Abstract
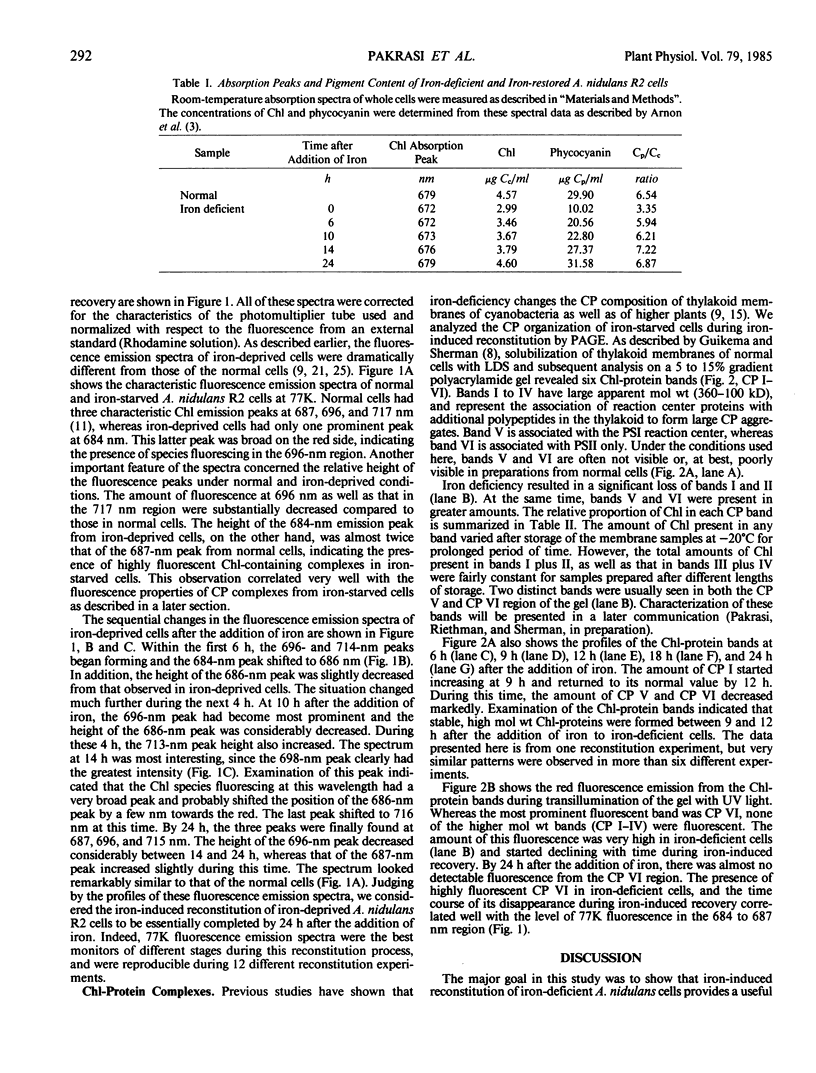
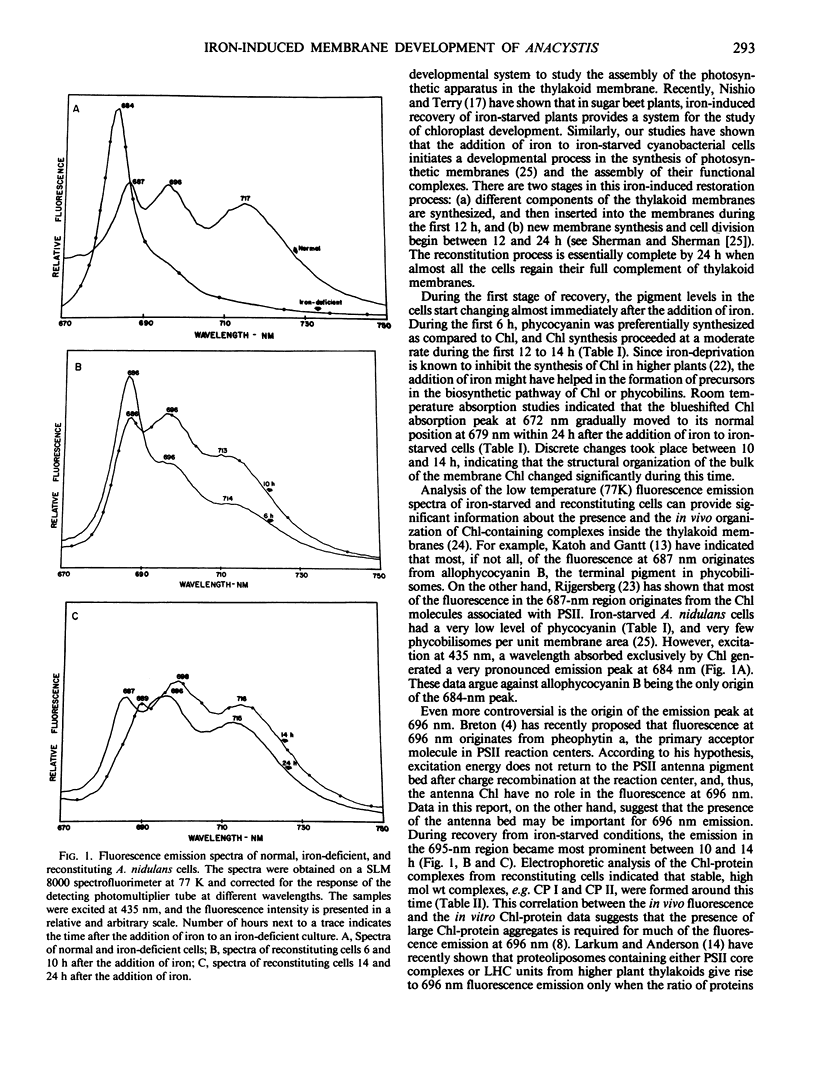
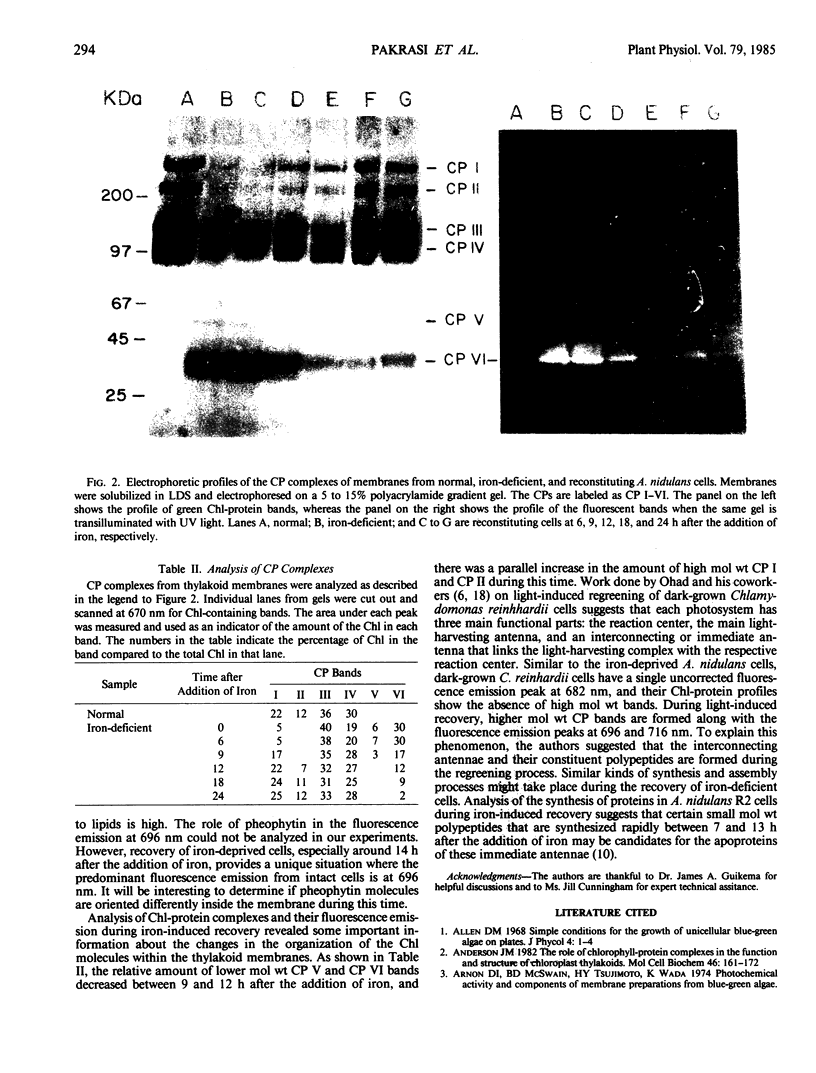
Deprivation of iron from the growth medium results in physiological as well as structural changes in the unicellular cyanobacterium Anacystis nidulans R2. Important among these changes are alterations in the composition and function of the photosynthetic membranes. Room-temperature absorption spectra of iron-starved cyanobacterial cells show a chlorophyll absorption peak at 672 nanometers, 7 nanometers blue-shifted from its normal position at 679 nanometers. Iron-starved cells have decreased amounts of chlorophyll and phycobilins. Their fluorescence spectra (77K) have one prominent chlorophyll emission peak at 684 nanometers as compared to three peaks at 687, 696, and 717 nanometers from normal cells. Chlorophyll-protein analysis of iron-deprived cells indicated the absence of high molecular weight bands. Addition of iron to iron-starved cells induced a restoration process in which new components were initially synthesized and integrated into preexisting membranes; at later times, new membranes were assembled and cell division commenced. Synthesis of chlorophyll and phycocyanins started almost immediately after the addition of iron. The absorption peak slowly returned to its normal wavelength within 24 to 28 hours. The fluorescence emission spectrum at 77K changed over a period of 14 to 24 hours during which the 696- and 717-nanometer peaks grew to their normal levels, and the 684 nanometer peak moved to 687 nanometers and its relative intensity decreased to its normal level. Analysis of chlorophyll-protein complexes on polyacrylamide gels showed that high molecular weight chlorophyll-protein bands were formed during this time, and that low molecular weight bands (related to photosystem II) disappeared. The origin of the fluorescence emission at 687 and 696 nanometers is discussed in relation to the specific chlorophyll-protein complexes formed during iron reconstitution.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. M. The role of chlorophyll-protein complexes in the function and structure of chloroplast thylakoids. Mol Cell Biochem. 1982 Aug 6;46(3):161–172. doi: 10.1007/BF00239665. [DOI] [PubMed] [Google Scholar]
- Delepelaire P., Chua N. H. Lithium dodecyl sulfate/polyacrylamide gel electrophoresis of thylakoid membranes at 4 degrees C: Characterizations of two additional chlorophyll a-protein complexes. Proc Natl Acad Sci U S A. 1979 Jan;76(1):111–115. doi: 10.1073/pnas.76.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershoni J. M., Shochat S., Malkin S., Ohad I. Functional Organization of the Chlorophyll-Containing Complexes of Chlamydomonas reinhardi: A Study of Their Formation and Interconnection with Reaction Centers in the Greening Process of the y-1 Mutant. Plant Physiol. 1982 Sep;70(3):637–644. doi: 10.1104/pp.70.3.637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Chlorophyll-protein organization of membranes from the cyanobacterium Anacystis nidulans. Arch Biochem Biophys. 1983 Jan;220(1):155–166. doi: 10.1016/0003-9861(83)90396-x. [DOI] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Influence of Iron Deprivation on the Membrane Composition of Anacystis nidulans. Plant Physiol. 1984 Jan;74(1):90–95. doi: 10.1104/pp.74.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guikema J. A., Sherman L. A. Organization and Function of Chlorophyll in Membranes of Cyanobacteria during Iron Starvation. Plant Physiol. 1983 Oct;73(2):250–256. doi: 10.1104/pp.73.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh T., Gantt E. Photosynthetic vesicles with bound phycobilisomes from Anabaena variabilis. Biochim Biophys Acta. 1979 Jun 5;546(3):383–393. doi: 10.1016/0005-2728(79)90075-6. [DOI] [PubMed] [Google Scholar]
- Machold O. Lamellar proteins of green and chlorotic chloroplasts as affected by iron deficiency and antibiotics. Biochim Biophys Acta. 1971 May 13;238(2):324–331. doi: 10.1016/0005-2787(71)90099-2. [DOI] [PubMed] [Google Scholar]
- Manodori A., Melis A. Photochemical Apparatus Organization in Anacystis nidulans (Cyanophyceae) : Effect of CO(2) Concentration during Cell Growth. Plant Physiol. 1984 Jan;74(1):67–71. doi: 10.1104/pp.74.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishio J. N., Terry N. Iron nutrition-mediated chloroplast development. Plant Physiol. 1983 Mar;71(3):688–691. doi: 10.1104/pp.71.3.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman D. M., Sherman L. A. Effect of iron deficiency and iron restoration on ultrastructure of Anacystis nidulans. J Bacteriol. 1983 Oct;156(1):393–401. doi: 10.1128/jb.156.1.393-401.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]



