Abstract
Introduction
Eggerthia catenaformis, a non-spore-forming anaerobic Gram-positive bacillus component of the human fecal microbiota has rarely been reported in human diseases. In almost every case described in current literature to date, dental diseases (abscesses, periodontitis, or caries), are the most common source of the infection which extends to the brain, cervical spaces, pulmonary parenchyma, the pleural cavity, the abdominal wall, and the abdominal cavity.
Case report
An 82-year-old male Caucasian patient was admitted to our Emergency Department (ED) with a painless, right submandibular mass, dyspnea, and inspiratory stridor. A CT scan of the head, neck, and chest with intravenous contrast material revealed a retrotonsillar fluid collection. Air bubbles and minimal fluid were present from the right sub-mandibular area to the lower mediastinum between the spine, the descending thoracic aorta, and the trachea. The patient underwent surgical treatment and a broad-spectrum antibiotic. The retropharyngeal fluid collection culture showed the presence of Eggerthia catenaformis. After a first period in the Intensive Care Unit, he was admitted to a Step-Down Unit (SDU) where he underwent respiratory weaning, motor rehabilitation, and gradual oral feeding resumption. At discharge, the patient maintained the tracheal cannula as he still had impaired swallowing of solid foods.
Conclusions
Here we report the first case of descending necrotizing mediastinitis in a patient with a retropharyngeal abscess, in the absence of dental diseases.
Keywords: Necrotizing mediastinitis, Eggerthia catenaformis, retropharyngeal abscess
Introduction
Descending necrotizing mediastinitis (DNM) is determined by an infection of oropharyngeal or cervical origin, which has spread to the mediastinum through three possible pathways: a pre-tracheal route to the anterior mediastinum, a lateral pharyngeal route to the middle mediastinum or a retro-pharyngeal route to the posterior mediastinum.1 Despite the absence of a real physical barrier between compartments, the mediastinum space can be anatomically divided into an anterior (bordered by the pericardium posteriorly and below the thoracic plane) and a posterior compartment (bordered by the pericardium anteriorly and the spine posteriorly. Furthermore, the mediastinum can be divided into superior and inferior mediastinum by the transverse thoracic plane, running through the level of the sternal angle.2
Endo et al. defined the clinical progression of DNM upon computed tomography (CT) evaluation as follows: type IA, when the infection is localized in the antero-superior mediastinum above the carina; type IIA, when the infection is characterized by diffuse anterior mediastinal involvement, type IIB, when the infection has diffused in the anterior and posterior mediastinum.3 Recently type IIC has been described: when the infection has spread only to the posterior mediastinum.4
Bacteriologic investigation often revealed polymicrobial infection, in particular infections originated from the orofacial, dental, or gastrointestinal tract are due to anaerobic bacteria. Streptococcus spp. are the most common aerobic bacteria while Bacteroides fragilis, Prevotella, and Fusobacterium are the most common anaerobic bacteria. The mortality rate is about 5.6%.5
Case report
Here we describe an unusual case of DNM infection caused by Eggerthia catenaformis, likely arising in the retropharyngeal space and spreading to the neck and mediastinum.
An 82-year-old Caucasian male patient addressed to our Emergency Department (ED) with an unpainful, right submandibular mass, dyspnea, and inspiratory stridor. Investigation of the patient’s medical history revealed obesity (body mass index of 36 kg/m2, weight 98 kg, height 165 cm), arterial chronic hypertension and permanent atrial fibrillation treated with warfarin.
Physical examination revealed pronounced painless swelling in the right cervical area, swelling of the right tonsillar pillar, complete lack of teeth and normal gums. Body temperature was 38°C.
Routine laboratory exams showed: white blood cell count 7.72 × 109/L [reference value 4-10] (neutrophils 73%, lymphocytes 16%), hemoglobin 14.7 g/dL [reference value 13.5-17.2], platelets 140 × 109/L [reference value 140-400], creatinine 1.14 mg/dL [reference value 0.7-1.2], C-reactive protein 189 mg/L [reference value <5], INR 3.1.
Due to respiratory failure (appearance of stridor, SaO2 82% on room air, respiratory rate 30/min) the patient underwent sedation and tracheal intubation.
A CT scan of the head, neck, and chest with intravenous contrast, revealed a retrotonsillar fluid collection, with a maximum diameter of 10 cm, extended anteriorly to the right lobe of the thyroid gland (Figure 1). Air bubbles and minimal fluid were present from the right submandibular area to the lower mediastinum between the spine, the descending thoracic aorta, and the trachea (Figure 2).
Figure 1.
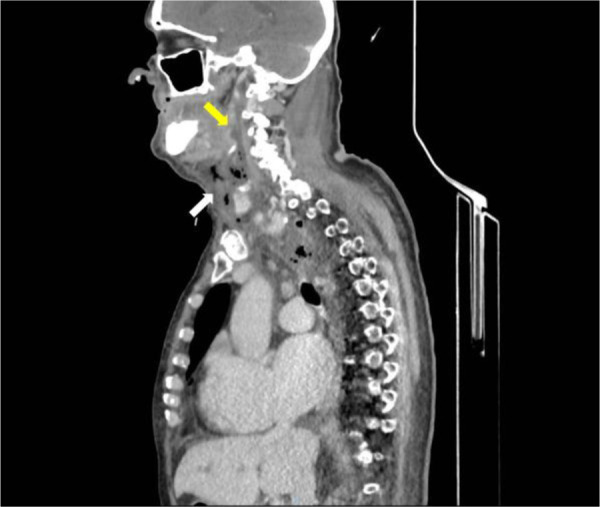
CT scan of head, neck, and chest with intravenous contrast material, shows a retropharyngeal collection (yellow arrow) and submandibular air bubbles (white arrow)
Figure 2.
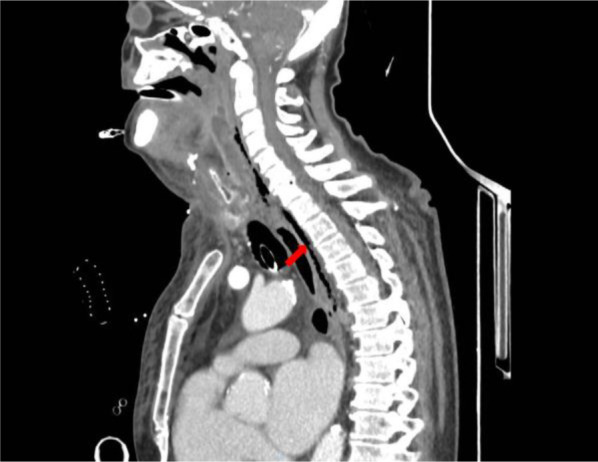
Chest CT scan with contrast material shows air bubbles and minimal fluid in the posterior mediastinum between the spine, the descending thoracic aorta, and the trachea (red arrow).
The patient underwent surgical treatment under general anesthesia, which consisted of a trans-cervical approach through an incision anteriorly to the sternocleidomastoid muscle, muscular debridement, removal of cervical necrotic tissues and placement of a drainage tube in the posterior mediastinum. The retropharyngeal fluid collection was drained trans-orally by direct laryngoscopy. Finally, a surgical tracheostomy was performed.
He was then admitted to the Intensive Care Unit (ICU) where treatment with fluid filling, noradrenaline, and broad-spectrum antibiotics (piperacillin/tazobactam, clindamycin and vancomycin) was started due to emerging septic shock.
An additional CT scan, on post-operative day three, revealed the presence of segmental pulmonary artery embolism and right jugular vein thrombosis. Doppler ultrasound of the lower extremities demonstrated a right femur-popliteal and iliac vein thrombosis. For this reason, was started parenteral anticoagulation therapy with enoxaparin.
Retropharyngeal fluid collection culture demonstrated the presence of E. catenaformis. The sample was seeded on Schaedler agar +5% sheep blood plates (BioMérieux, France), incubated at 37°C in an anaerobic atmosphere for 48 hours. Grown colonies were identified by automated VITEK® MS system (bioMérieux) using mass spectrometry with MALDI-TOF (matrix assisted laser desorption ionization time-of-flight) technology.
The antibiogram was performed on Mueller Hinton agar + 5% horse blood + 20 mg/l ß-NAD (MHF) plates (bioMérieux), with a 0.5 McFarland bacterial suspension. ETEST® strips were used to define the minimum inhibitory concentration (MIC). The results were evaluated after 24 hours of incubation at 37°C in anaerobiosis using interpretive criteria provided by EUCAST (European Committee on Antimicrobial Susceptibility Testing) to determine the antibiotic susceptibility of the isolated microorganism.6 The isolated E. catenaformis strain was susceptible in vitro to penicillin G (MIC of 0.064 mg/L), meropenem (MIC of 0.032 mg/L), metronidazole (MIC of 0.75 mg/L), clindamycin (MIC of 0.094 mg/L), and vancomycin (MIC of 0.38 mg/L). We did not perform genomic typing. The blood culture was positive for Staphylococcus hominis.
The previous antibiotic therapy was suspended, while meropenem (1 g IV every 8 hours) and daptomycin (6 mg/kg IV every 24 hours) were administered to the patient for 14 days.
During periodic surgical wound inspections following the removal of the mediastinal drain tube, a pharyngeal fistula was evident, which was closed by apposition of a pectoralis major muscle flap.
On day twenty the patient was transferred to a Step-Down Unit (SDU) where he underwent respiratory weaning, motor rehabilitation and gradual oral feeding resumption. After 20 days, at discharge, the patient maintained the tracheal cannula as he still had impaired swallowing for solid foods.
Discussion
E. catenaformis is a non-spore-forming anaerobic Gram-positive bacillus component of the human fecal microbiota, firstly described in 1935 by AH Eggerth. It was reassigned to the Eggerthia genus from Lactobacillus catenaformis by Salvetti et al. based on 16S rRNA gene sequencing.7
Kordjian et al. (2015) described the first case of E. catenaformis bacteremia in a patient with dental abscess and cervical fluid accumulation treated with antibiotic therapy, tonsillectomy, and neck surgical drainage.8
In almost every described case in current literature, dental diseases (abscesses, periodontitis, or caries) represent the most common source of the infection extending to brain, cervical spaces, pulmonary parenchyma, and pleural cavity, abdominal wall, and abdominal cavity. A worse prognosis is described for patients with encephalic and abdominal infection.9
Our patient was edentulous, with normal gums, and presented no mandibular bone lesions.
Rahman MA et al. identified through whole-genome shotgun sequencing on E. catenaformis genomic DNA extracted from saliva sample of healthy humans, two putative genes related to virulence and antibiotic resistance to tetracycline/aminoglycoside.10 Even in the absence of dental disease, oral pathogenic E. catenaformis strains may spread to deep tissues, especially if mucosal lesions are present.
A single case of DNM originating from dental E. catenaformis disease has been described in the past.
Conclusions
To the best of our knowledge, we report the first case of DNM, determined by retropharyngeal E. catenaformis infection that spread to the mediastinum, and not caused by dental diseases.
Footnotes
Conflicts of interest: All authors – none to declare.
Funding: None to declare.
Consent: Written informed consent was obtained from the patient for the publication of this case report and the accompanying images.
References
- 1.Sumi Y. Descending necrotizing mediastinitis: 5 years of published data in Japan. Acute Med Surg. 2014;2:1–12. doi: 10.1002/ams2.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ugalde PA, Pereira ST, Araujo C, Irion KL. Correlative anatomy for the mediastinum. Thorac Surg Clin. 2011;21:251–72, ix. doi: 10.1016/j.thorsurg.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 3.Endo S, Murayama F, Hasegawa T. Guideline of surgical management based on diffusion of descending necrotizing mediastinitis. Jpn J Thorac Cardiovasc Surg. 1999;47:14–9. doi: 10.1007/BF03217934. [DOI] [PubMed] [Google Scholar]
- 4.Estrera AS, Landay MJ, Grisham JM, Sinn DP, Platt MR. Descending necrotizing mediastinitis. Surg Gynecol Obstet. 1983;157:545–52. [PubMed] [Google Scholar]
- 5.Brook I, Frazier EH. Microbiology of mediastinitis. Arch Intern Med. 1996;156:333–6. doi: 10.1001/archinte.1996.00440030139017. [DOI] [PubMed] [Google Scholar]
- 6.European Committee on Antimicrobial Susceptibility Testing Breakpoint tables for interpretation of MICs and zone diameters. 2018. Version 8.0.
- 7.Salvetti E, Felis GE, Dellaglio F, Castioni A, Torriani S, Lawson PA. Reclassification of Lactobacillus catenaformis (Eggerth 1935) Moore and Holdeman 1970 and Lactobacillus vitulinus Sharpe et al. 1973 as Eggerthia catenaformis gen. nov., comb. nov. and Kandleria vitulina gen. nov., comb. nov., respectively. Int J Syst Evol Microbiol. 2011;61(Pt 10):2520–4. doi: 10.1099/ijs.0.029231-0. [DOI] [PubMed] [Google Scholar]
- 8.Kordjian HH, Schultz JD, Rosenvinge FS, Møller J, Pedersen RM. First clinical description of Eggerthia catenaformis bacteremia in a patient with dental abscess. Anaerobe. 2015;35(Pt B):38e40. doi: 10.1016/j.anaerobe.2015.07.002. [DOI] [PubMed] [Google Scholar]
- 9.Foronda C, Calatrava E, Casanovas I, Martín-Hita L, Navarro-Marí JM, Cobo F. Eggerthia catenaformis bacteremia in a patient with an odontogenic abscess. Anaerobe. 2019;57:115–6. doi: 10.1016/j.anaerobe.2019.04.007. [DOI] [PubMed] [Google Scholar]
- 10.Rahman MA, Mullany P, Roberts AP. Draft genome sequence of Eggerthia catenaformis strain MAR1 isolated from saliva of healthy humans. Genome Announc. 2017;5:e00638-17. doi: 10.1128/genomeA.00638-17. [DOI] [PMC free article] [PubMed] [Google Scholar]


