Abstract
Sequencing upstream of the Streptococcus mutans gene for a CcpA gene homolog, regM, revealed an open reading frame, named amy, with homology to genes encoding α-amylases. The deduced amino acid sequence showed a strong similarity (60% amino acid identity) to the intracellular α-amylase of Streptococcus bovis and, in common with this enzyme, lacked a signal sequence. Amylase activity was found only in S. mutans cell extracts, with no activity detected in culture supernatants. Inactivation of amy by insertion of an antibiotic resistance marker confirmed that S. mutans has a single α-amylase activity. The amylase activity was induced by maltose but not by starch, and no acid was produced from starch. S. mutans can, however, transport limit dextrins and maltooligosaccharides generated by salivary amylase, but inactivation of amy did not affect growth on these substrates or acid production. The amylase digested the glycogen-like intracellular polysaccharide (IPS) purified from S. mutans, but the amy mutant was able to digest and produce acid from IPS; thus, amylase does not appear to be essential for IPS breakdown. However, when grown on excess maltose, the amy mutant produced nearly threefold the amount of IPS produced by the parent strain. The role of Amy has not been established, but Amy appears to be important in the accumulation of IPS in S. mutans grown on maltose.
Dental caries arises as a consequence of enamel demineralization by organic acid end products formed by metabolism of dietary carbohydrates by bacteria in dental plaque. The organism believed to be the principal etiological agent of dental caries is Streptococcus mutans, and high levels of S. mutans at a tooth site are indicative of a high risk of subsequent caries development (15). Factors thought to determine the levels of S. mutans include the ability of this species to ferment a wide range of substrates and to withstand conditions of low pH. S. mutans can also accumulate a glycogen-like intracellular polysaccharide (IPS) containing mainly α-1,4-linked glucose units (11). This stored IPS is believed to be of significance, in the absence of fermentable dietary carbohydrates, in production of acid which can lead to enamel demineralization and contribute to S. mutans cariogenicity (35).
Dietary availability of carbohydrates is thus the major influence upon levels of S. mutans, and since starch is a major constituent of the human diet, it is important to investigate its metabolism by S. mutans. There are conflicting bodies of evidence from epidemiological studies on the cariogenicity of starch (25), though there is increasing concern that modern high-temperature processes for manufacturing starch-based food products may increase their fermentability. In North African countries, where the diet is extensively starch based, high numbers of S. mutans are found in plaque, though the fact that caries levels are generally low in these countries suggests that the means by which starch encourages S. mutans accumulation is not related to its fermentability (40).
Dental plaque exhibits starch-degrading activity, though this ability may be largely due to bound salivary α-amylase (8). In one report, S. mutans was demonstrated to have some starch-degrading activity but the enzymes involved were not characterized (10). Another study indicated that utilization of starch by S. mutans was dependent on the addition of exogenous α-amylase (4).
In this study, we report the nucleotide sequence of an intracellular α-amylase of S. mutans and our attempt to determine its function in the utilization of starch and in the synthesis and breakdown of IPS.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
Wild-type S. mutans (strain LT11) (36) was grown without agitation in either Todd-Hewitt broth (Oxoid) with 0.5% yeast extract (THYE), brain heart infusion (Oxoid), or the semidefined medium of Terleckyj et al. (38) modified by the replacement of individual amino acids with 0.5% casein hydrolysate (26). Carbohydrates were added as required at 0.5% unless otherwise stated. When required, kanamycin (500 μg ml−1) was used for selection in S. mutans, and ampicillin (100 μg ml−1), erythromycin (400 μg ml−1), or kanamycin (25 μg ml−1) was used for selection in Escherichia coli JM83 (44). Plasmid pOB209, used in a previous study (30), contains regM and also contains the amy gene in its entirety. Plasmid pOB219, which contains amy alone, was produced by ligating a 2.3-kbp EcoRI-EcoRV fragment from pOB209 into pUC19 digested with EcoRI and SmaI. This fragment extends from the EcoRV site at bp 1982 of pepQ regM (31) to the EcoRI site in the pBK-CMV phagemid (Stratagene) multiple cloning site.
DNA manipulations.
DNA manipulations and isolation of plasmid DNA were done by standard procedures (27). Southern blot analysis was carried out by using digoxigenin-labeled probes according to the manufacturer’s instructions (Boehringer Mannheim). Preparations of chromosomal DNA from S. mutans were obtained as previously described (39).
DNA sequencing.
Automated DNA sequencing (with a DNA sequencer from Applied Biosystems, Inc.) was performed by the Molecular Biology Facility, University of Newcastle upon Tyne. Sequencing was carried out with the universal and reverse primers or custom oligonucleotides on subclones in pUC vectors. All sequencing was done in both directions with overlapping clones. DNA and protein sequences were manipulated by using IBI-Pustell and PC-Gene software packages.
Insertional inactivation.
In order to inactivate the amy gene, the EcoRI-EcoRV fragment of pOB219 was cloned into appropriate sites in plasmid pVA8912 (derived from pVA8911 by removal of the PvuII-PvuII fragment [16]), which replicates in E. coli but not in streptococci. This intermediate vector was used to avoid any possibility of introducing ampicillin resistance from a pUC-based vector into S. mutans. The omega-Km2 element, encoding kanamycin resistance (20), was cloned into a KpnI site within the amy gene, and the plasmid was linearized before transformation into S. mutans to allow integration into the chromosome by a double-crossover event.
S. mutans LT11 was transformed by the addition of plasmid DNA to cells in early exponential growth phase in THYE. Cultures were incubated for a further 2 h before aliquots were plated on brain heart infusion agar supplemented with kanamycin. Southern blot analysis was used to confirm integration of the plasmid into the appropriate gene on the S. mutans chromosome.
Hydrolysis of starch.
The ability of S. mutans LT11 and the amy mutant to degrade starch was determined by culturing them on plates containing TH broth plus 1% starch agar. After anaerobic incubation at 37°C for 2 days, the plates were flooded with 0.2% iodine in 2% KI; a clear zone around the growth indicated hydrolysis of starch.
SDS-PAGE and detection of starch-hydrolyzing activity.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and zymography were carried out as previously described by Whitehead and Cotta (43).
Preparation of cell extracts.
Cells of S. mutans or E. coli were harvested by centrifugation, washed twice in 50 mM Tris-HCl (pH 7.5), and resuspended in a small volume of the same buffer. Cell extracts were prepared by sonicating the cells with 10 1-min pulses in the presence of 0.1-mm-diameter glass beads, with cooling on ice. Extracts were centrifuged in a microcentrifuge to remove beads and cell debris. Protein concentrations were determined by using Coomassie protein assay reagent (Pierce) with bovine serum albumin as a standard. Cell extracts were diluted with 50 mM Tris-HCl (pH 7.5) to a standard protein concentration and used in amylase activity assays.
Determination of amylase activity.
The amylase activity of cell extracts was determined by monitoring the release of reducing sugar from starch by using dinitrosalicylic acid reagent (5, 21). Cell extracts (50 μl) were mixed with an equal volume of either 50 mM Tris-HCl (pH 7.5) or starch solution (1% soluble potato starch in 50 mM Tris-HCl [pH 7.5]–0.06% CaCl2 · 2H2O) in a microtiter tray and incubated at 37°C. Enzyme activity was stopped by the addition of 150 μl of dinitrosalicylic acid (21), and the tray was heated at 100°C for 20 min. The tray was cooled to room temperature, and the absorbance at 620 nm was determined with a Titertek plate reader. Maltose was used to produce a standard curve. One enzyme unit equaled 1 nmol of maltose equivalent produced per min.
Identification of products.
Thin-layer chromatography (TLC) was used to analyze products resulting from the action of the cloned Amy enzyme and S. mutans cell extracts on starch, amylose, amylopectin, maltotriose, maltotetraose, maltopentaose, and maltooligosaccharide mixture (Pfanstiehl Laboratories, Inc.) and IPS purified from S. mutans LT11. Cell extracts (0.1 to 10 ml) were incubated with 50 μl of 10-mg ml−1 substrate in 50 mM Tris-HCl (pH 7.5)–0.03% CaCl2 at 37°C overnight. Digestion products were separated on Silica Gel 60 (Whatman) plates by two ascents in 1:1:6:3 (by volume) ammonia–ethyl acetate–propan-1-o1–water solvent (6). The sugars were detected by dipping the plate in 0.5% (wt/vol) α-naphthol–5% (vol/vol) H2SO4 in ethanol and heating to 120°C.
Acid production from extracellular carbohydrate.
The procedure for determining the level of acid production was essentially that described by Marsh et al. (17). S. mutans LT11 and the amy mutant were grown overnight in TH broth. Cells were harvested and washed in 135 mM KCl. Harvested cells were washed twice, resuspended in 135 mM KCl to an optical density at 620 nm (OD620) of 1.0, and incubated at 37°C for 30 min. After this starvation period, cells were washed twice in 135 mM KCl before resuspension in 1/10 volume of the same buffer. Resuspended cells (4.75 ml) were incubated at 37°C, and the pH was adjusted to approximately 7.00 with 0.01 M NaOH with a model PHM290 pH-Stat controller (Radiometer Copenhagen). Carbohydrate was then added to 0.5%, and the pH of the cell suspension was recorded for a further 5 min.
Growth curves.
Cells were grown overnight in semidefined medium containing 0.5% glucose. Overnight cultures were harvested, washed twice in sugar-free medium, and added to fresh medium containing either 0.1% glucose, 0.1% maltose, or 0.1% maltooligosaccharide mixture to a standard OD620. Cultures were incubated at 37°C and samples were taken every 30 min in order to monitor growth spectrophotometrically.
For determination of IPS concentration during growth on excess sugar, cells were grown overnight in THYE supplemented with 0.5% glucose or 0.5% maltose. Cultures were diluted in fresh medium, THYE with 2% glucose or maltose, to a standard OD620. Cultures were incubated at 37°C and samples were taken every 30 min in order to monitor growth spectrophotometrically. Samples were removed at two points during exponential growth, at early stationary phase, 2 h after stationary phase was reached, and at 24 h. These samples were used to determine IPS content.
Determination of IPS content.
The IPS content of S. mutans cultures was determined by alkaline hydrolysis followed by the alkaline complex method of DiPersio et al. (7, 34). Cells were harvested and washed in 150 mM KPO4 buffer (pH 7.0). The cells were resuspended in 150 mM KPO4 buffer (pH 7.0) to an OD620 of 1.0. The cells were collected by centrifugation and resuspended to 1/5 volume. Aliquots of 100 μl each were collected, and 30 μl of 5.3 M KOH was added before boiling for 90 min. After cooling of the aliquots, 30 ml of 5.3 M HCl, 150 μl of 1 M KPO4 buffer (pH 7.0), and 60 μl of freshly prepared 0.2% iodine in 2% KI solution were added. The sample was mixed, and 200 μl was transferred to a microtiter tray. The absorbance of the resulting polysaccharide-iodine complex was determined by measurement in a Titertek plate reader at 540 nm. Glycogen was used to prepare a standard curve.
For monitoring digestion of IPS, cells were harvested from cultures grown on THYE plus 2% glucose or maltose after 2 h in stationary phase and processed as described above. CaCl2 was added to 0.03% to the cell suspension before incubation at 37°C. Samples of 100 μl each were collected over a 1-h period, and the IPS content was determined.
Acid production from IPS.
S. mutans LT11 and the amy mutant were grown in THYE plus 2% glucose or maltose to stationary phase, and the cultures were then incubated at 37°C for a further 2 h. A 5-ml sample of culture was removed for determining IPS consumption, and the remaining cells were harvested, washed in 135 mM KCl, and resuspended in 135 mM KCl to an OD620 of 1.0. Cells were harvested and resuspended in 1/10 volume of 135 mM KCl. The cell suspension was placed in a 37°C water bath, and the pH was adjusted to about 7.3 with 0.01 M NaOH. Incubation was continued at 37°C, and the pH was monitored with a model PHM290 pH-Stat controller (Radiometer Copenhagen) over a 15-min period.
Purification of IPS.
S. mutans LT11 and the amy mutant were grown in 200 ml of THYE or semidefined medium with 2% glucose or maltose for 24 h. IPS was extracted and purified by the method of DiPersio et al. (7), as follows. Cells were harvested and washed twice with cold distilled water. A freshly made solution of 30% (wt/vol) KOH was added to make a final volume of 10 ml, and the suspension was boiled for 90 min. Extracts were centrifuged (8,000 × g, 15 min), and the pellet was discarded. IPS was purified by two precipitations with an equal volume of 95% ethanol, with cooling to −80°C, and then lyophilized.
Nucleotide sequence accession number.
The sequence shown in Fig. 1B has been assigned GenBank accession no. AF055987.
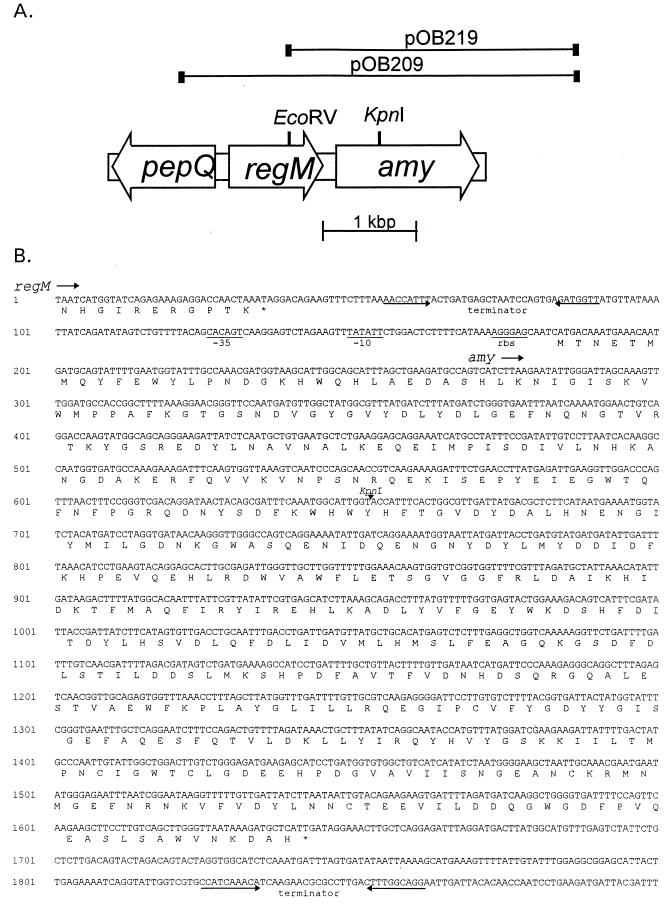
FIG. 1.
Sequence analysis of the amy locus. (A) Genetic organization of the amy region in S. mutans. Clones expressing amylase activity are shown, with the EcoRV site used for producing subclone pOB219 and the KpnI site used to inactivate the amy gene by insertion of a kanamycin resistance cassette. (B) Nucleotide sequence and deduced amino acid sequence of the amy region from the end of regM. Potential ribosome binding sites (rbs), −10 and −35 promoter elements, and terminators are indicated. The KpnI site used to inactivate the amy gene by insertion of a kanamycin resistance cassette is indicated.
RESULTS
Sequence analysis of the amy gene.
Plasmid pOB209 was used in a previous study where a homolog of CcpA in S. mutans, RegM, was identified (31). Nucleotide sequencing revealed an open reading frame downstream of regM, and a BLAST search (1, 9) revealed homology to amylase genes of Streptococcus bovis and Bacillus spp. The deduced protein sequence had a high degree of similarity to the amylase proteins of a number of other organisms, particularly to the intracellular amylase of S. bovis, with which it had 60% amino acid identity.
The gene, which we named amy, has the same polarity as regM, with 150 bp interposed between them (Fig. 1). A potential terminator of regM with a free energy value of −4.4 kcal was identified between the genes. Nucleotide sequencing confirmed that the plasmids contain the amy gene in its entirety. The nucleotide sequence of the amy gene was 1,461 bp in length and was preceded by a putative ribosome binding site (AGGAG) located 6 bp upstream of a methionine codon. A potential promoter was also identified, with a putative −10 (TATATT) sequence beginning at base 149 and a −35 (CACAGT) sequence beginning at base 126 (Fig. 1B). A potential terminator with a free energy value of −3.8 kcal was also identified. Unlike genes encoding amylases in Bacillus spp., the amy gene is not preceded by a catabolite-responsive element (CRE) sequence as determined by using the consensus sequence of Hueck et al. (13). The deduced amino acid sequence defined a protein of 486 amino acids, with a calculated molecular weight of 56,347. A protein of this size with starch-hydrolyzing activity was detected in whole-cell extracts of E. coli carrying pOB209 after SDS-PAGE and incubation with starch. No signal peptide could be identified with the SignalP program of Nielsen et al. (19), and no starch-binding domain could be identified by using the consensus sequence defined by Svensson et al. (33). We were also unable to experimentally demonstrate any binding of the amylase to cornstarch, α-cyclodextrin, or β-cyclodextrin (data not shown).
Inactivation of amy.
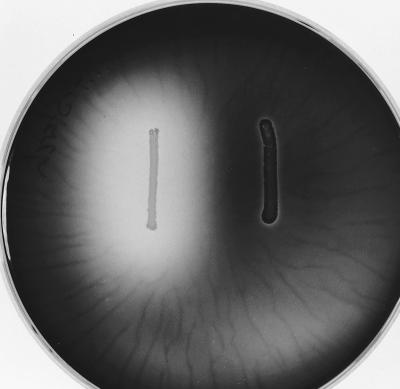
To determine the function of Amy in S. mutans, the amy gene was interrupted by the insertion of a kanamycin resistance cassette as described in Materials and Methods. The insertion was confirmed by Southern blot analysis, and the loss of starch-hydrolyzing activity was demonstrated by iodine staining after 2 days’ growth on plates containing TH broth plus 1% starch agar (Fig. 2). Inactivation of amy was confirmed by amylase assays of cell extracts. Despite the apparent release of enzyme on starch-agar plates, amylase activity could not be detected in 65% ammonium sulfate precipitates of culture supernatants of either the wild type or the amy mutant. These results suggest that S. mutans has a single amylase activity which is intracellular and is encoded by the amy gene.
FIG. 2.
Hydrolysis of starch in agar plates by S. mutans LT11 (left) and the amy mutant (right).
Regulation of amylase.
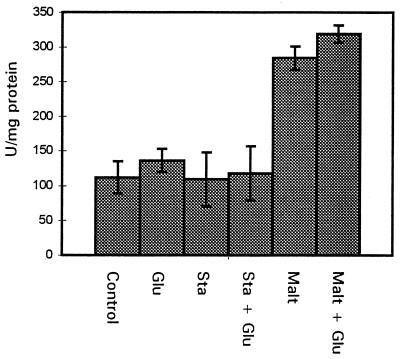
The amylases of S. bovis have been reported to be induced by starch and maltose and catabolite repressed by glucose (3, 5, 28). In order to determine if this was the case in S. mutans, cell extracts were prepared from overnight cultures of S. mutans LT11 grown in THYE with the addition of starch or maltose. Starch had no effect on amylase activity, but the addition of maltose increased amylase activity two- to threefold (Fig. 3). To test for catabolite repression, glucose was included in the media. This did not result in a reduction in amylase activity, indicating that the intracellular amylase is not catabolite repressed by glucose in S. mutans.
FIG. 3.
Amylase activities of S. mutans LT11 after overnight growth in THYE plus 0.5% maltose (Malt), starch (Sta), and/or glucose (Glu).
Analysis of products.
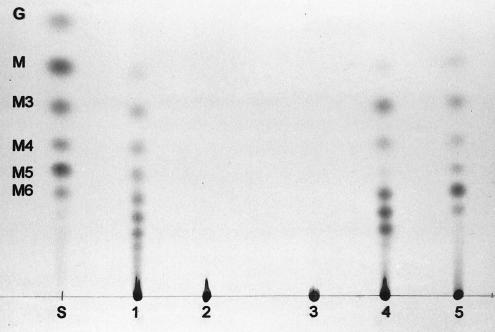
TLC analysis was used to determine the range of substrates the cloned amy activity was able to hydrolyze. Cell extracts of E. coli carrying pOB219, containing the entire amy gene, hydrolyzed soluble starch, amylose, and amylopectin. The products of hydrolysis were sugars ranging from glucose to maltohexaose and larger maltooligosaccharides (Fig. 4). Cell extracts of S. mutans LT11 produced the same pattern of products from starch, whereas the amy mutant was unable to act on starch at all. Cell extracts of recombinant E. coli expressing amy also hydrolyzed IPS produced by S. mutans, giving the same range of products as starch hydrolysis (Fig. 4), but greater amounts of enzyme activity were required to achieve complete digestion. The pattern of products was not affected by whether IPS was formed in the presence of glucose or maltose or whether it was synthesized by S. mutans LT11 or the amy mutant.
FIG. 4.
TLC analysis of starch and IPS digested by cell extracts overnight. Lane S, glucose (G), maltose (M), and maltooligosaccharide (M3 through M6) standards; lanes 1 to 4, starch digested by S. mutans LT11, the amy mutant, E. coli JM83, and E. coli JM83(pOB219), respectively; lane 5, IPS digested by E. coli JM83(pOB219).
Effect of amy on growth and IPS production.
In order to determine whether loss of amy affected growth rates, the growth of the wild-type S. mutans strain, LT11, was compared to that of the amy mutant. Growth rates in semidefined media with glucose, maltose, or maltooligosaccharides as the sole carbon source were not affected by the amy mutation.
Growth of the S. mutans wild type in THYE with excess sugar (2% glucose or maltose) was also compared with that of the amy mutant. Although growth rates were unaffected by loss of amy, the amy mutant reached a higher OD620 than the wild type when grown on maltose (Fig. 5). The IPS content of the cells was also monitored during exponential and stationary phases. The S. mutans wild type and amy mutant produced similar amounts of IPS when grown on excess glucose, but the amy mutant produced nearly three times as much IPS as the S. mutans wild type when grown in the presence of excess maltose.
FIG. 5.
Growth (⧫) and IPS production (▨) of S. mutans LT11 (wild type) and the amy mutant in THYE with 2% glucose and in THYE with 2% maltose.
Acid production from extracellular carbohydrates.
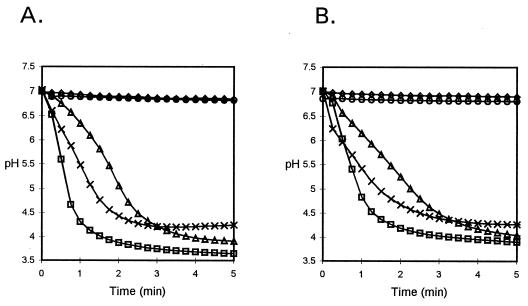
The ability of S. mutans LT11 and the amy mutant to ferment carbohydrates was determined by monitoring the drop in pH of an unbuffered cell suspension in the presence of 0.5% exogenous carbohydrate. Both the wild type and the mutant produced acid in the presence of glucose, maltose, and maltooligosaccharides, but neither produced acid from starch (Fig. 6).
FIG. 6.
Drop in pH of S. mutans LT11 (wild type) (A) and the amy mutant (B) in the absence of exogenous sugar (◊) and in the presence of 0.5% glucose (□), maltose (▵), maltooligosaccharides (×), and starch (○).
Acid production from intracellular carbohydrate.
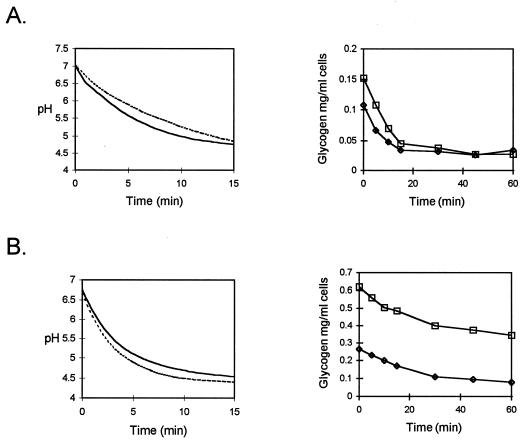
The ability of S. mutans LT11 and the amy mutant to ferment IPS was determined by monitoring the drop in pH of cells grown to stationary phase in excess glucose or maltose. The wild type and the amy mutant produced similar pH drops after prior growth in either 2% glucose or 2% maltose to accumulate IPS (Fig. 7). Determining the IPS content of cells from the same cultures during incubation at 37°C revealed that the production of acid was paralleled by the consumption of IPS (Fig. 7). The amy mutant retained the ability to digest IPS produced during growth on glucose or maltose. Despite much higher levels of IPS in the maltose-grown amy mutant culture, the rate of acid production and the final pH reached were unaffected (Fig. 7).
FIG. 7.
Drop in pH and digestion of IPS after growth of wild-type S. mutans (—, pH; ⧫, digestion of IPS) and amy mutant (----, pH; □, digestion of IPS) in 2% glucose (A) or maltose (B).
DISCUSSION
In this study a gene from S. mutans LT11 encoding an intracellular α-amylase has been sequenced and its product has been partially characterized. Genes encoding intracellular α-amylases have previously been reported for E. coli and S. bovis, and the α-amylase of S. mutans has 60% amino acid identity to the latter (24, 28, 29, 43). Although there has been some characterization of these activities, no clear physiological role for intracellular α-amylase has been established for either E. coli or S. bovis. However, in S. bovis inactivation of the gene encoding intracellular α-amylase resulted in growth rates of 15 to 20% of that of the wild type, leading to the postulation that the intracellular enzyme plays an important role in rapid cell growth (3). In contrast to these findings, inactivation of intracellular amylase in S. mutans did not affect growth, nor did it affect the rate of acid production from glucose, maltose, or maltooligosaccharides. Genes homologous to intracellular amylases can also be found in Salmonella typhimurium (24) and Streptococcus pneumoniae (14), and intracellular α-amylase activity has also been detected in Streptococcus salivarius (43), though for these organisms no information on function is available.
S. mutans has previously been reported to have extracellular amylase activity (10), on the basis of hydrolysis of starch in agar plates. The protein encoded by amy does not contain a signal peptide typical of secreted proteins and appears to be located predominantly intracellularly. However, it is responsible for the clearing of starch observed around colonies, because the mutant in which the amy gene had been insertionally inactivated was unable to hydrolyze starch in agar plates. The mechanism by which some of the amylase activity escapes the cell is unknown, and in liquid medium, S. mutans does not have sufficient extracellular amylase activity to enable it to produce acid from starch or to grow on starch as a sole carbon source. In agreement with a previous finding that S. mutans required exogenous α-amylase in order to hydrolyze starch (4), addition of salivary α-amylase (10 U/ml; Sigma) enabled S. mutans to produce acid from starch (data not shown). S. mutans can thus utilize limit dextrins from starch digestion, which can be taken up by the multiple sugar metabolism transport system (37).
The extracellular α-amylases from both Bacillus spp. and S. bovis are catabolite repressed by glucose (5, 12, 22). We at first thought that the S. mutans amylase was also catabolite repressed because no clearing was seen on starch-agar plates to which 1% glucose had been added (30, 31). However, determination of α-amylase activity in cell extracts of liquid cultures showed no evidence for catabolite repression. The effect seen on plates may therefore be due to some other factor, such as local pH or an effect on release of amylase from the cell. Catabolite-repressed genes in gram-positive bacteria are generally believed to have 14-bp palindromic sequences called CREs within or near their promoter regions, where binding of catabolite control proteins prevents transcription (13). CRE sequences could not be identified in the promoter region of the intracellular α-amylase gene of either S. mutans or S. bovis by the method of Hueck et al. (i.e., one mismatch to the consensus sequence within 200 bp of the translation start site [13]).
As S. mutans is unable to produce acid from starch, it seems unlikely that Amy plays a role in starch utilization. Furthermore, its spectrum of action on starch, amylose, and amylopectin is not substantially different from that of salivary amylase, so it would play no further role in the metabolism of low-molecular-weight starch degradation products once they are transported into the cell. The only high-molecular-weight substrate therefore available to amylase within the cell would be IPS, and it has previously been suggested that in E. coli the intracellular amylase AmyA may be involved in the breakdown of IPS (24). Purified AmyA from E. coli does show some activity against glycogen, although increased concentrations of enzyme and prolonged incubation times were required before digestion products were detected (24). A similar result was obtained with the cloned activity from S. mutans. The amylase is thus capable of degrading IPS, but this activity does not appear to be of physiological importance, because inactivation of amy had no effect on either the rate of breakdown or the rate of acid production from IPS.
The only clear difference between the amy mutant and the parent strain which we observed was in the amount of IPS accumulated during growth on maltose. This result seems paradoxical, since amylase can degrade IPS. When glucose is present in excess, IPS is synthesized from glucose-1-phosphate and ADP-glucose by the sequential action of ADP-glucose pyrophosphorylase and ADP-glucose-glycogen glucosyltransferase, a pathway which is found in a wide range of organisms, including S. mutans (2, 23, 32). Less is known about the synthesis of IPS from maltose. Some species, for example, Streptococcus pyogenes, can make IPS only from maltose (18), and the enzyme responsible is thought to be amylomaltase (22), which can synthesize a low-molecular-weight α-1,4-glucan chain from maltose. Another enzyme which might be involved is the transglucosylase identified in both S. bovis and Streptococcus mitis by Walker (41, 42), which synthesizes higher-molecular-weight maltodextrins from maltotriose. Neither of these activities has yet been reported for S. mutans, but if they are present and contribute to the accumulation of IPS during growth on maltose, or during growth on starch when exogenous amylase is present, they provide a substrate for the intracellular amylase and hence provide an explanation for the greater accumulation of IPS observed in the amy mutant than in the wild-type S. mutans. The function of the amylase might thus be to trim or in some way modulate the accumulation of IPS. Further work will be required to characterize the structure of IPS in S. mutans grown on maltose in order to resolve this issue.
ACKNOWLEDGMENT
This work was supported by Medical Research Council grant G9505672PB.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Birkhed D, Tanzer J M. Glycogen synthesis pathway in Streptococcus mutans strain NCTC 10449S and its glycogen synthesis-defective mutant 805. Arch Oral Biol. 1979;24:67–73. doi: 10.1016/0003-9969(79)90177-8. [DOI] [PubMed] [Google Scholar]
- 3.Brooker J D, McCarthy J M. Gene knockout of the intracellular amylase gene by homologous recombination in Streptococcus bovis. Curr Microbiol. 1997;35:133–138. doi: 10.1007/s002849900226. [DOI] [PubMed] [Google Scholar]
- 4.Clarkson B H, Krell D, Wefel J S, Crall J, Feagin F F. In vitro caries-like lesion production by Streptococcus mutans and Actinomyces viscosus using sucrose and starch. J Dent Res. 1987;66:795–798. doi: 10.1177/00220345870660031801. [DOI] [PubMed] [Google Scholar]
- 5.Cotta M A, Whitehead T R. Regulation and cloning of the gene encoding amylase activity of the ruminal bacterium Streptococcus bovis. Appl Environ Microbiol. 1993;59:189–196. doi: 10.1128/aem.59.1.189-196.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Defretin, S. Personal communication.
- 7.DiPersio J R, Mattingly S J, Higgins M L, Shockman G D. Measurement of intracellular iodophilic polysaccharide in two cariogenic strains of Streptococcus mutans by cytochemical and chemical methods. Infect Immun. 1974;10:597–604. doi: 10.1128/iai.10.3.597-604.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Douglas C W I. The binding of human salivary α-amylase by oral strains of streptococcal bacteria. Arch Oral Biol. 1983;28:567–573. doi: 10.1016/0003-9969(83)90003-1. [DOI] [PubMed] [Google Scholar]
- 9.Gish W, States D J. Identification of protein coding regions by database similarity search. Nat Genet. 1993;3:266–272. doi: 10.1038/ng0393-266. [DOI] [PubMed] [Google Scholar]
- 10.Glor E B, Miller C H, Spandau D F. Degradation of starch and its hydrolytic products by oral bacteria. J Dent Res. 1988;67:75–81. doi: 10.1177/00220345880670011501. [DOI] [PubMed] [Google Scholar]
- 11.Hamilton I R. Intracellular polysaccharide synthesis by cariogenic microorganisms. In: Stiles H M, Loesche W J, O’Brien T L, editors. Proceedings: Microbial Aspects of Dental Caries. Special supplement to Microbiology abstracts. Vol. 3. Washington, D.C: Information Retrieval, Inc.; 1976. pp. 683–701. [Google Scholar]
- 12.Heineken F G, O’Connor R J. Continuous culture studies on the biosynthesis of alkaline protease, neutral protease and α-amylase by Bacillus subtilis NRRL-B3411. J Gen Microbiol. 1972;73:35–44. doi: 10.1099/00221287-73-1-35. [DOI] [PubMed] [Google Scholar]
- 13.Hueck C J, Hillen W, Saier M H., Jr Analysis of a cis-active sequence mediating catabolite repression in Gram-positive bacteria. Res Microbiol. 1994;145:503–518. doi: 10.1016/0923-2508(94)90028-0. [DOI] [PubMed] [Google Scholar]
- 14.The Institute for Genomic Research.ftp://ftp.tigr.org/pub/data/s_pneumoniae/. The Institute for Genomic Research, Rockville, Md.
- 15.Loesche W J. Role of Streptococcus mutans in human dental decay. Microbiol Rev. 1986;50:353–380. doi: 10.1128/mr.50.4.353-380.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Malke H, Mechold U, Gase K, Gerlach D. Inactivation of the streptokinase gene prevents Streptococcus equisimilis H46A from acquiring cell-associated plasmin activity in the presence of plasminogen. FEMS Microbiol Lett. 1994;116:107–112. doi: 10.1111/j.1574-6968.1994.tb06683.x. [DOI] [PubMed] [Google Scholar]
- 17.Marsh P D, Williamson M I, Keevil C W, McDermid A S, Ellwood D C. Influence of sodium and potassium ions on acid production by washed cells of Streptococcus mutans Ingbritt and Streptococcus sanguis NCTC 7865 grown in a chemostat. Infect Immun. 1982;36:476–483. doi: 10.1128/iai.36.2.476-483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McFarland C R, Snyder T L, McKenzie R. Polysaccharide storage in different streptococci. Microbios. 1984;40:7–14. [PubMed] [Google Scholar]
- 19.Nielsen H, Engelbrecht J, Brunak S, von Heijne G. Identification of prokaryotic and eukaryotic signal peptides and prediction of their cleavage sites. Protein Eng. 1997;10:1–6. doi: 10.1093/protein/10.1.1. [DOI] [PubMed] [Google Scholar]
- 20.Perez-Casal J, Caparon M G, Scott J R. Mry, a trans-acting positive regulator of the M protein gene of Streptococcus pyogenes with similarity to the receptor proteins of two-component regulatory systems. J Bacteriol. 1991;173:2617–2624. doi: 10.1128/jb.173.8.2617-2624.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pettersson G, Porath J. A cellulolytic enzyme from Penicillium notatum. Methods Enzymol. 1966;8:603–607. [Google Scholar]
- 22.Preiss J, Romeo T. Physiology, biochemistry and genetics of bacterial glycogen synthesis. Adv Microb Physiol. 1989;30:183–238. doi: 10.1016/s0065-2911(08)60113-7. [DOI] [PubMed] [Google Scholar]
- 23.Priest F G. Effect of glucose and cyclic nucleotides on the transcription of α-amylase mRNA in Bacillus subtilis. Biochem Biophys Res Commun. 1974;63:606–607. doi: 10.1016/s0006-291x(75)80427-x. [DOI] [PubMed] [Google Scholar]
- 24.Raha M, Kawagishi I, Müller V, Kihara M, Macnab R M. Escherichia coli produces a cytoplasmic α-amylase, AmyA. J Bacteriol. 1992;174:6644–6652. doi: 10.1128/jb.174.20.6644-6652.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rugg-Gunn A J. Nutrition and dental health. Oxford, United Kingdom: Oxford University Press; 1993. pp. 194–222. [Google Scholar]
- 26.Russell R R B. Purification of Streptococcus mutans glucosyltransferase by polyethylene glycol precipitation. FEMS Microbiol Lett. 1979;6:197–199. [Google Scholar]
- 27.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 28.Satoh E, Uchimura T, Kudo T, Komagata K. Purification, characterization, and nucleotide sequence of an intracellular maltotriose-producing α-amylase from Streptococcus bovis 148. Appl Environ Microbiol. 1997;63:4941–4944. doi: 10.1128/aem.63.12.4941-4944.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Satoh E, Niimura Y, Uchimura T, Kozaki M, Komagata K. Molecular cloning and expression of two α-amylase genes from Streptococcus bovis 148 in Escherichia coli. Appl Environ Microbiol. 1993;59:3669–3673. doi: 10.1128/aem.59.11.3669-3673.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Simpson C L, Russell R R B. Catabolite repression in Streptococcus mutans. J Dent Res. 1996;75:1186. [Google Scholar]
- 31.Simpson C L, Russell R R B. Identification of a homolog of CcpA catabolite repressor protein in Streptococcus mutans. Infect Immun. 1998;66:2085–2092. doi: 10.1128/iai.66.5.2085-2092.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Spatafora Harris G, Michalek S M, Curtiss R., III Cloning of a locus involved in Streptococcus mutans intracellular polysaccharide accumulation and virulence testing of an intracellular polysaccharide-deficient mutant. Infect Immun. 1992;60:3175–3185. doi: 10.1128/iai.60.8.3175-3185.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Svensson B, Jespersen H, Sierks M R, MacGregor E A. Sequence homology between putative raw-starch binding domains from different starch-degrading enzymes. Biochem J. 1989;264:309–311. doi: 10.1042/bj2640309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Takahashi N, Iwami Y, Yamada T. Metabolism of intracellular polysaccharide in the cells of Streptococcus mutans under strictly anaerobic conditions. Oral Microbiol Immunol. 1991;6:299–304. doi: 10.1111/j.1399-302x.1991.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 35.Tanzer J M, Freedman M L, Woodiel R N, Eifert R L, Rinehimer L A. Association of Streptococcus mutans virulence with synthesis of intracellular polysaccharide. In: Stiles H M, Loesche W J, O’Brien T L, editors. Proceedings: Microbial Aspects of Dental Caries. Special supplement to Microbiology abstracts. Vol. 3. Washington, D.C: Information Retrieval, Inc.; 1976. pp. 597–616. [Google Scholar]
- 36.Tao L, MacAlister T J, Tanzer J M. Transformation efficiency of EMS-induced mutants of Streptococcus mutans of altered cell shape. J Dent Res. 1993;72:1032–1039. doi: 10.1177/00220345930720060701. [DOI] [PubMed] [Google Scholar]
- 37.Tao L, Sutcliffe I C, Russell R R B, Ferretti J J. Cloning and expression of the multiple sugar metabolism (msm) operon of Streptococcus mutans in heterologous streptococcal hosts. Infect Immun. 1993;61:1121–1125. doi: 10.1128/iai.61.3.1121-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Terleckyj B, Willett N P, Shockman G D. Growth of several cariogenic strains of oral streptococci in a chemically defined medium. Infect Immun. 1975;11:649–655. doi: 10.1128/iai.11.4.649-655.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ushiro I, Lumb S M, Aduse-Opoku J, Ferretti J J, Russell R R B. Chromosomal deletions in melibiose-negative isolates of Streptococcus mutans. J Dent Res. 1991;70:1422–1426. doi: 10.1177/00220345910700110501. [DOI] [PubMed] [Google Scholar]
- 40.van Palenstein Helderman W H, Matee M I N, van der Hoeven J S, Mikx F H M. Cariogenicity depends more on diet than the prevalent mutans streptococcal species. J Dent Res. 1996;75:535–545. doi: 10.1177/00220345960750010501. [DOI] [PubMed] [Google Scholar]
- 41.Walker G J. A transglucosylase of Streptococcus bovis. Biochem J. 1965;94:299–308. doi: 10.1042/bj0940299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Walker G J. Metabolism of the reserve polysaccharide of Streptococcus mitis: properties of a transglucosylase. Biochem J. 1966;101:861–872. doi: 10.1042/bj1010861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Whitehead T R, Cotta M A. Identification of intracellular amylase activity in Streptococcus bovis and Streptococcus salivarius. Curr Microbiol. 1995;30:143–148. doi: 10.1007/BF00296199. [DOI] [PubMed] [Google Scholar]
- 44.Yanisch-Perron C, Vieira J, Messing J. Improved M13 phage cloning vectors and host strains: nucleotide sequences of the M13mp18 and pUC19 vectors. Gene. 1985;33:103–119. doi: 10.1016/0378-1119(85)90120-9. [DOI] [PubMed] [Google Scholar]