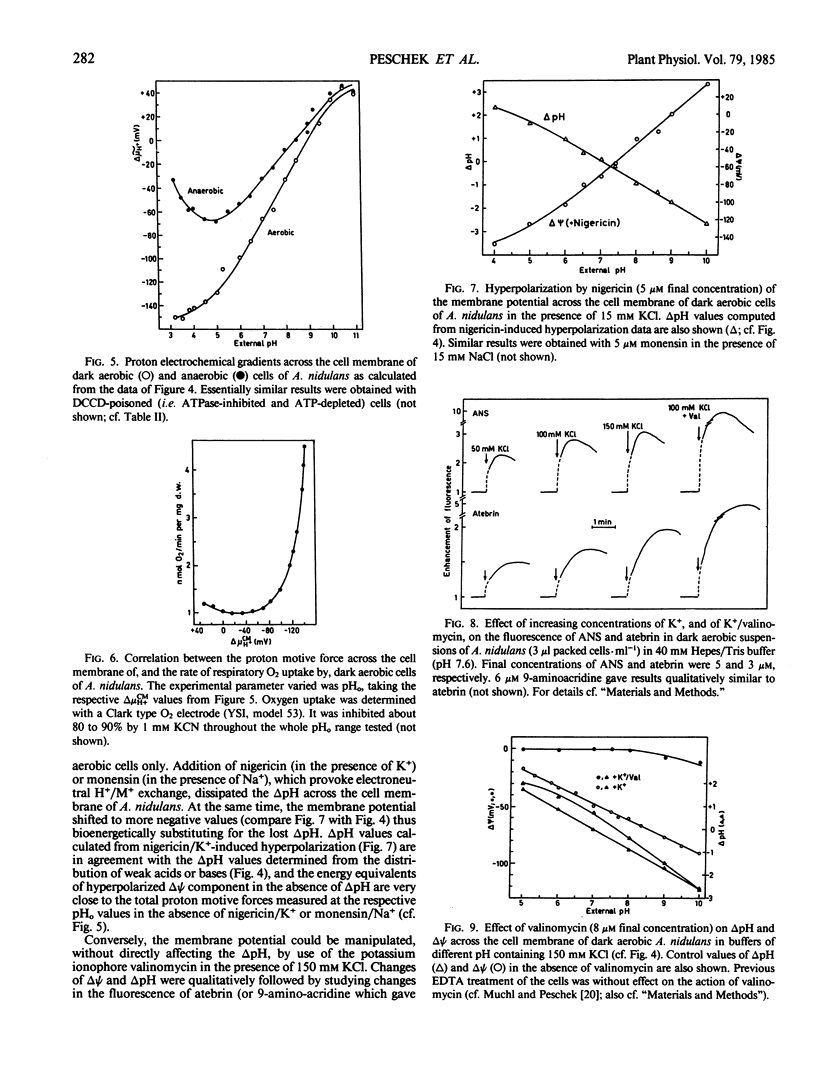
The transmembrane proton electrochemical potential gradient ΔμH+ in whole cells of Anacystis nidulans was measured in aerobic and anaerobic dark conditions using the distribution, between external medium and cell interior, of radioactively labeled weak acids (acetylsalicyclic acid, 5,5-dimethyloxazolidine-2,4-dione) or bases (imidazole, methylamine), and permeant ions (tetraphenylphosphonium cation, thiocyanate anion), as determined by flow dialysis. Alternatively, the movements across the plasma membrane of ΔpH-indicating atebrin or 9-aminoacridine, and of ΔΨ-indicating 8-anilino-l-naphthalenesulfonate were qualitatively followed by fluorescence measurements. Attempts were made to discriminate between the individual chemiosmotic gradients across the cytoplasmic (plasmalemma) and the intracytoplasmic (thylakoid) membranes. By use of the ionophores nigericin, monensin, and valinomycin, the components of the proton motive force, namely the proton concentration gradient ΔpH and the electric membrane potential ΔΨ were shown to be mutually exchangeable within the range of external pH values tested (3.2-11.0). Both components were depressed by the uncoupler carbonylcyanide m-chlorophenylhydrazone, though inhibition of ΔpH was much more pronounced than that of ΔΨ, notably in the alkaline pH0 range. The total proton electrochemical gradient across the plasma membrane was significantly higher in aerobic than in anaerobic cells and increased markedly (i.e. became more negative) towards lower pH0 values. This increase was paralleled by a similar increase in the rate of endogenous respiration of the cells. At the same time the ATPase inhibitor dicyclohexylcarbodiimide only slightly affected the proton motive force across the plasma membrane of aerobic cells. The results will be discussed in terms of a respiratorily competent plasma membrane in Anacystis nidulans.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen M. M. Photosynthetic membrane system in Anacystis nidulans. J Bacteriol. 1968 Sep;96(3):836–841. doi: 10.1128/jb.96.3.836-841.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E. P., Mangerich W. E. Interconversion of components of the bacterial proton motive force by electrogenic potassium transport. J Bacteriol. 1981 Sep;147(3):820–826. doi: 10.1128/jb.147.3.820-826.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., O'neill S. D., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: I. Identification and Characterization of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):538–544. doi: 10.1104/pp.74.3.538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett A. B., Spanswick R. M. H-ATPase Activity from Storage Tissue of Beta vulgaris: II. H/ATP Stoichiometry of an Anion-Sensitive H-ATPase. Plant Physiol. 1984 Mar;74(3):545–548. doi: 10.1104/pp.74.3.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falkner G., Horner F. pH Changes in the Cytoplasm of the Blue-Green Alga Anacystis nidulans Caused by Light-dependent Proton Flux into the Thylakoid Space. Plant Physiol. 1976 Dec;58(6):717–718. doi: 10.1104/pp.58.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldmann K. New devices for flow dialysis and ultrafiltration for the study of protein--ligand interactions. Anal Biochem. 1978 Jul 15;88(1):225–235. doi: 10.1016/0003-2697(78)90414-1. [DOI] [PubMed] [Google Scholar]
- Heldt W. H., Werdan K., Milovancev M., Geller G. Alkalization of the chloroplast stroma caused by light-dependent proton flux into the thylakoid space. Biochim Biophys Acta. 1973 Aug 31;314(2):224–241. doi: 10.1016/0005-2728(73)90137-0. [DOI] [PubMed] [Google Scholar]
- Kienzl P. F., Peschek G. A. Oxidation of c-Type Cytochromes by the Membrane-Bound Cytochrome Oxidase (Cytochrome aa(3)) of Blue-Green Algae. Plant Physiol. 1982 Mar;69(3):580–584. doi: 10.1104/pp.69.3.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Maloney P. C. Relationship between phosphorylation potential and electrochemical H+ gradient during glycolysis in Streptococcus lactis. J Bacteriol. 1983 Mar;153(3):1461–1470. doi: 10.1128/jb.153.3.1461-1470.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nitschmann W. H., Peschek G. A. Vanadate and dicyclohexylcarbodiimide insensitive proton extrusion from oxygen pulsed cells of the cyanobacterium Anacystis nidulans. Biochem Biophys Res Commun. 1984 Aug 30;123(1):358–364. doi: 10.1016/0006-291x(84)90421-2. [DOI] [PubMed] [Google Scholar]
- Padan E., Zilberstein D., Rottenberg H. The proton electrochemical gradient in Escherichia coli cells. Eur J Biochem. 1976 Apr 1;63(2):533–541. doi: 10.1111/j.1432-1033.1976.tb10257.x. [DOI] [PubMed] [Google Scholar]
- Peschek G. A. Characterization of the Proton-Translocating Cytochrome c Oxidase Activity in the Plasma Membrane of Intact Anacystis nidulans Spheroplasts. Plant Physiol. 1984 Aug;75(4):968–973. doi: 10.1104/pp.75.4.968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peschek G. A., Schmetterer G. Evidence for plastoquinol-cytochrome f/b-563 reductase as a common electron donor to P700 and cytochrome oxidase in cyanobacteria. Biochem Biophys Res Commun. 1982 Oct 15;108(3):1188–1195. doi: 10.1016/0006-291x(82)92126-x. [DOI] [PubMed] [Google Scholar]
- Peschek G. A. Structure and function of respiratory membranes in cyanobacteria (blue-green algae). Subcell Biochem. 1984;10:85–191. doi: 10.1007/978-1-4613-2709-7_2. [DOI] [PubMed] [Google Scholar]
- Ramos S., Schuldiner S., Kaback H. R. The use of flow dialysis for determinations of deltapH and active transport. Methods Enzymol. 1979;55:680–688. doi: 10.1016/0076-6879(79)55076-9. [DOI] [PubMed] [Google Scholar]
- Reed R. H., Rowell P., Stewart W. D. Components of the proton electrochemical potential gradient in Anabaena variabilis. Biochem Soc Trans. 1980 Dec;8(6):707–708. doi: 10.1042/bst0080707. [DOI] [PubMed] [Google Scholar]
- Scherer S., Stürzl E., Böger P. Oxygen-dependent proton efflux in cyanobacteria (blue-green algae). J Bacteriol. 1984 May;158(2):609–614. doi: 10.1128/jb.158.2.609-614.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scholes P., Mitchell P., Moyle J. The polarity of proton translocation in some photosynthetic microorganisms. Eur J Biochem. 1969 Apr;8(3):450–454. doi: 10.1111/j.1432-1033.1969.tb00548.x. [DOI] [PubMed] [Google Scholar]


