Abstract
In this report, we compared the effects on the growth of Lactobacillus plantarum of raising the medium molarity by high concentrations of KCl or NaCl and iso-osmotic concentrations of nonionic compounds. Analysis of cellular extracts for organic constituents by nuclear magnetic resonance spectroscopy showed that salt-stressed cells do not contain detectable amounts of organic osmolytes, whereas sugar-stressed cells contain sugar (and some sugar-derived) compounds. The cytoplasmic concentrations of lactose and sucrose in growing cells are always similar to the concentrations in the medium. By using the activity of the glycine betaine transport system as a measure of hyperosmotic conditions, we show that, in contrast to KCl and NaCl, high concentrations of sugars (lactose or sucrose) impose only a transient osmotic stress because external and internal sugars equilibrate after some time. Analysis of lactose (and sucrose) uptake also indicates that the corresponding transport systems are neither significantly induced nor activated directly by hyperosmotic conditions. The systems operate by facilitated diffusion and have very high apparent affinity constants for transport (>50 mM for lactose), which explains why low sugar concentrations do not protect against hyperosmotic conditions. We conclude that the more severe growth inhibition by salt stress than by equiosmolal concentrations of sugars reflects the inability of the cells to accumulate K+ (or Na+) to levels high enough to restore turgor as well as deleterious effects of the electrolytes intracellularly.
The responses of microorganisms to growth-inhibiting salt concentrations have been studied extensively (for reviews, see references 1 and 3). In a number of cases, the effects of both salt and sugar stress on the accumulation of individual compatible solutes such as K+ and glycine betaine have been determined (1, 5, 6, 10, 11). However, only in a few studies have the osmolytes accumulated in salt-stressed cells been compared with those in sugar (nonionic)-stressed cells (2, 12). In one study, it was reported that levels of accumulation of the major compatible solutes N-acetylglutaminyl-glutamine amide, glucosylglycerol, and glutamate were similar irrespective of the type of osmotic stress (2), whereas large differences between sugar- and salt-stressed cells were observed in another study (12).
Growth stimulation of osmotically stressed cells by exogenous glycine betaine is frequently observed, because most microorganisms cannot synthesize this compound and therefore must take up glycine betaine from the medium (4, 5, 8, 10, 16). The effects of glycine betaine on the growth of salt- and sugar-stressed cells are not always the same. In the family Enterobacteriaceae (Escherichia coli, Salmonella typhimurium, and Klebsiella pneumoniae), glycine betaine stimulated growth with both ionic and nonionic (sucrose) stresses (6), whereas in the lactic acid bacteria Lactococcus lactis and Lactobacillus plantarum, a stimulatory effect of glycine betaine was observed only when a salt (KCl or NaCl) stress was applied (5, 10). It should be emphasized that KCl and NaCl inhibited growth of the lactic acid bacteria much more than equiosmolar concentrations of sucrose.
In this study, the mechanisms underlying the responses of L. plantarum towards salt and sugar stress were investigated. The osmolyte pools in cellular extracts from cells cultured at high KCl, NaCl, sucrose, lactose, and sorbitol concentrations were determined by nuclear magnetic resonance (NMR) spectroscopy and by high-pressure liquid chromatography (HPLC). Our data provide a rationale for the different effects exerted on the organism by high concentrations of inorganic ions and sugars.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and media.
L. plantarum ATCC 14917 was grown at 30°C in a chemically defined medium (CDM; pH 6.7) containing 0.5% (wt/vol) glucose as described previously (5). High-osmolarity media were obtained by adding KCl (0.8 M), NaCl (0.8 M), sucrose (35% [wt/vol]), lactose (35% [wt/vol]), sorbitol (30% [wt/vol]), or xylitol (30% [wt/vol]) to the standard CDM at the concentrations indicated in parentheses. The osmolality of the solutions was measured by freezing-point depression with an Osmomat 030 (Gonotec, Berlin, Germany). The osmolality of 0.8 M KCl (or NaCl) equaled 1.35 osM/kg, whereas a similar osmolality of a sucrose (or lactose) solution extrapolated to a concentration of about 1 M (∼35% [wt/vol]) (data not shown).
Cellular K+ and Na+ concentrations.
Exponentially growing cells of L. plantarum (about 1 mg of total cell protein) were collected on cellulose acetate filters with a pore size of 0.45 μm (Schleicher & Schuell GmbH, Dassel, Germany) by a manifold filtration apparatus. The filters were washed twice with 50 mM sodium-free potassium phosphate (pH 6.5) (Na+ determination) or sodium phosphate (pH 6.5) (K+ determination), without (low-osmolarity cultures) or with (high-osmolarity cultures) 0.8 M KCl or NaCl. The filters were transferred to vials containing 0.3 ml of 5% (vol/vol) perchloric acid. After 30 min of incubation, the cellular extracts were centrifuged and the supernatant was used to determine the Na+ or K+ concentration by atomic absorption, using a Perkin-Elmer 3030 spectrophotometer.
NMR spectroscopy.
Exponentially growing cells of L. plantarum were harvested and washed with 50 mM potassium phosphate (pH 6.5) without (low-osmolarity cultures) or with (high-osmolarity cultures) 0.8 M KCl. Cellular extracts were obtained by incubating the cells (around 200 mg of protein per 10 ml) for 30 min with 5% perchloric acid containing 1 mM citric acid as the internal standard. The mixture was neutralized by adding 5 N KOH, and the precipitate was centrifuged for 15 min at 48,000 × g. Subsequently, the supernatant was lyophilized, resuspended in 3 to 5 ml of D2O, centrifuged, lyophilized, and resuspended in ca. 0.6 ml of D2O containing 0.5 to 3.0 mg of EDTA-d12 (fully deuterated EDTA; to complex paramagnetic metal ions). The recordings were performed on a Varian 400 Unity Plus spectrometer, using either a 5-mm probe for four nuclei or a pulsed-field gradient inverse broadband probe.
Mass spectrometry.
Electrospray mass spectra were recorded on an R 3010 quadrupole mass spectrometer (NERMAG, Argenteuil, France) equipped with a custom-built pneumatically assisted electrospray (IonSpray) ion source.
Transport assays.
Uptake assays and enzymatic synthesis of [14C]glycine betaine from [N-methyl-14C]choline (40 to 60 mCi/mmol) and uptake of [14C]lactose (55.8 mCi/mmol) and [14C]sucrose (540 mCi/mmol) were performed as described previously (5). To prevent carryover of osmolytes from the growth media, the cells were washed twice with a large excess (∼100 times the volume of the cell pellet) of buffer as specified in the figure legends.
Miscellaneous.
Protein was determined by the method of Lowry et al. (7), with bovine serum albumin as a standard. The osmolarities of media and buffers were measured by freezing-point depression with an Osmomat 030 (Gonotec). Growth experiments were performed in sterile low-protein-binding microplates. Plate wells containing 200 μl of culture were sealed by adding 75 μl of sterile silicone oil (1.03 g/ml), and growth rates were determined from A620 increases with a Multiscan MCC/340 MKII (Flow Laboratories, Lugano, Switzerland). For the calculation of intracellular concentrations, we used a value for the specific internal volume of 3 μl/mg of protein; the cytoplasmic volume was found to be constant under different culture and assay conditions (low and high osmolarities), as inferred from flow cytometry measurements (5).
RESULTS
Main osmolytes under salt or sugar stress.
Previous experiments demonstrated that addition of 2.5 mM glycine betaine to the medium was sufficient to alleviate growth inhibition caused by KCl or NaCl stress up to 1.2 M, whereas it did not affect the growth of sucrose-stressed L. plantarum (5). Glycine betaine also did not stimulate the specific growth rates of L. plantarum under lactose, sorbitol, and xylitol stress (data not shown). To study the adaptation to high-osmolarity media further, the main osmolytes in cellular extracts of L. plantarum grown at low osmolarity and under salt or sugar stress were measured by NMR spectroscopy.
(i) Salt-stressed cultures.
Organic osmolytes could not be detected by proton NMR spectroscopy in cells cultured in the presence of 0.8 M KCl or 0.8 M NaCl; however, increases in the accumulation of glutamate, alanine, and proline (additional accumulation of 223, 40, and 243 nmol/mg of protein, respectively) were measured by HPLC previously (5) (Table 1). When glycine betaine was added to the culture media, it accumulated to concentrations of 850 nmol per mg of protein (Fig. 1A), whereas the concentrations of the amino acids were reduced significantly (5). It was previously shown by atomic absorption spectrometry that large amounts (ca. 5,000 nmol/mg of protein; ∼1.7 M) of potassium were associated with 0.8 M KCl-stressed cells, irrespective of whether glycine betaine was present in the medium (5). We have now established that the Na+ concentration increased to ca. 2,000 nmol/mg of protein in 0.8 M NaCl-stressed cells but not in 0.8 M KCl-stressed cells. Increased concentrations of K+ and Na+ ions (about 2,000 nmol/mg of protein) were detected only when the cells were stressed with the corresponding salts. Overall, the data suggest that L. plantarum is unable to respond adequately to osmotic stress by accumulating K+, as the increased potassium concentration was not observed in NaCl-stressed cells, even though the growth medium contained 35 mM K+. As glycine betaine diminishes the growth inhibition by KCl and NaCl stress, whereas the amounts of K+ and Na+ associated with the cells are similar with or without this compatible solute (5), it may well be that the protective effect of glycine betaine is not only osmotic but also due to the stabilization of macromolecular structures at high cellular concentrations of ions.
TABLE 1.
Intracellular osmolyte concentrations in L. plantarum
| Osmolyte | Osmolyte concn (nmol/mg of protein) in CDM supplemented witha:
|
||||||
|---|---|---|---|---|---|---|---|
| No additions
|
0.8 M KCl
|
1 M lactose
|
1 M sucrose, −GBb | ||||
| −GB | +GB | −GB | +GB | −GB | +GB | ||
| Glycine betaine | 0c | ND | 0c | 850 | 0c | ∼200 | ∼300 |
| Glutamate | 57 | 36 | 280 | 110 | ∼550 | ∼500 | ∼550 |
| Alanine | 23 | 18 | 63 | 39 | ∼700 | ∼500 | ∼700 |
| Proline | 7 | 4 | 250 | 10 | ∼100 | <100 | ∼100 |
| Lactose (or derivative) | ND | ND | ND | ND | ∼3,800 | ∼3,200 | ND |
| Sucrose (or derivative) | ND | ND | ND | ND | ND | ND | ∼3,600 |
| Lactic acid | ∼70 | ∼65 | ∼40 | ∼65 | ∼1,200 | ∼1,200 | ∼800 |
Some of the data are from reference 5. −GB, no glycine betaine; +GB, glycine betaine added to a final concentration of 2.5 mM; ND, not detectable; ∼, based on NMR data (less precise than HPLC analysis).
Sucrose solutions were contaminated with >100 μM glycine betaine.
0 by definition because L. plantarum cannot synthesize glycine betaine.
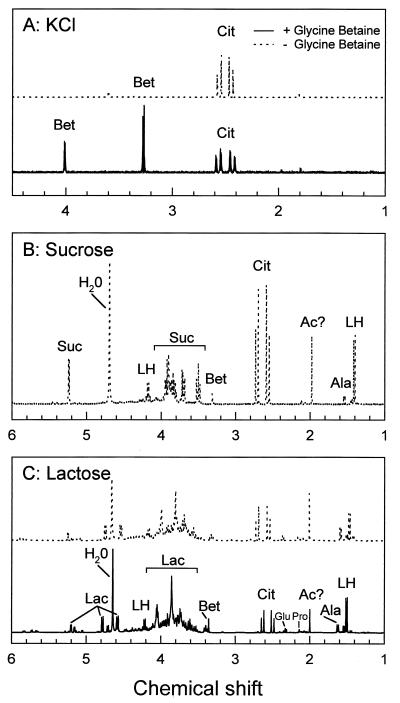
FIG. 1.
Proton NMR spectra of perchloric acid extracts of L. plantarum ATCC 14917 grown in CDM supplemented with 0.8 M KCl (A), 35% sucrose (B), or 35% lactose (C). The cells were grown in CDM without (−) or with (+) 2.5 mM glycine betaine. Ac?, acetyl-group-containing compound; Ala, alanine; Bet, glycine betaine; Cit, citric acid; Glu, glutamic acid; Lac, lactose; LH, lactic acid; Pro, proline; Suc, sucrose.
(ii) Sugar-stressed cultures.
Glycine betaine was detected at concentrations of 300 nmol per mg of protein in extracts from sucrose-stressed cells growing on 25 mM glucose, irrespective of whether glycine betaine was added to the culture medium (Fig. 1B; Table 1). This was surprising since L. plantarum is unable to synthesize glycine betaine (5). Mass spectrometry analysis of standard sucrose solutions (biochemical grade; Acros Organics), chromatographed on a Hypersil ammonium column, indicated that low amounts of glycine betaine were contaminating the sucrose. The concentration of glycine betaine present in a 35% (wt/vol) sucrose solution (the concentration used to stress the cells) could not be estimated accurately but fell in the range of several hundreds micromolar, which is sufficient to restore the turgor partially through the uptake of glycine betaine. In addition to the relatively small amounts of glycine betaine, glutamate, and alanine, the major osmolyte sucrose was present at ca. 3,600 nmol per mg of protein (∼1.2 M) in sucrose-stressed cells.
In the case of cells grown in the presence of lactose (35% [wt/vol]; ∼1.0 M) or sorbitol (35% [wt/vol]; ∼1.7 M) plus 25 mM glucose, about 3,800 or 6,100 nmol of the respective sugar per mg of protein was detected by NMR spectroscopy in cellular extracts. At a specific internal volume of 3 μl/mg of protein, this corresponds to an internal lactose and sorbitol concentration of ∼1.3 or ∼2.0 M, respectively, which is of the same order as the externally applied concentrations. Glycine betaine was detected in lactose- or sorbitol-stressed cells only when the compound was present in the medium (Table 1). In cellular extracts from L. plantarum cultured on CDM with 35% lactose, some phosphorylated sugar-like compounds were found in addition to the unmodified disaccharide (Fig. 1C). The proton-undecoupled phosphorus spectrum showed that the phosphate of one of the phosphorylated compounds forms a triplet and is therefore linked to a CH2 group (⩵6-phosphate), whereas the other is most likely a sugar-bisphosphate (data not shown). A comparison with reference spectra identified one compound as the glycolytic intermediate glucose-6-phosphate. The phosphorylated compounds were quantified by using the inorganic phosphate from the buffer as an internal standard. These compounds comprised less than 15% of the total amount of sugar (or derivatives) in the cellular extracts and are therefore thought to be of limited importance for maintaining the osmotic balance. Since L. plantarum ATCC 14917 does not grow on lactose but does grow on the monosaccharides tested, the phosphorylated sugars cannot be derived from lactose but must originate from glucose (carbon and energy source). Like sucrose-stressed cells, cells grown in the presence of 1 M lactose contained relatively large amounts of lactic acid (at least 1 order of magnitude higher than in control or KCl-stressed cells [Table 1]).
To substantiate the notion that L. plantarum cannot metabolize lactose or lactose-6-phosphate (after transport via a phosphoenolpyruvate-dependent sugar phosphotransferase system), the initial step in the breakdown, β-galactosidase or phospho-β-galactosidase activity, was measured by using the chromogenic substrate o-nitrophenyl-β-d-galactopyranoside (ONPG) or o-nitrophenyl-β-d-galactopyranoside-6-phosphate (ONPG-P), respectively. Neither activity was present in L. plantarum grown on CDM containing 0.5% glucose plus 0.5 or 30% lactose, whereas β-galactosidase (ONPG hydrolysis) and phospho-β-galactosidase (ONPG-P hydrolysis) activities were measured in Streptococcus thermophilus ST11 or L. lactis ML3, respectively. These results confirm that lactose is not metabolized by L. plantarum ATCC 14917. As also shown by the transport studies presented below, the intracellular concentration of lactose equilibrates with the extracellular concentration, which implies that lactose contributes significantly to the internal osmolality only when its concentration in the medium is high. Although L. plantarum can grow in the presence of sucrose or sorbitol as the sole energy source, the intracellular concentrations of these sugars were also close to the extracellular ones, which indicates that decreases in turgor pressure upon addition of these sugars to the medium will be only transient.
The addition of glycine betaine to CDM supplemented with 35% lactose resulted in the accumulation of glycine betaine up to concentrations of about 200 nmol/mg of protein. Moreover, cells cultured on CDM containing 35% lactose or sucrose contained relatively large amounts of glutamate (500 to 550 nmol/mg of protein), alanine (500 to 700 nmol/mg of protein), and lactate (800 to 1,200 nmol/mg of protein), irrespective of whether glycine betaine was present in the growth medium. These compounds were identified by means of gradient-selected heteronuclear single-quantum coherence and gradient-selected heteronuclear single-quantum coherence total correlated spectroscopy (18).
Restoration of osmotic imbalance.
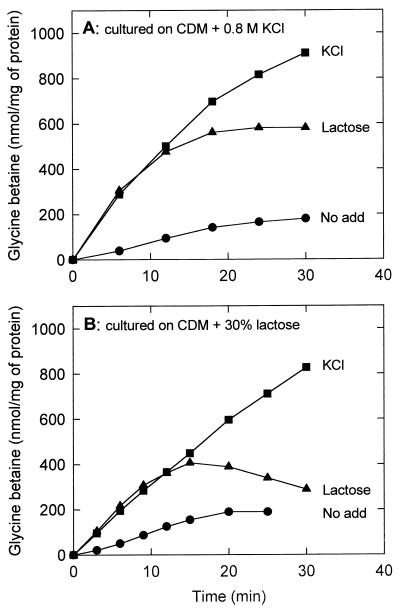
Since sugar stress resulted in much lower intracellular concentrations of glycine betaine than salt stress, we compared the uptake of glycine betaine under the two conditions. The rationale for the experiment is that the activated state of the glycine betaine uptake system can be used as a measure of osmotic imbalance following an osmotic upshift. Cells were grown on CDM supplemented with 0.8 M KCl plus 0.5% glucose, washed twice, and resuspended in potassium phosphate (pH 6.5), and uptake was assayed in CDM. The final accumulation levels of glycine betaine were significantly lower in the presence of 30% (wt/vol) lactose (or sucrose) than when iso-osmotic concentrations of KCl were present (Fig. 2A). The final level of glycine betaine was even lower when cells were cultured on CDM supplemented with 30% (wt/vol) lactose (iso-osmotic with 0.8 M KCl) plus 0.5% (wt/vol) glucose and assayed in the presence of 30% (wt/vol) lactose (Fig. 2B). The cytoplasmic glycine betaine concentrations determined in these assays correlate well with those estimated from the NMR spectra. Since the uptake rates under KCl and lactose (or sucrose) stress are initially the same, while the final accumulation levels differ, the data suggest that the osmotic inbalance is restored more rapidly in the presence of lactose (or sucrose) through uptake of these sugars. Hence, glycine betaine uptake is inhibited at an earlier time point by high concentrations of sugar (after ca. 12 min) than by high concentrations of KCl.
FIG. 2.
Glycine betaine uptake at high medium osmolarities imposed by KCl or lactose. Cells of L. plantarum ATCC 14917 were grown on CDM containing 0.8 M KCl (A) or 30% (wt/vol) lactose (B), washed, and resuspended in potassium phosphate (pH 6.5). The concentrated cell suspensions were diluted into CDM without (circles) or with 0.8 M KCl (squares) or 30% (wt/vol) lactose (triangles) to final protein concentrations of 0.23 to 0.75 mg/ml. After 6 min of preenergization with 10 mM glucose, uptake was initiated at time zero by the addition of [14C]glycine betaine (final concentration of 1.3 mM).
Regulation of sugar uptake by osmolarity.
Since L. plantarum has no detectable β-galactosidase and/or phospho-β-galactosidase activities, the uptake of lactose can be studied without interference of cellular metabolism. The lactose (and sucrose) uptake rates, assayed in 50 mM potassium phosphate (pH 6.5), were similar for cells cultured on CDM containing 0.5% glucose, 0.5% glucose plus 0.8 M KCl, or 0.5% glucose plus 0.5% lactose (or sucrose). However, when the cells were cultured in CDM containing 0.5% glucose plus 30% lactose (or sucrose), the rates of lactose (or sucrose) uptake increased two- to threefold (Fig. 3 and 4 and data not shown). This finding is consistent with induction of putative uptake systems for lactose and sucrose by high concentrations of the respective sugars.
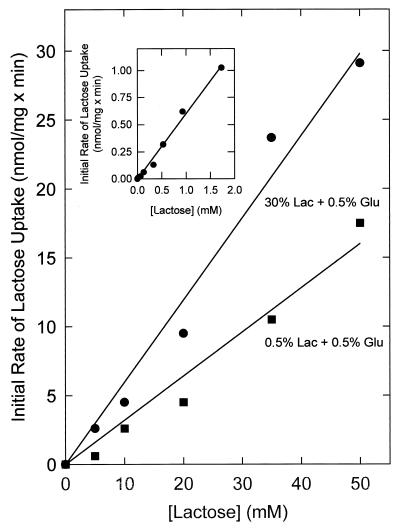
FIG. 3.
Dependence of the initial rate of lactose uptake on the lactose concentration. Cells of L. plantarum ATCC 14917 were grown on CDM containing 30% (wt/vol) lactose (Lac) plus 0.5% glucose (Glu) or CDM containing 0.5% lactose plus 0.5% glucose, washed, and resuspended in potassium phosphate (pH 6.5) to a final protein concentration of 1.5 mg/ml. After 6 min of preenergization with 10 mM glucose, uptake was initiated at time zero by the addition of [14C]lactose, and the initial uptake rates were determined after 30 s of uptake. The inset shows the initial rate of lactose uptake at low lactose concentrations.
FIG. 4.
Lactose counterflow in L. plantarum ATCC 14917. The cells were grown on CDM containing 0.5% (wt/vol) glucose (glu) plus 0.5% (wt/vol) lactose (lac) or 0.5% (wt/vol) glucose plus 30% (wt/vol) lactose. The cells were washed and resuspended in potassium phosphate (pH 6.5). After equilibration with 2.5 mM lactose on ice for 3 h, the cells were diluted 50-fold (final protein concentration, 0.3 to 0.4 mg/ml) into potassium phosphate containing 72 μM [14C]lactose (55.8 mCi/mmol) without (open symbols) or with (inset; closed symbols) 10 mM glucose.
As described previously for glycine betaine (4), the uptake of the sugars may be subject to osmotic regulation and the transport system(s) may be more active under hyperosmotic conditions. To study osmotic regulation at the protein level (activation of the transporter), lactose uptake was analyzed in cells cultured on CDM supplemented with 30% (wt/vol) lactose plus 0.5% (wt/vol) glucose (fully induced cells). The cells were washed and resuspended in potassium phosphate (pH 6.5), and lactose uptake was initiated by dilution into potassium phosphate (pH 6.5) plus 10 mM glucose and 1.3 mM [14C]lactose without further additions (low osmolarity) or with 30% (wt/vol) lactose or 0.8 M KCl (high osmolarity). Activation of lactose uptake by hyperosmotic conditions was not observed; i.e., 0.8 M KCl had no stimulating effect on the activity. However, the uptake rate of [14C]lactose in the presence of an additional 30% (wt/vol) lactose increased approximately 50-fold (to 60 nmol/min/mg of protein), which was more than expected if one assumes an affinity constant for lactose in the submillimolar range, i.e., as observed for lactose transport in other lactic acid bacteria (15). In fact, the data for L. plantarum point to an uptake system with a very high affinity constant for lactose (see below).
Kinetic characterization of lactose uptake.
The kinetic constants for lactose uptake by L. plantarum were determined for cells cultured on CDM supplemented with 0.5% glucose plus 0.5% lactose and CDM supplemented with 0.5% glucose plus 30% lactose. In both cases, the initial lactose uptake rates increased linearly up to a lactose concentration of at least 50 mM; the initial lactose uptake rates were highest in cells cultured in CDM containing 30% lactose (Fig. 3). Note that the uptake of lactose in Fig. 3 is downhill and involves no lactose metabolism. Measurements of uptake versus time showed that lactose was never taken up beyond the equilibration level (data not shown). A lactose counterflow assay (14) was used to test whether the observed low-affinity uptake of lactose is facilitated by an uptake system or due to passive diffusion. If facilitated diffusion occurs, one should observe a transient accumulation of [14C]lactose as a result of isotopic exchange with intracellular unlabeled lactose, whereas in case of passive influx, the intra- and extracellular pool should only equilibrate. Indeed, counterflow was observed in cells that were cultured on CDM supplemented with 30% lactose plus 0.5% glucose as well as in cells cultured on CDM supplemented with 0.5% lactose plus 0.5% glucose. For the cells grown in the presence of 30% lactose, [14C]lactose was taken up by counterflow to about 3 nmol/mg of protein (∼1 mM; after 4 to 5 min in Fig. 4), which is almost 15-fold higher than the extracellular concentration. As in the lactose uptake assays, the counterflow rates were approximately two- to threefold higher in cells cultured on 30% lactose than in cells cultured on medium containing 0.5% lactose (Fig. 4). Surprisingly, the counterflow activity was subject to inhibition by glucose (Fig. 4, inset). These results together with the NMR data suggest that lactose enters the cells by facilitated diffusion rather than by passive diffusion across the cytoplasmic membrane. Since lactose uptake in resting and glucose-metabolizing cells is observed to only the equilibration level, it seems that transport is not coupled to any form of metabolic energy (e.g., proton motive force or ATP).
DISCUSSION
In this report, we provide a rationale for the observation that raising the medium osmolality through the addition of electrolytes (KCl or NaCl) and of nonelectrolytes (various sugars), has different consequences for L. plantarum. The transport data indicate that osmotic upshocks elicited by the addition of KCl and by equiosmolar amounts of lactose enhance the rate of glycine betaine uptake to the same extent (Fig. 2), which reflects the activation of the uptake system as a result of loss of turgor pressure (see also our previous study [4]). As lactose diffuses into cells, the osmostatic conditions are restored and the net rate of glycine betaine uptake ceases. On the basis of the regulation of glycine betaine fluxes during osmostasis or during hyper- and hypo-osmotic shock (4), we infer that this decreased rate of uptake is due to the lowering of the unidirectional uptake rate from activated to basal, whereas the unidirectional efflux may increase somewhat. The uptake of sugar (to equilibration levels) together with the accumulation of glycine betaine may eventually result in hyperosmolarity of the cytoplasm, which will then be compensated by net exit of glycine betaine. The decrease in glycine betaine levels after 15 min of uptake in the presence of lactose (Fig. 2B) is a strong indication that the cytoplasm becomes hypertonic relative to the external medium and that the glycine betaine exit systems become activated. The properties of the osmotically regulated efflux systems in L. plantarum have recently been described (4).
Although electrolytes and nonelectrolytes impose an osmotic upshift initially, the facilitated influx of sugars diminishes the osmotic gradient in time. If one assumes an apparent affinity constant for lactose transport of 100 mM (the system is clearly not saturated at 50 mM; Fig. 3), the rate of lactose uptake at a medium concentration of 30% (0.83 M) lactose will be approximately 120 nmol/min/mg of protein. Taking this rate, the cytoplasmic concentration of lactose will increase by 40 mM/min and equilibrate with the external medium concentration after about 20 min. This value is in accordance with the transport data presented in Fig. 2B, given the uncertainty about the affinity constant for lactose transport.
The amount of K+ associated with the cells increases when the osmolarity of the medium is increased by the addition of 0.8 M KCl, but not 0.8 M NaCl, as a stress factor. The cellular levels of K+ (bound and/or free) are already high (∼1 M) in unstressed cells, and it seems that L. plantarum is unable to increase the K+ levels much further upon hyperosmotic (e.g., NaCl) stress. This observation implies that under conditions of salt stress, and in the absence of an alternative compatible solute, the cell turgor remains far from optimal. In fact, even in the presence of glycine betaine, the increase in organic compatible solutes is insufficient to restore the turgor pressure in KCl-stressed cells completely. Therefore, the growth inhibition by KCl and NaCl may not only have an osmotic origin, but high salt in the cytoplasm may become inhibitory by binding to intracellular macromolecules. Because glycine betaine is able to restore the growth of salt-stressed L. plantarum, whereas the amounts of K+ ions associated with the cells are similar in the presence and absence of glycine betaine in the medium (reference 5 and this study), it seems likely that glycine betaine offers (additional) protection through stabilization of enzymes or other macromolecules.
The facilitated influx of lactose and sucrose uptake at high sugar concentrations also occurred when glycine betaine was present (Fig. 1B and C), indicating that the lower levels of glycine betaine in sugar-stressed cells are most likely due to the equilibration of sugar. During salt (KCl or NaCl) stress, glycine betaine is taken up to higher levels than in sugar-stressed cells. Our interpretation of the data is that the cells are unable to increase the osmolality of the cytoplasm sufficiently by accumulating K+ or Na+. Support for this notion also comes from the experiments on the activated state of the glycine betaine uptake system upon salt and sugar stress (Fig. 2), as discussed above. Thus, the addition of glycine-betaine to media with high concentrations of salts leads to the accumulation of this osmolyte, partial restoration of the osmotic imbalance, possible stabilization of macromolecules, and consequently an increased specific growth rate. The addition of lactose, sucrose, or sorbitol (at a concentration of 14 mM) to KCl-stressed cells does not increase the specific growth rate of L. plantarum (unpublished results), because the organism fails to accumulate these sugars against their concentration gradients.
At this point, we cannot (and do not) discriminate between growth inhibition by an osmotic upshift as a result of a decrease in turgor pressure or perhaps plasmolysis, i.e., retraction of the cytoplasmic membrane from the cell wall. In contrast to gram-negative bacteria, gram-positive bacteria, in general, do not plasmolyse (9, 13, 19). The reason for the failure of gram-positive bacteria to plasmolyse might be the strong adhesion between the cytoplasmic membrane and peptidoglycan. Alternatively, these bacteria may not plasmolyse because of their very high internal osmotic pressure (turgor pressures of 15 to 25 atm), which means that external osmolalities needed before the turgor drops to zero are much higher than in gram-negative bacteria (turgor pressures of 1 to 5 atm).
A few other points relevant to this discussion require explanation. In a previous study, we observed accumulation of glycine betaine to levels as high as 1,500 nmol/mg of protein for cells cultured in the presence of 0.8 M KCl or NaCl (5), whereas levels of 850 nmol/mg of protein are reported here. The present measurements, however, were performed in CDM from which other osmolytes were accumulated, most notably glutamate (Table 1), rather than in phosphate buffer. The coaccumulation of these osmolytes will have its impact on the restoration of turgor pressure and consequently reduce the accumulation of glycine betaine.
To obtain equiosmolar conditions in case of salt or sugar addition to CDM or phosphate buffer, the osmolality was measured by freezing-point depression rather than calculated from the number of particles in the solution. We chose to measure the osmolality because the amount of solute added to a solvent does not usually produce a proportional increase in osmotic pressure due to interaction of the solute with the solvent, which in the case of CDM is likely to be very complex because several other solutes are present as well. Although measurements of osmolality by freezing-point depression (or any other method) has its limitations (17), deviations in the actual osmolality as experienced by the cells will not significantly affect the interpretation of the present data.
In conclusion, the response of L. plantarum to hyperosmotic conditions is different from that of other microorganisms. Mainly on the basis of studies in enteric bacteria, it was found that K+ uptake is activated and K+ ions accumulate to high levels upon an osmotic upshock (1). To maintain electroneutrality, the accumulation of K+ is accompanied by the uptake of anions (e.g., glutamate) and/or exit of other cations (e.g., protons). At later times during hyperosmotic stress, at least part of the potassium is replaced by neutral osmolytes that can either be synthesized or taken up from the medium (1). Our findings indicate that L. plantarum cells are unable to compensate for a decrease in turgor by increased accumulation of K+, and an exogenous organic osmolyte that can be accumulated to high levels (e.g., glycine betaine) is needed to restore growth by reversing at least partly the decrease in turgor pressure upon salt stress. Hyperosmotic conditions imposed by sugar stress are much less detrimental and only transient, because the cells are able to equilibrate the extra- and intracellular concentrations of lactose (and sucrose). The uptake of these sugars most likely occurs by facilitated diffusion via system(s) with a very low affinity for the substrates, which is consistent with the inability of the sugars to serve as compatible solute (at low substrate concentration) in L. plantarum.
ACKNOWLEDGMENTS
This research was funded by Unilever Research Laboratories, Vlaardingen, The Netherlands.
We thank C. M. Jeronimus-Stratingh and A. P. Bruins for mass spectrometry analysis, J. C. S. Niël for NMR spectroscopy, and J. P. P. M. Smelt for stimulating discussions.
REFERENCES
- 1.Csonka L N. Physiological and genetic responses of bacteria to osmotic stress. Microbiol Rev. 1989;53:121–147. doi: 10.1128/mr.53.1.121-147.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.D’Souza-Ault M, Smith L T, Smith G M. Roles of N-acetylglutaminylglutamine amide and glycine betaine in adaptation of Pseudomonas aeruginosa to osmotic stress. Appl Environ Microbiol. 1993;59:473–478. doi: 10.1128/aem.59.2.473-478.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galinski E A, Trüper H G. Microbial behaviour in salt-stressed ecosystems. FEMS Microbiol Rev. 1994;15:95–108. [Google Scholar]
- 4.Glaasker E, Konings W N, Poolman B. Glycine betaine fluxes in Lactobacillus plantarum during osmostasis and hyper- and hypo-osmotic shock. J Biol Chem. 1996;271:10060–10065. doi: 10.1074/jbc.271.17.10060. [DOI] [PubMed] [Google Scholar]
- 5.Glaasker E, Konings W N, Poolman B. Osmotic regulation of intracellular solute pools in Lactobacillus plantarum. J Bacteriol. 1996;178:575–582. doi: 10.1128/jb.178.3.575-582.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Le Rudulier D, Bouillard L. Glycine betaine, an osmotic effector in Klebsiella pneumoniae and other members of the Enterobacteriaceae. Appl Environ Microbiol. 1983;46:152–159. doi: 10.1128/aem.46.1.152-159.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lowry O H, Rosebrough N J, Farr A J, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 8.Millner J L, Grothe S, Wood J M. Proline porter II is activated by a hyperosmotic shift in both whole cells and membrane vesicles of Escherichia coli K12. J Biol Chem. 1988;263:14900–14905. [PubMed] [Google Scholar]
- 9.Mitchell P, Moyle J. Osmotic structure and function in bacteria. Symp Soc Gen Microbiol. 1956;6:150–180. [Google Scholar]
- 10.Molenaar D, Hagting A, Alkema H, Driessen A J M, Konings W N. Characteristics and osmoregulatory roles of uptake systems for proline and glycine betaine in Lactococcus lactis. J Bacteriol. 1993;175:5438–5444. doi: 10.1128/jb.175.17.5438-5444.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perroud B, Le Rudulier D. Glycine betaine transport in Escherichia coli: osmotic modulation. J Bacteriol. 1985;161:393–401. doi: 10.1128/jb.161.1.393-401.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pocard J-A, Smith L T, Smith G M, Le Rudulier D. A prominent role for glucosylglycerol in the adaptation of Pseudomonas mendocina SKB70 to osmotic stress. J Bacteriol. 1994;176:6877–6884. doi: 10.1128/jb.176.22.6877-6884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Poolman, B., and E. Glaasker. Regulation of compatible solute accumulation in bacteria. Mol. Microbiol., in press. [DOI] [PubMed]
- 14.Poolman B, Knol J, Mollet B, Nieuwenhuis B, Sulter G. Regulation of bacterial sugar-H+ symport by phosphoenolpyruvate-dependent enzyme I/HPr-mediated phosphorylation. Proc Natl Acad Sci USA. 1995;92:778–782. doi: 10.1073/pnas.92.3.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Poolman B, Modderman R, Reizer J. Lactose transport system of Streptococcus thermophilus: the role of histidine residues. J Biol Chem. 1992;267:9150–9157. [PubMed] [Google Scholar]
- 16.Stimeling K W, Graham J E, Kaenjak A, Wilkinson B J. Evidence for feedback (trans) regulation of, and two systems for, glycine betaine transport by Staphylococcus aureus. Microbiology. 1994;140:3139–3144. doi: 10.1099/13500872-140-11-3139. [DOI] [PubMed] [Google Scholar]
- 17.Sweeney T E, Beuchat C A. Limitations of methods of osmometry: measuring the osmolality of biological fluids. Am J Physiol. 1993;33:R469–R480. doi: 10.1152/ajpregu.1993.264.3.R469. [DOI] [PubMed] [Google Scholar]
- 18.Van de Ven F J M. Multidimensional NMR in liquids. New York, N.Y: VCH Publishers; 1995. [Google Scholar]
- 19.Whatmore A M, Chudek J A, Reed R H. Determination of turgor pressure in Bacillus subtilis: a possible role for K+ in turgor regulation. J Gen Microbiol. 1990;136:2521–2526. doi: 10.1099/00221287-136-12-2521. [DOI] [PubMed] [Google Scholar]